Abstract
A protein, designated as Sgl, showing a muramidase lytic activity to the cell wall of the Gram-positive bacterium Micrococcus lysodeikticus was isolated for the first time from plasma of Escherichia coli-immunized fifth instar Schistocerca gregaria. The isolated Sgl was detected as a single protein band, on both native- and SDS-PAGE, has a molecular weight of ∼15.7 kDa and an isoelectric point (pI) of ca 9.3 and its antiserum has specifically recognized its isolated form. Fifty-nine percentage of Sgl lytic activity was recovered in the isolated fractions and yielded ca 126-fold increase in specific activity than that of the crude. The partial N-terminal amino acid sequence of the Sgl has 55 and 40% maximum identity with Bombyx mori and Gallus gallus c-type lysozymes, respectively. The antibacterial activity against the Gram-positive and the Gram-negative bacteria were comparatively stronger than that of the hen egg white lysozyme (HEWL). The detected Sgl poration to the inner membrane that reach a maximum ability after 3 h was suggested to operate as a nonenzymatic mechanism for Gram-negative bacterial cell lysis, as tested in a permease-deficient E. coli, ML-35 strain. Sgl showed a maximal muramidase activity at pH 6.2, 30–50°C, and 0.05 M Ca2+ or Mg2+; and has a Km of 0.5 μg/ml and a Vmax of 0.518 with M. lysodeikticus as a substrate. The Sgl displayed a chitinase activity against chitin with a Km of 0.93 mg/ml and a Vmax of 1.63.
Keywords: lysozyme c, muramidase, kinetics, antibacterial activity, Schistocerca gregaria
Insects lack an acquired immunity and have an innate immune system. This system is highly developed and comprises cellular and humoral components (Gillespie et al. 2000). Lysozymes (E.C. 3.2.1.17), 1, 4-N-acetylmuramidases, are of the humoral immune system components with which insects respond to bacterial invasion through its lytic activity. It hydrolyzes the β-1,4-glycosidic linkage between the alternating linked residues of N-acetylmuramic acid (MurNAc) and N-acetylglucosamine (GlcNAc) of peptidoglycan (PGN), the major bacterial cell wall polymer, resulting in its depolymerization (Vocadlo et al. 2001). Several classes of lysozymes have been identified and their biochemical properties vary between species; the best known one is the c-type (chicken-type or GH22). Lysozyme generally exhibits greater bactericidal lytic activity against Gram-positive bacteria (Jollès and Jollès 1984). However, some other insect lysozymes were reported to have moderate activities against Gram-negative bacteria (Chapelle et al. 2009). Some insect lysozymes also exhibits antifungal activity by hydrolyzing the β-1,4-linkages of chitooligosaccharides in the fungal cell wall (Fiolka et al. 2005). On the other hand, some c-type lysozymes have been evolutionarily adapted to digestive functions and are found in the midgut of larval cyclorrhaphans that live in highly contaminated decomposing matter (Regel et al. 1998, Cançado et al. 2007). To our knowledge, few works have been performed on the lysozymes of hemimetabolous insects as opposed to well-studied holometabolous ones, e.g. the lepidoptrans Spodoptera littoralis (Jollès et al. 1979), Hyalophora cecropia (Hultmark et al. 1980, Engström et al. 1985), Manduca sexta (Mulnix et al. 1994), Bombyx mori (Powning and Davidson 1973, Abraham et al. 1995), Trichoplusia ni (Kang et al. 1996), Heliothis virescens (Lockey and Ourth 1996), Hyphantria cunea (Kang et al. 1996), Antheraea mylitta (Jain et al. 2001), Samia cynthia ricini (Fujimoto et al. 2001), S. litura (Kim and Yoe 2003), Artogeia rapae (Yoe et al. 1996), Ostrinia furnacalis (Wang et al. 2009), and Spodoptera frugiperda (Chapelle et al. 2009).
The desert locust, Schistocerca gregaria (Forskål), and possibly other hemimetabolous insects, differ in biological and developmental aspects from holometabolous insects, and therefore, different defense mechanisms against pathogens are expected. Recently, in order to understand the defense mechanisms of the hemimetabolous insect, S. gregaria against pathogens, we have shown that the activation and biosynthesis of hemolymph lysozyme is following the mechanism associated with the recognition of invading pathogen through pathogens elicitor molecules, and not the regulatory mechanism associated with epidermis wounding and disintegration or even the spiking effect (Mohamed et al. 2013). However, basic knowledge of biochemical and antimicrobial properties of this enzyme in the isolated form is still lacking. Understanding of the insect immune defenses may ultimately contribute to wider utilization and development of bio-pesticides for this insect when invading crops area.
The present work aimed to isolate, and biochemically characterize the protein that displayed the mentioned lysozyme activity, for the first time, from the plasma of S. gregaria. The assessment of its enzymatic and nonenzymatic microbicidal activities against various Gram-positive and Gram-negative bacteria, yeasts, and filamentous fungi was accomplished.
Materials and Methods
Insect Rearing, Immunization, and Sample Collection
The fifth instar S. gregaria were from a well-established laboratory colony at the Entomology Department, Faculty of Science, Cairo University. Immune-induced lysozymes were prepared as described previously (Mohamed et al. 2013). Briefly, immune-challenging was accomplished by spicking 5–7-d-old fifth instar in the intersegmental membrane between the meta-thorax and first abdominal segment with a thin needle previously dipped into a concentrated pellet (2 × 108 cells/ml) of Escherichia coli. Plasma was separated and collected 6 h postchallenge by centrifugation of hemolymph at 8,000 × g for 5 min at 4°C and stored at −20°C until used for protein isolation. In total 40 ml of plasma, corresponding to ∼392 mg of proteins, were collected from ∼320 individuals.
Protein Salting Out and Ion-Exchange and Size-Exclusion Chromatography
The plasma, 40 ml, was diluted (1: 1, v/v) with 0.05 M Na-acetate buffer (SAB), pH 6.5. The proteins in the mixture were fractionated with (NH4)2SO4 (Merck) at 60% saturation level (Scopes 1982). The mixture was stirred for 2 h, and left overnight at 4°C to produce the precipitate. The precipitate was separated by centrifugation at 10,000 rpm for 10 min at 4°C, dissolved in a minimal volume of 0.05 M Na-acetate, and subsequently dialyzed, in VISKING dialysis tubing (SERVA Electrophoresis GmbH, Heidelberg, Germany) (MWCO 12000-14000; pore diameter ∼25 Å). After dialysis, the solution inside the bag was concentrated by freeze-drying. On the obtained fraction-soluble proteins, ion-exchange chromatography was carried out (Zachary and Hoffmann 1984) with modifications.
The lyophilized pellet was dissolved in 25 ml of 0.5 M Na-acetate buffer, pH 6.5 and applied onto a column (20 × 2 cm) of CM-Sepharose Fast Flow ion-exchange resin (Sigma-Aldrich) equilibrated with the same buffer. The column was washed with two volumes of the starting buffer until complete removal of a substance with absorption at 280 nm (baseline achieved). The protein bound to the cation-exchanger was eluted with a gradient of 200 ml 0.5–3.0 M Na-acetate buffer (pH 6.5) at a flow rate of 1 ml/min. Eluted fractions (4.5 ml each) were collected and their lytic activity was measured by the radial diffusion assay against Micrococcus lysodeikticus. The fractions showing lytic activity were pooled, lyophilized, and dialyzed against double distilled water, pH 7.0, to remove Na-acetate (designated as Sgl), and stored at −20°C until further used. The total protein concentration of plasma or collected fractions was determined spectrophotometrically at 595 nm (Bradford 1976). Bovine serum albumin (BSA) fraction V (Sigma-Aldrich) was used as a protein standard.
Molecular weight of the isolated protein in native form was measured by molecular size-exclusion chromatography (Andrews 1965) on a column of Sephadex G-75 (super fine; Sigma-Aldrich) (2.6 × 60 cm; void volume 318 ml), previously equilibrated with 0.5 M Na-acetate buffer, pH 6.5. The sample was applied onto the column and eluted at a flow rate of 2.6 ml/min. Marker proteins (Biolabs) used were as follows: BSA, 68 kDa; ovalbumin, 45 kDa; α-chymotrypsinogen, 25 kDa; cytochrome c, 12.5 kDa.
Polyacrylamide Gel Electrophoresis
The purity and integrity of the isolated S. gregaria lysozyme were assessed by both native (10%) and denaturing sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (Laemmli 1970). The molecular weight of the protein was estimated with Gel-pro Analyzer software (version 4.5; Media Cybernetics, Inc., USA) against the protein molecular weight marker (Amresco). Lytic activity was detected directly in the gel by incubating 0.5 cm slices overnight at 4°C, of the gel area containing protein of interest, in 50 mM Na-acetate (pH 6.2) (assay buffer). Afterwards, incubated gel pieces were centrifuged at 12,000 × g for 5 min at 4°C and the lytic activity against M. lysodeikticus in lysoplates was measured.
Isoelectric Focusing
Isoelectric focusing (IEF) (Robertson et al. 1987) of the Sgl was performed for 1 h at 200 V, then 1.5 h at 400 V at 4°C with the electrode solutions 1 M phosphoric acid at the anode and 1 M sodium hydroxide at the cathode. The isoelectric point (pI) of the isolated Sgl was determined by running proteins (5 µg each) of known pI (IEF Mix 3.6-9.3, Sigma) in an IEF gel. The pI of the isolated Sgl was determined from a graph of relative mobility (Rm) versus pI values of standard proteins. For this purpose, Gel-pro Analyzer software (version 4.5; Media Cybernetics, Inc.) was used.
N-Terminal Amino Acid Sequencing
The isolated Sgl was run on 12% SDS-PAGE and transferred to grade polyvinyidene fluoride, PVDF, membrane (Bio-Rad, Tokyo, Japan). The transferred band was detected by staining with Coomassie blue R250 for a few seconds and washed with water. The stained protein band was excised and the N-terminal amino acid sequence of protein was determined by automated Edman degradation using an ABI Procise model 491 protein sequencer connected to an online ABI analyzer (Applied Biosystems, CA, USA).
Preparation of Polyclonal Anti-Sgl Antibodies
Polyclonal antibodies against the isolated Sgl were raised in rabbits. An 0.5 ml aliquot containing 300 µg of the Sgl in phosphate buffered saline (PBS: 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4) was added to 0.5 ml Freund’s complete adjuvant (Sigma-Aldrich, MO, USA), mixed thoroughly and injected into the hypodermis of New Zealand White albino rabbits weighing 2.0–2.5 kg. Three booster injections were given at 2-wk intervals, using Freund’s incomplete adjuvant. Blood samples were collected from the ear vein of rabbits 1 wk after the final injection. The blood was incubated for 1 h at room temperature, overnight at 4°C, and centrifuged at 10,000 × g for 30 min to remove coagulated blood cells. The supernatant was collected and stored in aliquots at −20°C until used.
Double Immunodiffusion Assay
The specificity of the antiserum against the isolated Sgl was determined by using the double radial immunodiffusion technique as described by Ouchterlony (1949). Briefly, antiserum and the isolated Sgl were loaded into individual wells made of a 1% agarose gel, 2 mm thick, in PBS containing 0.1 % sodium azide as preservative. Purified aliquots of Sgl were loaded in the peripheral wells and the central well contained the polyclonal antibody against Sgl (anti-Sgl serum). The gel was kept in a moist chamber until opaque precipitin lines between the antigen and the antiserum wells were observed. The gel was then soaked in a 0.1 M NaCl solution for 12 h to fix the immunoprecipitate; the diffused proteins were stained with COBB R-250, then photographed.
Microbicidal Activity Assay (Sensitivity Tests) of Sgl
The microbicidal activity of the isolated Sgl was determined by the radial diffusion assay against M. lysodeikticus on agarose plates inoculated with this bacterial species as described before (Mohamed et al. 2013). The activity was recorded by measuring the diameter (mm) of the clearance zone after incubating the plates at 37°C for 24–48 h. Activity was obtained from a standard curve made with HEWL (Sigma-Aldrich, 40,000 units/mg) and expressed in μg/ml HEWL. In this study, one unit of lysozyme activity corresponds to ∼4 µg of HEWL. Microbicidal activity was determined by a modified Kirby–Bauer disc diffusion method (Bauer et al. 1966). The standard methods M7-A3, M38-A, and M44-P were used for evaluating the susceptibilities of bacteria, filamentous fungi, and yeasts, respectively, to the isolated Sgl (Vilcinskas and Matha 1997; Wayne, 2002, 2003). Plates were incubated at 37°C for 24 h for bacteria, at 25°C for 72 h for filamentous fungi, and at 33°C for 48 h for yeasts. Inhibition zones were expressed as diameter measured in millimeters (after subtracting the disk diameter). Tetracycline and amphotericin B, broad spectrum antibacterial and antifungal agents, respectively, were used as positive controls and PBS alone as a negative control.
Minimal inhibitory concentration (MIC) was determined by the agar dilution method (Wayne 1997). Briefly, stationary-phase cultures of Staphylococcus aureus and Pseudomonas aeruginosa, were prepared at 37°C and used to inoculate fresh 5.0 ml cultures to an OD600 of 0.05. Cultures (5.0 ml) were then incubated at 37°C until an optical density (OD) OD600 of 0.10 was achieved from which standardized bacterial suspensions were prepared to a final cell density of 6 × 106 CFU/ml. Serial dilutions from the Sgl aliquots 0–32 μl/ml, were prepared, mixed with 5.0 ml of the standardized bacteria suspension, then added to the plates and incubated for 24 h at 37°C. The colony forming units (CFUs) were counted for each dilution.
The following test microorganisms were employed for investigating their sensitivities toward Sgl; the Gram-positive Bacillus subtilis (ATCC 6051), B. thuringiensis var. kurstaki (EG 2348) (purchased from Intrachem Bio Italia S.p.A, Italy), Enterococcus faecalis (ATCC 19433), Micrococcus luteus (ATCC 49732) (purchased from Biomerieux, Canada), and St. aureus (ATCC 12600); the Gram-negative E. coli (ATCC 11775), Neisseria gonorrhoeae (ATCC 19424), P. aeruginosa (ATCC 10145), and Serratia marcescens (EMCC 1247) [obtained from the Microbiological Resource Center (MIRCEN), Faculty of Agriculture, Ain Shams University, Cairo, Egypt]; the yeasts Candida albicans (ATCC 26555), Candida tropicalis (ATCC 750), and Saccharomyces cerevisiae (ATCC 2180-1 A); and the filamentous fungi Aspergillus niger (IMI 31276) and Aspergillus flavus (IMI 111023).
Effects of pH, Temperature, and Divalent Cations on the Sgl Muramidase Activity
Effects of changes in pH and temperature on the activity of the isolated Sgl were measured by a turbidimetric standard assay (Schneider 1985) with slight modifications. The effect of pH of reaction media on the lytic activity of the Sgl (in triplicates) was examined in the following buffers at pH ranging from 4.0 to 10.0 in 0.5 pH units: 0.2 M sodium acetate buffer (pH 4.0 to 6.5), 0.2 M sodium phosphate buffer (pH 7 to 8.5), and 0.1 M Tris-HCl buffer (pH 9.0 to 10.0). A suspension of 0.1% (w/v) freeze-dried M. lysodeikticus (Sigma) in the corresponding buffer at each pH-level was used for the assay; 100 μl aliquots of the Sgl to be tested were added to 500 μl of bacterial suspension in corresponding buffer the reaction was stopped by placing the suspension into ice bath. Cleavage of the substrate was monitored spectrophotometrically at 450 nm after 30 min incubation at 37°C. A unit of lysozyme activity was defined as the quantity of enzyme that decreases the absorbance (A450) by 0.001/min under the specified conditions. The specific activity of the lysozyme in the samples is defined as units of activity divided by protein concentration (mg). Finally, the muramidase activity was calculated in relative units as percentage of the maximal activity.
For determination of the effect of temperature on lytic activity of the Sgl, 16 aliquots of the isolated lysozyme were mixed with 0.1% suspension (w/v) of freeze-dried M. lysodeikticus in 0.2 M Na-acetate buffer at pH 6.2. After incubation in a water bath for 10 min at different temperatures, ranging from 25 to 100°C, lytic activity of the samples was measured by the turbidimetric assay.
Effects of Ca2+ and Mg2+ on Sgl activity were determined by adding CaCl2 or MgCl2 at different concentrations to prepare a 0.1% suspension (w/v) of freeze-dried M. lysodeikticus in 0.2 M Na-acetate buffer at pH 6.2. CaCl2 or MgCl2 were added to the series of concentrations of 0.01–0.09 M. Control was prepared by omitting CaCl2 or MgCl2. The lytic activity, measured in triplicates, was calculated as the relative activities expressed in percentage in comparison with the 100% activity. Muramidase lytic activity of Sgl on M. lysodeikticus in 0.2 M Na-acetate buffer at pH 6.2 was taken to represent 100%.
Kinetic Analysis
The maximal velocity (Vmax) and the Michaelis constant (Km) were estimated for the Sgl with lyophilized M. lysodeikticus as substrate. The substrate was re-suspended with different concentrations in 0.2 M Na-acetate buffer at pH 6.2 (the pH at which Sgl lytic activities have typically been measured). Assays were carried out in triplicate and lytic activity was measured as the decrease in turbidity at OD450 nm during 30 min incubation at 37°C without shaking. Kinetic constants were estimated from linear regression of reciprocal rates (Lineweaver-Burk analysis) data with the program Enzfitter (Version 2.0; Biosoft, Ferguson, MO, USA).
Assay of Sgl Chitinase Activity
Chitinase activity was measured (Ohtakara 1988) by determining the rate of degradation of colloidal chitin into GlcNAc. Colloidal chitin was prepared by washing chitin powder (Sigma) with 100 mM Na-acetate buffer, pH 5.5 (2 mg powder/ml buffer) to remove colored materials that would interfere with the assay. Briefly, 250 μl samples of Sgl and 500 μl of the substrate (0.5%) were added to 500 μl of 0.2 M sodium acetate buffer (pH 6). The mixture was incubated in a shaking water-bath at 37°C for 1 h. The reaction was stopped by the addition of 100 µl of 0.8 M boric acid, followed by boiling for 3 min at 100°C. After cooling and centrifugation at 10,000 g for 30 min, 300 µl of the clear supernatant were mixed with 500 μl of p-dimethyl-amino-benzaldehyde reagent and incubated for 20 min at 37°C. The absorbance of the mixture was measured at 585 nm. The change in absorbance is correlated with the GlcNAc concentrations.
Assay of Inner Membrane Permeabilization by Sgl
Determination of the inner membrane permeabilization was performed by measuring the β-galactosidase activity of E. coli ML-35, a lactose permease-deficient strain with constitutive cytoplasmic β-galactosidase activity, using o-nitrophenyl-β-D galactopyranoside (ONPG) as substrate at 37°C (Skerlavaj et al. 1990). The β-galactosidase activity of the samples was assayed by tracing the production of o-nitrophenol from ONPG over time (1–4 h post incubation) that was monitored spectrophotometrically at 405 nm. An equivalent volume of sterile distilled water replaced the Sgl solution in the control assay, with HEWL (150 μg/ml) used as control.
Prophenoloxidase (proPO) Activation and Phenoloxidase (PO) Activity
Phenoloxidase (PO) activity in plasma was assayed principally according to (Dularay and Lackie 1992), in 10 mM Na-acetate buffer (SAB-Ca2+, pH 6.0). Calcium, supplied as CaCl2.2H2O, is crucial for activating the prophenoloxidase (proPO) system of S. gregaria. The β-1,3-glucans laminarin (Sigma) was used to in vitro activation of PO, and 0.1 mg/ml was added to plasma. Subsequently, L-dopamine [4-(2-aminoethyl) benzene-1,2-diol] (Sigma-Aldrich) was added as a substrate (2 mg/ml) in SAB. The dopachrome formation was determined spectrophometrically at OD490 nm, 30 min later. One enzyme activity unit was defined as the amount of enzyme which causes a change of 0.001 OD490 per minute. Specific activity was calculated in terms of units/mg total plasma protein determined by Bradford Assay.
To study the effect of Sgl on PO activity and proPO activation in plasma, method in (Rao et al. 2010) was employed with slight modifications. Briefly, 100 µl aliquots of native plasma were preactivated with 10 µg laminarin, each in 500 µl SAB-Ca2+ buffer in sterile eppendorf tubes at 25°C, and then incubated with 50 µg each of BSA, Sgl, HEWL for 30 min. The same conditions were repeated for a second group, in which native plasma aliquots were incubated without laminarin, with the proteins. Subsequently, 200 µl L-dopamine were added, and absorbance was monitored at 470 nm. Data from four replicas of each group were analyzed, and the significance of difference was determined.
Statistical Analysis and Multiple Sequence Alignments
Statistical analysis was performed using SPSS software (version 15; SPSS, Chicago, IL, USA). Data are expressed as means ± SE. N-terminal sequence of S. gregaria lysozyme, Sgl, was compared with GeneBank database using the BLAST software from NCBI site: http://www.ncbi.nlm.nih.gov/. Multiple sequence alignments were done using the ClustalW2 program: www.ebi.ac.uk/Tools/msa/clustalw2/.
Results and Discussion
Lysozyme Isolation, Molecular Mass, and pI
A protein with high lytic activity against M. lysodeikticus was isolated from the plasma of E. coli immunized fifth instar S. gregaria, by a combination of (NH4)2SO4 fractionation at a 60% saturation and ion-exchange chromatography on CM-Sepharose column (Fig. 1). Fifty-nine percentage of the lytic protein activity from the crude plasma content was recovered in the isolated fractions and yielded ca 126-fold increase in specific activity, than that of the crude plasma, as revealed by the radial diffusion assay (Table 1).
Fig. 1.
Purification of lysozyme from plasma of fifth instar Schistocerca gregaria with cation-exchange chromatography on CM-Sepharose Fast Flow ion-exchange column. Proteins obtained with 60% (NH4)2SO4 saturation of hemolymph fifth instars S. gregaria immunized with pricking by a thin needle previously dipped into a concentrated pellet (2 × 108 cells/ml) of Escherichia coli were applied to column (20 × 2 cm) equilibrated with 0.5 M Na-acetate (pH 6.5) buffer. Elution was carried out with a gradient of 0.5–3.0 M of the same buffer at a flow rate of 1 ml/min (——, A280;…….., Na-acetate concentration; ○- - - -○, anti-Micrococcus lysodeikticus activity). Five microliter of the fraction samples were used for the inhibition zone assay. Active fractions (anti-M. lysodeikticus fractions), tubes 31–39, were pooled, desalted, lyophilized, and stored at −20°C for further use.
Table 1.
Purification of Schistocerca gregaria lysozyme (Sgl)
| Step | Volume (ml) | Total activity (U) | Total protein (mg) | Specific activity (U/mg protein) | Yield (%) | Purification factor |
|---|---|---|---|---|---|---|
| Crude plasma | 40 | 384,000 | 500 | 768 | 100 | – |
| (NH4)2SO4 fractionation | 25 | 225,000 | 197 | 1142 | 59 | 1.5 |
| CM-Sepharose ion-exchange chromatography | 40.5 | 165,240 | 1.7 | 97200 | 74 | 85 |
The electrophoretic profile, under denaturating conditions, of whole plasma proteins and 60%-saturated-(NH4)2SO4 fraction protein is shown in Fig. 2A-1. Fractions with lytic activity were eluted from the cation-exchange column; and only one distinct peak (fractions 31–39) was indicated to exhibit strong lytic activity against M. lysodeikticus which was assayed separately for each fraction (Fig. 2A-2). The homogeneity of the isolated protein was monitored on SDS-PAGE (Fig. 2A-2). Each fraction exhibited only a single band with the same molecular weight; indicating that, they are homogenous fractions. The molecular weight of each band is consistent with the corresponding band from the crude whole plasma (Fig. 2A-1). Accordingly, the fractions exhibiting lytic activity from the cation-exchange column were collected into a single pool, and designated as isolated Sgl.
Fig. 2.
Electrophoresis. (A) SDS/PAGE (12%), under reducing condition, of fractions obtained during purification of the Sgl. Samples were taken of each step during the purification and electrophoresed. The following samples were separated: (A-1) Lane 1, AMRESCO mid molecular weight standards; lane 2, crude hemolymph; lane 3, the salted out proteins from 60% (NH4)2SO4 saturation. (A-2) Lane 1, mid molecular weight standards; Lanes 2–10, pooled active fractions from CM-Sepharose Fast Flow column. (B) SDS-PAGE, 12%, under reducing conditions. Lane 1, marker proteins; lane 2, the isolated Sgl. The molecular mass of Sgl under reducing conditions was estimated to be 15.7 kDa. (C) Native PAGE, 10% acrylamide, of the isolated Sgl. (D) Native IEF/PAGE patterns (pH range 3-10), 10%, of the isolated Sgl. The pH gradient was obtained with a mixture containing 4% (v/v) ampholytes 3-10 (Sigma Ampholyte high resolution). Lane 1, IEF Mix 3.6-9.3, Sigma; lane 2, the isolated Sgl. Proteins in the gels were stained with COBB R-250.
For further confirmation, the isolated Sgl was run on 12% SDS-PAGE (Fig. 2B) and appeared as a single protein band of ∼15.7 kDa, suggesting that Sgl is represented by single protein subunit. In addition, when Sgl was separated on 10% native PAGE several times under the same conditions (Fig. 2C), it displayed as a single protein band of an Rm of 0.63. This reveals that the Sgl was isolated to homogeneity, established by its single identity.
The results of IEF showed that Sgl has a pI of 9.3, and migrated to near the end of the basic region of the gel (Fig. 2D). This basic pI value of Sgl (>9) is comparable to pI of lysozymes of H. cecropia (Hultmark et al. 1980), He. virescens (Lockey and Ourth 1996), and Helicoverpa zea (Liu et al. 2004). These values are also comparable to those values of lysozymes reported from chicken egg white and typical basic lysozymes (Kylsten et al. 1992, Kang et al. 1996, Knubovets et al. 1999). This high pI values is typical for most lysozymes which were reported to usually be basic proteins (Jollès and Jollès 1984).
The molecular weight of the isolated Sgl was further estimated by gel filtration on Sephadex G-75 column (Fig. 3) and was calculated to be 15.7 kDa, which is similar to its detected size on SDS-PAGE. The molecular weight of the Sgl is comparable with that of other orthopterans; i.e. 16.5 kDa in Locusta migratoria (Zachary and Hoffmann 1984) and 15.4, 15 kDa for lysozyme I and II, respectively, in Gryllus bimaculatus (Schneider 1985). It is also comparable with that of certain lepidopterans, i.e. 16.5 kDa in B. mori (Powning and Davidson 1973), 15.3 kDa in both Galleria mellonella and S. littorals (Jollès et al. 1979), and 16 kDa in He. virescens (Lockey and Ourth 1996). This range is also comparable to that, 14.4 kDa, of the HEWL (Jollès and Jollès 1984).
Fig. 3.

Estimation of Sgl molecular weight by gel filtration on Sephadex G-75 column. A column (2.6 × 60 cm, void volume 318 ml) was equilibrated with 0.5 M Na-acetate buffer, pH 6.5; then calibrated with a set of marker proteins at 25°C and a regression correlation between elution volume and the logarithm of the molecular weight of each marker protein was made. The Sgl and marker proteins were eluted at a flow rate of 2.6 ml/min. Marker proteins, inset of figure, (Biolabs) were used. Mr, relative molecular weight.
Though lysozymes have been reported in a quite number of insect species, only a few attempts at isolation have been made. For orthopterans other than S. gregaria, isolation and characterization of lysozymes from hemolymph have been previously reported from L. migratoria (Zachary and Hoffmann 1984) and Gr. bimaculatus (Schneider 1985). In other insects, particularly Lepidoptera, lysozymes were isolated and studied, e.g. G. mellonella (Powning and Davidson 1973, Yu et al. 2002), B. mori (Abraham et al. 1995), He. virescens (Lockey and Ourth 1996), Ar. rapae (Yoe et al. 1996), Hy. cunea (Park et al. 1997), S. cynthia ricini (Fujimoto et al. 2001), Agrius convolvuli (Yu et al. 2002), S. litura (Kim and Yoe 2003), O. furnacalis (Wang et al. 2009), and Sp. frugiperda (Chapelle et al. 2009).
Immunological Identity of the Sgl
The specificity of the polyclonal antiserum raised against the Sgl was determined by double radial immunodiffusion. The latter was performed with two aliquots of the Sgl that were loaded in peripheral wells and the polyclonal antibody against the Sgl (anti-Sgl serum) were loaded in a central well as shown in Fig. 4. The results show that antiserum produced a single precipitin arc with the isolated Sgl (i.e. genuine pattern of complete antigenic identity). This is a confirmation for the specificity of the antiserum directed against the Sgl; i.e. the antiserum has specifically recognized the purified Sgl.
Fig. 4.

Ouchterlony double immunodiffusion showing cross-reactivity of Sgl antiserum with the purified Sgl. Center well (anti-Sgl) containing Sgl antiserum. Upper peripheral wells, Sgl, containing purified Sgl. Sgl and anti-Sgl were stained with COBB.
N-Terminal Amino Acid Sequence
The N-terminal amino acid sequencing of the isolated S. gregaria plasma lysozyme, Sgl, by automated Edman degradation identified 47 amino acid residues (i.e. KLQRCEIVSALKRHGITSDLRNWVCL V E S E S G G R T D K R G P R N K N G S Y). The obtained sequence (Fig. 5) showed homology with N-terminal sequence of the c-type lysozymes of L. migratoria (GenBank accession number: AHE76131), G. mellonella (P82174), Ma. sexta (AAB31190), Triatoma brasiliensis (AAU04569), Gallus gallus (HEWL) (AAA48943; Palmiter et al. 1977) with 91, 61, 57, 51, 43% identity, respectively. However, no similarity was found between the N-terminal amino acid sequence of the isolated Sgl and the N-terminal amino acid sequences of the other purified i-type: Nilaparvata lugens (AGK40910) (Bao et al. 2013) and g-type: Azumapecten farreri (AGA95494) (Li et al. 2013) lysozymes. These obtained results suggest that S. gregaria isolated antibacterial protein might be of a c-type lysozyme.
Fig. 5.

N-terminal amino acid sequence of the isolated S. gregaria lysozyme (Sgl) aligned with some c-, i-, and g- type lysozymes. Accession numbers are as follows: c-type: Locusta migratoria (AHE76131); Galleria mellonella (P82174); Manduca sexta (AAB31190); Triatoma brasiliensis (AAU04569), G. gallus (HEWL) (AAA48943); g-type: Azumapecten farreri (AGA95494); i-type: Nilaparvata lugens (AGK40910). Similar amino acids are marked with a dot (.).
Microbicidal Activity of the Sgl
The results show that isolated Sgl has antibacterial activities against both Gram-positive and Gram-negative bacteria (Table 2). The activity against the tested bacteria is comparatively higher than that of HEWL; it may reach 1.5 and 4 times in the two groups, respectively.
Table 2.
Antimicrobial activities of purified Sgl against different bacteria, yeasts, and filamentous fungi
| Microorganism | Inhibition zone diameter (mm/sample)* |
|||
|---|---|---|---|---|
| Standard |
Sample |
|||
| †Tetracycline | ‡Amphotericin B | Sgl | HEWL | |
| Gram-positive bacteria | ||||
| Bacillus subtilis (ATCC 6051) | *23 | – | 11 | 9 |
| Bacillus thuringiensis var. kurstaki (EG 2348) | 21 | – | 3 | 2 |
| Enterococcus faecalis (ATCC 19433) | 22 | – | 12 | 9 |
| Micrococcus luteus (ATCC 49732) | 24 | – | 13 | 10 |
| Staphylococcus aureus (ATCC 12600) | 24 | – | 18 | 14 |
| Gram-negative bacteria | ||||
| Escherichia coli (ATCC 11775) | 24 | – | 12 | 5 |
| Neisseria gonorrhoeae (ATCC 19424) | 23 | – | 8 | 2 |
| Pseudomonas aeruginosa (ATCC 10145) | 24 | – | 15 | 6 |
| Serratia marcescens (EMCC 1247) | 22 | – | 2 | –ve |
| Yeasts | ||||
| Candida albicans (ATCC 26555) | – | 13 | –ve | –ve |
| Candida tropicalis (ATCC 750) | – | 14 | 4 | –ve |
| Saccharomyces cerevisiae (ATCC 2180-1 A) | – | 11 | –ve | –ve |
| Filamentous fungi | ||||
| Aspergillus niger (IMI 31276) | – | 9 | –ve | –ve |
| Aspergillus flavus (IMI 111023) | – | 10 | –ve | –ve |
The antimicrobial spectra of purified Sgl were tested by radial diffusion assay (disc diffusion method). Positive control: †tetracyclin (antibacterial agent) and ‡amphotericin b (antifungal agent), negative control: sterile double distilled water. Each data record represents the mean of triplicates (n = 3). *Full diameter of clear inhibition zones expressed in millimeters, after subtracting the disk diameter.
It was observed that a limited growth inhibition of the Sgl was detected against the Gram-positive bacterium B. thuringiensis or against the Gram-negative bacterium Se. marcescens even at the maximum concentration tested (10 μl of 250 µg/ml; Table 2). Therefore, these bacteria are highly resistant to the Sgl action. Furthermore, the MIC of the Sgl against the most sensitive Gram-positive and Gram-negative tested bacteria St. aureus and P. aeruginosa has been assessed through radial diffusion assay and was observed to correspond to 1 and 2 μg/ml, respectively. The resistance of both Gram-positive and Gram-negative bacteria to microbicidal peptides and proteins, including lysozymes, was reported to involve secretion of impeding factors to these substances; these factors are mostly peptidases that hydrolyze these proteinaceous substances (Fedhila et al. 2002). This was reported in the Gram-positive bacteria B. thuringiensis (Hultmark et al. 1982, Dalhammar and Steiner 1984, Boman and Hultmark 1987, Boman 1987) and the Gram-negative bacteria Se. marcescens (Tao et al. 2006, Mohan et al. 2011).
So far, for the antibacterial activity against Gram-negative bacteria, among the known isolated lysozymes, five lysozymes have been reported to display this activity. These are the lysozymes isolated from the lepidopterans B. mori, G. mellonella, Ag. convolvuli, Sp. frugiperda, and O. furnacalis (Abraham et al. 1995, Vilcinskas and Matha 1997, Yu et al. 2002, Chapelle et al. 2009). As far as we know, the Sgl that isolated from the fifth instar S. gregaria, as a hemimetabolous insect, is the first immune-lysozyme displaying such property from an insect outside Lepidoptera.
Interestingly, the isolated Sgl displays antifungal activity, demonstrated through lytic activity against the yeast C. tropicalis, but it has no such activity against the other tested yeast C. albicans and the filamentous fungus A. niger (Table 2). This observed antifungal activity for a lysozyme was also reported by other authors (Gillespie et al. 2000).
Enzymatic and Nonenzymatic Activities of the Sgl
The degradation of PGN of the bacterial cell wall by the muramidase activity of lysozyme leads to rapid killing of Gram-positive bacteria (Navarre and Schneewind 1999, Nash et al. 2006). Consequently, lysozymes are generally believed to be more active against Gram-positive bacteria because their cell walls are largely made of PGN (∼90%) that forms an exposed external layer of the cell wall (Navarre and Schneewind 1999). However, this mechanism cannot account for the bactericidal activity of Sgl against Gram-negative bacteria because the PGN layer of the cell wall is covered by an outer membrane with subjected surface molecules of lipopolysaccharide (LPS) (Beveridge 1999). This membrane constitutes a permeability barrier. Therefore, other mechanisms of action were proposed, with growing evidences that the activity of lysozymes against Gram-negative bacteria include both enzymatic and nonenzymatic activity (Nash et al. 2006). These activities were tested for the Sgl; the enzymatic activity was measured as a muramidase activity, and the nonenzymatic one was tested as membrane permeabilization.
Muramidase Activity and the Effect of pH and Temperature, and the Divalent Cations
The muramidase activity of Sgl catalyzing hydrolysis of the β-1,4-linkage between MurNAc and GlcNAc of the PGN was assayed by measuring the decrease in turbidity (OD450 nm) of suspensions of dried M. lysodeikticus cells. It was found to be maximal at the slightly acidic conditions, at pH 6.2 (Fig. 6A). This slightly acidic optimum pH is comparable to lysozymes from G. mellonella and B. mori (Powning and Davidson 1973), H. cecropia (Hultmark et al. 1980), L. migratoria (Zachary and Hoffmann 1984), Gr. bimaculatus (Schneider 1985), Hy. cunea (Park et al. 1997), S. cynthia ricini (Fujimoto et al. 2001), which all have pH optima of mildly to slightly acidic values (5.0 and 6.4). The Sgl activity was also observed to increase with elevating temperature, reaching a maximum and fairly constant level at the temperature range 30–50°C, then after starts to decline afterward (Fig. 6B). The decline reaches ∼75% of the original value at 65°C, and up to 90% at 80–100°C. The stability of Sgl at high temperatures is in agreement with those of other insect lysozymes. This relative thermal stability at elevated temperature is variable between insects, but most of them show relative activities of 20–50% at 100°C (Yoe et al. 1996) and this is also a characteristic of all c-type lysozymes (Jollès and Jollès 1984). In this context, the effect of temperature on lysozyme activity has been found to be correlated to the pH and concentration of the protein (Lemos et al. 1993). The similarity of the general shape of the presented activity curve compared with those of the lysozymes from G. mellonella and B. mori (Powning and Davidson 1973, Demura 2006), H. cecropia (Hultmark et al. 1980), and Hy. cunea (Park et al. 1997) show a close resemblance of the tested physicochemical properties of the Sgl to those of lysozymes of these insects.
Fig. 6.

(A) Effect of pH on the lytic activity of the Sgl. The buffers used were 0.2 M sodium acetate (pH 4.0 to 6.5), 0.2 M sodium phosphate (pH 7 to 8.5), and 0.1 M Tris-HCl (pH 9.0 to 10.0). The muramidase activity of lysozyme samples was tested using a turbidimetric standard assay against M. lysodeikticus. Lysozyme activity was shown as percentage of the highest activity. (B) Heat stability of the isolated Sgl. Lysozyme aliquots were adjusted to pH 6.2, placed at the indicated temperatures for 10 min, and tested for lytic activity using a turbidimetric standard assay against M. lysodeikticus. Aliquots were removed at the different temperatures and assayed for lysozyme activity which is shown as percentage of the highest activity. Data represent the mean of three replicates. (C) Effects of the divalent cations Ca2+ and Mg2+ on the lytic, muramidase, activity of the Sgl at ionic strength 0.1. The ionic strength was regulated with NaCl. Effects were observed by measuring the activity of lysozyme in 0.1 M sodium acetate buffer at pH 6.2 supplemented with CaCl2 or MgCl2. The 100% activity represents a specific lysozyme activity of U/mg protein in 0.1 M sodium acetate buffer at pH 6.2. Lysozyme activity was shown as percentage of the lowest activity.
The presented results show also that the muramidase activity of the Sgl increases steadily as the divalent cation concentration was increased from 0 to 0.05 M, reaching a maximum, whether Ca2+ or Mg2+ was added (Fig. 6C). It was also observed that this activity was increased by 150–200% in Ca2+-containing buffers relative to its activity in control-reference (without divalent cation addition); whereas in MgCl2-containing buffers, it was relatively increased by 130–180%. However, the activity has been decreased from this maximum value with the further increment of concentrations of the divalent cations above 0.05 M. In this respect, it was reported (Jensen and Kleppe 1972, Demura 2006) that Ca2+ is effective on the conformational stability and folding of the calcium-binding lysozymes. The Km and Vmax of the Sgl (as determined by Michaelis-Menten plot of 1/V against 1/[S], using the freeze-dried M. lysodeikticus as a substrate) in comparison to that of the HEWL is shown in Fig. 7A.
Fig. 7.

(A) Determination of Michaelis-Menten constant (Km) of the muramidase activity of the Sgl and HEWL (as a standard reference) by Lineweaver-Burk plot. Enzymatic assay (turbidemetric standard assay) was carried out in 0.2 M sodium acetate buffer, pH 6.2 at 37°C. M. lysodeikticus was used as substrate. S is expressed in mg/ml. Each point is the mean of three replicates. Km was calculated from the reciprocal of the x intercept and measured as OD/mg protein/30 min at 450 nm. (B) Determination of Michaelis-Menten constant (Km) of chitinase activity (at pH 6.0) of the isolated Sgl and HEWL. Km was determined by Lineweaver-Burk plot, (S is expressed in mg/ml), using colloidal chitin as a substrate and measured as OD/mg protein/h at 585 nm.
Chitinase Activity
The Sgl and HEWL have a chitinase activity; the Km value using colloidal chitin substrate is lower than of the HEWL (Fig. 7B), i.e. it has more affinity for this substrate. Some lysozymes have a considerable chitinase activity; for instance, HEWL hydrolyze chitin with ∼50% of its efficiency to hydrolyze PGN (Monzingo et al. 1996). The displayed chitinase activity of Sgl on pure chitin, however, does not explain its antifungal activity against chitin-containing cell wall of the yeast C. tropicalis (Table 2). This is because it has no such activity against both the other tested yeast C. albicans and the filamentous fungus A. niger (Table 2). The muramidase and chitinase activities of lysozyme are glycosidic hydrolases. The first catalyze hydrolysis of the β-1,4-glycosidic bonds of alternating copolymers of MurNAc and GlcNAc of PGN, and the second hydrolyzes the β-1,4 link of the homopolymer of GlcNAc of chitin.
Membrane Permeabilizability (Nonenzymatic Bactericidal Activity) of Sgl
Antibacterial nonenzymatic activity of the Sgl against both Gram-negative and Gram-positive bacteria was determined in the present work. This was supposed to be through disruption of functions of the inner membrane by their permeabilization and pore formation in, and disintegration of, this membrane that could result in lethal events. Monitoring of the permeabilization of the inner membrane due to pore formation by the Sgl was accomplished by observing release of the intracellular β-galactosidase molecules from a lactose-permease deficient E. coli ML-35 p into an incubation medium containing the Sgl and o-nitrophenyl-β-D-galactopyranoside, the specific substrate β-galactosidase. The obtained results (Fig. 8) show that the activity of the β-galactosidase which released from these bacteria into the surrounding medium increases with the time, reaching a maximum after 3 h, then became constant. This implies that Sgl has crossed the outer membrane of the Gram-negative bacteria, then hydrolyzed the PGN layer, and reached the vicinity of the inner membrane where it causes its poration releasing the internal β-galactosidase to outside.
Fig. 8.

Kinetics of permeabilization of the inner membrane (in parallel to antibacterial activity) by the Sgl against E. coli ML-35 p. The release of β-galactosidase from the lactose permease-deficient E. coli ML-35 p under the effect of the Sgl in an incubation medium and monitored by tracing the production of o-nitrophenol over time (1, 2, 3, and 4 h post incubation) from the specific substrate o-nitrophenyl-β-D-galactopyranoside. The absorbance (which is proportional to the β-galactosidase activity) was plotted against the incubation time. Values shown represent the mean of three experiments. In the control, an equivalent volume of sterile ddw replaced the Sgl solution. HEWL was used as a standard reference.
Role of Sgl in Regulation of proPO Activation
In a trial to elucidate any possible interaction between the Sgl and the proPO and/or PO of the fifth instar S. gregaria, simple in vitro experiments concerning the Sgl in comparison to the HEWL and other proteins were conducted. The obtained results show that the Sgl and HEWL greatly inhibit (up to ∼ 60 %) of the proPO activation in plasma of the native (unimmunized) fifth instar S. gregaria; but do not inhibit PO activity (preactivated by laminarin) in native plasma (Fig. 9). This may indicate that Sgl plays a probable regulatory role in the proPO cascade activation. In this respect, Ma. sexta lysozyme was observed to inhibit proPO activation by preventing its conversion to PO, possibly via direct protein interaction between these proteins, and probably also by degradation of certain regulatory proteins (Rao et al. 2010).
Fig. 9.
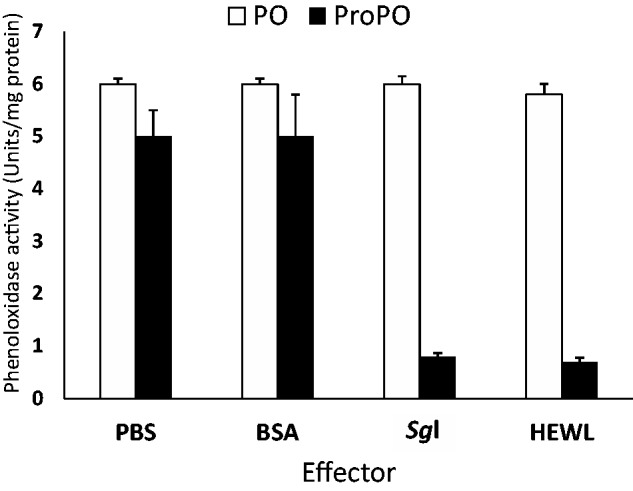
The effect of Sgl on activity of prophenoloxidase and phenoloxidase (PO) in plasma. Aliquots of preactivated plasma, using laminarin, were incubated with the Sgl purified from plasma, HWEL, BSA, or PBS. L-dopamine substrate was added to each sample and PO activity was monitored after certain intervals of time at 470 nm for 30 min. The bars represent the SE of mean of four replicates.
In insects, activation of the proPO cascade (as well as induction of expression of antimicrobial peptides, including lysozymes) is an important component of the humoral immune defense responses (Lemaitre and Hoffmann 2007, Jiang 2008, Kanost and Gorman 2008). This activation can be positively and negatively regulated and modulated by different factors, including serine peptidases and their homologs and inhibitors (Zou and Jiang 2005, Colinet et al. 2009), C-type lectins and some other proteins (Ling et al. 2009). It can also be triggered by microbial cell wall components including PGNs (Lee et al. 2004; Jiang et al. 2004, 2011). In particular, the soluble fragments of lysozyme-mediated partial digestion of lys-type PGN represent a potent activator to proPO cascade through their formation of a complex by clustering with certain proteins, including its recognition protein, which leads to activation of proPO cascade (Dularay and Lackie 1992). In certain holometabolous insects, this inhibition phenomenon was observed, and a trade-off phenomenon between lysozyme activity and PO activity has been assumed (Rao et al. 2010). For this phenomenon, it was suggested (Rao et al. 2010) that insects can select effective immune mechanisms against variable pathogens; the selected mechanisms depend upon the nature of each pathogen.
Conclusions
This is the first report on the isolation and characterization of a c-type lysozyme from the plasma of the hemimetabolous insect S. gregaria retaining 126-fold increase in specific activity of the crude plasma lysozyme against M. lysodeikticus. The antibacterial (Gram-positive and Gram-negative) and antifungal mechanisms of action were elucidated. Future identification of the lysozyme gene and characterization of its expression will assist in identifying the protein function in the S. gregaria. The Sgl might not only be valuable as a model for understanding the immune response mechanism but also useful for future therapeutic purposes.
Acknowledgments
We are indebted to Dr. James Nation, Professor Emeritus, University of Florida, Gainesville, USA, for critically reading the manuscript and making many useful suggestions. We wish to thank Dr. Shuo-Yang Wen, Department of Entomology, South China Agricultural University, Wushan, China and Ramesh Gunaratna, Department of Molecular and Cellular Medicine, Texas A&M University, USA, for their help and support. Dr. Rasha Naguib, the Microbiology Division, Microanalytical Center, Faculty of Science, Cairo University, Egypt, is thanked for her enthusiastic work and assistance in microbiological assays.
References Cited
- Abraham E. G., Nagaraju J., Salunke D. M., Gupta H. M., Dutta R. K. 1995. Purification and partial characterization of an induced antibacterial protein in the silkworm, Bombyx mori. J. Invert. Pathol. 65: 17–24. [DOI] [PubMed] [Google Scholar]
- Andrews P. 1965. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem. J. 96: 595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y. Y., Qu L. Y., Zhao D., Chen L. B., Jin H. Y., Xu L. M., Cheng J. A., Zhang C. X. 2013. The genome- and transcriptome-wide analysis of innate immunity in the brown planthopper, Nilaparvata lugens . BMC Genomics 14: 160–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris C., Turck M. 1966. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 45: 493–496. [PubMed] [Google Scholar]
- Beveridge T. J. 1999. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 181: 4725–4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman H. G., Huhtmark D. 1987. Cell-free immunity in insects. Annu. Rev. Microbiol. 41: 103–126. [DOI] [PubMed] [Google Scholar]
- Boman H. G. 1987. Assaying antimicrobial peptides, pp. 54–61. In Marsh J., Goode J. A. (eds.), Antimicrobial peptides. Ciba foundation symposium 186. John Wiley and Sons, New York. [Google Scholar]
- Bradford M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Cançado F. C., Valerio A. A., Marana S. R., Barbosa J. A. 2007. The crystal structure of a lysozyme c from housefly Musca domestica, the first structure of a digestive lysozyme. J. Struct. Biol. 160: 83–92. [DOI] [PubMed] [Google Scholar]
- Chapelle M., Girard P. A., Cousserans F., Volkoff N. A., Duvic B. 2009. Lysozymes and lysozyme-like proteins from the fall armyworm, Spodoptera frugiperda. Mol. Immunol. 47: 261–269. [DOI] [PubMed] [Google Scholar]
- Colinet D., Dubuffet A., Cazes D., Moreau S., Drezen J. M., Poirié M. 2009. A serpin from the parasitoid wasp Leptopilina boulardi targets the Drosophila phenoloxidase cascade. Dev. Comp. Immunol. 33: 681–689. [DOI] [PubMed] [Google Scholar]
- Dalhammar G., Steiner H. 1984. Characterization of inhibitor A, a protease from Bacillus thuringiensis which degrades attacins and cecropins, two classes of antibacterial proteins in insects. Eur. J. Biochem. 139: 247–252. [DOI] [PubMed] [Google Scholar]
- Demura M. 2006. NMR insight of structural stability and folding of calcium-binding lysozyme, pp. 493–497. In: Modern magnetic resonance, part I application in chemistry, biological and maine sciences; Springer, the Netherlands. [Google Scholar]
- Dularay B., Lackie A. M. 1992. Haemocytic encapsulation and the prophenoloxidase-activation pathway in the locust Schistocerca gregaria Forsk. Insect Biochem. 15: 827–834. [Google Scholar]
- Engström A., Xanthopoulos K. G., Boman H. G., Bennich H. 1985. Amino acid and cDNA sequences of lysozyme from Hyalophora cecropia. EMBO J. 4: 2119–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedhila S., Nel P., Lereclus D. 2002. The InhA2 metalloprotease of Bacillus thuringiensis strain 407 is required for pathogenicity in insects infected via the oral route. J. Bacteriol. 184: 3296–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiolka M. J., Ptaszynska A. A., Czarniawski W. 2005. Antibacterial and antifungal lysozyme-type activity in Cameraria ohridella pupae. J. Invertebr. Pathol. 90: 1–9. [DOI] [PubMed] [Google Scholar]
- Fujimoto S., Toshimori-Tsuda I., Kishimoto K., Yamano Y., Morishima I. 2001. Protein purification, cDNA cloning and gene expression of lysozyme from eri-silkworm, Samia cynthia ricini. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 128: 709–718. [DOI] [PubMed] [Google Scholar]
- Gillespie J. P., Burnett C., Charnley A. K. 2000. The immune response of the desert locust Schistocerca gregaria during mycosis of the entomopathogenic fungus, Metarhizium anisopliae var acridum. J. Insect Physiol. 46: 429–437. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Engström A., Bennich H., Kapur R., Boman H. G. 1982. Insect immunity: isolation and structure of cecropin D and four minor antibacterial components from Cecropia pupae. Eur. J. Biochem. 127: 207–217. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Steiner H., Rasmuson T., Boman H. G. 1980. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur. J. Biochem. 106: 7–16. [DOI] [PubMed] [Google Scholar]
- Jain D., Nair D. T., Swaminathan G. J., Abraham E. G., Nagaraju J., Salunke D. M. 2001. Structure of the induced antibacterial protein from tasar silkworm, Antheraea mylitta. Implications to molecular evolution. J. Biol. Chem. 276: 41377–41382. [DOI] [PubMed] [Google Scholar]
- Jensen H. B., Kleppe K. 1972. Effect of ionic strength, pH, amines and divalent cations on the lytic activity of T4 lysozyme. Eur. J. Biochem. 28: 116–122. [DOI] [PubMed] [Google Scholar]
- Jiang H. 2008. The biochemical basis of antimicrobial responses in Manduca sexta. Insect Sci. 15: 53–66. [Google Scholar]
- Jiang H., Vilcinskas A., Kanost M. R. 2011. Immunity in lepidopteran insects. Adv. Exp. Med. Biol. 708: 181–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Ma C., Lu Z. Q., Kanost M. R. 2004. Beta-1,3-glucan recognition protein-2 (betaGRP-2) from Manduca sexta; an acute-phase protein that binds beta-1,3-glucan and lipoteichoic acid to aggregate fungi and bacteria and stimulate prophenoloxidase activation. Insect Biochem. Mol. Biol. 34: 89–100. [DOI] [PubMed] [Google Scholar]
- Jollès J., Schoentgen F., Croizier G., Croizier L., Jollès P. 1979. Insect lysozymes from three species of Lepidoptera: their structural relatedness to the C (chicken) type lysozyme. J. Mol. Evol. 14: 267–271. [DOI] [PubMed] [Google Scholar]
- Jollès P., Jollès J. 1984. What’s new in lysozyme research? Always a model system, today as yesterday. Mol. Cell. Biochem. 63: 165–189. [DOI] [PubMed] [Google Scholar]
- Kang D., Liu G., Gunne H., Steiner H. 1996. PCR differential display of immune gene expression in Trichoplusia ni. Insect Biochem. Mol. Biol. 26: 177–184. [DOI] [PubMed] [Google Scholar]
- Kanost M. R., Gorman M. J. 2008. Phenoloxidase in insect immunity, pp. 69–96. In Beckage N. (ed.), Insect immunology. Academic Press, San Diego. [Google Scholar]
- Kim J. W., Yoe S. M. 2003. Purification of lysozyme from hemolymph of tobacco cutworm, Spodoptera litura. Korean J. Entomol. 33: 287–291. [Google Scholar]
- Knubovets T., Osterhout J. J., Connolly P. J., Klibanov A. M. 1999. Structure, thermostability, and conformational flexibility of hen egg-white lysozyme dissolved in glycerol. Proc. Natl. Acad. Sci. U.S.A. 96: 1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kylsten P., Kimbrell D. A., Daffre S., Samakovlis C., Hultmark D. 1992. The lysozyme locus in Drosophila melanogaster: different genes are expressed in midgut and salivary glands. Mol. Gen. Genet. 232: 335–343. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. 1970. Cleavage of structural proteins during the assembly of bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- Lee M. H., Osaki T., Lee J. Y., Baek M. J., Zhang R., Park J. W., Kawabata S., Söderhäll K., Lee B. L. 2004. Peptidoglycan recognition proteins involved in 1,3-beta-D-glucan-dependent prophenoloxidase activation system of insect. J. Biol. Chem. 279: 3218–3227. [DOI] [PubMed] [Google Scholar]
- Lemaitre B., Hoffmann J. 2007. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 25: 697–743. [DOI] [PubMed] [Google Scholar]
- Lemos F.J.A., Ribeiro A. F., Terra W. R. 1993. A bacteria-digesting midgut-lysozyme from Musca domestica (Diptera) larvae. Purification, properties and secretory mechanism. Insect Biochem. Mol. Biol. 23: 533–541. [Google Scholar]
- Li L., Zhao J., Wang L., Qiu L., Song L. 2013. Genomic organization, polymorphisms and molecular evolution of the goose-type lysozyme gene from Zhikong scallop Chlamys farreri. Gene 513: 40–52. [DOI] [PubMed] [Google Scholar]
- Ling E., Rao X. J., Ao J. Q., Yu X. Q. 2009. Purification and characterization of a small cationic protein from the tobacco hornworm Manduca sexta. Insect Biochem. Mol. Biol. 39: 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Cui L., Cox-Foster D., Felton G. W. 2004. Characterization of a salivary lysozyme in larval Helicoverpa zea. J. Chem. Ecol. 30: 2439–2457. [DOI] [PubMed] [Google Scholar]
- Lockey T. D., Ourth D. D. 1996. Purification and characterization of lysozyme from hemolymph of Heliothis virescens larvae. Biochem. Biophys. Res. Comm. 220: 502–508. [DOI] [PubMed] [Google Scholar]
- Mohamed A. A., Elmogy M., Dorrah M. A., Yousef H. A., Bassal T.T.M. 2013. Antibacterial activity of lysozyme in the desert locust, Schistocerca gregaria (Orthoptera: Acrididae). Eur. J. Entomol. 110: 559–565. [Google Scholar]
- Mohan M., Selvakumar G., Sushil S. N., Bhatt J. C., Gupta H. S. 2011. Entomopathogenicity of endophytic Serratia marcescens strain SRM against larvae of Helicoverpa armigera (Noctuidae: Lepidoptera). World J. Microbiol. Biotechnol. 27: 2545–2551. [Google Scholar]
- Monzingo A. F., Marcotte E. M., Hart P. J., Robertus J. D. 1996. Chitinases, chitosanases, and lysozymes can be divided into procaryotic and eucaryotic families sharing a conserved core. Nat. Struct. Biol. 3: 133–140. [DOI] [PubMed] [Google Scholar]
- Mulnix A. B., Dunn P. E. 1994. Structure and induction of a lysozyme gene from the tabacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 24: 271–281. [DOI] [PubMed] [Google Scholar]
- Nash J. A., Ballard T. N., Weaver T. E., Akinbi H. T. 2006. The peptidoglycan-degrading property of lysozyme is not required for bactericidal activity in vivo. J. Immunol. 177: 519–526. [DOI] [PubMed] [Google Scholar]
- Navarre W. W., Schneewind O. 1999. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 63: 174–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtakara A. 1988. Chitinase and β-N-acetylhexosaminidase from Pycnoporus cinnabarinus, pp. 462–470. In Wood W. A., Kellogg S. T. (eds.), Methods in enzymology. Academic Press, New York. [Google Scholar]
- Ouchterlony Ö. 1949. Antigen-antibody reactions in gels. Acta pathologica et microbiologica Scandinavica 26: 507–515. [DOI] [PubMed] [Google Scholar]
- Palmiter D., Gagnon J., Ericsson L. H., Walsh K.A. 1977. Precursor of egg white lysozyme, amino acid sequence of an NH2 terminal extension. J. Biol. Chem. 252: 6386–6393. [PubMed] [Google Scholar]
- Park H. Y., Park S. S., Shin S. W., Park D. S., Kim M. G., Oh H. W., Joo C. K. 1997. Protein purification and nucleotide sequence of a lysozyme from the bacteria-induced larvae of the fall webworm, Hyphantria cunea. Arch. Insect Biochem. Physiol. 35: 335–345. [DOI] [PubMed] [Google Scholar]
- Powning R. F., Davidson W. J. 1973. Studies on insect bacteriolytic enzymes: I. Lysozyme in haemolymph of Galleria mellonella and Bombyx mori. Comp. Biochem. Physiol. 45B: 669–681. [PubMed] [Google Scholar]
- Rao X. J., Ling E., Yu X. Q. 2010. The role of lysozyme in the prophenoloxidase activation system of Manduca sexta: an in vitro approach. Dev. Comp. Immunol. 34: 264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regel R., Matioli S. R., Terra W. R. 1998. Molecular adaptation of Drosophila melanogaster lysozymes to a digestive function. Insect Biochem. Mol. Biol. 28: 309–319. [DOI] [PubMed] [Google Scholar]
- Robertson E. F., Dannelly H. K., Malloy P. J., Reeves H. C. 1987. Rapid isoelectric focusing in a vertical polyacrylamide minigel system. Anal. Biochem. 167: 290–294. [DOI] [PubMed] [Google Scholar]
- Schneider P. M. 1985. Purification and properties of three lysozymes from hemolymph of the cricket, Gryllus bimaculatus (De Geer). Insect Biochem. 15: 463–470. [Google Scholar]
- Scopes R. K. 1982. Protein purification. In Principles and practice. Springer-Verlag, New York. [Google Scholar]
- Skerlavaj B., Domenico R., Gennaro R. 1990. Rapid permeabilization and inhibition of vital functions of gram-negative bacteria by bactenicins. Infect. Immun. 58: 3724–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao K., Long Z., Liu K., Tao Y., Liu S. 2006. Purification and properties of a novel insecticidal protein from the locust pathogen Serratia marcescens HR-3. Curr. Microbiol. 52: 45–49. [DOI] [PubMed] [Google Scholar]
- Vilcinskas A., Matha V. 1997. Antimycotic activity of lysozyme and its contribution to antifungal humoral defense reactions in Galleria mellonella. Anim. Biol. 6: 19–29. [Google Scholar]
- Vocadlo D. J., Davies G. J., Laine R., Withers S. G. 2001. Catalysis by hen egg-white lysozyme proceeds via a covalent intermediate. Nature 412: 835–838. [DOI] [PubMed] [Google Scholar]
- Wayne P. A. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. In Approved standard, 4th ed. M7-A4. NCCLS. National Committee for Clinical Laboratory Standards, USA. [Google Scholar]
- Wayne P. A. 2002. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. In Proposed standard M38-A. NCCLS, National Committee for Clinical Laboratory Standards, USA. [Google Scholar]
- Wayne P. A. 2003. Method for antifungal disk diffusion susceptibility testing of yeast. In Proposed guideline M44-P. NCCLS, National Committee for Clinical Laboratory Standards, USA. [Google Scholar]
- Wang W. X., Wang Y. P., Deng X. J., Dang X. L., Tian J. H., Yi H. Y., Li Y. F., He X. F., Cao Y., Xia Q. Y., et al. 2009. Molecular and functional characterization of a c-type lysozyme from the Asian corn borer, Ostrinia furnacalis, J. Insect. Sci. 9: 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoe S. M., Bang I. S., Kang C. S., Kim H. J. 1996. Purification and characterization of two lysozymes from larval haemolymph of cabbage butterfly, Artogeia rapae. Mol. Cells 6: 609–614. [Google Scholar]
- Yu K. H., Kim K. N., Lee J. H., Lee H. S., Kim S. H., Cho K. Y., Nam M. H., Lee I. H. 2002. Comparative study on characteristics of lysozymes from the hemolymph of three lepidopteran larvae, Galleria mellonella, Bombyx mori, Agrius convolvuli. Dev. Comp. Immunol. 26: 707–713. [DOI] [PubMed] [Google Scholar]
- Zachary D., Hoffmann D. 1984. Lysozyme is stored in the granules of certain hemocyte types in Locusta. J. Insect Physiol. 30: 405–411. [Google Scholar]
- Zou Z., Jiang H. 2005. Manduca sexta serpin-6 regulates immune serine proteinases PAP-3 and HP8. cDNA cloning, protein expression, inhibition kinetics, and function elucidation. J. Biol. Chem. 280: 14341–14348. [DOI] [PMC free article] [PubMed] [Google Scholar]




