Abstract
An important characteristic of cancer is that the disease can overcome the surveillance of the immune system. A possible explanation for this resistance arises from the ability of tumor cells to block the tumoricidal activity of host immune cells such as natural killer (NK) cells by inducing the localized accumulation of regulatory T (Treg) cells. Evidence exists that components in commonly consumed foods including vitamins A, D, and E, water-soluble constituents of mushrooms, polyphenolics in fruits and vegetables, and n-3 fatty acids in fish oil can modulate NK cell activities, Treg cell properties, and the interactions between those two cell types. Thus, it is extremely important for cancer prevention to understand the involvement of dietary components with the early stage dynamics of interactions among these immune cells. This review addresses the potential significance of diet in supporting the function of NK cells, Treg cells, and the balance between those two cell types, which ultimately results in decreased cancer risk.
1. Introduction
NK cells are large granular lymphocytes without B or T cell characteristics and highly effective in destroying tumor cells and virally infected cells without the need for prior sensitization or recognition of a specific antigen (1–3). These cells represent innate immune cells that secrete cytokines participating in the adaptive immune response. For example, NK cells are a major source of protective cytokine IFN-γ that is critical for the development of an appropriate cytotoxic T cell response to the pathogen. The direct and indirect tumoridical properties of NK cells equip them with the ability to serve as a critical sentinel against invading pathogens. Both experimental and clinical data indicate an important role for NK cells in early neoplastic development, possibly by either responding to pathogen-associated molecular patterns (PAMPs) or to various types of extracellular or cell-associated proteinases (4, 5). Cancer cells often evade NK-cell surveillance by producing immunosuppressive molecules and through the recruitment of tolerance-related Treg cells (6, 7).
Treg cells (CD4+, CD25+, fork head box p3 [Foxp3] +) that characteristically express the nuclear transcription factorFoxp3, are known to down-regulate the tumoricidal activity of NK cells and thus maintain immunological self-tolerance and homeostasis. No doubt, it is important to understand the early stage(s) of pathogen-host interactions, and redirect these events from a pro-tumor to an anti-tumor state. Diet may represent a subtle approach to regulating NK cells without losing their homeostasis maintained by regulatory T (Treg) cells. Here, we will discuss our current understanding of the mechanism by which dietary components modulate the function and balance between NK cells and Treg cells for cancer prevention. Papers that do not provide evidence dealing with the effects of specific dietary constituents on the targeted immune-prevention are not included for the discussion.
2. Dietary influence on NK cell properties
Several lines of evidence suggest that a number of bioactive food components can induce tumor cell death, possibly by enhancing NK cell activity. For example, water-soluble extracts of the dried Brazilian sun- (Agaricus Blazei) and Maitake- (Grifola frondosa) mushrooms can enhance the cytolytic activity of NK cells in BALB/c mice (8–10). Likewise, dietary supplementation with vitamin E (250 mg daily for 2 weeks) can enhance NK cell cytolytic activity in advanced colorectal cancer cells obtained from patients (11). Interestingly, the supplementation of vitamin E (administered at 100 mg/day for 8 weeks) restored NK cell activity in a 16 month-old boy with Shwachman-Diamond syndrome which is classically associated with a persistent reduction in NK cytolytic activity (12). Collectively, these findings suggest the involvement of dietary components in the regulation of NK cell tumoricidal activity. In this review, we propose three distinct processes: receptor-ligand interactions, the release of cytokines, and the secretion of lytic enzymes (Figure 1) as possible mechanisms explaining their actions.
Figure 1.
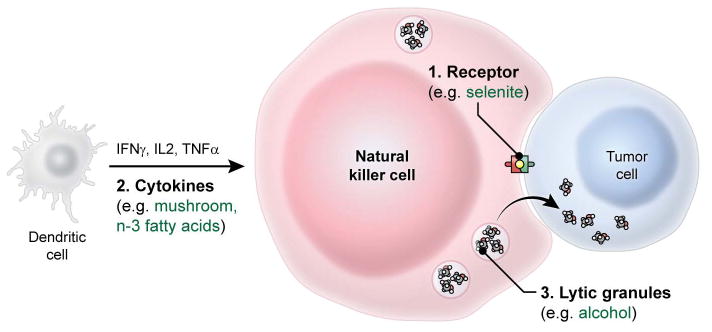
Dietary components modulate tumoricidal activity of NK cells by three distinct processes including receptor-ligand interactions, the release of cytokines, and the secretion of lytic enzymes. Specific examples are discussed in the text under section 2. This figure does not reflect the actual size of cells.
2.1 Interaction of bioactive food components with NK cell receptors and their ligands
NK cells are known to exhibit their activity through a diverse repertoire of activating (e.g., NKG2 receptor family) and inhibitory (e.g., killer immunoglobulin-like receptor [KIR] family) receptors that recognize specific ligands on the surface of target cells (13–15). Many of the KIRs recognize major histocompatibility complex (MHC) class I molecules, which in humans are human leukocyte antigen (HLA) class I molecules (16). The inhibitory KIRs block NK cytotoxicity for cells that express normal levels of MHC class 1 molecules on their surface (17).
There is evidence, admittedly limited, that suggests certain dietary components may modulate the NK cell activity in response to tumor antigen stimuli. For example, when STAV-AB malignant mesothelioma cells are exposed to 7.5 μM selenite for 24 hours, these cells become highly sensitive to NK cells (18). This event is possibly caused by the observed selenite-mediated loss of tumor antigen HLA-E on the surface of STAV-AB cells as this molecule can bind to the CD94/NKG2A receptor on NK cells and thereby suppress their tumoricidal activities.
The tumor antigen, HLA-G has tolerogenic properties and provides tumor cells with an efficient way to escape from NK tumoricidal function. This effect of HLA-G appears to be independent of lipid raft integrity that could be modulated by a variety of lipid soluble dietary factors including n-3 polyunsaturated fatty acids (PUFAs) abundant in fish oil and conjugated linoleic acids (CLAs) found in dairy products (19–22). For example, n-3 PUFAs and cis-9, trans-11-CLAs target lipid raft organization in membrane to influence immune cell function (21, 22). However, HLA-G is mainly localized outside the lipid rafts of tumor cells during this process and so remains unaffected by these dietary components.
2.2 Influence of bioactive food components on cytokine release from NK cells
Circulating NK cells, as opposed to dendritic cells, only mature during inflammation or infection (23–25). NK cells, which can lyse tumor cells, provide antigenic cellular debris for mature dendritic cells to present to T cells; in later stages of an immune response, NK cells terminate the process by lysing the dendritic cells and halting their ability for antigen presentation (26, 27). During early onset of inflammation, immature dendritic cells secrete a variety of cytokines including tumor necrosis factor-α (TNF-α), IL-2, IL-12, and IL-18 (24, 25). These cytokines can induce a rapid expression of IFN-γ and subsequently enhance the intrinsic cytolytic activity of NK cells (27). However the response is complex, since T-helper 2 (Th2) cytokines such as IL-4, which are generally viewed as an antagonist of IFN-γ expression in T cells, can induce signal transducer and activator of transcription 6 (STAT6)-dependent IFN-γ secretion by NK cells (28).
Dietary supplementation of 4 month old C57BL/6 mice with 10% (Wt/Wt) white button mushroom powders for 10 weeks increased the production of TNF-α, IL-2, and IFN-γ, which correlated with the increased tumoricidal activity of splenic NK cells (29). Unfortunately, this study does not allow for the determination if the increased production of cytokines is mediated via dendritic cells or from the direct effect of mushroom powders on NK cell function.
Recently, LPS stimulated dendritic cells were found to increase the release of IFN-γ following isoflavone (100 μM genistein or daidzein), which in turn enhanced the cytolytic effects of NK cells in culture (30). While it appears that soy isoflavones stimulate the release of cytokines from matured dendritic cells, it remains unclear how these cytokines activate the NK cell function.
It is reported that supplementation of mice with galacto-oligosaccharides (5 g/kg b.w. for 2 weeks), a soluble fiber found in human milk, increases the NK cell-mediated protection against Helicobacter infection in a Smad3-deficient inflammation model (31). This effect of a milk component on NK cell activity is possibly associated with the increased expression of the NK chemokine receptor CCR 9 as the binding by its ligand CCL25 is known to decrease the severity of pathogen-induced inflammation in the colon. While these results suggest that specific bioactive food components can modulate the ability of NK cells to respond to various cytokines, the precise mechanism(s) remains unresolved.
2.3 Dietary modulation of release of lytic granules from NK cells
The lysosomal release of cytotoxic granules from NK cells, including two membrane-perturbing proteins such as perforin and granulysin, and a family of serine proteases (also known as granzymes), constitutes the main pathway for the NK-mediated elimination of tumor cells (27, 32–34). A number of studies indicate that dietary habits, including alcohol consumption and caloric restriction, may influence the cytolytic activity of NK cells by down-regulating the release, activity, and expression of perforin and granular proteases (35–38). Restricting calorie intake (starting with 10% restriction at the age of 10 weeks and gradually increased up to 40% at the age of 14 weeks and after) in mice was reported to reduce NK cell number and function particularly cytolytic activity as evident by their decreased ability to resist against influenza virus infection (37). While the mechanism for these changes in NK cells remains unclear, the significance of the PI3K signaling cascade in NK cell biology (39) and the recent finding that inositol (1, 3, 4, 5) tetrakisphosphate (IP4) suppresses NK cell granule exocytosis and target-cell killing (40), may explain a possible mechanism. IP4 is a soluble metabolite generated from the membrane lipid phosphatidylinositol- 4, 5-diphosphate [PI(4,5)P2]. This compound is reported to be critical in immune cell development and function in hematopoietic cells (41). Knocking-out of ItpkB, an enzyme that is essential for the production of IP4, revealed that this phosphorylated inositol metabolite promotes NK cell maturation and limits NK cell responsiveness (40). It is known that caloric restriction reduces tumor incidence and numbers in animal models. Further studies are warranted to examine how NK cells play a role in these dynamics involving the tumor micro-environment, energy homeostasis, and host immunity.
3. Dietary components modulate Treg cell induction
Treg cells represent 5–10% of total CD4+ T lymphocytes in humans and mice, these cells constitute about one fifth of tumor T-cell infiltrates (2, 42, 43) suggesting their active role in the tumor microenvironment. Under normal conditions, Treg cells are known to have a role in developing tolerance to nonpathogenic foreign antigens, (e.g. foods or commensal bacteria) to avoid inappropriate immune responses. Within a tumor site, these regulatory T cells can suppress cancer cell-specific immune reactions, resulting in the cytokine profile that is typically immunosuppressive. For example, transforming growth factor-β (TGF-β), a tumor cell secreted cytokine that can convert antigen-activated peripheral CD4+ T cells to CD4+CD25+ Treg cells, suppresses tumoricidal activity of CD8 (+) cytotoxic T lymphocyte (CTL) and NK cells (44). Through this mechanism, tumor cells escape from either CTL or NK cell-attacks by utilizing Treg cells that are recruited to the tumor by the chemokine ligand CCL22 that is produced by tumor cells and/or tumor-associated macrophages (45).
There are two different types of Treg cells, naturally occurring Treg (nTreg) and inducible Treg (iTreg) (46). The former suppresses T-cell responses by cell contact-dependent mechanisms, whereas the latter secretes inhibitory cytokines, including TGF-β and interleukin-10 (IL-10). While both nTreg cells and iTreg cells are known to modulate NK cell cytotoxicity in vitro and in vivo (47, 48), the precise mechanism explaining how this works largely remains unclear.
A few bioactive food components including vitamin A (49, 50), vitamin D (51–53), n-3 fatty acids (54, 55), dietary polyphenolics such as naringenin (56), and epigallocatechin-3-gallate (EGCG, 57) are shown to induce peripheral iTregcells to escape from immune effector cells including NK cells and thereby prevent autoimmunity and allergy. The effects of iTreg cells are carried out by modulating several processes including the inhibition of dendritic cell (DC) differentiation/maturation (48), stimulation of TGF-β release from the membrane of Treg cells (58), and the modulation of NK-cell perforin and granzyme B expression (59). However, it remains to be revealed which pathway(s) is modulated by these dietary constituents to exert their preventive effects on autoimmunity and allergy. With that limit, this section will focus on the currently known role of dietary constituents in the properties of iTreg cells at peripheral tissues.
3.1 Vitamin A modulates the induction of regulatory T cells in intestine, which promotes NK tolerance against foods and commensal gut bacteria
Antigen presenting cells (APCs) such as DCs harbor retinaldehyde dehydrogenase that metabolizes vitamin A to retinoic acid (RA) (60, 61). RA is reported to be critical for TGF-β-dependent iTreg generation in the intestine where immune tolerance towards foods and commensal microbes is important to maintain homeostasis (62, Figure 2). The conversion to iTreg (CD4+Foxp3+ Treg) cells from naïve T (CD4+Foxp3−) cells may be mediated by a specific population of dendritic cells that are identified by a CD103 marker in the lamina propria of the small intestine (49, 63). It is notable that a conserved non-coding sequence 1 (CNS1) in the Foxp3 locus, which contains TGF-β-Smad/NFAT response elements, has a critical role in iTreg cell generation in gut-associated lymphoid tissues (64, 65). In addition, the inhibitory effects of retinoic acids on the release of pro-inflammatory cytokines such as IL-4, IL-21, and IFN-γ from CD44(hi)CD4(+) memory T cells appears to contribute to the process (50, Figure 2). Interestingly, this cytokine-mediated indirect effect of RA is shown to require the expression of its nuclear receptor retinoic acid receptor-α (RAR-α) (66). The binding of RA to RAR-α can block cytokine production by effector T cells, which in turn facilitates Treg induction and down-regulates NK function. Finally, RA is reported to actively participate in the TGF-β-dependent reciprocal regulation of iTreg (anti-inflammatory) versus Th17 (pro-inflammatory) cells by inhibiting the IL6-driven induction of the latter (67, Figure 2). The priming of naïve T cells by DC in the presence of TGF-β leads to either anti-inflammatory Treg cells or pro-inflammatory Th17 cells (67). Vitamin A metabolite RA was demonstrated as a key determinant driving this pathway toward anti-inflammatory Treg cell induction mediated by mucosal DC. This property of RA seems to be particularly important in the intestine, where efficient immune protection is required to maintain homeostasis in response to constant exposure to exogenous stimuli. Overall, the experimental results suggest that vitamin A may be involved with the fine-tuning of T cell differentiation into Treg in the intestine, which generates NK tolerance against external and internal antigens.
Figure 2.
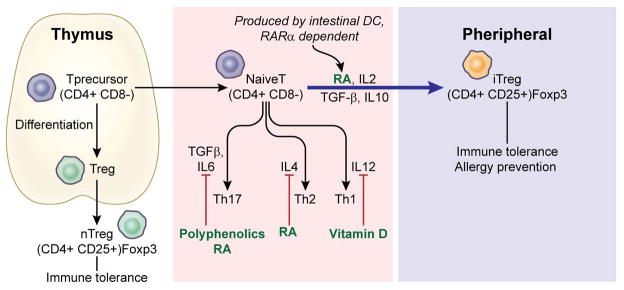
A naïve T cell can be differentiated into any Th effector cells depending on the cytokines released into the microenvironment. Dietary constituents including vitamin A metabolite retinoic acids, vitamin D, and polyphenolics are shown to inhibit the expression of various cytokines such as IL4, IL6, and IL12, and thereby increase TGFβ-dependent peripheral Treg induction.
3.2 Vitamin D inhibits dendritic cell maturation and thereby increases the induction of Treg cells
Studies have consistently shown that exposure of DCs to 1,25(OH)2D3, the active form of vitamin D, leads to the inhibition of differentiation and maturation of these cells (68–70). In vitro treatment of DCs with 1,25(OH)2D3 has been shown to decrease IL-12 and enhance IL-10 production, resulting in decreased T-cell activation (68, Figure 2). This effect of vitamin D seems to be mediated by vitamin D receptor (VDR) as shown in the study utilizing VDR knockouts (69). The modulating activities of 1,25(OH)2D3 on DCs and T cells were abolished in these mice with limited expression of VDR. Furthermore, 1,25(OH)2D3 selectively works on myeloid DCs but not plasmacytoid DCs, suggesting that the differentially produced cytokines in former cells such as IL-12 may play a significant role throughout the process (70, 71). Therefore, the VDR mediated inhibition of myeloid DC maturation, coupled with changes in cytokines such as IL-10 and IL-12 appears to ultimately render the myeloid DCs to be either inactive or tolerogenic (68, 70).
The changes in anti- and pro-inflammatory cytokines in the 1,25(OH)2D3 or VDR deficient mice has a key role in favoring Treg induction in peripheral tissues (72). For example, DCs secreting IL-10 induce iTreg cells that do not express Foxp3 (Fox3−) (51). These Foxp3− IL-10+ Treg cells possess immune suppressive properties and commonly named Tr1 (T regulatory type-1) or Tr1-like cells (73). Vitamin D has also been shown to be able to induce Foxp3+ Treg cells though it remains unclear if the changes in cytokines contribute to these events (52, 53). The dynamics between vitamin D and FoxP3+ Treg cells was confirmed in the study reporting that Treg induction in peripheral lymphocytes was abolished in VDR knockout mice in which the utilization of this nutrient is limited (74).
3.3 N-3 fatty acids may modulate both antigen-specific Th1/Th17 differentiation and iTreg function
N-3 fatty acids are commonly known to produce anti-inflammatory metabolites and thereby reduce cancer risks (75, 76). Recently, modulating effects of these fish oil components on immune cell development including T cell differentiation have been extensively investigated as a part of the mechanism(s) explaining the observed efficacy of n-3 fatty acids on the inflammatory processes (54, 55, 77). While these findings appear positive, the study results are mixed for which the reason remains unclear. For example, in Fat-1 transgenic mice where n-6 fatty acids are genetically converted to n-3 fatty acids (78), n-3 fatty acids are shown to suppress differentiation of pro-inflammatory Th1/Th17 cells without altering the expression of Treg cells (55). On the other hand, in Smad3−/− inflammatory colon cancer mice, feeding these animals with diet containing various concentrations (0.75% to 6%) of n-3 fatty acids suppressed CD4+ CD8+T effector cell population and increased Treg cell activity (79). These results suggest that one of the main functions of n-3 fatty acids may be the maintenance of homeostasis between effector T cells and Treg cells that inhibit CD4+CD8+ T cell function and thereby prevent autoimmunity.
3.4 Modulating effects of dietary polyphenolics on the suppressive activity of iTreg cells
TGFβ, one of the anti-inflammatory cytokines, is actively induced by myeloid immature DCs within the tumor microenvironment (80). When TGFβ works with proinflammatory cytokine IL6, it primes the differentiation of naïve CD4+ T cells into IL17-producing Th17 cells (81, Figure 2). Interestingly, TGFβ is also key to the conversion of peripheral naïve CD4+T cells into Foxp3+ iTregs with regulatory capacity (82). This fate decision between Th17 and Treg cells during differentiation seems to be determined by a ligand activated transcription factor aryl hydrocarbon receptor (AhR) (83–88) and hypoxia-inducible factor-1α (HIF-1α) (89, 90). Recently, it was shown that AhR-mediated stability and suppressive activity of iTreg cells require both the expression of a lineage-determining transcription factor Foxp3 and epigenetic reprogramming through T cell receptor (TCR) stimulation (91). On the other hand, HIF-1α was reported to mitigate Foxp3 expression by increasing its degradation in the proteasome and thereby fostering Th17 lineage commitment (89, 90). Nevertheless, the relevance of HIF-1α to the T cell differentiation has not been fully elucidated yet.
The AhR-related Treg induction and epigenetic regulation may be modulated by various dietary polyphenolics including naringenin found in citrus fruits and epigallocatechin-3-gallate (EGCG) in green tea (56, 57). These polyphenolics are known to possess the ability to inhibit the expression of proinflammatory cytokine IL6 (92, 93). Naringenin is a natural ligand for AhR and the complex stimulates T cell differentiation toward suppressive Treg cells (56, Figure 2). While this event seems to occur independently from TGFβ, the availability of unoccupied AhR is considered critical for such effects (56). Another dietary polyphenol compound found in tea, EGCG, was shown to significantly increase Treg frequencies (1.8 fold) in Balb/c mice when injected (i.p., 50 mg/kg) for 7 days (57). This effect of EGCG may involve its ability to inhibit DNA methyl transferase (DNMT) activity and thereby increase the expression of Foxp3. The similar effects of other dietary inhibitors of DNMT such as sulforaphane (94) and γ-tochopherol (95) may support this possibility. Regardless, further research on the mechanism elucidating the modulating effects of these dietary components on Treg expression and their ability to suppress other effector cells is warranted.
4. Discussion
The growth and spread of cancer depend not only on the biological characteristics of the tumor per se but also on the host responses. Adaptive immune cells such as B and T cells respond to a specific antigen while innate lymphocytes including NK cells recognize target cells based on the absence of specific cell surface determinants and affect their functions by simple kill/do not kill decisions. Although innate immune cells are less sophisticated than adaptive immune cells in terms of the effector function, these cells nevertheless are early responders and a front-line defense against invading pathogens or malignant non-self molecules. While this tumoricidal activity of NK cells is critical to protect hosts from invading pathogens, these cells could also cause autoimmunity that has been reported to be regulated by Treg cells. No doubt, it is extremely important to understand the early stage(s) of pathogen-host interactions, and redirect these events from a pro-tumor to an anti-tumor state. Diet may represent a subtle approach to regulating NK cells without losing their homeostasis maintained by Treg cells.
The evidence for the antagonizing effect of Treg cells on NK cell function was first reviewed by Zimmer et al. in 2008 (96). Treg cells are produced in the thymus (nTreg) and are also induced from conventional CD4(+) T cells at peripheral sites (iTreg). It has been reported that dietary constituents could modulate iTreg cell function through which they enhance immune responses without causing autoimmunity. However, the mechanism explaining the controlling role of diet-induced Treg cells in NK cell tumoricital activity remains unclear. Furthermore, considering the main focus of this paper is on cancer prevention which usually deals with normal cells and/or pre-malignant stage of normal cells, studies involving cancer animal models that could be used to examine or detect the modulating effects of dietary components on these immune cells have not been covered in this paper.
Available data suggest that certain sites such as in the gastrointestinal tract that is continuously exposed to foods and other exogenous stimuli are dominated by induced Treg cells that arise from peripheral conversion, rather than T cell differentiation in the thymus (6). These iTreg cells appear to be the main target for immune-modulating dietary components such as vitamins A (67), vitamin D (72), n-3 fatty acids (55), and plant polyphenolics (56), which contribute to the fine tuning of immune cells to maintain homeostasis. While these studies suggest the role of diet-derived nutrients in modulating gastrointestinal immunity, several questions including if the effects of dietary components are achievable within the range of physiological concentrations remain unanswered. To clarify this gap existing in research area of nutrition and immunity, the dietary levels along with experimental models adopted in the reported studies are listed with physiological concentrations of the effective bioactive food components in human plasma in Table 1. Interestingly, the effects of RAs and vitamin D on iTreg cells were examined with the physiologically achievable concentrations in humans. In contrast, the influence of other dietary components such as n-3 fatty acids, naringenin, and EGCG was evaluated with exaggerated concentrations either in vitro or in vivo. Thus, it is suggested to evaluate the study results with caution because it remains unclear how the data generated with high doses in experimental models translate into humans.
TABLE 1.
Dietary modulation of Treg cell induction with a focus on the concentrations adopted for studies and their human plasma levels
| BFCs* | Effects | Concentration used in studies | Model | Human plasma levels | Reference |
|---|---|---|---|---|---|
| Retinoic acids | TGF-β-dependent iTreg generation at the intestine | 10–100 nM | C57BL/6 mice | 4–14 nM | 49, 50, 97 |
| Vitamin D | Inhibits the maturation of myeloid DCs† and thereby increases iTreg | 15–20 μM [1, 25 (OH)2D3] | C57BL/6 Rag−/− mice |
30 μM [25(OH)D3] | 51–53, 98 |
| N-3 fatty acids | Modulate Th1/Th17 differentiation and iTreg function | 4–6% of diet | Rag−/− mice | DHA‡ ~3.5 mol/% EPA§ ~1.1 mol/% |
54, 55, 99, 100 |
| Naringenin | Stimulates AhR-mediated Treg induction | 50 μM | Murine splenic CD4+ T cells | ~ 1.0 μM | 56, 101 |
| EGCG^ | Inhibits DNMT and thereby increases Foxp4 expression | 7.8 μM | Balb/C mice | ~ 0.3 μM | 57, 102 |
Bioactive food components (BFCs)
Dendritic cells (DCs)
Docosahexaenoic acid (DHA, 22:6n-3): The value is presented as mol/% of phospholipid
Eicosapentaenoic acid (EPA, 20:5n-3): The value is presented as mol/% of phospholipid
Epigallocatechin 3-gallate (EGCG)
In conclusion, immunoprevention of cancer may occur by promoting antitumor effector cells such as NK cells for tumor suppression as well as by counteracting the accompanied autoimmunity through the enhancement of the immunoregulatory mechanisms such as Treg activity that inhibits effector cell cytotoxic properties. When the balance between immunity and autoimmunity is not maintained appropriately as in tumors, the Treg cells alter their polarity and stability to adjust to a new microenvironment. Through these changes, Treg cells are adopted by malignant cells as a mediator to escape from immunity and increase their survival (103, 104). While some studies support the general concept that specific dietary components contribute to immunoprevention by enhancing tumoricidal activity of NK cells and inducing the controlling properties of Treg cells (Figure 3), the evidence largely remains indirect. Therefore, the underlying mechanisms explaining the precise role of dietary components in maintaining the balance between immune surveillance and immune tolerance remain as an emerging research agenda which warrants further investigation.
Figure 3.
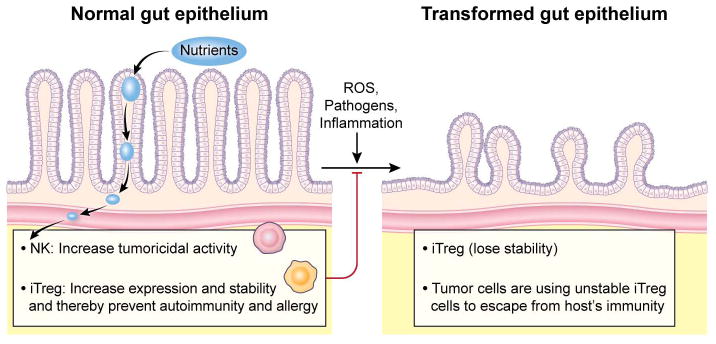
Nutrients are metabolized to small molecular weight compounds which increase tumoricidal activity of NK cells and induce the controlling properties of iTreg cells. The modulating effects of nutrients on the expression and function of iTreg cells are critical to prevent autoimmunity and allergy. When the gut epithelium is transformed by external stimuli including reactive oxygen species (ROS), pathogens, and inflammatory molecules, iTreg cells lose their stability and function. However, the role of these iTreg cells in tumor microenvironment remains unclear.
Acknowledgments
We would like to thank Dr. Margherita Cantorna and Dr. Mark Steven Miller for their critical review of this manuscript.
Abbreviation
- AhR
aryl hydrocarbon receptor
- APC
antigen presenting cell
- CCL
C-C motif chemokine ligand
- CCR
C-C motif chemokine receptor
- CNS1
conserved non-coding sequence 1
- CLA
conjugated linoleic acid
- CTL
cytotic T lymphocyte
- DC
dendritic cell
- DNMT
DNA methyl transferase
- EGCG
epigallocatechin-3-gallate
- Foxp3
forkhead box protein 3
- HIF1α
hyposia-inducible factor 1α
- HLA
human leukocyte antigen
- IFN
interferon
- IL
interleukin
- IP3
inositol (1, 4, 5)-triphosphate
- ItpkB
inositol triphosphate 3-kinase B
- iTreg
inducible regulatory T cells
- KIR
killer immunoglobulin-like receptor
- LPS
lipopolysaccharide
- MHC
major histocompatibility complex
- NFAT
nuclear factor of activated T cell
- NK
natural killer
- NKG2
natural killer group 2
- nTreg
naturally occurring regulatory T cells
- PAMP
pathogen-associated molecular pattern
- PI(3,4,5)P3
phophatidylinosytol (3, 4, 5)-triphosphate
- PUFA
polyunsaturated fatty acid
- PI3K
phosphoinositide 3-kinase
- RA
retinoic acid
- RAR-α
retinoic acid receptor-α
- ROS
reactive oxygen species
- STAT
signal transducer and activator of transcription
- TCR
T cell receptor
- TGF-β
transforming growth factor-β
- Th
T-helper
- TNF-α
tumor necrosis factor-α
- Treg
regulatory T
- Tr1
T regulatory type-1
- VDR
vitamin D receptor
- Wt
weight
Footnotes
Disclosure of Potential Conflicts of Interest
The authors declare no conflict of interest.
Authors’ Contributions
Conception and design: Y.S. Kim and H.A. Young
Writing of the manuscript: Y.S. Kim and H.A. Young
Review of the manuscript: T.J. Sayers, N.H. Colburn, and J.A. Milner
References
- 1.Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. 2012;12:239–52. doi: 10.1038/nri3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terme M, Fridman WH, Tartour E. NK cells from pleural effusions are potent antitumor effector cells. Eur J Immunol. 2013;43:331–4. doi: 10.1002/eji.201243264. [DOI] [PubMed] [Google Scholar]
- 3.Chang YH, Connolly J, Shimasaki N, Mimura K, Kono K, Campana D. A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res. 2013;73:1777–86. doi: 10.1158/0008-5472.CAN-12-3558. [DOI] [PubMed] [Google Scholar]
- 4.Saini RV, Wilson C, Finn MW, Wang T, Krensky AM, Clayberger C. Granulysin delivered by cytotoxic cells damages endoplasmic reticulum and activates caspase-7 in target cells. J Immunol. 2011;186:3497–504. doi: 10.4049/jimmunol.1003409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao S, Wang H, Nie Y, Mi Q, Chen X, Hou Y. Midkine upregulates MICA/B expression in human gastric cancer cells and decreases natural killer cell cytotoxicity. Cancer Immunol Immunother. 2012;61:1745–53. doi: 10.1007/s00262-012-1235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol. 2006;176:1582–7. doi: 10.4049/jimmunol.176.3.1582. [DOI] [PubMed] [Google Scholar]
- 7.Bergmann C, Wild CA, Narwan M, Lotfi R, Lang S, Brandau S. Human tumor-induced and naturally occurring Treg cells differentially affect NK cells activated by either IL-2 or target cells. Eur J Immunol. 2011;41:3564–73. doi: 10.1002/eji.201141532. [DOI] [PubMed] [Google Scholar]
- 8.Lin JG, Fan MJ, Tang NY, Yang JS, Hsia TC, Lin JJ, Lai KC, Wu RS, Ma CY, Wood WG, Chung JG. An extract of Agaricus blazei Murill administered orally promotes immune responses in murine leukemia BALB/c mice in vivo. Integr Cancer Ther. 2012;11:29–36. doi: 10.1177/1534735411400314. [DOI] [PubMed] [Google Scholar]
- 9.Yuminamochi E, Koike T, Takeda K, Horiuchi I, Okumura K. Interleukin-12- and interferon-gamma-mediated natural killer cell activation by Agaricus blazei Murill. Immunology. 2007;121:197–206. doi: 10.1111/j.1365-2567.2006.02558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kodama N, Mizuno S, Nanba H, Saito N. Potential antitumor activity of a low-molecular-weight protein fraction from Grifola frondosa through enhancement of cytokine production. J Med Food. 2010;13:20–30. doi: 10.1089/jmf.2009.1029. [DOI] [PubMed] [Google Scholar]
- 11.Hanson MG, Ozenci V, Carlsten MC, Glimelius BL, Frodin JE, Masucci G, Malmberg KJ, Kiessling RV. A short-term dietary supplementation with high doses of vitamin E increases NK cell cytolytic activity in advanced colorectal cancer patients. Cancer Immunol Immunother. 2007;56:973–84. doi: 10.1007/s00262-006-0261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adachi N, Migita M, Ohta T, Higashi A, Matsuda I. Depressed natural killer cell activity due to decreased natural killer cell population in a vitamin E-deficient patient with Shwachman syndrome: reversible natural killer cell abnormality by alpha-tocopherol supplementation. Eur J Pediatr. 1997;156:444–8. doi: 10.1007/s004310050634. [DOI] [PubMed] [Google Scholar]
- 13.Carbone E, Neri P, Mesuraca M, Fulciniti MT, Otsuki T, Pende D, Groh V, Spies T, Pollio G, Cosman D, Catalano L, Tassone P, Rotoli B, Venuta S. HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood. 2005;105:251–8. doi: 10.1182/blood-2004-04-1422. [DOI] [PubMed] [Google Scholar]
- 14.Andrews DM, Sullivan LC, Baschuk N, Chan CJ, Berry R, Cotterell CL, Lin J, Halse H, Watt SV, Poursine-Laurent J, Wang CR, Scalzo AA, Yokoyama WM, Rossjohn J, Brooks AG, Smyth MJ. Recognition of the nonclassical MHC class I molecule H2-M3 by the receptor Ly49A regulates the licensing and activation of NK cells. Nat Immunol. 2012;13:1171–7. doi: 10.1038/ni.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raulet DH. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat Immunol. 2004;5:996–1002. doi: 10.1038/ni1114. [DOI] [PubMed] [Google Scholar]
- 16.Older Aguilar AM, Guethlein LA, Adams EJ, Abi-Rached L, Moesta AK, Parham P. Coevolution of killer cell Ig-like receptors with HLA-C to become the major variable regulators of human NK cells. J Immunol. 2010;185:4238–51. doi: 10.4049/jimmunol.1001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonsson AH, Yokoyama WM. Natural killer cell tolerance licensing and other mechanisms. Adv Immunol. 2009;101:27–79. doi: 10.1016/S0065-2776(08)01002-X. [DOI] [PubMed] [Google Scholar]
- 18.Enqvist M, Nilsonne G, Hammarfjord O, Wallin RP, Björkström NK, Björnstedt M, Hjerpe A, Ljunggren HG, Dobra K, Malmberg KJ, Carlsten M. Selenite induces posttranscriptional blockade of HLA-E expression and sensitizes tumor cells to CD94/NKG2A-positive NK cells. J Immunol. 2011;187:3546–54. doi: 10.4049/jimmunol.1100610. [DOI] [PubMed] [Google Scholar]
- 19.Baudhuin J, Lesport E, Sousa S, Migraine J, Vigneron J, Lemaoult J, Carosella ED, Mooney N, Favier B. HLA-G inhibition of NK-cell cytolytic function is uncoupled from tumor cell lipid raft reorganization. Eur J Immunol. 2012;42:700–9. doi: 10.1002/eji.201141930. [DOI] [PubMed] [Google Scholar]
- 20.Chapkin RS, Wang N, Fan YY, Lupton JR, Prior IA. Docosaheaenoic acid alters the size and distribution of cell surface microdomains. Biochim Biophys Acta. 2008;1778:466–71. doi: 10.1016/j.bbamem.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rockett BD, Teague H, Harris M, Melton M, Williams J, Wassall SR, Shaikh SR. Fish oil increases raft size and membrane order of B cells accompanied by differential effects on function. J Lipid Res. 2012;53:674–85. doi: 10.1194/jlr.M021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grądzka I, Sochanowicz B, Brzóska K, Wójciuk G, Sommer S, Wojewódzka M, Gasińska A, Degen C, Jahreis G, Szumiel I. Cis-9, trans-11-conjugated linoleic acid affects lipid raft composition and sensitizes human colorectal adenocarcinoma HT-29 cells to X-radiation. Biochim Biophys Acta. 2013;1830:2233–42. doi: 10.1016/j.bbagen.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Montaldo E, Vitale C, Cottalasso F, Conte R, Glatzer T, Ambrosini P, Moretta L, Mingari MC. Human NK cells at early stages of differentiation produce CXCL8 and express CD161 molecule that functions as an activating receptor. Blood. 2012;119:3987–96. doi: 10.1182/blood-2011-09-379693. [DOI] [PubMed] [Google Scholar]
- 24.Satpathy AT, Wu X, Albring JC, Murphy KM. Re(de)fining the dendritic cell lineage. Nat Immunol. 2012;13:1145–54. doi: 10.1038/ni.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hespel C, Moser M. Role of inflammatory dendritic cells in innate and adaptive immunity. Eur J Immunol. 2012;42:2535–43. doi: 10.1002/eji.201242480. [DOI] [PubMed] [Google Scholar]
- 26.Morandi B, Mortara L, Chiossone L, Accolla RS, Mingari MC, Moretta L, Moretta A, Ferlazzo G. Dendritic cell editing by activated natural killer cells results in a more protective cancer-specific immune response. PLoS One. 2012;7:e39170. doi: 10.1371/journal.pone.0039170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colonna M, Jonjic S, Watzl C. Natural killer cells: fighting viruses and much more. Nat Immunol. 2011;12:107–10. doi: 10.1038/ni0211-107. [DOI] [PubMed] [Google Scholar]
- 28.Bix M, Kim S, Rao A. Opposites Attract in Differentiating T Cells. Science. 2005;308:1563–1565. doi: 10.1126/science.1114167. [DOI] [PubMed] [Google Scholar]
- 29.Wu D, Pae M, Ren Z, Guo Z, Smith D, Meydani SN. Dietary supplementation with white button mushroom enhances natural killer cell activity in C57BL/6 mice. J Nutr. 2007;137:1472–7. doi: 10.1093/jn/137.6.1472. [DOI] [PubMed] [Google Scholar]
- 30.Wei J, Bhatt S, Chang LM, Sampson HA, Masilamani M. Isoflavones, genistein and daidzein, regulate mucosal immune response by suppressing dendritic cell function. PLoS One. 2012;7:e47979. doi: 10.1371/journal.pone.0047979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gopalakrishnan A, Clinthorne JF, Rondini EA, McCaskey SJ, Gurzell EA, Langohr IM, Gardner EM, Fenton JI. Supplementation with galacto-oligosaccharides increases the percentage of NK cells and reduces colitis severity in Smad3-deficient mice. J Nutr. 2012;142:1336–42. doi: 10.3945/jn.111.154732. [DOI] [PubMed] [Google Scholar]
- 32.Vanherberghen B, Olofsson PE, Forslund E, Sternberg-Simon M, Khorshidi MA, Pacouret S, Guldevall K, Enqvist M, Malmberg KJ, Mehr R, Onfelt B. Classification of human natural killer cells based on migration behavior and cytotoxic response. Blood. 2013;121:1326–34. doi: 10.1182/blood-2012-06-439851. [DOI] [PubMed] [Google Scholar]
- 33.Wang P, Gu Y, Zhang Q, Han Y, Hou J, Lin L, Wu C, Bao Y, Su X, Jiang M, Wang Q, Li N, Cao X. Identification of resting and type I IFN-activated human NK cell miRNomes reveals microRNA-378 and microRNA-30e as negative regulators of NK cell cytotoxicity. J Immunol. 2012;189:211–21. doi: 10.4049/jimmunol.1200609. [DOI] [PubMed] [Google Scholar]
- 34.Bao Y, Zheng J, Han C, Jin J, Han H, Liu Y, Lau YL, Tu W, Cao X. Tyrosine kinase Btk is required for NK cell activation. J Biol Chem. 2012;287:23769–78. doi: 10.1074/jbc.M112.372425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen CP, Boyadjieva NI, Advis JP, Sarkar DK. Ethanol suppression of the hypothalamic proopiomelanocortin level and the splenic NK cell cytolytic activity is associated with a reduction in the expression of proinflammatory cytokines but not anti-inflammatory cytokines in neuroendocrine and immune cells. Alcohol Clin Exp Res. 2006;30:1925–32. doi: 10.1111/j.1530-0277.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- 36.Ballas ZK, Cook RT, Shey MR, Coleman RA. A dynamic flux in natural killer cell subsets as a function of the duration of alcohol ingestion. Alcohol Clin Exp Res. 2012;36:826–34. doi: 10.1111/j.1530-0277.2011.01678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clinthorne JF, Beli E, Duriancik DM, Gardner EM. NK cell maturation and function in C57BL/6 mice are altered by caloric restriction. J Immunol. 2013;190:712–22. doi: 10.4049/jimmunol.1201837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gardner EM. Caloric restriction decreases survival of aged mice in response to primary influenza infection. J Gerontol A Biol Sci Med Sci. 2005;60:688–94. doi: 10.1093/gerona/60.6.688. [DOI] [PubMed] [Google Scholar]
- 39.Tassi I, Cella M, Gilfillan S, Turnbull I, Diacovo TG, Penninger JM, Colonna M. p110gamma and p110delta phosphoinositide 3-kinase signaling pathways synergize to control development and functions of murine NK cells. Immunity. 2007;27:214–27. doi: 10.1016/j.immuni.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 40.Sauer K, Park E, Siegemund S, French AR, Wahle JA, Sternberg L, Rigaud S, Jonsson AH, Yokoyama WM, Huang YH. Inositol tetrakisphosphate limits NK cell effector functions by controlling phosphoinositide 3-kinase signaling. Blood. 2013;121:286–97. doi: 10.1182/blood-2012-05-429241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sauer K, Cooke MP. Regulation of immune cell development through soluble inositol-1,3,4,5-tetrakisphosphates. Nat Rev Immunol. 2010;10:257–71. doi: 10.1038/nri2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL, Kaiser LR, June CH. Cutting Edge: Regulatory T Cells from Lung Cancer Patients Directly Inhibit Autologous T Cell Proliferation. J Immunol. 2002;168:4272–6. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 43.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–67. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 44.Chen YL, Chang MC, Chen CA, Lin HW, Cheng WF, Chien CL. Depletion of regulatory T lymphocytes reverses the imbalance between pro- and anti-tumor immunities via enhancing antigen-specific T cell immune responses. PLoS One. 2012;7:e47190. doi: 10.1371/journal.pone.0047190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ménétrier-Caux C, Faget J, Biota C, Gobert M, Blay JY, Caux C. Innate immune recognition of breast tumor cells mediates CCL22 secretion favoring Treg recruitment within tumor environment. Oncoimmunology. 2012;1:759–761. doi: 10.4161/onci.19680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 47.Bergmann C, Wild CA, Narwan M, Lotfi R, Lang S, Brandau S. Human tumor-induced and naturally occurring Treg cells differentially affect NK cells activated by either IL-2 or target cells. Eur J Immunol. 2011;41:3564–73. doi: 10.1002/eji.201141532. [DOI] [PubMed] [Google Scholar]
- 48.Idoyaga J, Fiorese C, Zbytnuik L, Lubkin A, Miller J, Malissen B, Mucida D, Merad M, Steinman RM. Specialized role of migratory dendritic cells in peripheral tolerance induction. J Clin Invest. 2013;123:844–54. doi: 10.1172/JCI65260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, Mathis D, Benoist C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–70. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Urry Z, Chambers ES, Xystrakis E, Dimeloe S, Richards DF, Gabryšová L, Christensen J, Gupta A, Saglani S, Bush A, O’Garra A, Brown Z, Hawrylowicz CM. The role of 1α,25-dihydroxyvitamin D3 and cytokines in the promotion of distinct Foxp3+ and IL-10+ CD4+ T cells. Eur J Immunol. 2012;42:2697–708. doi: 10.1002/eji.201242370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeffery LE, Wood AM, Qureshi OS, Hou TZ, Gardner D, Briggs Z, Kaur S, Raza K, Sansom DM. Availability of 25-Hydroxyvitamin D3 to APCs Controls the Balance between Regulatory and Inflammatory T cell Responses. J Immunol. 2012;189:5155–64. doi: 10.4049/jimmunol.1200786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Penna G, Roncari A, Amuchastegui S, Daniel KC, Berti, Colonna M, Adorini L. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood. 2005;106:3490–3497. doi: 10.1182/blood-2005-05-2044. [DOI] [PubMed] [Google Scholar]
- 54.Zhang P, Kim W, Zhou L, Wang N, Ly LH, McMurray DN, Chapkin RS. Dietary fish oil inhibits antigen-specific murine Th1 cell development by suppression of clonal expansion. J Nutr. 2006;136:2391–8. doi: 10.1093/jn/136.9.2391. [DOI] [PubMed] [Google Scholar]
- 55.Monk JM, Jia Q, Callaway E, Weeks B, Alaniz RC, McMurray DN, Chapkin RS. Th17 cell accumulation is decreased during chronic experimental colitis by (n-3) PUFA in Fat-1 mice. J Nutr. 2012;142:117–24. doi: 10.3945/jn.111.147058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang HK, Yeh CH, Iwamoto T, Satsu H, Shimizu M, Totsuka M. Dietary flavonoid naringenin induces regulatory T cells via an aryl hydrocarbon receptor mediated pathway. J Agric Food Chem. 2012;60:2171–8. doi: 10.1021/jf204625y. [DOI] [PubMed] [Google Scholar]
- 57.Wong CP, Nguyen LP, Noh SK, Bray TM, Bruno RS, Ho E. Induction of regulatory T cells by green tea polyphenol EGCG. Immunol Lett. 2011;139:7–13. doi: 10.1016/j.imlet.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghiringhelli F, Ménard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E, Lecesne A, Robert C, Blay JY, Bernard J, Caillat-Zucman S, Freitas A, Tursz T, Wagner-Ballon O, Capron C, Vainchencker W, Martin F, Zitvogel L. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. 2005;202:1075–85. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mittelbronn M, Schittenhelm J, Bakos G, de Vos RA, Wehrmann M, Meyermann R, Bürk K. CD8(+)/perforin/granzyme B(+) effector cells infiltrating cerebellum and inferior olives in gluten ataxia. Neuropathology. 2010;30:92–6. doi: 10.1111/j.1440-1789.2009.01042.x. [DOI] [PubMed] [Google Scholar]
- 60.Molenaar R, Knippenberg M, Goverse G, Olivier BJ, de Vos AF, O’Toole T, Mebius RE. Expression of retinaldehyde dehydrogenase enzymes in mucosal dendritic cells and gut-draining lymph node stromal cells is controlled by dietary vitamin A. J Immunol. 2011;186:1934–42. doi: 10.4049/jimmunol.1001672. [DOI] [PubMed] [Google Scholar]
- 61.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J Immunol. 2007;179:3724–33. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 62.Duriancik DM, Lackey DE, Hoag KA. Vitamin A as a regulator of antigen presenting cells. J Nutr. 2010;140:1395–9. doi: 10.3945/jn.110.124461. [DOI] [PubMed] [Google Scholar]
- 63.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–74. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schlenner SM, Weigmann B, Ruan Q, Chen Y, von Boehmer H. Smad3 binding to the foxp3 enhancer is dispensable for the development of regulatory T cells with the exception of the gut. J Exp Med. 2012;209:1529–35. doi: 10.1084/jem.20112646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–12. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hall JA, Grainger JR, Spencer SP, Belkaid Y. The role of retinoic acid in tolerance and immunity. Immunity. 2011;35:13–22. doi: 10.1016/j.immuni.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 68.Adorini L, Penna G. Dendritic cell tolerogenicity: a key mechanism in immunomodulation by vitamin D receptor agonists. Hum Immunol. 2009;70:345–52. doi: 10.1016/j.humimm.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 69.Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: A vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci USA. 2001;98:6800–6805. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Széles L, Keresztes G, Töröcsik D, Balajthy Z, Krenács L, Póliska S, Steinmeyer A, Zuegel U, Pruenster M, Rot A, Nagy L. 1,25-dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J Immunol. 2009;182:2074–83. doi: 10.4049/jimmunol.0803345. [DOI] [PubMed] [Google Scholar]
- 71.Penna G, Amuchastegui S, Laverny G, Adorini L. Vitamin D receptor agonists in the treatment of autoimmune diseases: selective targeting of myeloid but not plasmacytoid dendritic cells. J Bone Miner Res. 2007;(Suppl 2):V69–73. doi: 10.1359/jbmr.07s217. [DOI] [PubMed] [Google Scholar]
- 72.Unger WW, Laban S, Kleijwegt FS, van der Slik AR, Roep BO. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: differential role for PD-L1. Eur J Immunol. 2009;39:3147–59. doi: 10.1002/eji.200839103. [DOI] [PubMed] [Google Scholar]
- 73.Pletinckx K, Döhler A, Pavlovic V, Lutz MB. Role of dendritic cell maturity/costimulation for generation, homeostasis, and suppressive activity of regulatory T cells. Front Immunol. 2011;2:39. doi: 10.3389/fimmu.2011.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ghoreishi M, Bach P, Obst J, Komba M, Fleet JC, Dutz JP. Expansion of antigen-specific regulatory T cells with the topical vitamin D analog calcipotriol. J Immunol. 2009;182:6071–78. doi: 10.4049/jimmunol.0804064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:e60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morisseau C, Inceoglu B, Schmelzer K, Tsai HJ, Jinks SL, Hegedus CM, Hammock BD. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J Lipid Res. 2010;51:3481–90. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yessoufou A, Ple A, Moutairou K, Hichami A, Khan NA. Docosahexaenoic acid reduces suppressive and migratory functions of CD4+CD25+ regulatory T-cells. J Lipid Res. 2009;50:2377–88. doi: 10.1194/jlr.M900101-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bilal S, Haworth O, Wu L, Weylandt KH, Levy BD, Kang JX. Fat-1 transgenic mice with elevated omega-3 fatty acids are protected from allergic airway responses. Biochim Biophys Acta. 2011;1812:1164–9. doi: 10.1016/j.bbadis.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Woodworth HL, McCaskey SJ, Duriancik DM, Clinthorne JF, Langohr IM, Gardner EM, Fenton JI. Dietary fish oil alters T lymphocyte cell populations and exacerbates disease in a mouse model of inflammatory colitis. Cancer Res. 2010;70:7960–9. doi: 10.1158/0008-5472.CAN-10-1396. [DOI] [PubMed] [Google Scholar]
- 80.Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919–29. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zuniga LA, Jain R, Haines C, Cua DJ. Th17 cell development: from the cradle to the grave Immunol. Rev. 2013;252:78–88. doi: 10.1111/imr.12036. [DOI] [PubMed] [Google Scholar]
- 82.Yamane H, Paul WE. Early signaling events that underlie fate decisions of naive CD4(+) T cells toward distinct T-helper cell subsets. Immunol Rev. 2013;252:12–23. doi: 10.1111/imr.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schulz VJ, Smit JJ, Bol-Schoenmakers M, van Duursen MB, van den Berg M, Pieters RH. Activation of the aryl hydrocarbon receptor reduces the number of precursor and effector T cells, but preserves thymic CD4+CD25+Foxp3+ regulatory T cells. Toxicol Lett. 2012;215:100–9. doi: 10.1016/j.toxlet.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 84.Rohlman D, Pham D, Yu Z, Steppan LB, Kerkvliet NI. Aryl Hydrocarbon Receptor-Mediated Perturbations in Gene Expression during Early Stages of CD4(+) T-cell Differentiation. Front Immunol. 2012;3:223. doi: 10.3389/fimmu.2012.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singh NP, Singh UP, Singh B, Price RL, Nagarkatti M, Nagarkatti PS. Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PLoS One. 2011;6:e23522. doi: 10.1371/journal.pone.0023522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stevens EA, Mezrich JD, Bradfield CA. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology. 2009;127:299–311. doi: 10.1111/j.1365-2567.2009.03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gandhi R, Kumar D, Burns EJ, Nadeau M, Dake B, Laroni A, Kozoriz D, Weiner HL, Quintana FJ. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol. 2010;11:846–53. doi: 10.1038/ni.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 89.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, Luo W, Zeller K, Shimoda L, Topalian SL, Semenza GL, Dang CV, Pardoll DM, Pan F. Control of T(H) 17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–84. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–76. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ohkura N, Hamaguchi M, Morikawa H, Sugimura K, Tanaka A, Ito Y, Osaki M, Tanaka Y, Yamashita R, Nakano N, Huehn J, Fehling HJ, Sparwasser T, Nakai K, Sakaguchi S. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012;37:785–99. doi: 10.1016/j.immuni.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 92.Bodet C, La VD, Epifano F, Grenier D. Naringenin has anti-inflammatory properties in macrophage and ex vivo human whole-blood models. J Periodontal Res. 2008;43:400–7. doi: 10.1111/j.1600-0765.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 93.Hosokawa Y, Hosokawa I, Ozaki K, Nakanishi T, Nakae H, Matsuo T. Tea polyphenols inhibit IL-6 production in tumor necrosis factor superfamily 14-stimulated human gingival fibroblasts. Mol Nutr Food Res. 2010;54 (Suppl 2):S151–8. doi: 10.1002/mnfr.200900549. [DOI] [PubMed] [Google Scholar]
- 94.Fan H, Zhang R, Tesfaye D, Tholen E, Looft C, Hölker M, Schellander K, Cinar MU. Sulforaphane causes a major epigenetic repression of myostatin in porcine satellite cells. Epigenetics. 2012;7:1379–90. doi: 10.4161/epi.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang Y, Khor TO, Shu L, Saw CL, Wu TY, Suh N, Yang CS, Kong AN. A γ-tocopherol-rich mixture of tocopherols maintains Nrf2 expression in prostate tumors of TRAMP mice via epigenetic inhibition of CpG methylation. J Nutr. 2012;142:818–23. doi: 10.3945/jn.111.153114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zimmer J, Andrès E, Hentges F. NK cells and Treg cells: a fascinating dance cheek to cheek. Eur J Immunol. 2008;38:2942–5. doi: 10.1002/eji.200838813. [DOI] [PubMed] [Google Scholar]
- 97.Kurlandsky SB, Gamble MV, Ramarkrishnan R, Blaner WS. Plasma delivery of retinoic acid to tissues in the rat. J Biol Chem. 1995;270:17850–7. doi: 10.1074/jbc.270.30.17850. [DOI] [PubMed] [Google Scholar]
- 98.Chen P, Li M, Gu X, Liu Y, Li X, Li C, Wang Y, Xie D, Wang F, Yu C, Li J, Chen X, Chu R, Zhu J, Ou Z, Wang H. Higher Blood 25(OH)D Level May Reduce the Breast Cancer Risk: Evidence from a Chinese Population Based Case-Control Study and Meta-Analysis of the Observational Studies. PLoS One. 2013;8:e49312. doi: 10.1371/journal.pone.0049312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Linderborg KM, Kaur G, Miller E, Meikle PJ, Larsen AE, Weir JM, Nuora A, Barlow CK, Kallio HP, Cameron-Smith D, Sinclair AJ. Postprandial metabolism of docosapentaenoic acid (DPA, 22:5n-3) and eicosapentaenoic acid (EPA, 20:5n-3) in humans. Prostaglandins Leukot Essent Fatty Acids. 2013;88:313–9. doi: 10.1016/j.plefa.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 100.Macášek J, Vecka M, Žák A, Urbánek M, Krechler T, Petruželka L, Staňková B, Zeman M. Plasma fatty acid composition in patients with pancreatic cancer: correlations to clinical parameters. Nutr Cancer. 2012;64:946–55. doi: 10.1080/01635581.2012.716138. [DOI] [PubMed] [Google Scholar]
- 101.Khan MK, Rakotomanomana N, Dufour C, Dangles O. Binding of citrus flavanones and their glucuronides and chalcones to human serum albumin. Food Funct. 2011;2:617–26. doi: 10.1039/c1fo10077g. [DOI] [PubMed] [Google Scholar]
- 102.Fung ST, Ho CK, Choi SW, Chung WY, Benzie IF. Comparison of catechin profiles in human plasma and urine after single dosing and regular intake of green tea (Camellia sinensis) Br J Nutr. 2013;109:2199–207. doi: 10.1017/S0007114512004370. [DOI] [PubMed] [Google Scholar]
- 103.Savage PA, Malchow S, Leventhal DS. Basic principles of tumor-associated regulatory T cell biology. Trends Immunol. 2013;34:33–40. doi: 10.1016/j.it.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–64. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]


