Abstract
Neurons and especially their synapses often project long thin processes that can invaginate neighboring neuronal or glial cells. These “invaginating projections” can occur in almost any combination of postsynaptic, presynaptic, and glial processes. Invaginating projections provide a precise mechanism for one neuron to communicate or exchange material exclusively at a highly localized site on another neuron, e.g., to regulate synaptic plasticity. The best-known types are postsynaptic projections called “spinules” that invaginate into presynaptic terminals. Spinules seem to be most prevalent at large very active synapses. Here, we present a comprehensive review of all kinds of invaginating projections associated with both neurons in general and more specifically with synapses; we describe them in all animals including simple, basal metazoans. These structures may have evolved into more elaborate structures in some higher animal groups exhibiting greater synaptic plasticity. In addition to classic spinules and filopodial invaginations, we describe a variety of lesser-known structures such as amphid microvilli, spinules in giant mossy terminals and en marron/brush synapses, the highly specialized fish retinal spinules, the trophospongium, capitate projections, and fly gnarls, as well as examples in which the entire presynaptic or postsynaptic process is invaginated. These various invaginating projections have evolved to modify the function of a particular synapse, or to channel an effect to one specific synapse or neuron, without affecting those nearby. We discuss how they function in membrane recycling, nourishment, and cell signaling and explore how they might change in aging and disease.
Keywords: Capitate projections, Filopodia, Spinule, Synapse, Synaptic plasticity, Trophospongium
Introduction
Neuronal synapses function chiefly for rapid neurotransmission of sensory input, associative processing, and motor output, mediated by chains and complexes of neurons interconnected by multiple chemical, and to a lesser extent, electrical synapses. The formation and maintenance of this neuronal organization and interconnectivity depends on additional communication of information involving both long distance and short distance or paracrine signaling between the processes of neurons, as well as with the processes of associated glial cells. These paracrine mechanisms are probably crucial to normal brain function and may be central to the etiology of many neurological disorders. Paracrine communication often involves cell surface projections from the signaling cell. These projections can be very short and release vesicles called shedding vesicles or ectosomes (Cocucci et al. 2009; Muralidharan-Chari et al. 2009; Cocucci and Meldolesi 2015; ciliary ectosomes-Wood and Rosenbaum 2015), or they can form long filopodia that can make a direct contact with other associated cells in the local environment. Filopodia typically are thin cell processes, about 100–400 nm thick, with a core of actin, and vary in length from one to more than 200 μm (Kornberg and Roy 2014). These often are called cytonemes, although this name originally was used to define a particular kind of filopodia associated with Drosophila wing imaginal disk cells (Ramirez-Weber and Kornberg 1999; Fairchild and Barna 2014; Kornberg and Roy 2014; chapter 11 in Mueller et al. 2015). Not only are these filopodia involved in normal cell signaling, but they can develop also for intercellular transfer of viruses via “infectious or viral synapses” (Sherer et al. 2007; Sherer and Mothes 2008).
One of the most striking forms of paracrine cellular communication among cells is via invaginating projections; these structures invaginate into and thus are surrounded by the invaginated membrane of the receiving cell, and this arrangement may be the key to specialized, focused communication between neurons. Although filopodial contacts often remain only on the surface of the target cell, in some cases, the tip of the filopodium projects into a surface invagination. In addition to definitive filopodia, cells may contact other cells via shorter processes that appear to be either irregular varicosities or narrow, elongate structures reminiscent of synaptic spines and called “spinules.” Spinules typically are less than 100 nm in diameter and less than 1 μm in length, but the term generally is defined loosely in the literature. These various structures can mediate the transfer of proteins (transcytosis) involved in cell development (Cagan et al. 1992; Greco et al. 2001; Marston et al. 2003) as well as the spread of bacterial pathogens (Robbins et al. 1999).
In neurons, invaginating projections may mediate paracrine cellular communication and include a number of kinds of short cell surface projections that are associated with synapses or other parts of the neurons, and these often form a distinctive ultrastructure, where these projections invaginate into the adjacent cell structures. Various authors use the terms spinule, filopodium, and varicosity, and several other terms arbitrarily, and understandably, there is some vagueness in this terminology (Table 1). In this review, we will use “filopodia” to describe regular, finger-like processes more than 100 nm in diameter (implying some regular organization of cytoskeletal components such as actin filaments) and usually over 1 μm in length and “spinule” to describe thin processes usually less than 100 nm in diameter and under 1 μm long. Where these various terms were not used by the original citations, we will choose the most appropriate term based on the appearance of the structures. As an example of the latter, in the large mushroom spines in vertebrates, the postsynaptic density (PSD) often is perforated or segmented with thin areas of membrane between the PSDs. As we will see in “Small Postsynaptic Invaginating Projections: Spinules” section, these thin areas often invaginate into the presynaptic terminals as long, thin spinules. However, many authors still designate these as “spinules” even if the membrane area only bulges slightly into the presynaptic terminal and it does not form a long finger-like or spine-like projection. In another example, Popov et al. (2011) describe invaginating projections as “spinules,” “filopodia,” “spinule-like protrusions,” or “filopodia-like protrusions” in the mossy terminals of the CA3 region of the hippocampus (see “Small Postsynaptic Invaginating Projections: Spinules” and “Synaptic Spinules in Aging and Disease” sections), and these authors alternate among the terms throughout the publication. It probably is best to think of spinules, in general, as thinner forms of filopodia, with no absolute division between them.
Table 1.
Special terminology for invaginating projections, used in this review
| Appendages | This general term is used by some authors to describe dendritic projections that vary in shape from knob-like to filiform, emanating from unipolar brush cells and other related cell types of the cerebellum and cochlear nuclei (“Small Postsynaptic Invaginating Projections: Spinules” section). Those “appendages” that invaginate into mossy terminals look similar to some of the larger spinules described in this review for other neurons and synapses |
| Boutons | French term (means button, bud, spot, knob, pimple) used to describe the short invaginating projections from neuron-like cells in sponges (Pavans de Ceccatty 1966; “Synapse/Neuronal Invaginating Projections in the Simplest Animals” section); commonly also used to describe presynaptic axon terminals in other animals |
| Capitate projections | Specialized glial spinules invaginating into the presynaptic terminals of photoreceptor/retinular cells of fly eyes; they have a unique intercellular substance or “third membrane” in the spinule head (“Glia-Derived Invaginating Projections” section) |
| Filopodia | Finger-like, actin-filled processes, typically>100 to 400 nm in diameter and of varying length from 1 to>200 μm |
| Gnarls | Specialized short, glial projections, sometimes with a stalk and head region, associated with α and β fibers of fly eyes (“Glia-Derived Invaginating Projections” section) |
| Invaginating projections | An arbitrary term used in this review to define all kinds of these processes |
| Microvilli | Similar to filopodia in structure but tend to form more regular, parallel bundles on a cell surface. Here, they are described from dendrites in nematode peripheral sense organs including amphids (“Large Postsynaptic Invaginating Projections: Filopodia and Spines” section) and from the apical end of the developing R8 retinal cell in Drosophila (“Function of Invaginating Projections in Cell Signaling” section) |
| Probosci | Thin spinules arising from parallel fiber axons and invaginating into Purkinje cell dendrites in the cerebellum of the early postnatal rat (Altman and Bayer 1997; “Presynaptic Terminal/Axon-Derived Invaginating Projections” section). These authors also refer to this structure as growing “like a tongue” |
| Pseudopodial interdigitations (PSIs) |
“PSIs” are large invaginating projections between abutting presynaptic terminals in the torpedo ray electrical organ (Boyne and McLeod 1979) and mammalian limbic system (Boyne and Tarrant 1982). They can be short or long, finger-like or highly irregular, and simple or compound (in which PSIs may interdigitate with each other), and they usually contain some synaptic vesicles |
| Spine protrusions | Synonym for spinule, used by Erisir and Dreusicke (2005) for invaginating projections in the ferret visual cortex (“Small Postsynaptic Invaginating Projections: Spinules” section) |
| Spinules | Typically slender processes, mainly <100 nm in diameter, although often with an enlarged head region, and usually less than 1 μm long |
| Tongues | Small spinules from the presynaptic terminal membrane invaginating into the postsynaptic process (here described by Waxman et al. 1980, in a lizard and gymnotid fish; “Presynaptic Terminal/Axon-Derived Invaginating Projections” section). The analogy to a tongue has been used for other projections, including the protrusions of membranes of vesicles at synapses in the simple, flatworm-like animal, Xenoturbella westbladi (Raikova et al. 2000; “Synapse/Neuronal Invaginating Projections in the Simplest Animals” section), and for projections of parallel fibers in the developing cerebellum (“Presynaptic Terminal/Axon-Derived Invaginating Projections” section; Altman and Bayer 1997; see definition of proboscis) |
| Trophic prolongations | Long processes with enlarged heads containing a desmosome attachment; parenchymal cells in an ectocommensal flatworm invaginate these into adhesive secretion gland cells; the end lies very close to the gland cell nucleus (Williams 1994; “Synapse/Neuronal Invaginating Projections in the Simplest Animals” section) |
| Trophospongium | Elongate glial processes of varying lengths and shapes that invaginate into neuron cell bodies or axons, and are believed to have a trophic function (“Glia-Derived Invaginating Projections” section) |
| Tunnel fibers | Fibers formed from the trunks of small neurons called microneurons, and that invaginate, often in groups of three or more, into presynaptic terminals in the optic lobe of octopi (these terminals also have invaginating postsynaptic spines; Dilly et al. 1963; Case et al. 1972; “Large Postsynaptic Invaginating Projections: Filopodia and Spines” section) |
| Varicosities | Generally used to describe highly irregular and often swollen projections. The parallel fiber terminals that enwrap Purkinje spines of the developing cerebellum also are described by Altman and Bayer (1997) as varicosities (“Large Postsynaptic Invaginating Projections: Filopodia and Spines” section) |
Spinules and other invaginating projections associated with synapses can involve any combination of the three main components of the synapse, the presynaptic terminal (as well as axon), postsynaptic spine or dendrite shaft (or muscle cell for neuromuscular junctions; NMJs), and the associated glial cells (Table 2). They include: (1) those derived from the spines and projecting into invaginations in the presynaptic terminal or adjacent glial processes; (2) projections from the presynaptic terminals that extend into invaginations in the dendritic process; and (3) projections from the surrounding glial cell processes. In some cases, these processes simply may be a means to remove and degrade excess cell membrane (membrane retrieval) or to remove certain associated components such as specific receptor proteins, but this could also be a method of close, controlled signaling between the cells that together produce the synapse (Spacek and Harris 2004; Tao-Cheng et al. 2009).
Table 2.
Dendritic, axonal, and glial invaginating projections: major ones mentioned in this review, classified by animal groups
| Animal group | Dendritic projection (“Synapse/ Neuronal Invaginating Projections in the Simplest Animals”, “Spine/ Dendrite-Derived Invaginating Projections” sections) |
Axonal projection (“Synapse/Neuronal Invaginating Projections in the Simplest Animals”, “Presynaptic Terminal/Axon- Derived Invaginating Projections” sections) |
Glial projection (“Synapse/Neuronal Invaginating Projections in the Simplest Animals”, “Glia-Derived Invaginating Projections” sections) |
|---|---|---|---|
| Porifera (sponges) |
Possibly (spine-like process on neuron-like cell into another cell) |
Possibly (terminal-like process on neuron-like cell into another cell) |
|
| Cnidaria (jellyfish) |
Spine into terminal Nematocyte process into axon |
Axon into nematocyte | |
| Platyhelminthes (flatworms) |
Presumptive postsynaptic process into terminal |
Terminal into presumptive postsynaptic process |
Trophospongium [also trophic prolongations of parenchymal cells (not glia)] |
| Nematoda | Amphid microvilli into sheath cell | ||
| Annelida | Trophospongium | ||
| Mollusca (snails, squid, octopi, chitons) |
Spines and tunnel fibers into terminal Spinules into chiton sensory hair supporting cell |
Axon–axon spinules Axon–axon projections associated with the squid eye |
Trophospongium |
| Crustacea (crayfish) |
Trophospongium | ||
| Arachnida (scorpion) |
Between two nerve fibers or a nerve fiber and a terminal in the cardiac ganglion |
||
| Insecta | Axon–axon projections associated with fly, bee, butterfly eyes Invaginating axon terminals in larval NMJ |
Trophospongium Capitate projections (fly eye) Gnarls (fly eye) |
|
| Fish | Lamprey-presumptive postsynaptic invaginations into terminal Fish retinal spinules |
PSIs in synapses with electrical organ of torpedo ray Spinule of presynaptic membrane into postsynaptic process in electromotor n. of gymnotid fish |
|
| Amphibia (frogs) |
Filopodia into terminals | PSIs in NMJ | Filopodia into terminals Schwann cell processes at frog NMJ |
| Reptiles (lizard) | Spinule of presynaptic membrane into postsynaptic process in oculomotor nucleus |
||
| Birds | Axon spinules in Purkinje dendrites | ||
| Mammals (mouse, rat, cat, primate, etc.) |
Spines, filopodia, and spinules into terminals (including regular and giant mossy terminals) |
PSIs on the sides of synaptic terminals PSI-like projections of GABAergic terminals into the postsynaptic neuron Axon spinules in Purkinje dendrites Spinules in dendritic processes at mossy fibers Invaginating terminals into spines |
Oligodendrocyte processes into spinal motor axons Schwann cell processes into spinal ganglion neurons |
Thus, invaginating neuronal projections, as described in this review, may play key roles in neuronal communication. For example, a recent study shows how one kind of inhibitory presynaptic terminal in the amygdala and entorhinal cortex invaginates short projections used for precise regulation of tonic synaptic inhibition via focused retrograde cannabinoid signaling (Omiya et al. 2015). Apparently, a high concentration of the cannabinoid signaling in an invaginated projection is necessary for this particular functional response. And here is a classic example: In the development of the eight specific photoreceptor cells forming a functional unit in the Drosophila eye, the R8 cell probably utilizes transcytosis via invaginating projections to transfer the receptor tyrosine kinase ligand, bride of sevenless; this induces the receiving cell to develop into the R7 cell (Cagan et al. 1992; chapter 10 in Mueller et al. 2015). Thus, the invaginating projections allow the signal to affect only one neuron and not its close neighbors, to achieve the proper arrangement of photoreceptor cells.
This review will concentrate on describing the various kinds of invaginating projections found at synapses, as well as in neurons in general, in all kinds of animals, from the simplest to the most complex. We will discuss their possible functions, especially where they may play a role in cell signaling, in normal function as well as in aging and disease.
Synapse/Neuronal Invaginating Projections in the Simplest Animals
Before the nervous system: protists and sponges
Even the simplest single-celled organisms may communicate via extended cell processes. Many kinds of bacteria communicate with adjacent bacteria via a variety of tubular structures, secreted outer–inner membrane vesicles, and tubules made of outer membrane vesicle chains (Wanner et al. 2008; Pérez-Cruz et al. 2015; Remis et al. 2014), suggesting that animal filopodia and spinules have their functional origins in a basic mechanism of intercellular communication that evolved in the early evolution of cells. Also, eukaryote protists and the simplest metazoans can have elongate cellular processes with varied structures and functions (Sebé-Pedrós et al. 2013). Individual cells in colonial choanoflagellates, which are protists that form into colonies resembling (and maybe homologous to) the choanocyte filter cell assemblages of the simple animals—the sponges or Porifera (reviewed in Petralia et al. 2014b), contact each other via intercellular bridges and in some cases, filopodia (Dayel et al. 2011). Dayel et al. (2011) suggests that filopodia may serve in cell signaling among the cells of these colonies, functioning like the epithelial cytonemes discussed in the Introduction.
Even the simplest of multicellular animals (metazoans) may have neuron-like cells that contact other cells via elongate cellular processes, and these processes also may bear the first and simplest examples of synapse-like contacts as well as invaginating projections. Simple metazoans called placozoans have fiber cells that superficially resemble neurons and extend filopodia-sized processes that make close contacts with other cells (Jorgensen 2014; Smith et al. 2014). Sponges, another very simple metazoan group, may have cells like this (Pavans de Ceccatty 1966; Renard et al. 2009; Nickel 2010). In the sponge, Tethya lyncurium, these elongate cells form partially invaginated contacts with at least two other kinds of cells, i.e., an enlarged end (“bouton”) of the filopodial process fits into a shallow pocket on the receiving cell (Fig. 1a, b; Pavans de Ceccatty 1966). In addition, filopodia-like invaginations are found among groups of sponge choanocytes, lining the specialized filter chambers of sponges (Gonobobleva and Maldonado 2009). While choanocytes are not neurons, they may represent an early pre-stage in the evolution of metazoan sensory hair cells (Fritzsch and Straka 2014; also see discussion of cochlear hair cells in “Spine/Dendrite-Derived Invaginating Projections” and “Presynaptic Terminal/Axon-Derived Invaginating Projections” sections).
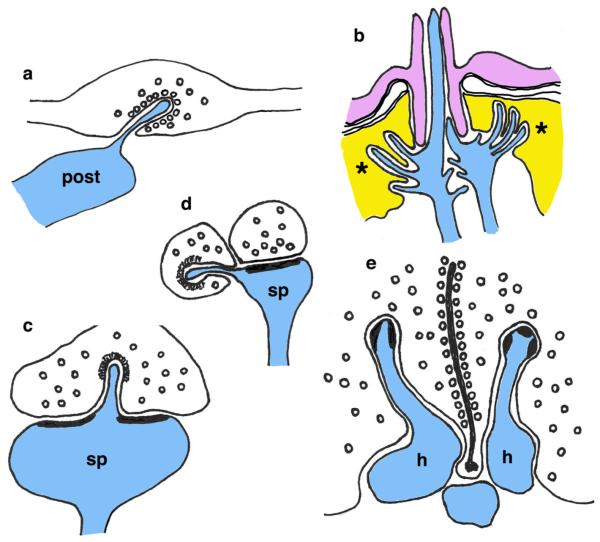
Fig. 1.

Invaginating projections in simple animals. a, b In the sponge, Tethya lyncurium, elongate processes with many fine filaments, from neuron-like cells (a network of elongate cells with thin, often branched processes that may interconnect among the cells), project “boutons” (asterisks) that can make invaginated contacts with other cells, including scleroblasts (a; sc) and flagellated endopinacocytes (b; ep). The processes contacting the latter kind of cell are 100–250 nm in diameter, and the intercellular space at the contact is about 20 nm (Pavans de Ceccatty 1966). c “Trophic prolongation” from a parenchymal cell that extends into an adhesive gland cell in the flatworm, Temnocephala novaezealandiae; the swollen end forms a desmosome attachment (arrow) near the gland cell nucleus (nucleus not shown; Williams 1994). All drawings in Figs. 1, 3, 4, 7, 8, and 9 are original and are based on micrographs and illustrations in the original publications
The first nervous systems
Among the basal metazoan groups, definitive neuronal tissue and synapses are found in the cnidarians (jellyfish, corals, sea anemones, hydras) and ctenophores (comb jellies); no definitive neuronal tissue is yet known for placozoans and sponges (Jorgensen 2014; Halanych 2015; Leys 2015). The simplest bilaterians, the flatworms (acoels, planarians, flukes, tapeworms), have definitive brains. Yet there does not seem to be any special development or abundance of spinules or similar invaginating projections associated with the synapses of these simple animals. Other animals with relatively simple nervous systems (nematodes, chaetognaths, tunicates, echinoderms, etc.) also do not show any particular preponderance of synaptic spinules and other projections; at least we could not find clear evidence of this in a perusal of the relevant literature. In addition, in a detailed examination of the ultrastructure of the brain of a planarian flatworm (probably Dugesia tigrina; brown planaria from Carolina Biological Supplies Company), no definitive spinules or other long synaptic invaginating projections were found (R.S.P. and Y.-X.W. unpublished data). Planaria have abundant, well-developed synapses with a variety of vesicle types and often with two postsynaptic processes. Various processes in the neuropil often project slightly into shallow depressions on the surface of other processes where they contact each other (as in the neuropil of almost any animal); a few of these processes may go deep enough into the other processes to be considered short invaginating projections (Fig. 2).
Fig. 2.
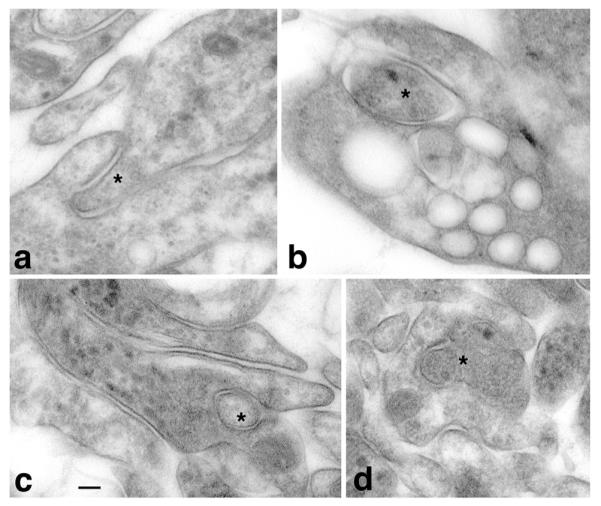
Apparent short invaginating projections (asterisks) in the cephalic nervous tissue of the brown planaria. a Two unidentified processes. b An invaginating projection in a possible growth cone. c An invaginating, presumptive postsynaptic process in a presynaptic terminal (note the many clear and dense-cored synaptic vesicles in the terminal). d An invaginating presynaptic terminal filled with clear synaptic vesicles, in a presumptive postsynaptic process. Scale bar is 100 nm (unpublished data: general methods similar to those in Petralia et al. 2011, 2012)
There are a few examples described in the literature of various invaginating projections associated with the neuronal processes and synapses in the simplest nervous systems. A variety of such structures are associated with neuronematocyte synapses in the medusoid (“jellyfish”) cnidarian, Gonionemus vertans (Westfall 1970). These projections occur both ways in the contact between presynaptic terminals plus their proximal axonal region and postsynaptic processes of nematocytes (stinging cells bearing nematocysts). They vary from filopodia to irregular varicosities to short spinules (see figures 3 and 4 in Westfall 1970). Satterlie and Case (1978) show a micrograph of neuron processes that penetrate within cells of the comb plates (specialized ciliated swimming structures) in the ctenophore, Pleurobrachia bachei. In the intraepidermal nerve net of one of the simplest, flatworm-type animals, Xenoturbella westbladi (probably belonging to the acoel flatworm group), Raikova et al. (2000) describe and illustrate “paracrine release sites with tongue-like protrusions of the membranes of the vesicles penetrating the membrane of…” synaptic terminals. It is difficult for us to tell from the micrographs whether they are a type of spinule or some unique structure. In addition, representatives of all of the major groups of flatworms including planarians, flukes, and tapeworms have thin glial processes that extend into invaginations in some neuron cell bodies and nerve processes (Sukhdeo and Sukhdeo 1994; Mäntylä et al. 1998; Biserova 2008; Biserova et al. 2010). Similar glial invaginating projections may be present also in the brain of an acanthocephalan worm, although the glial origins of these are not clearly described (Budziakowski and Mettrick 1985). Such structures may be relevant to the early evolution of glia-derived synaptic processes that are described in “Glia-Derived Invaginating Projections” section. Other kinds of invaginating cell processes may be relevant to this evolution. In the ectocommensal flatworm Temnocephala novaezealandiae that lives on crayfish (Williams 1994), parenchymal cells send long “trophic prolongations” deep within adhesive secretion gland cells (of the sucker used for commensal attachment) (Fig. 1c); portions of these processes can be thick and contain microtubules and slender mitochondria, but the tapering ends of these resemble spinules, especially when compared to capitate glial projections of insects (see “Glia-Derived Invaginating Projections” section). They appear to be less than 100 nm in diameter in thin parts and have a swollen tip forming a desmosome attachment lying very close to the nucleus of the invaginated gland cell. In this case, these processes probably serve mainly as anchors to stabilize this flatworm’s sucker attachment to the host crayfish.
Fig. 3.
Spine/dendrite-derived invaginating projections. a In the mollusk, Aplysia californica, an elongate spine from a postsynaptic process (post) invaginates deep into a large presynaptic terminal to form a synapse (Bailey et al. 1979). b Nematodes have multisensory peripheral sense organs in which the dendrites of some of the sensory neurons form microvilli or microvillus-like structures (asterisks) that invaginate into a surrounding sheath cell (yellow; pore cuticle is violet; Wright and Hui 1976; Wright 1992). c A large mushroom spine (sp) in the CA1 region of the hippocampus can project a spinule from the central area of the postsynaptic membrane where the spinule perforates the PSD and may even separate the PSD into segments. In the drawing, the spinule tip is surrounded by a clathrin-coated pit of the presynaptic terminal. d A spinule (clathrin-coated pit at end) from a thin spine (sp) in the CA1 region of the hippocampus typically invaginates a nearby axon terminal rather than the presynaptic terminal of that spine; this drawing is based largely on our micrograph in Fig. 6. e Horizontal cells (h) of cone photoreceptor cell synapses (note the elongate presynaptic ribbon, cut on edge, lined with synaptic vesicles) in fish can form specialized spinules with thickened dense regions. Note that dendritic processes are blue and glial processes are red in all drawings in Figs. 3, 4, 7, 8 and 9. Presynaptic terminals are not colored and contain small, round circles representing synaptic vesicles (Color figure online)
Fig. 4.
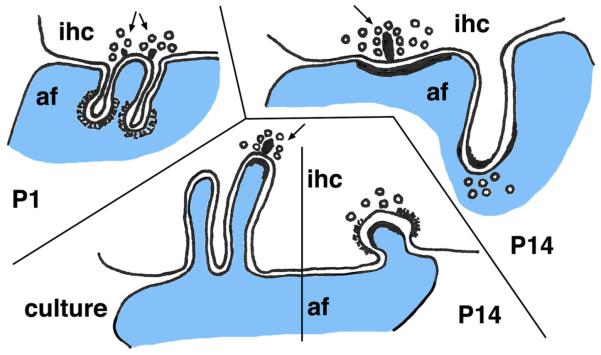
In the cochlea, inner hair cells (ihc) form specialized synapses with presynaptic bodies or ribbons surrounded by vesicles (arrows), apposed to the postsynaptic membrane and PSD of an afferent dendrite process (af); various invaginating projections can form at this synapse (Sobkowicz et al. 1986, 2002, 2003). Examples shown include thin spinules invaginating into the postsynaptic afferent dendrite process at P1 (postnatal day 1; note the clathrin-coated end; upper left drawing). At about P14, large filopodia from the hair cell can invaginate into the afferent ending and form a spine synapse (upper right). At this age, other short filopodia from the afferent dendrite can invaginate into the hair cell (lower right). Note the partial coated (probably a clathrin coat) membrane around the invagination. If slices are placed in culture on P5 (lower left), the afferents can grow multiple filopodia that invaginate into the hair cell (over several days in culture), with the tips eventually being sites of synapse formation (Sobkowicz et al. 1998) (dendritic processes in blue) (Color figure online)
Evolution of invaginating projections
The examples given above demonstrate that animal cells developed the ability to interact via invaginating projections early in metazoan evolution. Yet these simple animals appear to lack any kinds of elaborate invaginating projections in their nervous systems. We suggest that more elongate and specialized invaginating projections were either not a major contributor to the early evolution of the nervous system, or perhaps if important in some immature life stage, then they might form and regress over brief times during neuronal differentiation. Indeed, even synaptogenesis in the highly complex CA1 region of the hippocampus of the early postnatal rat involves extensive development and interactions of transient filopodia, yet invaginating projections have not been described in this process (Fiala et al. 1998). More likely, synaptic spinules and other specialized invaginating projections have increased in frequency and importance as nervous systems evolved to mediate more complex behavior patterns of higher animal groups such as insects and vertebrates, as we shall describe in the following sections of this review.
It is difficult at this point to theorize in any detail on evolutionary trends for invaginating projections because the literature is not complete. It is unclear whether the known distribution (as we shall cover in this review) is due to a real trend in evolution, or just an emphasis on animals and parts of the brain that are of most interest. Scientists who study the ultrastructure of the brains of different animals often ignore invaginated projections in their micrographs. Often there are hints of these structures on the edges of micrographs or in low-magnification micrographs, but the authors make no mention of them. Thus, broad evolutionary speculation from what is probably sketchy data is problematic. This is one reason that we decided to compile this thorough review of what is published so far on the subject.
Synaptic/Neuronal Invaginating Projections in More Complex Animals
Spine/Dendrite-Derived Invaginating Projections
Overall, the best-documented studies of synaptic spinules and related projections describe postsynaptic processes, especially from spines, projecting into invaginations in presynaptic terminals. Since spines themselves are a kind of postsynaptic process, and spinules resemble miniature spines in general appearance, we will consider here the entire continuum of postsynaptic dendritic processes, especially those that interact with and may project into invaginations in presynaptic terminals.
Large Postsynaptic Invaginating Projections: Filopodia and Spines
Filopodia profiles in micrographs may be difficult to distinguish from profiles of the thinner spines (i.e., the latter having synaptic contacts or active zones), as both are elongate structures filled with actin filaments. Filopodia are common in the early postnatal CA1 area of the hippocampus and may form an initial contact in the development of many synapses (Fiala et al. 1998), especially excitatory synapses (Wierenga et al. 2008). These filopodial contacts are probably the equivalent of the filopodia/cytonemes used by some types of epithelial cells for intimate contact with adjacent cells (described in the “Introduction” section). Like cytonemes, these filopodial contacts may be surface contacts only and may not involve invagination of the end of the filopodia into the terminal (Fiala et al. 1998). And some types of synapses such as GABAergic ones may form directly from pre–post contacts without intervening filopodia (Wierenga et al. 2008). When isolated bullfrog brains are stimulated with potassium chloride, synapses in the forebrain often develop filopodia from the postsynaptic membrane or an adjacent glial process, and these invaginate into the presynaptic terminals (Harreveld and Trubatch 1975). They are about 100–200 nm in diameter and extend about 3–4 μm into the terminal and may have a pinocytotic vesicular pit (coated?) at the tip of the surrounding presynaptic membrane. These filopodia may be functionally comparable to the thinner spinules that are induced by potassium stimulation in the hippocampus (see “Small Postsynaptic Invaginating Projections: Spinules” section; Tao-Cheng et al. 2009).
Invaginating spine synapses
Just as some cytonemes form deep invaginations, some large postsynaptic processes extend into deep invaginations in presynaptic terminals. These can form definitive synaptic contacts, although it is not clear whether these formed first as filopodial invaginations or whether the terminal enlarged around a developed synaptic spine. Examples are widespread among animal types, including in the simple nervous systems of cnidarians (Holtmann and Thurm 2001) and echinoderms (Mashanov et al. 2006). In the mollusk, Aplysia californica, synaptic contacts deep within large terminals form at the tips of thin, elongate spines that look more like filopodia than spines; these are typically 100–250 nm in diameter and can be over a micrometer in length (Fig. 3a; Bailey et al. 1979). Some presynaptic terminals in the optic lobe of cephalopod mollusks, including the squid, Loligo pealei (Cohen 1973) and octopi, Eledone and Octopus (Dilly et al. 1963; Case et al. 1972), have similar invaginated postsynaptic dendritic processes. Octopi also have enigmatic “tunnel fibers” that invaginate into the same terminals that contain the definitive invaginated spine synapses; often three or more tunnel fibers may run together in a single “tunnel” in a terminal (Dilly et al. 1963; Case et al. 1972; not found in the squid, Cohen 1973). Often the large “carrot”-shaped terminals in octopi contain several invaginated spine synapses and tunnels with tunnel fibers. Tunnel fibers are formed from the “trunk” of small neurons called microneurons, believed to be “amacrine”-type neurons involved in local circuitry; they may represent postsynaptic elements, but their putative synaptic structure is not as definitive as that of the invaginated spine synapses (Case et al. 1972).
Elongate spines enwrapped in terminals are seen also in the early postnatal development of parallel fiber–Purkinje spine synapse in the rat cerebellum (Altman and Bayer 1997; Zhao et al. 1998; and in the adult—Gray 1961). Altman and Bayer (1997) describe its development as follows: The undifferentiated spine penetrates a parallel fiber varicosity, then presynaptic vesicles and pre- and postsynaptic dense membranes form, and later the parallel fiber varicosity matures into a bulbous or kidney-shaped structure. Purkinje cell spines that form synapses with climbing fibers also sometimes may invaginate almost completely into the climbing fiber terminal (Palay and Chan-Palay 1974). In the giant mossy fiber terminals of the hippocampus (see “Small Postsynaptic Invaginating Projections: Spinules” section), the postsynaptic thorny excrescences, although not technically spines, can be nearly completely invaginated into the mossy terminals (Nitsch and Rinne 1981; Chicurel and Harris 1992; Petralia and Wenthold 1992; Petralia et al. 2011). Inner hair cells of the mouse cochlea (see subsequent sections below) can be involved in a three-way circuit among a hair cell body, and afferent and efferent terminals, and all three processes may include some invaginating postsynaptic spine processes (Sobkowicz et al. 2003).
Cochlea hair cell synapses
A variety of invaginating spinules and larger filopodia are associated with the inner hair cell ribbon synapse of the mouse cochlea, especially during development (Fig. 4). These include both the processes going from post to pre (this section) and from pre to post (see “Presynaptic Terminal/Axon-Derived Invaginating Projections” section). In these synapses, the main presynaptic process is actually the base of the inner hair cell body, which transduces sound signals into chemical synaptic transmission at this synapse. It bears a ribbon synapse, although unlike the ribbon synapses of the retina, the shape of the “ribbon” in the inner ear can be spherical, planar, or oblong and often is called a synaptic “dense body” (Matthews and Fuchs 2010). Functionally, the hair cell soma base acts as the axonal terminal of the synapse. The postsynaptic process is a dendritic afferent fiber derived from a spiral ganglion neuron that sends its axon into the brain via the eighth cranial nerve. Efferent axonal terminals from brain neurons also are associated with the hair cell synapses and afferent fibers. The synaptic organization is further complicated in some cases by reciprocal synapses, especially between the afferent ending and the hair cell base (Sobkowicz et al. 2003). During postnatal development, especially around the second week (onset of hearing), the dendritic afferent terminal extends invaginating projections into the presynaptic hair cell, typically adjacent to the synapse active zone or its precursor region (Sobkowicz et al. 1986, 2002, 2003). These can be elongate filopodia several micrometers long (Sobkowicz et al. 1986), but the ones illustrated in Sobkowicz et al. (P14–16; 2002) are mainly short, thick spinules or filopodia with enlarged heads that may have a “dense fuzzy coat of postsynaptic density.” The surrounding presynaptic membrane of the hair cell is often coated, presumably by clathrin; these “coated pits” are about twice the size of standard coated pits seen on the adjacent hair cell synaptic membrane region. Alternatively, there can be several regular coated pits surrounding the process. In some cases, the invading filopodium actually forms a postsynaptic spine process opposite presynaptic ribbons. Development of these processes is enhanced if the organ of Corti (part of the cochlea containing the inner hair cells) is transplanted into culture at P5 (Sobkowicz et al. 1998). After 1 day in culture, the afferent dendrites invaginate numerous filopodia; their ends have enlarged clathrin coats or bud off regular clathrin-coated pits. True immature synapses also form and increase in number. These have presynaptic vesicles and developing ribbon structures; the postsynaptic structure in these synapses appears to be a filopodium that is developing a PSD. After about 5 days, most of the filopodia have degenerated, leaving some remnants appearing as “fibrous cytoplasmic plaques”; some of the micrographs appear to show degradation of filopodia in autophagosomes, although a few may persist. The authors also noted some evidence of filopodial growth into outer hair cells (Sobkowicz et al. 1998). In another study using cultures made from newborn mice and laser ablation of spiral ganglion neurons, filopodia from the surviving afferent fibers can invaginate into both inner and outer hair cells (Sobkowicz et al. 1999).
Amphid microvilli
Interestingly, many nematodes have specialized cell process invaginations equivalent to the dendritic filopodia/spine invaginations described above. Within multisensory peripheral sense organs in the head of the nematode, dendrites of some neurons form a large array of microvilli or microvillus-like structures that invaginate into a sheath cell surrounding the central receptor cavity (Fig. 3b; Wright and Hui 1976; Wright 1992). Microvilli are similar in structure to filopodia but tend to form more regular, parallel bundles on a cell surface; both structure and molecular composition vary among filopodia and microvilli from different animals (Passey et al. 2004; Sebé-Pedrós et al. 2013). In the primary sensory organs of the head, called amphids, the dendrite with the invaginating microvilli appears to be thermosensory (Ashton et al. 1999; Bumbarger et al. 2009). This dendrite bears ~75 to 100 microvilli embedded in the sheath cell in the first-stage juvenile, J1, and 400–500 in the adult that terminate either within the sheath cell or between dendrites in the sensory channel (Bumbarger et al. 2009). Interestingly, in the adult, the microvillar membrane adjacent to the sheath cell membrane appears to be modified and shows dense patches, while these modifications are not seen in the juvenile stage (Bumbarger et al. 2009). Could this be a parallel development to the maturational change from filopodial to synaptic spine contact that may occur at some animal synapses?
Small Postsynaptic Invaginating Projections: Spinules
Hippocampus
Postsynaptic spinules have been studied best in the hippocampus (Figs. 3, 5, 6, and 7). Westrum and Blackstad (1962) found that the spinules in synapses from the CA1 stratum radiatum of adult rats ranged from 25 to 100 nm in width and 75 to 150 nm long, while the surrounding presynaptic membrane was 60 to 100 nm wide and 100 to 250 nm long. Spacek and Harris (2004) found that spinules from the CA1 stratum radiatum of the adult rat hippocampus can be <8 to 80 nm at the base and vary from 27 to 150 nm in width. Those from dendritic spines range from 33 to 765 nm in length, while those derived from axons range from 40 to 240 nm (Spacek and Harris 2004). Postsynaptic spinules in hippocampal slice cultures (from 6 to 8 day postnatal [P6–8] rats and cultured for 10 to 14 days) range in length from ~80 to ~500 nm (Tao-Cheng et al. 2009). These spinules are distinctly smaller than filopodia, and so their internal structure is more difficult to discern; they can contain filamentous structures, often continuous with those in the spine, as well as occasional small dense puncta that resemble ribosomes (Tarrant and Routtenberg 1977, 1979). Often the tip of the spinule is surrounded by a coated pit at the end of the presynaptic membrane invagination (Westrum and Blackstad 1962; Tarrant and Routtenberg 1977; Spacek and Harris 2004; Yao et al. 2005; Tao-Cheng et al. 2009). Spacek and Harris (2004) found coated pits on the tips of ~70 % of the spinules.
Fig. 5.
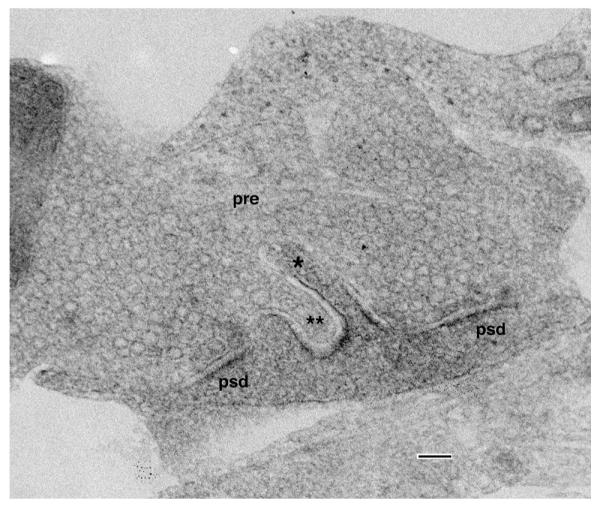
Reciprocal spinules in a synaptic terminal from a hippocampal cell culture; one spinule (**) invaginates into the postsynaptic process, and another (*) invaginates into the presynaptic terminal (pre). psd, postsynaptic density. Scale bar is 100 nm (unpublished data; for methods, see Mitchell et al. 2012)
Fig. 6.

A spinule (small open arrowheads) is seen emerging from a spine into a neighboring axon in the CA1 stratum radiatum region of the hippocampus of a young adult rat. The spinule is located at the side of a synaptic junction as characterized by the presence of synaptic vesicles (SV) and the PSD. The invaginated membrane of the neighboring axon (probably part of a developing terminal) surrounds the tip of the spinule and is clathrin-coated (black arrow), and the endocytic accessory protein AP180 (immunogold indicated by white arrowheads) is associated with the spinule. This section is a little thicker than normal, probably more than 90 nm thick; this allows more of the spinule profile to be visible in the section but causes some of the structural details of the synapse to be obscured. Scale bar is 50 nm. Reprinted from figure 7D of Yao et al. (2005)
Fig. 7.
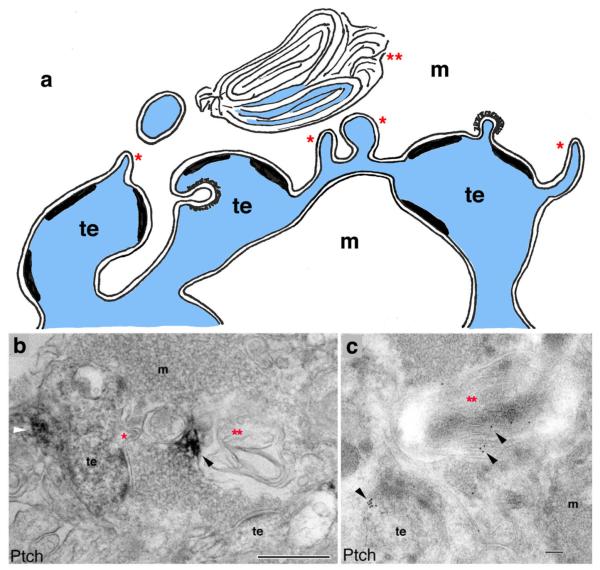
Invaginating projections in hippocampal mossy terminals. a Mossy terminals (m) are very large and filled with large, round vesicles as well as some dense-cored vesicles (for clarity, vesicles and other organelles are not drawn) and form synapses on modified dendritic processes called thorny excrescences (te; Petralia et al. 2011). Spinules (single red asterisks) of various shapes form from the dendrites and appear to pinch off in places. Association of some spinule processes with large autophagosomes (double red asterisks) in the terminal suggests that spinules may be acquired and degraded in the terminal (Petralia et al. 2011). Spinules may become more abundant in water maze-trained rats and even connect between thorny excrescences (center of diagram; Stewart et al. 2005a, b). In mossy terminals in rabbits during epileptiform seizures, invaginated spinules with coated membrane on their apex can form both into the terminal and into the thorny excrescences (Nitsch and Rinne 1981). b, c. Association of the sonic hedgehog receptor, patched (Ptch), with synaptic spinules and autophagosomes in the CA3 mossy terminal area of the adult hippocampus. b Ptch immunoperoxidase/DAB labeling (white arrowhead) in a thorny excrescence with a spinule. Ptch labeling (black arrowhead) is also seen in a nearby irregularly shaped autophagosome. c Ptch immunogold labeling (arrowheads) in a thorny excrescence and in a nearby large autophagosome. Scale bar 500 nm in b and 100 nm in c. (b and c reprinted from part of figure 8 of Petralia et al. 2011) (dendritic processes in blue) (Color figure online)
Spacek and Harris (2004; also Sorra et al. 1998) found, in the CA1 region of the adult rat hippocampus, that postsynaptic spinules from the large mushroom spines originated from the central area of the synaptic contact and usually extended into invaginations in the main presynaptic terminal (Figs. 3c), while spinules from the thin spines originated from the side of the head or neck of the spine and extended into invaginations in side terminals or glial processes (Figs. 3d, 6; Yao et al. 2005). Thin spine spinules may be involved in a competition for synapses with neighboring axons, while mushroom spine spinules are involved in remodeling and retrograde signaling. Large mushroom terminals typically have a perforated, or segmented, completely partitioned PSD, and the spinule in many cases may become elongated both into the terminal and also along the active zone membrane; initially its appearance perforates the PSD, and then, as it grows, it completely segments the PSD (Geinisman et al. 1994; Ganeshina et al. 2004a, b; Stewart et al. 2005b; Petralia et al. 2014a). As the spinule projection grows, its cross-sectional shape becomes more ridge-like as it divides the PSD (Calverley and Jones 1987; Geinisman et al. 1994; Petralia et al. 2014a). This appears to be a key component in synaptic plasticity, such as occurs during long-term potentiation (LTP), with many spine synapses going through cycles of structural enlargement and shrinkage related to activity, aging, and changing levels of neurotransmitter receptors (reviewed in Geinisman et al. 1994; Geinisman 2000; Stewart et al. 2005b; Huganir and Nicoll 2013; Meyer et al. 2014; Petralia et al. 2014a). There is some evidence that synaptic spines could divide into two under certain circumstances, following enlargement of the spine, segmentation of the density, and formation of the spinule into a presynaptic partition, but this still is controversial (Carlin and Siekevitz 1983; Sorra et al. 1998; Toni et al. 1999; Fiala et al. 2002; Richards et al. 2005; Stewart et al. 2005b; Medvedev et al. 2014).
Several studies have experimentally stimulated neurons of the hippocampus to produce spinules, including those that show the increase in perforated and complex PSDs separated by spinule structures. Examples include incubation of high-density neuron cell cultures with estradiol (a form of the hormone estrogen; Murphy and Andrews 2000), stimulation of hippocampal slice cultures with high potassium to induce depolarization (Tao-Cheng et al. 2009), incubation of hippocampal slice cultures in a modified chemical medium designed to induce LTP (Stewart et al. 2005b), induction of LTP in hippocampal slices using a high-frequency stimulation method (Toni et al. 1999), and induction of LTP via implanted stimulating electrodes in live rats (Schuster et al. 1990; Geinisman et al. 1994). Ueda and Hayashi (2013) used two-photon glutamate uncaging to induce LTP in targeted spines; this caused a persistent enlargement of the spines and the generation of filopodia-like protrusions that they call “spinules.” However, they did not do electron microscopy, and so, it is not known whether any of these filopodia invaginated into terminals. Interestingly, PIP3 (phosphatidylinositol-3,4,5-triphosphate), which plays important roles in cell motility via effects on filopodia and lamellipodia, shows a decrease in the whole spines after potentiation, due to an accumulation of the PIP3 in the filopodia (or “spinules”). Ueda (2014) suggests that there are three possible roles for this accumulation of PIP3 in the “spinules”: (1) induction of new synapses; (2) internalization of the PIP3-containing tips of the “spinules” into the presynaptic terminal, where PIP3 can act as a second messenger for retrograde signaling; or (3) the “spinule” tip may be discarded extracellularly as part of spine reorganization.
Giant mossy terminals
The giant mossy terminals of the hippocampus and cerebellum commonly have spinules or slightly larger, more irregular invaginating projections. In both cases, these processes originate from specialized dendritic processes that invaginate deeply into the terminal. In the cerebellar glomeruli of the granular layer, the central irregular mossy terminal receives numerous granule cell dendritic protrusions (“dendritic claws”; and also some postsynaptic Golgi cell dendrite processes) that bear the synaptic active zones. In the immature cerebellar glomerulus of the mouse, the granule cell dendritic protrusions invaginate numerous spinules into the mossy terminal (Eckenhoff and Pysh 1979). These are capped with one or several coated pits, and others appear to have pinched off recently as coated vesicles. These authors called them “double-walled coated vesicles” because they contain a pinched-off portion of the dendrite membrane in their center. There also are some that proceed in the opposite direction, from the terminal membrane to invaginate into the dendrites (see “Presynaptic Terminal/Axon-Derived Invaginating Projections” section), as well as some spinules directly between two dendritic processes. Spinule numbers peak at about 20 days postnatal and are decreased markedly in the adult. But in a study of the rat cerebellum, large spinule-like projections of various shapes are fairly common in young adults (Petralia et al. 2012). The latter study looked at the distribution of immunolabeling for the sonic hedgehog (Shh) growth factor receptors, patched and smoothened. These mossy fiber spinule-like projections showed labeling for both of these.
Another type of neuron, the unipolar brush cell, makes synapses with invaginating projections into mossy terminals; these are located in the granule cell layer of the cerebellum (Palay and Chan-Palay 1974; Floris et al. 1994; Mugnaini et al. 1994; Dino et al. 2000) and similar granule cell regions of the cochlear nuclei (Mugnaini et al. 1980; Floris et al. 1994; Weedman et al. 1996). Unipolar brush cells can make “brush” or “en marron” synaptic contacts with mossy fibers; these are elongate, crenated contacts with multiple synaptic active zones interspersed with invaginating “appendages” varying from knob-like to filiform (Palay and Chan-Palay 1974; Mugnaini et al. 1980; Floris et al. 1994; Mugnaini et al. 1994; Dino et al. 2000; Weedman et al. 1996; Mugnaini et al. 2011). The identification of neurons that have this kind of complex synapse has been problematic, with various classifications including “chestnut” and “mitt” cells, as well as Golgi cells (the classification of these various cell types in the cochlear nuclei may be somewhat different than that of the cerebellum, and there were some misunderstandings in the older literature about the kinds of synapses made with the dendrites of true Golgi cells; Weedman et al. 1996; Mugnaini et al. 2011). The invaginating projections of unipolar brush cell–mossy fiber synapses are larger overall than the coated pit-associated spinules in developing granule cell dendrite–mossy terminal synapses described by Eckenhoff and Pysh (1979). But spinule-like projections described in granule cell–mossy terminal synapses in young adult rats by Petralia et al. (2012) also are larger than spinules described by the latter authors.
Similarly, in the mossy terminals of the rat hippocampus, numerous spinules form from the convoluted postsynaptic dendrite protrusions (“thorny excrescences”; Chicurel and Harris 1992; Petralia et al. 2011; Fig. 7). Spinules are prevalent in mossy terminals of the rabbit hippocampus during epileptiform seizures (Nitsch and Rinne 1981), and these can go both ways, from the dendrite to the terminal and from the terminal to the dendrite (for more on the latter, see “Presynaptic Terminal/Axon-Derived Invaginating Projections” section). Stewart et al. (2005a) compared the effects of chronic stress (restraint) to spatial training (water maze) on the structure of thorny excrescences and found that stress induces some retraction of the thorny excrescences. In contrast, water maze training increases thorny excrescence volume and the number of thorns per excrescence. Also, there are many more perforated PSDs and spinules after training; many of these spinules form a connection between two thorns. Petralia et al. (2011) found that the spinule structures in the mossy terminals were labeled abundantly with immunogold for the sonic hedgehog (Shh) receptors, patched and smoothened (Fig. 7). Labeling for the Shh receptors could be followed in a possible pathway from the postsynaptic dendrite structures to small and then large profiles of putative spinules/filopodia (these may be termed filopodia because of their large size; see also Popov et al. 2011) and ultimately into autophagosome structures adjacent to the spinules in the terminals. This suggested that Shh receptors on the postsynaptic membranes are being taken up into the terminal and processed through the autophagosomes. This may help regulate Shh signaling by degrading its postsynaptic receptors via autophagosomes; similarly, autophagy is used to regulate the levels of the receptor (dishevelled) of the signaling and developmental factor, Wnt, to inhibit Wnt function (Gao et al. 2010). Perhaps, Shh activation and regulation are part of the mechanism for formation and degradation of synaptic sites in the thorny excrescences, since these invaginated spinules/filopodia may represent intermediate stages in synapse formation within the complex thorny excrescences (Popov et al. 2011).
Cerebral cortex
Perforated synapses with central spinules invaginating into the presynaptic terminal have been described in the cerebral cortex of the cat (Friedlander et al. 1991), rat (Dyson and Jones 1984; Jones and Calverley 1991; Bozhilova-Pastirova and Ovtscharoff 1999). and ferret (Erisir and Dreusicke 2005). These examples show perforated PSDs with a finger-like or irregular membranous process invaginating into the presynaptic terminal, and without coated pits. Erisir and Dreusicke (2005) compared spinules (“spine protrusions”) in the ferret visual cortex during the critical period (P35–58; stage of anatomical changes in thalamic input due to unbalanced binocular visual stimulation) and in the adult. They were surprised to see large numbers of spinules both in the critical period, a time of greater synaptic plasticity, and in the adult, when plasticity was expected to be reduced; thus, they challenged the idea that perforated synapses and spinules are a sign of greater plasticity and suggested that spinule formation is due to greater spine motility rather than spine plasticity. In the prefrontal cortex of the primate, Macaca mulatta, short invaginating projections called spinules by the authors and corresponding double-walled vesicles are found in dendrites and originate from other dendrites or from axons; some of these are labeled with immunogold for D2 dopamine receptors (Paspalas et al. 2006). Familtsev (2013) used a combination of tracer injections and immunohistochemistry to identify geniculocortical terminals in the striate (primary visual) cortex of the tree shrew, Tupaia belangeri (tree shrews could represent an ancestral group to the prosimian primates), and compared these to pulvinocortical terminals in the temporal cortex. Fifty-eight percent of geniculocortical terminals have spinules, whereas the pulvinocortical ones have none; based on what is known about spinules as discussed in this review, the author suggested that the presence of spinules reflects the higher level of activity of the geniculocortical terminals. Interestingly, Pappas and Purpura (1961) noted frequent thin processes that they called “dendritic terminals” in the superficial (upper 400–500 nm) cerebral cortex of the adult cat. These are similar in size to spinules (as small as 30 nm in diameter) and can extend from the side of postsynaptic spine and wrap around part of the presynaptic terminal, but they do not seem to invaginate within the terminal.
It was recently reported that cerebral cortical neurons from KIBRA knockout mice exhibit reduced numbers of perforated synapses and synaptic spinules, without evident alterations in synapse numbers or overall ultrastructure; these mice also have more filopodial-like long dendritic spines in both the cerebral cortex and hippocampus (Blanque et al. 2015). These findings are interesting with regard to the normal functions of synaptic spinules because KIBRA is believed to play roles in cell membrane trafficking, including endosomal vesicle sorting, and to affect learning and memory; in fact, polymorphisms in the gene that encodes KIBRA are associated with cognitive performance in humans (Papassotiropoulos et al. 2006).
Other regions
While there are relatively few studies of spinules in the vertebrate brain outside of the hippocampus, cerebellum, and cerebral cortex, this is probably because not enough researchers have looked actively for spinules in other regions. Curcio et al. (1985) noted spinules associated with perforated synapses in the specialized (for olfaction) piriform cortex of the rat. Boyne and Tarrant (1982) found spinules in synapses of parts of the limbic system of the rat, including the hippocampus, nucleus accumbens, and caudate nucleus (part of the striatum). Perforated synapses with spinules also have been described in the caudate nucleus in elderly humans (Anglade et al. 1996; Muriel et al. 2001; see “Synaptic Spinules in Aging and Disease” section). Spinules also are found in more than 12 % of synapses in the rat medial habenular nucleus; a notable decrease in the abundance of synaptic spinules as well as degeneration of presynaptic terminals in this nucleus occurs following transection of the stria medullaris in the live animal (Zimmer et al. 1982). In giant reticulospinal axon synapses in the lamprey (Lampetra fluviatilis), a primitive jawless vertebrate fish, endophilin-associated endocytosis via clathrin-coated vesicles is often found at presynaptic membrane invaginations after stimulation (Shupliakov et al. 1997; Gad et al. 1998; Ringstad et al. 1999), and these typically have included an invaginating projection, probably from the postsynaptic cell. Presynaptic invaginations may be an important functional component for membrane endocytosis associated with synaptic vesicle cycling (Schmidt et al. 1997; Shupliakov et al. 1997; Gad et al. 1998).
Fish retinal spinules
In the retina of fish, a unique kind of small invaginating projection extends from horizontal cell dendrite as postsynaptic endings (also called “horizontal cell terminals”) that are one kind of postsynaptic process of retinal ribbon synapses, which are long, dense vertical structures lined with synaptic vesicles; the ribbon synapses apposing the horizontal cell dendrites are components of the specialized presynaptic terminal (pedicle) of cone photoreceptor cells (for color vision) (Fig. 3e). The small invaginating projections that project from horizontal cell dendrites into the presynaptic terminal were named “spinules” by Wagner (1980), but their structure is significantly different from other kinds of spinules described in this review. The main kind of retinal spinule is slender and finger shaped (average 94 × 298 nm). Typically, the distal end of the spinule has an intracellular dense material subjacent to the membrane, resembling that of a PSD, but this is absent from the very tip of the spinule. The wrapping of the presynaptic membrane around the distal, coated end makes the end appear somewhat rounded. But other spinules have a distinctly enlarged, rounded (spherical) head with several dense patches of the intracellular dense material and only a short stalk or neck region. Spinules contain fine filaments, occasional microtubules, and a few irregular vesicles. Spinules are numerous during the day, but most or all disappear at night. Rounded ones are most common 30 min after first light and 30 min after darkness begins, with the slender kind being most common throughout the day. Wagner (1980) compared spinules from five species of fish and found that there are some differences in shape and persistence after darkness; for example, while most fish lose most of their spinules by about 1 h after darkness, trout retain a large number of spinules 4 h after darkness. In the latter study, no spinules were found in an amphibian, turtle, rat, cat, dog, or macaque monkey. Retinal spinules appear to expand and retract daily from the horizontal dendrite endings. Wagner (1980) also found that multivesicular bodies appear in the presynaptic cone terminals at night; perhaps, these represent the reclamation of the presynaptic membrane that covers the spinules during the day. Kröger and Wagner (1996) define the round spinules as “immature spinules,” and also describe unusual bulbous protrusions without membrane-associated densities and assume these to be “vestigial spinules.”
Fish retinal spinule function is studied widely, and the literature can only be summarized here. A number of factors control spinule formation including dopamine and protein kinase C (reviewed in Wagner and Djamgoz 1993; Popova 2014), as well as nerve growth factor (Haamedi et al. 2001) and retinoic acid (Dirks et al. 2004). Spinules may mediate “…feedback activity essential for the coding of antagonistic color information” (Popova 2014). Some believe that the spinules mediate a subset of postsynaptic responses, as they possess structures similar to PSDs. Treatment with high-potassium Ringer solution induces the formation of large vesicles that seem to be fusing with the presynaptic membrane surrounding the spinule at the regions containing the postsynaptic dense material (Weiler et al. 1996). Spinules contain the calcium-binding protein caldendrin and CaMKII (calcium/calmodulin-dependent protein kinase II), and caldendrin appears to shift from the main horizontal cell dendrite endings into the spinules during light adaptation (Schultz et al. 2004); the authors conclude that calcium sensing via caldendrin affects the dynamic regulation (via CaMKII) of spinules. In addition, AMPA-type ionotropic glutamate receptors (at least the GluR2 subunit) are found in horizontal cell dendrite endings and to a lesser extent in spinules (Klooster et al. 2009), and activation of this kind of glutamate receptor, presumably by glutamate release at the cone terminal, induces spinule retraction (Weiler and Schultz 1993).
Invertebrates
There do not appear to be any examples, described in the literature, of prominent postsynaptic spinules among invertebrate groups. Of course, study of the literature on synapse ultrastructure of any animal group, vertebrate or invertebrate, will uncover some examples where irregular postsynaptic profiles might include an occasional protuberance that pushes slightly into shallow invaginations on equally irregular presynaptic structures. In addition, there are some examples of invaginating projections in invertebrates where one or both of the cell processes forming a synapse are not well defined (see “Presynaptic Terminal/Axon-Derived Invaginating Projections” and “Glia-Derived Invaginating Projections” sections). One important caveat of this possible lack of definitive postsynaptic spinules in invertebrates is that the vertebrate spinule structure involves two components—the postsynaptic spinule and the presynaptic invagination that outlines it nearly perfectly. At least one group of invertebrates, the insects, can have highly developed spinule-like profiles in presynaptic terminals, but the spinules are formed from glial processes (for details, see “Glia-Derived Invaginating Projections” section). Since the presynaptic invaginations around spinules are very similar in structure in the more complex synaptic circuits of both insects and vertebrates, it suggests that spinule function in some cases could center more on the presynaptic component of these organelles (Glia-Derived Invaginating Projections section), i.e., the postsynaptic spinule perhaps acts as an internal support structure for the surrounding invaginated presynaptic membrane.
An example of an enigmatic invaginating projection in an invertebrate is found in the medullary dendritic bundles in sensory hairs on the dorsal integument of the chiton, Mopalia muscosa (chitons are mollusks with a dorsal shell composed of eight articulating plates; Leise and Cloney 1982). In cross section, dendrites form a single projection, about 100 nm or less in diameter and less than a micrometer long. This invaginates into the surrounding supporting cell that wraps over the dendrites in the bundle; in the supporting cell’s cytoplasm, a subsurface cistern surrounds the end of its invaginated membrane. These projections appear to be identical in cross-sectional structure to adjacent interdigitating projections of the supporting cell, suggesting that these structures are ridges or folds rather than finger-like projections.
Presynaptic Terminal/Axon-Derived Invaginating Projections
Invertebrates
Axonal and presynaptic membranes also are capable of forming invaginating projections under various conditions. As noted in “Synapse/Neuronal Invaginating Projections in the Simplest Animals” section, axons near synapses in the simplest metazoans that possess nervous systems, the cnidarians, can send invaginating projections into adjacent cell processes; in this case, the latter are nematocyte processes (Westfall 1970). In cultures of neurons from the mollusk, Aplysia, treated with 5-HT (serotonin) and immunogold labeled for a group of immunoglobulin (Ig)-related cell adhesion molecules, the apCAMs, invaginating projections form between sensory neuron axons in fascicles. These show that the gold labeling for apCAM on one membrane is taken up into the double-membrane clathrin-coated pit in the other (essentially a short spinule invaginated into a coated pit; Bailey et al. 1992). This “…removal of apCAM may destabilize adhesive contacts between axonal processes and facilitate defasciculation.” Similarly, in the nervous system of another mollusk, Planorbis corneus, these kinds of short spinules that directly project into coated pits are found in axons and possible axon terminals, but the source process of the spinules is not well defined as being dendritic or axonal (Pentreath et al. 1975). In one micrograph in the latter study (their figure 8), an axon invaginates a spinule into an adjacent process and also receives one from another adjacent process. In another micrograph (their figure 2), a spinule invaginates from the edge of a synaptic terminal into a postsynaptic axon. Nerve fibers in the cardiac ganglion of a scorpion, Paruroctonus mesaensis, treated with lanthanum phosphate to highlight junctions, show invaginating projections ending in coated pits, between the nerve fibers and between nerve fibers and terminals (Farley and Chan 1985). The authors suggest that these structures represent the endocytosis of cell junctions (possibly gap junctions); this is consistent with studies in other kinds of cells that suggest that invaginating projections serve to endocytose various cell–cell junctional complexes (Risinger and Larsen 1981; Larsen 1983).
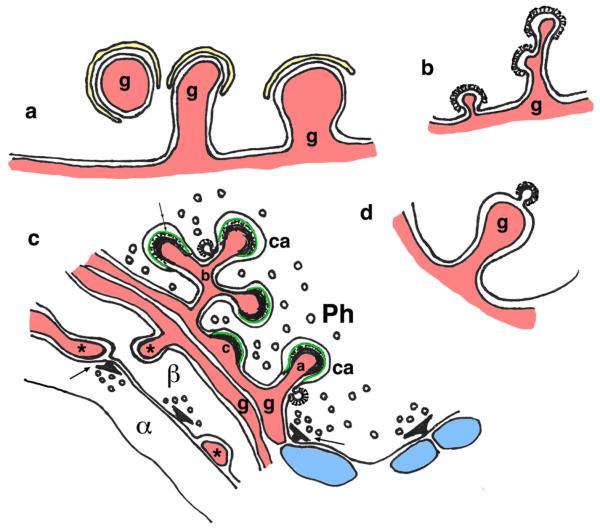
Groups of axons or axon terminals associated with the eyes of some invertebrates may be interlocked by short, finger-like projections. In the optic lobe of the squid, Loligo pealei, each terminal forms and may receive one or two invaginated projections from adjacent terminals (Cohen 1973). In some cases, subsurface cisterns may line part of the invagination on both sides (paired cisterns); between them, the space between the two membranes of the invagination are reduced to ~2 to 4 nm, and Cohen (1973) suggests that this is a gap junction for electrotonic neurotransmission. Nerve fibers in the visual system in insect, associated with both the compound (housefly—Chi and Carlson 1976; butterfly—Gordon 1985) and simple (ocelli; honeybee—Toh and Kuwabara 1974; flesh fly—Toh and Kuwabara 1975), eyes sometimes have small invaginating projections between them. In the butterfly, Agraulis vanillae, these are described as “finger-like projections,” from 0.2 to 0.7 μm in diameter (Gordon 1985), and appear to be about the same size in the housefly, Musca domestica (Chi and Carlson 1976); they may represent sites of electrical coupling between the fibers (Chi and Carlson 1976; Gordon 1985). Interestingly, although not described, close examination of the high-magnification image in figure 3C of Gordon (1985) appears to show a regular layer of microtubules (these axon profiles are all cross sections) lining the invaginated portion of the membrane of the fiber that receives the invaginating projection (Fig. 8a); the latter also has some microtubules lining it, although not as well organized; many microtubules in cross section also fill the axon profiles as would be expected. Thus, we suggest that these invaginating projections, always shown in axonal cross section, actually could be elongate ridges rather than “fingers.” Note that we also discuss in “Small Postsynaptic Invaginating Projections: Spinules” section the development of spinules into ridge-like projections in formation of partitions of perforated/segmented synapses, but both development and function between these two examples of ridges are probably very different.
Fig. 8.
Presynaptic terminal/axon-derived invaginating projections. a In the butterfly, Agraulis vanillae, invaginating projections form between axons in the compound eye units or ommatidia; each ommatidium has nine photoreceptor (retinula) cell axons including two close triads of axons, each from two diagonal (d) and one horizontal (h) retinula cells (Gordon 1985). The diagram shows a diagonal and horizontal axon from one ommatidium on the left, and a horizontal axon from an adjacent ommatidium on the right. The invaginating projections link diagonal and horizontal cell axons within a triad and also form between the horizontal axons from triads in two adjacent ommatidia. Note how the invaginated membrane is lined with a regular array of microtubules (indicated by black dots in all three drawings); only the microtubules associated with the invaginations are illustrated, although the entire axon cross sections are filled with microtubules and other filamentous structures. b Pseudopodial interdigitations (PSIs) between axon terminals in the globus pallidus. Only the sides of the terminals where they interdigitate are illustrated. PSIs can be simple and short (b1 example from the squirrel monkey; Fox et al. 1974) or long and compound (b2 example from the rat; Boyne and Tarrant 1982). Note that some synaptic vesicles are found within the PSIs. c In the cerebellum of chick embryos (Palacios-Prü et al. 1981) and early postnatal rats (Altman 1971, 1993; Altman and Bayer 1997), first contacts between parallel fiber axons (ax) and Purkinje cell dendrites (Pj de) appear to induce formation of thin spinules from the axons and these invaginate into coated pits on the dendrite. d In some synapses of the molecular layer of the dentate gyrus of adult rats, a central presynaptic terminal can be enwrapped completely by a concave spine to form a synapse (Desmond and Levy 1983) (dendritic processes in blue and glial processes in red) (Color figure online)
Pseudopodial indentations
A similar arrangement of interlocking invaginating projections between axon terminals occurs in some parts of the vertebrate nervous system. These are large processes called “pseudopodial indentations” or “PSIs” that are found on the lateral surfaces of some kinds of presynaptic terminals, where they abut with adjacent terminals (Boyne and McLeod 1979; Boyne and Tarrant 1982). PSIs vary in shape from filopodia-like to highly irregular, and they may be simple or compound; compound PSIs involve PSIs from both terminals that interdigitate between the two terminals, sometimes in complex patterns (Fig. 8b). PSIs are true continuations of the terminal cytoplasm and often contain presynaptic vesicles. In the cholinergic nerve terminals forming synapses with the electrical organ of the torpedo ray fish, Narcine brasiliensis, PSIs are normally rare, but following electrical stimulation to fatigue, membrane involved in the PSIs increases almost 27× and they are found in 62 % of terminal profiles; these PSIs can be up to 3 μm long, and sometimes sections of the PSI may pinch off into the terminal (Boyne and McLeod 1979). PSIs also may be present in stimulated NMJs of the frog (Smith et al. 1977; Gennaro et al. 1978; along with invaginating Schwann cell processes—see “Glia-Derived Invaginating Projections” section). PSIs are found throughout the limbic system of normal rats including the central nucleus of the amygdala, hippocampus, globus pallidus, substantia nigra, and caudate nucleus (Boyne and Tarrant 1982). In most cases, the terminals forming PSIs have pleomorphic vesicles and symmetrical densities and synapse directly on the dendrite shaft, unlike typical excitatory terminals on spines (round vesicles and asymmetrical densities); thus, they likely are inhibitory terminals. However, examples of PSIs in terminals on spines were seen in the CA4 hilar region of the hippocampus and the caudate nucleus. They show a micrograph of a PSI-bearing, large terminal on the neck of a spine in the hilar region (Boyne and Tarrant 1982). In the caudate nucleus, they also found a terminal with both a PSI and “shallow” spinule, but they did not illustrate it or describe it in any detail. PSIs are particularly abundant in the globus pallidus (Fig. 8b) of the rat (Boyne and Tarrant 1982) and squirrel monkey, Saimiri sciureus (Fox et al. 1974). Boyne and Tarrant (1982) discuss how the PSIs may act as “variable diffusion traps,” controlling levels of ions and other substances in the extracellular space between the processes, and thus influencing direct or receptor-mediated effects on membrane potential among adjacent synapses.
While mammalian PSIs typically may form on the sides of inhibitory terminals, similar short invaginating projections may form on the synaptic side of presumptive GABAergic, inhibitory terminals that contain the peptide, cholecystokinin (CCK). These have been described in the dentate gyrus (Leranth and Frotscher 1986; Acsády et al. 2000), basal nucleus of the basolateral amygdala (BA), and entorhinal cortex (EC; Yoshida et al. 2011; Omiya et al. 2015). They are best studied in the BA and EC, where the invagination forms just perisynaptic to symmetrical synaptic active zone(s) containing GABA receptors. In addition to GABA and CCK, this type of presynaptic terminal of the BA and EC contains the vesicular glutamate transporter, VGluT3, and the membrane contains the cannabinoid receptor, CB1. Interestingly, the postsynaptic membrane at the tip of the invaginating projection is rich in the enzyme, DGLα (diacylglycerol lipase-α), which synthesizes the endogenous cannabinoid 2-AG (2-arachidonoylglycerol). In this model, release of glutamate and CCK from the terminal activates receptors that promote 2-AG-mediated retrograde signaling confined by the invagination; this produces a specific, enhanced tonic inhibition of the synaptic activity.
Hippocampus
In the CA1 stratum radiatum of the mature rat hippocampus, about 12 % of the spinules originate from axons and invaginate into other axons or glia (Spacek and Harris 2004). In addition, these authors found two spinules arising from an axon and invaginating into a growth cone (possibly a dendritic growth cone, based on similarities to dendritic growth cones in the literature, but this was not confirmed). The giant irregular mossy terminals of the hippocampus and cerebellum can have spinules going both from dendrite process to axon terminal and from axon terminal to dendrite process (as well as between dendrite processes for the cerebellar mossy terminals). We already have discussed these synapses in detail in “Small Postsynaptic Invaginating Projections: Spinules” section, specifically regarding the spinules originating from the dendrite processes, but here we mention only the processes going in the opposite direction. Thus, Eckenhoff and Pysh (1979) describe short spinules forming coated pits arising from mossy terminals of the immature cerebellar glomerulus of the mouse and invaginating into the postsynaptic granule cell dendrite processes. Nitsch and Rinne (1981) examined the mossy terminals of the rabbit hippocampus during epileptiform seizures and found invaginating projections going both ways (see “Small Postsynaptic Invaginating Projections: Spinules” section also). Processes invaginating into the postsynaptic thorny excrescences from the mossy terminal include finger-like ones about 150 nm in diameter and covered with a dense coat, and some larger ones that appear to represent the internalization of part of the synaptic active zones.
Synaptogenesis/dendritogenesis
Initial invagination of processes from axons may be part of the mechanism for synaptogenesis in parts of the nervous system. Small spinule-like invaginations from axons into dendrites and ending in coated pits occur in early contacts in the embryonic mouse spinal cord (Vaughn 1989) and in the parallel fiber contacts onto Purkinje cell dendrites in chick embryos (Palacios-Prü et al. 1981) and early postnatal rats (Altman 1971, 1993; Altman and Bayer 1997) (Fig. 8c). Vaughn (1989) hypothesized that early contacts between axons and dendrites begin with a low-affinity contact stage followed by a stage of information exchange via small invaginating projections going both ways between axon and dendrite, later followed by formation of the stable synapse (he also suggested the same early stages for the development of adhaerens junctions between axons and radial glial processes). Altman and Bayer (1997) rather hypothesized that the invaginating projection from a parallel fiber axon (they call this structure a “proboscis”) is drawn into a coated pit on the main dendrite of the immature Purkinje cell and thus initiates (through a complex mechanism that we will not describe here) the formation of the dendrite branches; thus, these spinule-like contacts would be involved directly in dendrite differentiation rather than the later stages of spinogenesis/synaptogenesis.
Cochlea
In the inner hair cells of the cochlea of the P1 mouse (see “Large Postsynaptic Invaginating Projections: Filopodia and Spines” section), small spinules from the inner hair cells can form adjacent to the synaptic active zones and invaginate into the afferent dendrite endings; each is capped with a coated pit (Fig. 4; Sobkowicz et al. 2003). Over the next 2 weeks of postnatal development, invaginating projections may persist, but most are thicker filopodial structures. Interestingly, in studies of laser ablation of ganglion cell neurons in organ of Corti cultures from newborn mice (injured at 8 days in vitro and fixed at 11 days in vitro), numerous inner hair cell filopodia sometimes invaginate into the degenerating afferent endings that are also often enwrapped entirely by the base of the hair cell.
Other regions
We already described PSIs in the rat limbic system above. Waxman et al. (1980) described small spinules from presynaptic terminals entering the postsynaptic process adjacent to the synaptic zone in chemical synapses from the oculomotor nucleus of the lizard, Anolis carolinensis, as well as in electrotonic synapses from the electromotor nucleus of the gymnotid fish, Sternopygus sp. These consist of a “thin tongue” of the presynaptic membrane 20–100 nm in diameter, invaginating into a coated pit on the postsynaptic process. Also, as noted in “Small Postsynaptic Invaginating Projections: Spinules” section, short spinules in dendrites in the prefrontal cortex of a primate originate from either axons or other dendrites (Paspalas et al. 2006). In the prefrontal cortex of alcohol-fed rats, there are more complex forms of synapses including perforated synapses, compared to controls, (Cadete-Leite et al. 1986). In their figure 1, an example of a “multiple ‘perforated’ synapse” bears a short presynaptic filopodium or spinule that invaginates into the postsynaptic spine between two PSD/active zones; the invaginated portion of the spine membrane has a dense coating that appears to be another PSD, thus giving this synapse at least three PSD profiles.
Invaginating terminal synapses
Invaginating presynaptic terminals are the opposite of the invaginating spine synapses discussed in “Large Postsynaptic Invaginating Projections: Filopodia and Spines” section. Desmond and Levy (1983) describe some synapses in the molecular layer of the dentate gyrus of adult rats, in which a small central presynaptic terminal can be completely enwrapped in a concave spine (Fig. 8d). Application of brief, high-frequency electrical conditioning trains to the entorhinal cortical input to the dentate gyrus potentiates the excitatory postsynaptic potentials and increases the number of concave spine synapses in the dentate gyrus. Spinules (see “Small Postsynaptic Invaginating Projections: Spinules” section) also are found at many synapses in this region.
Presynaptic terminals of the larval neuromuscular junction of Drosophila and other flies are partly or entirely enclosed in an invagination of the muscle junctional membranes (Osborne 1967; Atwood et al. 1993; Jia et al. 1993). Typically, the invaginated terminals are large boutons with many vesicles and synaptic active zones and are surrounded by an elaborate subsynaptic reticulum in the muscle cell. As noted in the Introduction, within this enclosed space, the terminal releases exosome vesicles (see “Other Types of Short-Distance Intercellular Communication” section) containing the Wnt, wingless (Wg); this is aided by the Wnt-binding protein, evenness interrupted (Evi), so that Wg can be delivered to the postsynaptic Wg receptor, DFrizzled-2 (DFz2), ultimately signaling to the muscle cell to regulate neuromuscular function (Korkut et al. 2009; Kornberg and Roy 2014). These exosomes also transfer synaptotagmin 4 (Syt4) to the postsynaptic cell, where it mediates a retrograde signal needed for synaptic growth and function (Korkut et al. 2013).
Glia-Derived Invaginating Projections
Trophospongium
We discussed in “Synapse/Neuronal Invaginating Projections in the Simplest Animals” section how neuron cell bodies and axons of the simplest bilaterian metazoa, the flatworms, are often invaginated by glial processes. Such an arrangement actually is common throughout most of the major invertebrate groups such as mollusks, annelid worms, insects, and crustaceans, and these structures usually are designated (in numerous publications) as a “trophospongium.” The term derives from the belief that these glial processes have a trophic function. Hoyle et al. (1986) suggest that “…feeding of neurons seems to be by invagination and transfer, directly, via pinocytosis or by direct ingestion of the budded off tips of glial cell process[es].” Hoyle et al. (1986) describe four kinds of glial invaginations into neuron somata and axon hillocks of neurons of the metathoracic ganglia of locusts (regular, chunky, filigree, and ridge). In this case, processes from the neurons also make reciprocal invaginations into the glial cells; Hoyle et al. suggests that these could be “…acting in the reverse direction, dumping waste by direct loss into the glial cells.” For the glial-derived processes, often the outer neuron membrane surrounding the invaginating projection from the glial cell is capped by one or more endoplasmic reticulum cisternae covering the swollen end of the gial cell processes (snail—Bonga 1970; tobacco hornworm [insect]—Hartfelder et al. 1994; crayfish—Nordlander et al. 1975; Fedorenko and Uzdensky 2009). In fact, a subsurface cistern can initiate the formation of the invagination (Fig. 9a; Fedorenko and Uzdensky 2009). These cisternae also could represent the early stage in autophagosome formation (see also our discussion related to this in “Small Postsynaptic Invaginating Projections: Spinules” section for postsynaptic spinule processing). For the squid axon, Buchheit and Tytell (1992) used a fluorescent compound to demonstrate that the surrounding glial cells transfer vesicles into the axoplasm, probably via “…phagocytosis by the axon of small portions of the glial cells.”
Fig. 9.
Glia-derived invaginating projections. a Glial processes (g) invaginate into a stretch receptor neuron from the crayfish, Astacus leptodactylus (Fedorenko and Uzdensky 2009). Note how a subsurface cisterna (yellow) wraps the end of the invagination. b Glial processes from a surrounding myelin sheath (g; derived from a Schwann cell) invaginate into a neuron of the spiral ganglion of the cat; the tips are surrounded by coated pits (Adamo and Daigneault 1973a). c A composite drawing of the photoreceptor synaptic plexus in eyes of flies showing how glia (g) form specialized invaginating projections called capitate projections (ca) into the photoreceptor (Ph; or retinula) terminals and gnarls (asterisks) associated with β/α fiber pairs. Typically, capitate projections are singular (a; right one), but they also occasionally can be compound (b; left one) or stalkless (c; middle). The head of the projection includes a unique feature—the intercellular substance or “third membrane” (double arrow; green). Synapses are indicated by the specialized T-bar on the presynaptic side. Sometimes, the invaginating projection may be directly part of the synapse; these are called gliapses (arrows) and can involve either a capitate projection or a gnarl. Most of the diagram is based on micrographs from the compound eye (see text for references), but the gliapse involving the capitate projection is based on an image from a retinula terminal of an ocellus (Stark et al. 1989). Note also how coated pits may form along the capitate projections (Fabian-Fine et al. 2003). d Some glial processes that invaginate into terminals can form coated pits on the invaginated membrane without inclusion of the invaginating glial process (g). This is a generalized example representing those found in both the frog neuromuscular junction (Heuser and Reese 1973) and frog brain (Harreveld and Trubatch 1975) (dendritic processes in blue and glial processes in red) (Color figure online)
Trophospongium projections within axons (leech [annelid]—Coggeshall and Fawcett 1964; crayfish—Nordlander et al. 1975; cockroach—Blanco 1988) often are shorter than those found in neuron somata. Some of these processes resemble classic spinules with very narrow necks and swollen heads. One shown in a micrograph of a neuron from the snail, Lymnaea stagnalis, is less than 100 nm wide and extends about 3 μm into the neuron (Bonga 1970); a shorter one in a micrograph of a large axon in the leech, Hirudo medicinalis, also looks very spinule-like in shape (Coggeshall and Fawcett 1964). Tracer studies in the crayfish suggested that some of the tracer is passed on to the axon via vesicles pinching off of the glial processes via pinocytosis (Nordlander et al. 1975). An indication of the importance of the trophospongium in supporting neuronal function is seen in changes in neurosecretory neuron structure during the quiescent stage (diapause) of the tobacco hornworm, Manduca sexta, when there is a substantial reduction in the trophospongium (Hartfelder et al. 1994).
Related vertebrate glial processes
While the trophospongium is considered a specialized invertebrate structure, there are a few examples of similar glial processes invaginating into neurons of vertebrates, including satellite glial processes penetrating neurosecretory cells in the preoptic area of the goldfish, Carassius auratus (Gregory et al. 1988), oligodendrial processes projecting into spinal motor axons in the chicken, Gallus domesticus (Li et al. 2005), and Schwann cell processes into spiral ganglion neurons in the cat (Fig. 9b; Adamo and Daigneault 1973a, b). As described above for invertebrates, surface reticular cisternae can be involved in this invagination process (Li et al. 2005). However, clathrin-coated pits also may be involved in other cases; Adamo and Daigneault (1973a) include a micrograph (their figure 11) showing an invaginating projection from the cell membrane of the compact myelin layer with the surrounding neuronal membrane bearing two clathrin-coated pits. Thus, these various examples suggest at least three mechanisms for transfer of material from glial invaginating projections into the neuron soma or axon, including subsurface cistern-mediated invagination followed by phagocytosis, pinocytosis (non-clathrin), and clathrin-coated pit formation. Could these various mechanisms also reflect different functions for the invaginating glial processes?
Capitate projections
There are few examples of glial invaginating projections in synaptic terminals, but one of the most striking examples of an invaginating synaptic process is the capitate projection, found in the visual system of flies (Fig. 9c; Trujillo-Cenóz 1965). These are projections from one type of glial cell (the epithelial glial cell) into the synaptic terminals of the main photoreceptor/retinular cells (R1–6) in the synaptic plexus of the first optic neuropile of the compound eyes (Stark and Carlson 1986; Prokop and Meinertzhagen 2006). They also may be present in the terminals of the other two kinds of retinular cells (R7/8) in the second optic neuropile (Prokop and Meinertzhagen 2006). Among insect orders, capitate projections appear to be unique to true flies (Diptera); possible precursors to these structures may be found in the probable ancestors to true flies, the scorpion flies (Mecoptera; Shaw and Meinertzhagen 1986). Capitate projections have a large, round head and a narrow stalk (neck), and there is a distinctive, regular layer of intercellular substance coating the glial membrane in the head and adjacent to the surrounding terminal membrane (Trujillo-Cenóz 1965). Stark and Carlson (1986) note that in Drosophila, the head is 200 nm in diameter (stalk ~80 nm diameter), while the head is about 100 nm in the housefly (Musca; see also Saint Marie and Carlson 1982). Trujillo-Cenóz (1965) describes the head of the capitate projections of a flesh fly, Sarcophaga bullata, as being 100 nm in diameter, and separated from the surrounding terminal membrane by a 25-nm intercellular space, including the 10-nm thick layer of intercellular substance (“amorphous substance” or “third membrane”); the stalk is ~50 nm in diameter and between 100 and 500 nm long. These structures are abundant, with three per square micrometer of axon terminal in the housefly, so that these specialized, elongate photoreceptor terminals can have ~800 capitate projections (almost 5× the number of chemical synaptic active zones; Stark and Carlson 1986). In addition to the stalked form, there can be a hemispheric, indenting depression without a stalk (is this an early stage in genesis of the projection?), and there can be compound, stalked capitate projections, at least in Drosophila (Fröhlich and Meinertzhagen 1982; Stark and Carlson 1986). Capitate projections also are found in the retinular cell terminals in the simple eyes (ocelli; typically, insects have three ocelli in addition to their main, compound eyes) of Drosophila (Stark et al. 1989). In some cases, the retinular cell terminal makes a synapse on two postsynaptic processes including an interneuron and a glial cell that is sending a capitate projection into the retinular terminal at the synapse; the latter is called a “gliapse” (Carlson 1987; Stark et al. 1989).
The function of capitate projections is not fully understood, but they might be involved in neurotransmitter uptake into terminals and for presynaptic membrane retrieval to maintain synaptic vesicle recycling (Fabian-Fine et al. 2003). Related to the latter function, Fabian-Fine et al. (2003) localized immunogold labeling for two endocytosis-related proteins, endophilin and clathrin heavy chain, to capitate projections, and using temperature-sensitive dynamin mutants (and double mutants for dynamin and endophilin), they induced the formation of clathrin-coated pits and vesicles along capitate projection stalks. Thus, invaginating glial processes may participate in synaptic vesicle cycling, as discussed in “Small Postsynaptic Invaginating Projections: Spinules” section for invaginating postsynaptic neuronal processes.
Related vertebrate glial processes at synapses
Invaginating Schwann cell processes are seen also at the frog NMJ (Heuser and Reese 1973; Smith et al. 1977; Gennaro et al. 1978) and in the frog forebrain (Harreveld and Trubatch 1975), following stimulation of neurotransmission; these invaginations are rare or absent in controls. They often are associated with a clathrin-coated pit that mediates endocytosis of the presynaptic membrane; the invaginating projections in these examples are Schwann cell processes in the NMJ studies and either glial or postsynaptic neuronal processes in the forebrain study (Fig. 9d; see also “Synaptic/Neuronal Invaginating Projections in more Complex Animals” section). Schwann cell invaginations also are seen occasionally in the stimulated electrical ray organ terminals described in “Presynaptic Terminal/Axon-Derived Invaginating Projections” section (Boyne and McLeod 1979). A related process may occur in giant reticulospinal axon terminals in the lamprey, and as for the capitate projections described in the previous section, this involves an endophilin-associated endocytosis via clathrin-coated vesicles; in this case, though, the source of the invaginating postsynaptic process is not described (see “Small Postsynaptic Invaginating Projections: Spinules” section).
Fly Gnarls
Fly compound eyes also have another kind of glial invaginating projection called a “gnarl” (Fig. 9c). Gnarls are somewhat similar to capitate projections and arise from the same type of glial cell; they can have a stalk or neck and enlarged head, but they look more like spinules, without the elaborate head design of the capitate projection (Trujillo-Cenóz 1965; Boschek 1971; Campos-Ortega and Strausfeld 1973; Carlson 1987). Gnarls are found in small fibers, called α and β fibers, adjacent to the retinular terminals that contain the capitate projections; typically, the glial gnarls are associated with one side of synaptic terminal areas that form reciprocally between these two types of fibers [a glial process may also be directly part of these terminals, forming a gliapse (see above); the structural relationships are too complicated to describe in any detail here].
Structure and Function of Invaginating Projections
In the above section, we have described in detail the various kinds of neuronal invaginating projections in terms of their origin and composition. In this section, we first will focus on discussing their morphology and ultrastructure. And then we will give an overview of possible functions.
Structural Types of Invaginating Projections
The overall shape of invaginating projections varies from regular, finger-like, to those with an enlarged head with either a short or a long neck or stalk, to those that have elongate but rather irregular outlines, often tapering, and finally to those that are short and swollen. They also can be classified according to their associated structures. While many types of invaginating projections may have no particular association with other organelles, other kinds are associated with either coated pits or subsurface reticular cisternae.
Coated pits
Coated pits are commonly associated with invaginating projections, and we cite more than a dozen different kinds of associations in the sections of this review. A single coated pit may form at the distal end of the spinule, or there may be several pits along the shaft (Figs. 3c, d, 4, 6, 7, 8c, 9b). Some of these spinules can be very short and thin, no more than a projection from the parent cell entering directly into a coated pit on the cell surface membrane of the receiving cell. The best examples of these are spinules from axons that enter other axons or dendrites (e.g., postnatal mammalian cerebellar spinules) and from presynaptic terminals into the postsynaptic cell body. Other more elongate spinules also may have a coated pit surrounding their distal end, such as in spine-derived spinules of the hippocampus (Spacek and Harris 2004) or the presynaptic hair cell body spinules invaginated into the postsynaptic afferent dendrites in the early postnatal cochlea (Sobkowicz et al. 2003). This invagination of the spinule membrane into the coated pit can occur even when there are several coated pits around the spinule, as in some spine-derived spinules in the hippocampus (Tao-Cheng et al. 2009), in dendrite process spinules in immature cerebellar mossy terminals (Eckenhoff and Pysh 1979), and in glial cell-derived spinules invaginating into a neuron (Adamo and Daigneault 1973a).
In other cases, the coated pit does not draw in the spinule; rather in these cases, the coated pit or pits form along the receiving cell membrane that surrounds the elongate spinule (Fig. 9c, d). As described above, this is seen best in some kinds of invaginating glial processes, as well as some dendritic spinules. Glial capitate projections have a large, round head, but the sites of clathrin-mediated endocytosis seem to occur only on the sides of the stalk portion (Fabian-Fine et al. 2003). This suggests then that there are some cases, such as the capitate projections, where the only function may be recycling of the presynaptic terminal membrane following increased synaptic activity, i.e., resulting in recycling after increased fusion and incorporation of synaptic vesicle membranes with the terminal membrane. Perhaps in these cases, the invaginating projection serves a mainly mechanical or guidance function to help invaginate the presynaptic membrane in order to increase vesicle recycling, while maintaining the overall structural integrity of the synapse in the most efficient way possible. In other cases of high synaptic activity, where the coated pit engulfs the tip of the invaginating postsynaptic cell projection, there could be recycling of the enlarged postsynaptic membrane (enlarged with extra receptors and associated membrane proteins during potentiation), as may happen in the cycling of perforated and segmented mushroom synapses. Or there could be direct cell signaling from the source cell of the invaginating projection to the receiving cell, as will be discussed in more detail in “Function of Invaginating Projections in Cell Signaling” section.
Cisternae
In invaginating projections associated with cisternae, a cisterna may envelope or closely approach the distal end of the projection, and/or the projection will be associated with nearby autophagosomes (Fig. 9a). The invaginating glial projections of the trophospongium of many invertebrates often have these subsurface cisterns on their distal ends, and these cisternae may initiate the invagination as well as lead to phagocytosis of the invaginated processes. Similar subsurface cisternae are associated with invaginating oligodendroglial processes of spinal motor axons of the chicken (Li et al. 2005). Perhaps this subsurface cisterna represents an early phagophore structure, leading to formation of an autophagosome (Fader and Colombo 2009). Petralia et al. (2011) describe an apparent autophagy of spinules in the giant mossy terminals in the hippocampus (Fig. 7). In this case, this may be regulating the levels of sonic hedgehog receptors at this synapse, as noted above. We also have discussed how invaginating afferent dendrite filopodia, some forming synapses, in the inner hair cells of the cochlea appear to be degraded by autophagosomes in order to adjust the normal structural–functional arrangement of synapses in this area (Sobkowicz et al. 1998). A related phenomenon may be the daily phagocytosis of distal membrane disks from the outer segment (dendrite end; a modified cilium) of vertebrate photoreceptor cells, by retinal pigment epithelium cells (reviewed in Wood and Rosenbaum 2015). Tao-Cheng et al. (2009) show examples where the spinule becomes vesiculated (i.e., it appears to break into several vesicle-like portions) and suggest that such structures could become multivesicular bodies targeted for degradation by the receiving neuron. We suggest that it is more likely that vesiculated spinules are processed through autophagosomes, but in any case, the pathways of multivesicular bodies and autophagosomes are interconnected (Fader and Colombo 2009).
While these examples appear to involve phagocytosis of both the invaginating projection and the surrounding membrane of the receiving cell, in other cases, membrane retrieval and degradation may involve only the receiving cell membrane while the invaginating projection retracts. This appears to be the mechanism for fish retinal spinules where the spinules, which in this case may be involved in a form of direct neurotransmission, retract and the surrounding cone photoreceptor cell membranes are collected and degraded in multivesicular bodies (Wagner 1980; or more likely, this more directly involves autophagocytosis, as discussed in the previous paragraph).
An additional example of subsurface cisternae associated with an invaginated projection is found between axon terminals in the optic lobe of the squid, as noted in “Presynaptic Terminal/Axon-Derived Invaginating Projections” section. But in this case, the cisternae may be associated with a gap junction and perhaps are involved in synaptic neurotransmission.
Function of Invaginating Projections in Cell Signaling
Because there is a large variety in the form of invaginating projections, there also likely are several different functions, as we have described throughout this review. For example, Spacek and Harris (2004) note that spinules that originate from postsynaptic spines have two different organizations: those from thin spines invaginate into nearby axonal or glial processes, while those from the large mushroom spines invaginate into the main presynaptic terminal. They suggest that the thin spine spinules may be involved in a competition for synapses with neighboring axons, while the mushroom spine spinules are involved in remodeling and retrograde signaling (as noted above). Spinules forming from large mushroom spines and invaginating into the presynaptic terminals likely are linked to regulating the size of the synaptic membranes that increase in area during long-term potentiation or other forms of synaptic plasticity. Capitate projections in fly eyes probably help regulate the size of the photoreceptor terminal during high visual activity. The trophospongium of many invertebrates invaginate glial processes into cell bodies and axons and likely have some trophic function for support and maybe nourishment of the neuron. Other possible functions of invaginating projections discussed in this review include electrical coupling between nerve fibers in insect eyes, thermoreception in amphids, and mechanical stabilization (might this also be a subsidiary function of the capitate projections in fly eyes?). But in addition to these various physical and regulatory functions, might some invaginating projections mediate cell signaling?
One simple case that seems likely to involve cell signaling is the fish retinal spinules, in which these structure actually may mediate a form of neurotransmission at the cone cell photoreceptor ribbon synapse, as discussed in “Small Postsynaptic Invaginating Projections: Spinules” section. We also have described in “Presynaptic Terminal/Axon-Derived Invaginating Projections” section how, in the mollusk, Aplysia, the cell adhesion molecules called apCAMs are removed from the membrane in an axon–axon cell contact by sequestering of the apCAM molecules into a spinule that is consumed by the adjacent axon via coated pit-mediated endocytosis (Bailey et al. 1992); this might destabilize the contacts between the axons and lead to the growth of new synaptic connections. Sonic hedgehog receptors appear to be removed by recycling of spinules via autophagosomes in hippocampal mossy terminals (Petralia et al. 2011), and this could regulate the effect of sonic hedgehog signaling on these neurons (since autophagy regulates signaling; Gao et al. 2010). This could be a component of remodeling of synaptic sites of the thorny excrescences (Popov et al. 2011). Similarly, signaling from the contact of invaginated spinules/filopodia might mediate formation of new synaptic contacts as we have discussed for other synapses, such as the thin spines of the hippocampus (Spacek and Harris 2004) and the inner hair cell synapse of the cochlea (Sobkowicz et al. 1998), as well as several examples of spinules from axons that may initiate synaptogenesis or dendritogenesis.
While there is little direct evidence of a role of cell signaling by invaginating cell processes in neurons, there is a large body of the literature on cell signaling using exosomes/ectosomes or filopodia in various other cells and tissues, as noted in the Introduction and “Other Types of Short-Distance Intercellular Communication” section (as well as exosome release within invaginations—see our discussions on wingless (a Wnt) signaling in the larval neuromuscular junction of Drosophila; “Presynaptic Terminal/Axon-Derived Invaginating Projections” section). Of particular relevance here may be studies that suggest that cell signaling can occur via transcytosis when one cell invaginates a filopodium or spinule into another cell and this receiving cell pinches off part of the process, taking it up into its cytoplasm; this of course is what we have described here in many cases of invaginating projections in neurons. This is one method where a morphogen such as the Wnt, wingless, may be taken up in cells in membrane exovesicles called argosomes (Greco et al. 2001; sonic hedgehog is another morphogen that we discussed in the previous paragraph). Some signaling molecules that are directly involved in the development of the nervous system may be exchanged via transcytosis of parts of filopodia or spinules, such as in neurogenic signaling via Delta and Notch (Klueg and Muskavitch 1999), and the rac-dependent transcytosis of ephrinB, which is a membrane bound ligand of Eph RTKs (receptor tyrosine kinases; Marston et al. 2003). Eph receptor signaling is involved in axon guidance, neuronal growth cone survival, neural crest cell migration, establishing boundaries between rhombomeres in the hindbrain, etc.
Another RTK signaling mechanism is important in the early development of the retina in Drosophila (Krämer et al. 1991; Cagan et al. 1992; chapter 10 in Mueller et al. 2015). In this case, the ligand, bride of sevenless (“boss”), on the R8 photoreceptor cell membrane may be transferred and internalized using transcytosis via this kind of invaginating projection to a neighboring cell where it binds to the RTK, sevenless; this induces the receiving cell to become an R7 photoreceptor cell. In this case, the transfer is believed to involve the R8 apical microvilli (Cagan et al. 1992), which label intensely for boss (Krämer et al. 1991). This last example perhaps best illustrates the evolutionary advantage of developing a highly restricted process, such as spinule endocytosis, to signal between two specific cells; in this case, an R8 cell must provide a specific signal only to one adjacent cell, to induce it to become an R7 cell. As Cagan et al. (1992) note: “It is important for normal development that other competent cells located only a single cell diameter away from R8 do not have access to the inductive cue.” Perhaps, many kinds of neuronal spinules and other invaginating projections have this kind of specific neuron-to-neuron signaling mechanism.
Synaptic Spinules in Aging and Disease
There is little evidence in the literature on the effects of diseases on spinules and other invaginating projections. Patients with Parkinson’s disease seemed to have more perforated synapses with longer spinules in the caudate nucleus, compared to controls, although this trend of change was not quantitatively analyzed (Anglade et al. 1996). Sasaki and Iwata (1999) reported an increase in spinules in the Betz (large pyramidal) neurons of layer V in the motor cortex of patients with amyotrophic lateral sclerosis (ALS) compared to controls. In contrast, they did not see differences in spinule frequency in neurons of the anterior horn of the spinal cord in patients with ALS or lower motor neuron disease (Sasaki and Iwata 1995, 1996). Popov et al. (2011) examined mossy terminal–thorny excrescence synapses of the hippocampus of a mouse model for Down syndrome and found a significant decrease in the number of invaginated spinules/filopodia from the thorny excrescences, possibly contributing to decreased synaptic plasticity in Down syndrome.
While there is little direct information on changes in spinules with aging, there is substantial literature on the changes in perforated synapses with aging (for a recent review, see Petralia et al. 2014a). Since these kinds of synapses often produce spinules that invaginate into the presynaptic terminal, changes in perforated synapses can be expected to reflect, to some extent, changes in spinules. The effect of age on spinules was addressed specifically in the aging rat parietal cortex from 0.5 to 22 months (Jones and Calverley 1991). Spinules are most prominent in middle-aged rats around 12 months old, when perforated synapses also are most common, and are considerably less conspicuous in the young and old rats.
Other Types of Short-Distance Intercellular Communication
There are several additional mechanisms used by cells for intercellular communications. The first is the exchange of free molecules that are released via exocytosis from the signaling cell and then impinge onto associated receptor molecules on the nearby target cell. We refer the readers to chapter 11 in Mueller et al. (2015) for a comprehensive review.
The second mechanism is somewhat extreme, where a long, tubular conduit called a “tunneling nanotube” completely connects the two cells; this allows for exchange both of surface molecules and molecules trafficking in the cytoplasm (Gurke et al. 2008; Sherer and Mothes 2008; chapter 11 in Mueller et al. 2015).
The third mechanism relies on the release of small vesicles called exosomes. These vesicles are formed in specialized multivesicular bodies (MVB’s) and presumably are released mainly by fusion of the MVB’s with the cell surface membrane. For cells in a typical epithelial layer, the exosomes may be released from the free apical surface of epithelial cells into the surrounding medium, and subsequently travel to the apical surfaces of adjacent cells (Matsuo et al. 2014). Or the exosomes are released from the lateral surface of the cell where there is minimal intercellular space between the donor and target cell (planar transcytosis; Matsuo et al. 2014; Mueller et al. 2015). In addition, exosomes can be released directly from enclosed presynaptic terminals at the Drosophila NMJ (see “Presynaptic Terminal/Axon-Derived Invaginating Projections” section). The enclosed environment helps restrict uptake of the exosomes to the postsynaptic region of the muscle cell (Korkut et al. 2009, 2013; Kornberg and Roy 2014). Currently, exosomes are being studied intensely; we refer the readers to Chivet et al. (2012), Rajendran et al. (2014), and Joshi et al. (2015) for comprehensive reviews of exosomes in the normal nervous system and in Alzheimer’s disease.
Summary and Conclusions
Invaginating projections in neurons and associated glia of the nervous system include a wide range of structures that have been given many names in the literature, generating much confusion (Table 1). Most commonly, larger, finger-shaped invaginating projections more than about 100 nm in diameter and often more than 1 μm in length are called filopodia, while thinner and shorter ones are called spinules. The reader needs to be aware that authors of published research may interchange these terms or use entirely different terms. These structures in the nervous system resemble (and some could be derived from) similar processes that invaginate between cells in other animal tissue types, especially the long filopodia or cytonemes that can form contacts (sometimes invaginating) among epithelial cells, sometimes over several cell widths, as well as shorter spinule-like structures that may invaginate from close cell contacts such as the lateral walls of epithelial cells (transcytosis or transendocytosis). A number of these kinds of cell contacts have been implicated in signaling between two cells, such as the transfer of morphogens and other factors controlling growth and development. Similarly, some kinds of signaling seem to occur between neurons (or neurons and glia) via invaginating projections. Other functions of invaginating projections in the nervous system are more utilitarian functions such as removal of excess cell membrane and associated proteins following increased activity at the synapse, and nourishment of a neuron by associated glia. A greater complexity and prevalence of invaginating projections in some kinds of animals may reflect the evolution of greater synaptic and neuronal plasticity associated with more complex behaviors.
Invaginating projections have different associated structures that reflect the function and fate of the projection. In particular, many have either reticular cisterns or coated pits formed in the cell that receives the invaginating projection. The reticular cistern appears to be a precursor for a phagosome that will ingest part of the structure, including the invaginated internal portion derived from the invaginating cell. In other cases, where the invaginating inner process appears to be retracted rather than eaten (as for fish retinal spinules), the receiving cell’s membrane is resorbed in some way that is not clear, leading to the formation of multivesicular bodies to degrade the membrane. Coated pits may or may not consume part of the invaginated process from the other cell, and many varieties exist in different animals and different parts of the nervous system. The variation may depend on whether they are recycling one or both apposing cell membranes, and/or whether they are picking up some signaling molecule from the other cell.
While these various invaginating projections clearly have diverse functions, the most interesting questions now concern their role in cell signaling. Even a spinule or filopodium that clearly is involved in membrane recycling or some other utilitarian function may be involved in signaling also. The invaginating projection is the most intimate way that a neuron or glial cell can interact with another neuron or glial cell. It assures a direct transmission of a potential signal from one cell to another with minimal background spread of information to other neighboring cells in a region of the nervous system. It seems natural that the nervous system would evolve to take the maximum functional advantage of such a structural association, i.e., another level of cell communication to coordinate better a system designed overall for communication.
Acknowledgments
This work was supported by the Intramural Research Programs of NIDCD/NIH and NIA/NIH.
References
- Acsády L, Katona I, Martinez-Guijarro FJ, Buzsaki G, Freund TF. Unusual target selectivity of perisomatic inhibitory cells in the hilar region of the rat hippocampus. Journal of Neuroscience. 2000;20(18):6907–6919. doi: 10.1523/JNEUROSCI.20-18-06907.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamo NJ, Daigneault EA. Ultrastructural features of neurons and nerve fibres in the spiral ganglia of cats. Journal of Neurocytology. 1973a;2(1):91–103. doi: 10.1007/BF01099211. [DOI] [PubMed] [Google Scholar]
- Adamo NJ, Daigneault EA. Ultrastructural morphology of Schwann cell-neuronal relationships in the spiral ganglia of cats. American Journal of Anatomy. 1973b;138(1):73–77. doi: 10.1002/aja.1001380105. doi:10.1002/aja.1001380105. [DOI] [PubMed] [Google Scholar]
- Altman J. Coated vesicles and synaptogenesis. A developmental study in the cerebellar cortex of the rat. Brain Research. 1971;30(2):311–322. doi: 10.1016/0006-8993(71)90081-3. [DOI] [PubMed] [Google Scholar]
- Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. Journal of Comparative Neurology. 1993;145:399–464. doi: 10.1002/cne.901450402. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the cerebellar system in relation to its evolution, structure, and functions. CRC Press; New York: 1997. [Google Scholar]
- Anglade P, Mouatt-Prigent A, Agid Y, Hirsch E. Synaptic plasticity in the caudate nucleus of patients with Parkinson’s disease. Neurodegeneration. 1996;5(2):121–128. doi: 10.1006/neur.1996.0018. [DOI] [PubMed] [Google Scholar]
- Ashton FT, Li J, Schad GA. Chemo- and thermosensory neurons: Structure and function in animal parasitic nematodes. Veterinary Parasitology. 1999;84(3–4):297–316. doi: 10.1016/s0304-4017(99)00037-0. [DOI] [PubMed] [Google Scholar]
- Atwood HL, Govind CK, Wu CF. Differential ultrastructure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. Journal of Neurobiology. 1993;24(8):1008–1024. doi: 10.1002/neu.480240803. doi:10.1002/neu.480240803. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Chen M, Keller F, Kandel ER. Serotonin-mediated endocytosis of apCAM: An early step of learning-related synaptic growth in Aplysia. Science. 1992;256(5057):645–649. doi: 10.1126/science.1585177. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Thompson EB, Castellucci VF, Kandel ER. Ultrastructure of the synapses of sensory neurons that mediate the gill-withdrawal reflex in Aplysia. Journal of Neurocytology. 1979;8(4):415–444. doi: 10.1007/BF01214801. [DOI] [PubMed] [Google Scholar]
- Biserova NM. Do glial cells exist in the nervous system of parasitic and free-living flatworms? An ultrastructural and immunocytochemical investigation. Acta Biologica Hungarica. 2008;59(Suppl):209–219. doi: 10.1556/ABiol.59.2008.Suppl.30. doi:10.1556/ABiol.59.2008.Suppl.30. [DOI] [PubMed] [Google Scholar]
- Biserova NM, Gordeev II, Korneva JV, Salnikova MM. Structure of the glial cells in the nervous system of parasitic and free-living flatworms. Biology Bulletin. 2010;37(3):277–287. [PubMed] [Google Scholar]
- Blanco RE. Glial cells in peripheral nerves of the cockroach, Periplaneta americana. Tissue and Cell. 1988;20(5):771–782. doi: 10.1016/0040-8166(88)90022-5. [DOI] [PubMed] [Google Scholar]
- Blanque A, Repetto D, Rohlmann A, Brockhaus J, Duning K, Pavenstädt H, et al. Deletion of KIBRA, protein expressed in kidney and brain, increases filopodial-like long dendritic spines in neocortical and hippocampal neurons in vivo and in vitro. Frontiers in Neuroanatomy. 2015;9:13. doi: 10.3389/fnana.2015.00013. doi:10.3389/fnana.2015.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonga SEW. Ultrastructure and histochemistry of neurosecretory cells and neurohaemal areas in the pond snail Lymnaea stagnalis. Zeitschrift für Zellforschung und Mikroskopische Anatomie. 1970;108:190–224. doi: 10.1007/BF00335295. [DOI] [PubMed] [Google Scholar]
- Boschek CB. On the fine structure of the peripheral retina and lamina ganglionaris of the fly, Musca domestica. Zeitschrift für Zellforschung und Mikroskopische Anatomie. 1971;118(3):369–409. doi: 10.1007/BF00331193. [DOI] [PubMed] [Google Scholar]
- Boyne AF, McLeod S. Ultrastructural plasticity in stimulated nerve terminals: Pseudopodial invasions of abutted terminals in Torpedine ray electric organ. Neuroscience. 1979;4(5):615–624. doi: 10.1016/0306-4522(79)90138-6. [DOI] [PubMed] [Google Scholar]
- Boyne AF, Tarrant SB. Pseudopodial interdigitations between abutted nerve terminals: diffusion traps which occur in several nuclei of the rat limbic system. Journal of Neuroscience. 1982;2(4):463–469. doi: 10.1523/JNEUROSCI.02-04-00463.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozhilova-Pastirova A, Ovtscharoff W. Intramembranous structure of synaptic membranes with special reference to spinules in the rat sensorimotor cortex. European Journal of Neuroscience. 1999;11(5):1843–1846. doi: 10.1046/j.1460-9568.1999.00608.x. [DOI] [PubMed] [Google Scholar]
- Buchheit TE, Tytell M. Transfer of molecules from glia to axon in the squid may be mediated by glial vesicles. Journal of Neurobiology. 1992;23(3):217–230. doi: 10.1002/neu.480230303. doi:10.1002/neu.480230303. [DOI] [PubMed] [Google Scholar]
- Budziakowski ME, Mettrick DF. Ultrastructural morphology of the neuropile of the cerebral ganglion of Moniliformis moniliformis (Acanthocephala) Journal of Parasitology. 1985;71(1):75–85. [PubMed] [Google Scholar]
- Bumbarger DJ, Wijeratne S, Carter C, Crum J, Ellisman MH, Baldwin JG. Three-dimensional reconstruction of the amphid sensilla in the microbial feeding nematode, Acrobeles complexus (Nematoda: Rhabditida) Journal of Comparative Neurology. 2009;512(2):271–281. doi: 10.1002/cne.21882. doi:10.1002/cne.21882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadete-Leite A, Tavares MA, Paula-Barbosa MM, Gray EG. ‘Perforated’ synapses in frontal cortex of chronic alcohol-fed rats. Journal of Submicroscopic Cytology. 1986;18(3):495–499. [PubMed] [Google Scholar]
- Cagan RL, Kramer H, Hart AC, Zipursky SL. The bride of sevenless and sevenless interaction: Internalization of a transmembrane ligand. Cell. 1992;69(3):393–399. doi: 10.1016/0092-8674(92)90442-f. [DOI] [PubMed] [Google Scholar]
- Calverley RK, Jones DG. A serial-section study of perforated synapses in rat neocortex. Cell and Tissue Research. 1987;247(3):565–572. doi: 10.1007/BF00215750. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega JA, Strausfeld NJ. Synaptic connections of intrinsic cells and basket arborizations in the external plexiform layer of the fly’s eye. Brain Research. 1973;59:119–136. doi: 10.1016/0006-8993(73)90255-2. [DOI] [PubMed] [Google Scholar]
- Carlin RK, Siekevitz P. Plasticity in the central nervous system: Do synapses divide? Proceedings of the National Academy of Sciences of the USA. 1983;80(11):3517–3521. doi: 10.1073/pnas.80.11.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SD. Ultrastructure of the arthropod neuroglia and neuropil. In: Gupta AP, editor. Arthropod brain: Its evolution, development, structure, and functions. Wiley; New York: 1987. pp. 323–346. [Google Scholar]
- Case NM, Gray EG, Young JZ. Ultrastructure and synaptic relations in the optic lobe of the brain of Eledone and Octopus. Journal of Ultrastructure Research. 1972;39(1):115–123. doi: 10.1016/s0022-5320(72)80012-1. [DOI] [PubMed] [Google Scholar]
- Chi C, Carlson SD. Close apposition of photoreceptor cell axons in the house fly. Journal of Insect Physiology. 1976;22(8):1153–1157. doi: 10.1016/0022-1910(76)90126-8. [DOI] [PubMed] [Google Scholar]
- Chicurel ME, Harris KM. Three-dimensional analysis of the structure and composition of CA3 branched dendritic spines and their synaptic relationships with mossy fiber boutons in the rat hippocampus. Journal of Comparative Neurology. 1992;325(2):169–182. doi: 10.1002/cne.903250204. doi:10.1002/cne.903250204. [DOI] [PubMed] [Google Scholar]
- Chivet M, Hemming F, Pernet-Gallay K, Fraboulet S, Sadoul R. Emerging role of neuronal exosomes in the central nervous system. Frontiers in Physiology. 2012;3:145. doi: 10.3389/fphys.2012.00145. doi:10.3389/fphys.2012.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocucci E, Meldolesi J. Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends in Cell Biology. 2015;25(6):364–372. doi: 10.1016/j.tcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: Artefacts no more. Trends in Cell Biology. 2009;19(2):43–51. doi: 10.1016/j.tcb.2008.11.003. doi:10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Fawcett DW. The fine structure of the central nervous system of the leech, Hirudo medicinalis. Journal of Neurophysiology. 1964;27:229–289. doi: 10.1152/jn.1964.27.2.229. [DOI] [PubMed] [Google Scholar]
- Cohen AI. An ultrastructural analysis of the photoreceptors of the squid and their synaptic connections. 3. Photoreceptor terminations in the optic lobes. Journal of Comparative Neurology. 1973;147(3):399–426. doi: 10.1002/cne.901470306. doi:10.1002/cne.901470306. [DOI] [PubMed] [Google Scholar]
- Curcio CA, McNelly NA, Hinds JW. Aging in the rat olfactory system: Relative stability of piriform cortex contrasts with changes in olfactory bulb and olfactory epithelium. Journal of Comparative Neurology. 1985;235(4):519–528. doi: 10.1002/cne.902350409. doi:10.1002/cne.902350409. [DOI] [PubMed] [Google Scholar]
- Dayel MJ, Alegado RA, Fairclough SR, Levin TC, Nichols SA, McDonald K, et al. Cell differentiation and morphogenesis in the colony-forming choanoflagellate Salpingoeca rosetta. Development Biology. 2011;357(1):73–82. doi: 10.1016/j.ydbio.2011.06.003. doi:10.1016/j.ydbio.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond NL, Levy WB. Synaptic correlates of associative potentiation/depression: An ultrastructural study in the hippocampus. Brain Research. 1983;265(1):21–30. doi: 10.1016/0006-8993(83)91329-x. [DOI] [PubMed] [Google Scholar]
- Dilly PN, Gray EG, Young JZ. Electron microscopy of optic nerves and optic lobes of Octopus and Eledone. Proceedings of the Royal Society of London. Series B: Biological Sciences. 1963;158:446–456. doi: 10.1098/rspb.1963.0057. [DOI] [PubMed] [Google Scholar]
- Dino MR, Nunzi MG, Anelli R, Mugnaini E. Unipolar brush cells of the vestibulocerebellum: Afferents and targets. Progress in Brain Research. 2000;124:123–137. doi: 10.1016/S0079-6123(00)24013-2. doi:10.1016/S0079-6123(00)24013-2. [DOI] [PubMed] [Google Scholar]
- Dirks P, Tieding S, Schneider I, Mey J, Weiler R. Characterization of retinoic acid neuromodulation in the carp retina. Journal of Neuroscience Research. 2004;78(2):177–185. doi: 10.1002/jnr.20253. doi:10.1002/jnr.20253. [DOI] [PubMed] [Google Scholar]
- Dyson SE, Jones DG. Synaptic remodelling during development and maturation: Junction differentiation and splitting as a mechanism for modifying connectivity. Brain Research. 1984;315(1):125–137. doi: 10.1016/0165-3806(84)90084-1. [DOI] [PubMed] [Google Scholar]
- Eckenhoff MF, Pysh JJ. Double-walled coated vesicle formation: Evidence for massive and transient conjugate internalization of plasma membranes during cerebellar development. Journal of Neurocytology. 1979;8(5):623–638. doi: 10.1007/BF01208513. [DOI] [PubMed] [Google Scholar]
- Erisir A, Dreusicke M. Quantitative morphology and postsynaptic targets of thalamocortical axons in critical period and adult ferret visual cortex. Journal of Comparative Neurology. 2005;485(1):11–31. doi: 10.1002/cne.20507. doi:10.1002/cne.20507. [DOI] [PubMed] [Google Scholar]
- Fabian-Fine R, Verstreken P, Hiesinger PR, Horne JA, Kostyleva R, Zhou Y, et al. Endophilin promotes a late step in endocytosis at glial invaginations in Drosophila photoreceptor terminals. Journal of Neuroscience. 2003;23(33):10732–10744. doi: 10.1523/JNEUROSCI.23-33-10732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader CM, Colombo MI. Autophagy and multi-vesicular bodies: Two closely related partners. Cell Death and Differentiation. 2009;16(1):70–78. doi: 10.1038/cdd.2008.168. doi:10.1038/cdd.2008.168. [DOI] [PubMed] [Google Scholar]
- Fairchild CL, Barna M. Specialized filopodia: At the “tip” of morphogen transport and vertebrate tissue patterning. Current Opinion in Genetics & Development. 2014;27:67–73. doi: 10.1016/j.gde.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Familtsev D. Synapses, spines, zinc and pathology of Alzheimer’s disease. University of Louisville; Louisville, Kentucky: 2013. [Google Scholar]
- Farley RD, Chan DJ. The ultrastructure of the cardiac ganglion of the desert scorpion, Paruroctonus mesaensis (Scorpionida: Vaejovidae) J Morph. 1985;184:231–252. doi: 10.1002/jmor.1051840212. [DOI] [PubMed] [Google Scholar]
- Fedorenko GM, Uzdensky AB. Ultrastructure of neuroglial contacts in crayfish stretch receptor. Cell and Tissue Research. 2009;337(3):477–490. doi: 10.1007/s00441-009-0825-7. doi:10.1007/s00441-009-0825-7. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Allwardt B, Harris KM. Dendritic spines do not split during hippocampal LTP or maturation. Nature Neuroscience. 2002;5(4):297–298. doi: 10.1038/nn830. doi:10.1038/nn830. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. Journal of Neuroscience. 1998;18(21):8900–8911. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floris A, Dino M, Jacobowitz DM, Mugnaini E. The unipolar brush cells of the rat cerebellar cortex and cochlear nucleus are calretinin-positive: A study by light and electron microscopic immunocytochemistry. Anatomy and Embryology (Berl) 1994;189(6):495–520. doi: 10.1007/BF00186824. [DOI] [PubMed] [Google Scholar]
- Fox CA, Andrade AN, Lu Qui IJ, Rafols JA. The primate globus pallidus: A Golgi and electron microscopic study. Journal fur Hirnforschung. 1974;15(1):75–93. [PubMed] [Google Scholar]
- Friedlander MJ, Martin KAC, Wassenhove-McCarthy D. Effects of monocular visual deprivation on geniculocortical innervation of area 18 in cat. Journal of Neuroscience. 1991;11(10):3268–3288. doi: 10.1523/JNEUROSCI.11-10-03268.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Straka H. Evolution of vertebrate mechanosensory hair cells and inner ears: Toward identifying stimuli that select mutation driven altered morphologies. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology. 2014;200(1):5–18. doi: 10.1007/s00359-013-0865-z. doi:10.1007/s00359-013-0865-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich A, Meinertzhagen IA. Synaptogenesis in the first optic neuropile of the fly’s visual system. Journal of Neurocytology. 1982;11(1):159–180. doi: 10.1007/BF01258010. [DOI] [PubMed] [Google Scholar]
- Gad H, Low P, Zotova E, Brodin L, Shupliakov O. Dissociation between Ca2+-triggered synaptic vesicle exocytosis and clathrin-mediated endocytosis at a central synapse. Neuron. 1998;21(3):607–616. doi: 10.1016/s0896-6273(00)80570-x. [DOI] [PubMed] [Google Scholar]
- Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y. Synapses with a segmented, completely partitioned postsynaptic density express more AMPA receptors than other axospinous synaptic junctions. Neuroscience. 2004a;125(3):615–623. doi: 10.1016/j.neuroscience.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y. Differences in the expression of AMPA and NMDA receptors between axospinous perforated and nonperforated synapses are related to the configuration and size of postsynaptic densities. Journal of Comparative Neurology. 2004b;468(1):86–95. doi: 10.1002/cne.10950. [DOI] [PubMed] [Google Scholar]
- Gao C, Cao W, Bao L, Zuo W, Xie G, Cai T, et al. Autophagy negatively regulates Wnt signalling by promoting Dishevelled degradation. Nature Cell Biology. 2010;12(8):781–790. doi: 10.1038/ncb2082. doi:10.1038/ncb2082. [DOI] [PubMed] [Google Scholar]
- Geinisman Y. Structural synaptic modifications associated with hippocampal LTP and behavioral learning. Cerebral Cortex. 2000;10(10):952–962. doi: 10.1093/cercor/10.10.952. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, deToledo-Morrell L, Morrell F. Comparison of structural synaptic modifications induced by long-term potentiation in the hippocampal dentate gyrus of young adult and aged rats. Annals of the New York Academy of Sciences. 1994;747:452–466. doi: 10.1111/j.1749-6632.1994.tb44428.x. [DOI] [PubMed] [Google Scholar]
- Gennaro JF, Jr, Nastuk WL, Rutherford DT. Reversible depletion of synaptic vesicles induced by application of high external potassium to the frog neuromuscular junction. Journal of Physiology. 1978;280:237–247. doi: 10.1113/jphysiol.1978.sp012382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonobobleva E, Maldonado M. Choanocyte ultrastructure in Halisarca dujardini (Demospongiae, Halisarcida) Journal of Morphology. 2009;270(5):615–627. doi: 10.1002/jmor.10709. doi:10.1002/jmor.10709. [DOI] [PubMed] [Google Scholar]
- Gordon WC. Nonconventional interactions between photoreceptor axons in the butterfly lamina ganglionaris. Zeitschrift für Naturforschung. 1985;40c:460–463. [Google Scholar]
- Gray EG. The granule cells, mossy synapses and Purkinje spine synapses of the cerebellum: Light and electron microscope observations. Journal of Anatomy. 1961;95:345–356. [PMC free article] [PubMed] [Google Scholar]
- Greco V, Hannus M, Eaton S. Argosomes: A potential vehicle for the spread of morphogens through epithelia. Cell. 2001;106(5):633–645. doi: 10.1016/s0092-8674(01)00484-6. [DOI] [PubMed] [Google Scholar]
- Gregory WA, Hall DH, Bennett MV. Satellite glial cells penetrate neurosecretory cells to perinuclear position in the goldfish preoptic area. Developmental Brain Research. 1988;44(1):1–8. doi: 10.1016/0165-3806(88)90113-7. [DOI] [PubMed] [Google Scholar]
- Gurke S, Barroso JF, Gerdes HH. The art of cellular communication: Tunneling nanotubes bridge the divide. Histochemistry and Cell Biology. 2008;129(5):539–550. doi: 10.1007/s00418-008-0412-0. doi:10.1007/s00418-008-0412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haamedi SN, Karten HJ, Djamgoz MB. Nerve growth factor induces light adaptive cellular and synaptic plasticity in the outer retina of fish. Journal of Comparative Neurology. 2001;431(4):397–404. doi: 10.1002/1096-9861(20010319)431:4<397::aid-cne1078>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Halanych KM. The ctenophore lineage is older than sponges? That cannot be right! Or can it? Journal of Experimental Biology. 2015;218(Pt 4):592–597. doi: 10.1242/jeb.111872. doi:10.1242/jeb.111872. [DOI] [PubMed] [Google Scholar]
- Harreveld AV, Trubatch J. Synaptic changes in frog brain after stimulation with potassium chloride. Journal of Neurocytology. 1975;4(1):33–46. doi: 10.1007/BF01099093. [DOI] [PubMed] [Google Scholar]
- Hartfelder K, Hanton WK, Bollenbacher WE. Diapause-dependent changes in prothoracicotropic hormone-producing neurons of the tobacco hornworm, Manduca sexta. Cell and Tissue Research. 1994;277(1):69–78. doi: 10.1007/BF00303082. [DOI] [PubMed] [Google Scholar]
- Heuser JE, Reese TS. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. Journal of Cell Biology. 1973;57(2):315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmann M, Thurm U. Mono- and oligo-vesicular synapses and their connectivity in a Cnidarian sensory epithelium (Coryne tubulosa) J Comp Neurol. 2001;432(4):537–549. doi: 10.1002/cne.1118. [DOI] [PubMed] [Google Scholar]
- Hoyle G, Williams M, Philips C. Functional morphology of insect neuronal cell-surface/glial contacts: The trophospongium. Journal of Comparative Neurology. 1986;246:113–128. doi: 10.1002/cne.902460108. [DOI] [PubMed] [Google Scholar]
- Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: The last 25 years. Neuron. 2013;80(3):704–717. doi: 10.1016/j.neuron.2013.10.025. doi:10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia XX, Gorczyca M, Budnik V. Ultrastructure of neuromuscular junctions in Drosophila: Comparison of wild type and mutants with increased excitability. Journal of Neurobiology. 1993;24(8):1025–1044. doi: 10.1002/neu.480240804. doi:10.1002/neu.480240804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DG, Calverley RK. Perforated and nonperforated synapses in rat neocortex: Three-dimensional reconstructions. Brain Research. 1991;556(2):247–258. doi: 10.1016/0006-8993(91)90312-j. [DOI] [PubMed] [Google Scholar]
- Jorgensen EM. Animal evolution: Looking for the first nervous system. Current Biology. 2014;24(14):R655–R658. doi: 10.1016/j.cub.2014.06.036. doi:10.1016/j.cub.2014.06.036. [DOI] [PubMed] [Google Scholar]
- Joshi P, Benussi L, Furlan R, Ghidoni R, Verderio C. Extracellular Vesicles in Alzheimer’s Disease: Friends or Foes? Focus on Abeta-Vesicle Interaction. International Journal of Molecular Sciences. 2015;16(3):4800–4813. doi: 10.3390/ijms16034800. doi:10.3390/ijms16034800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klooster J, Yazulla S, Kamermans M. Ultrastructural analysis of the glutamatergic system in the outer plexiform layer of zebrafish retina. Journal of Chemical Neuroanatomy. 2009;37(4):254–265. doi: 10.1016/j.jchemneu.2009.02.004. doi:10.1016/j.jchemneu.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Klueg KM, Muskavitch MA. Ligand-receptor interactions and trans-endocytosis of Delta, Serrate and Notch: Members of the Notch signalling pathway in Drosophila. Journal of Cell Science. 1999;112(Pt 19):3289–3297. doi: 10.1242/jcs.112.19.3289. [DOI] [PubMed] [Google Scholar]
- Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, et al. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139(2):393–404. doi: 10.1016/j.cell.2009.07.051. doi:10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkut C, Li Y, Koles K, Brewer C, Ashley J, Yoshihara M, et al. Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron. 2013;77(6):1039–1046. doi: 10.1016/j.neuron.2013.01.013. doi:10.1016/j.neuron.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg TB, Roy S. Cytonemes as specialized signaling filopodia. Development. 2014;141(4):729–736. doi: 10.1242/dev.086223. doi:10.1242/dev.086223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer H, Cagan RL, Zipursky SL. Interaction of bride of sevenless membrane-bound ligand and the sevenless tyrosine-kinase receptor. Nature. 1991;352(6332):207–212. doi: 10.1038/352207a0. doi:10.1038/352207a0. [DOI] [PubMed] [Google Scholar]
- Kröger RH, Wagner HJ. Horizontal cell spinule dynamics in fish are affected by rearing in monochromatic light. Vision Research. 1996;36(24):3879–3889. doi: 10.1016/s0042-6989(96)00132-0. [DOI] [PubMed] [Google Scholar]
- Larsen WJ. Biological implications of gap junction structure, distribution and composition: A review. Tissue and Cell. 1983;15(5):645–671. doi: 10.1016/0040-8166(83)90041-1. [DOI] [PubMed] [Google Scholar]
- Leise EM, Cloney RA. Chiton integument: Ultrastructure of the sensory hairs of Mopalia muscosa (Mollusca: Polyplacophora) Cell and Tissue Research. 1982;223(1):43–59. doi: 10.1007/BF00221498. [DOI] [PubMed] [Google Scholar]
- Leranth C, Frotscher M. Synaptic connections of cholecystokinin-immunoreactive neurons and terminals in the rat fascia dentata: A combined light and electron microscopic study. Journal of Comparative Neurology. 1986;254(1):51–64. doi: 10.1002/cne.902540105. doi:10.1002/cne.902540105. [DOI] [PubMed] [Google Scholar]
- Leys SP. Elements of a ‘nervous system’ in sponges. Journal of Experimental Biology. 2015;218(Pt 4):581–591. doi: 10.1242/jeb.110817. doi:10.1242/jeb.110817. [DOI] [PubMed] [Google Scholar]
- Li YC, Li YN, Cheng CX, Sakamoto H, Kawate T, Shimada O, et al. Subsurface cisterna-lined axonal invaginations and double-walled vesicles at the axonal-myelin sheath interface. Neuroscience Research. 2005;53(3):298–303. doi: 10.1016/j.neures.2005.07.006. doi:10.1016/j.neures.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Mäntylä K, Reuter M, Halton DW, Maule AG, Brennan GP, Shaw C, et al. The nervous system of Procerodes littoralis (Maricola, Tricladida). An ultrastructural and immunoelectron microscopical study. Acta Zoologica. 1998;79(1):1–8. [Google Scholar]
- Marston DJ, Dickinson S, Nobes CD. Rac-dependent trans-endocytosis of ephrinBs regulates Eph–ephrin contact repulsion. Nature Cell Biology. 2003;5(10):879–888. doi: 10.1038/ncb1044. doi:10.1038/ncb1044. [DOI] [PubMed] [Google Scholar]
- Mashanov VS, Zueva OR, Heinzeller T, Dolmatov IY. Ultrastructure of the circumoral nerve ring and the radial nerve cords in holothurians (Echinodermata) Zoomorphology. 2006;125:27–38. [Google Scholar]
- Matsuo I, Kimura-Yoshida C, Shimokawa K. Divergent roles of heparan sulfate in regulation of FGF signaling during mammalian embryogenesis. In: Kondoh H, Kuroiwa A, editors. New principles in developmental processes. Springer; Japan: 2014. pp. 239–251. [Google Scholar]
- Matthews G, Fuchs P. The diverse roles of ribbon synapses in sensory neurotransmission. Nature Reviews Neuro-science. 2010;11(12):812–822. doi: 10.1038/nrn2924. doi:10.1038/nrn2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev NI, Dallérac G, Popov VI, Rodriguez Arellano JJ, Davies HA, Kraev IV, et al. Multiple spine boutons are formed after long-lasting LTP in the awake rat. Brain Structure and Function. 2014;219(1):407–414. doi: 10.1007/s00429-012-0488-0. doi:10.1007/s00429-012-0488-0. [DOI] [PubMed] [Google Scholar]
- Meyer D, Bonhoeffer T, Scheuss V. Balance and stability of synaptic structures during synaptic plasticity. Neuron. 2014;82(2):430–443. doi: 10.1016/j.neuron.2014.02.031. doi:10.1016/j.neuron.2014.02.031. [DOI] [PubMed] [Google Scholar]
- Mitchell N, Petralia RS, Currier DG, Wang YX, Kim A, Mattson MP, et al. Sonic hedgehog regulates presynaptic terminal size, ultrastructure and function in hippocampal neurons. Journal of Cell Science. 2012;125(Pt 18):4207–4213. doi: 10.1242/jcs.105080. doi:10.1242/jcs.105080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller WA, Hassel M, Grealy M. Development and reproduction in humans and animal model species. Springer; Berlin: 2015. [Google Scholar]
- Mugnaini E, Floris A, Wright-Goss M. Extraordinary synapses of the unipolar brush cell: An electron microscopic study in the rat cerebellum. Synapse. 1994;16(4):284–311. doi: 10.1002/syn.890160406. doi:10.1002/syn.890160406. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Osen KK, Dahl AL, Friedrich VL, Jr, Korte G. Fine structure of granule cells and related interneurons (termed Golgi cells) in the cochlear nuclear complex of cat, rat and mouse. Journal of Neurocytology. 1980;9(4):537–570. doi: 10.1007/BF01204841. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Sekerkova G, Martina M. The unipolar brush cell: A remarkable neuron finally receiving deserved attention. Brain Research Reviews. 2011;66(1–2):220–245. doi: 10.1016/j.brainresrev.2010.10.001. doi:10.1016/j.brainresrev.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, et al. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Current Biology. 2009;19(22):1875–1885. doi: 10.1016/j.cub.2009.09.059. doi:10.1016/j.cub.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muriel MP, Agid Y, Hirsch E. Plasticity of afferent fibers to striatal neurons bearing D1 dopamine receptors in Parkinson’s disease. Movement Disorders. 2001;16(3):435–441. doi: 10.1002/mds.1103. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Andrews SB. Culture models for the study of estradiol-induced synaptic plasticity. Journal of Neurocytology. 2000;29(5–6):411–417. doi: 10.1023/a:1007121525399. [DOI] [PubMed] [Google Scholar]
- Nickel M. Evolutionary emergence of synaptic nervous systems: What can we learn from the non-synaptic, nerveless Porifera? Invertebrate Biology. 2010;129(1):1–16. [Google Scholar]
- Nitsch C, Rinne U. Large dense-core vesicle exocytosis and membrane recycling in the mossy fibre synapses of the rabbit hippocampus during epileptiform seizures. Journal of Neurocytology. 1981;10(2):201–209. doi: 10.1007/BF01257967. [DOI] [PubMed] [Google Scholar]
- Nordlander RH, Masnyi JA, Singer M. Distribution of ultrastructural tracers in crustacean axons. Journal of Comparative Neurology. 1975;161:499–514. doi: 10.1002/cne.901610403. [DOI] [PubMed] [Google Scholar]
- Omiya Y, Uchigashima M, Konno K, Yamasaki M, Miyazaki T, Yoshida T, et al. VGluT3-Expressing CCK-positive basket cells construct invaginating synapses enriched with endocannabinoid signaling proteins in particular cortical and cortex-like amygdaloid regions of mouse brains. Journal of Neuroscience. 2015;35(10):4215–4228. doi: 10.1523/JNEUROSCI.4681-14.2015. doi:10.1523/JNEUROSCI.4681-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne MP. The fine structure of neuromuscular junctions in the segmental muscles of the blowfly larva. Journal of Insect Physiology. 1967;13:827–833. [Google Scholar]
- Palacios-Prü EL, Palacios L, Mendoza RV. Synaptogenetic mechanisms during chick cerebellar cortex development. Journal of Submicroscopic Cytology. 1981;13(2):145–167. [PubMed] [Google Scholar]
- Palay SL, Chan-Palay V. Cytology and organization. Springer; New York: 1974. Cerebellar cortex. [Google Scholar]
- Papassotiropoulos A, Stephan DA, Huentelman MJ, Hoerndli FJ, Craig DW, Pearson JV, et al. Common Kibra alleles are associated with human memory performance. Science. 2006;314(5798):475–478. doi: 10.1126/science.1129837. doi:10.1126/science.1129837. [DOI] [PubMed] [Google Scholar]
- Pappas GD, Purpura DP. Fine structure of dendrites in the superficial neocortical neuropil. Experimental Neurology. 1961;4:507–530. doi: 10.1016/0014-4886(61)90049-8. [DOI] [PubMed] [Google Scholar]
- Paspalas CD, Rakic P, Goldman-Rakic PS. Internalization of D2 dopamine receptors is clathrin-dependent and select to dendro-axonic appositions in primate prefrontal cortex. European Journal of Neuroscience. 2006;24(5):1395–1403. doi: 10.1111/j.1460-9568.2006.05023.x. doi:10.1111/j.1460-9568.2006.05023.x. [DOI] [PubMed] [Google Scholar]
- Passey S, Pellegrin S, Mellor H. What is in a filopodium? Starfish versus hedgehogs. Biochemical Society Transactions. 2004;32(Pt 6):1115–1117. doi: 10.1042/BST0321115. doi:10.1042/BST0321115. [DOI] [PubMed] [Google Scholar]
- Pavans de Ceccatty M. Ultrastructures et rapports des cellules mesenchymateuses de type nerveux de l’eponge Tethya lyncurium Link. Annales des Sciences Naturelles—Zoologie et Biologie Animale. 1966;8:577–614. [Google Scholar]
- Pentreath VW, Berry MS, Cobb JL. Nerve-ending specializations in the central ganglia of Planorbis corneus. Cell and Tissue Research. 1975;163(1):99–110. doi: 10.1007/BF00218593. [DOI] [PubMed] [Google Scholar]
- Pérez-Cruz C, Delgado L, Lopez-Iglesias C, Mercade E. Outer-inner membrane vesicles naturally secreted by gram-negative pathogenic bacteria. PLoS One. 2015;10(1):e0116896. doi: 10.1371/journal.pone.0116896. doi:10.1371/journal.pone.0116896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Mattson MP, Yao PJ. Communication breakdown: The impact of ageing on synapse structure. Ageing Res Rev. 2014a;14:31–42. doi: 10.1016/j.arr.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Mattson MP, Yao PJ. Aging and longevity in the simplest animals and the quest for immortality. Ageing Research Reviews. 2014b;16:66–82. doi: 10.1016/j.arr.2014.05.003. doi:10.1016/j.arr.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Schwartz CM, Wang YX, Mattson MP, Yao PJ. Subcellular localization of patched and smoothened, the receptors for sonic hedgehog signaling, in the hippocampal neuron. Journal of Comparative Neurology. 2011;519(18):3684–3699. doi: 10.1002/cne.22681. doi:10.1002/cne.22681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Mattson MP, Yao PJ. Subcellular distribution of patched and smoothened in the cerebellar neurons. Cerebellum. 2012;11(4):972–981. doi: 10.1007/s12311-012-0374-6. doi:10.1007/s12311-012-0374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wenthold RJ. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. Journal of Comparative Neurology. 1992;318(3):329–354. doi: 10.1002/cne.903180309. doi:10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- Popov VI, Kleschevnikov AM, Klimenko OA, Stewart MG, Belichenko PV. Three-dimensional synaptic ultra-structure in the dentate gyrus and hippocampal area CA3 in the Ts65Dn mouse model of down syndrome. Journal of Comparative Neurology. 2011;519(7):1338–1354. doi: 10.1002/cne.22573. doi:10.1002/cne.22573. [DOI] [PubMed] [Google Scholar]
- Popova E. Role of dopamine in distal retina. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology. 2014;200(5):333–358. doi: 10.1007/s00359-014-0906-2. doi:10.1007/s00359-014-0906-2. [DOI] [PubMed] [Google Scholar]
- Prokop A, Meinertzhagen IA. Development and structure of synaptic contacts in Drosophila. Seminars in Cell and Developmental Biology. 2006;17(1):20–30. doi: 10.1016/j.semcdb.2005.11.010. doi:10.1016/j.semcdb.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Raikova OI, Reuter M, Jondelius U, Gustafsson MKS. An immunocytochemical and ultrastructural study of the nervous and muscular systems of Xenoturbella westbladi (Bilateria inc. sed.) Zoomorphology. 2000;120:107–118. [Google Scholar]
- Rajendran L, Bali J, Barr MM, Court FA, Kramer-Albers EM, Picou F, et al. Emerging roles of extracellular vesicles in the nervous system. Journal of Neuroscience. 2014;34(46):15482–15489. doi: 10.1523/JNEUROSCI.3258-14.2014. doi:10.1523/JNEUROSCI.3258-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Weber FA, Kornberg TB. Cytonemes: Cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell. 1999;97(5):599–607. doi: 10.1016/s0092-8674(00)80771-0. [DOI] [PubMed] [Google Scholar]
- Remis JP, Wei D, Gorur A, Zemla M, Haraga J, Allen S, et al. Bacterial social networks: Structure and composition of Myxococcus xanthus outer membrane vesicle chains. Environmental Microbiology. 2014;16(2):598–610. doi: 10.1111/1462-2920.12187. doi:10.1111/1462-2920.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard E, Vacelet J, Gazave E, Lapebie P, Borchiellini C, Ereskovsky AV. Origin of the neuro-sensory system: New and expected insights from sponges. Integrative Zoology. 2009;4(3):294–308. doi: 10.1111/j.1749-4877.2009.00167.x. doi:10.1111/j.1749-4877.2009.00167.x. [DOI] [PubMed] [Google Scholar]
- Richards DA, Mateos JM, Hugel S, de Paola V, Caroni P, Gahwiler BH, et al. Glutamate induces the rapid formation of spine head protrusions in hippocampal slice cultures. Proceedings of the National Academy of Sciences of the USA. 2005;102(17):6166–6171. doi: 10.1073/pnas.0501881102. doi:10.1073/pnas.0501881102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringstad N, Gad H, Low P, Di Paolo G, Brodin L, Shupliakov O, et al. Endophilin/SH3p4 is required for the transition from early to late stages in clathrin-mediated synaptic vesicle endocytosis. Neuron. 1999;24(1):143–154. doi: 10.1016/s0896-6273(00)80828-4. [DOI] [PubMed] [Google Scholar]
- Risinger MA, Larsen WJ. Endocytosis of cell-cell junctions and spontaneous cell disaggregation in a cultured human ovarian adenocarcinoma. (COLO 316) Tissue and Cell. 1981;13(2):413–430. doi: 10.1016/0040-8166(81)90015-x. [DOI] [PubMed] [Google Scholar]
- Robbins JR, Barth AI, Marquis H, de Hostos EL, Nelson WJ, Theriot JA. Listeria monocytogenes exploits normal host cell processes to spread from cell to cell. Journal of Cell Biology. 1999;146(6):1333–1350. doi: 10.1083/jcb.146.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint Marie RL, Carlson SD. Synaptic vesicle activity in stimulated and unstimulated photoreceptor axons in the housefly. A freeze-fracture study. Journal of Neurocytology. 1982;11(5):747–761. doi: 10.1007/BF01153517. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Iwata M. Synaptic loss in the proximal axon of anterior horn neurons in motor neuron disease. Acta Neuropathologica. 1995;90(2):170–175. doi: 10.1007/BF00294317. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Iwata M. Synaptic loss in anterior horn neurons in lower motor neuron disease. Acta Neuropathologica. 1996;91(4):416–421. doi: 10.1007/s004010050444. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Iwata M. Ultrastructural change of synapses of Betz cells in patients with amyotrophic lateral sclerosis. Neuroscience Letters. 1999;268(1):29–32. doi: 10.1016/s0304-3940(99)00374-2. [DOI] [PubMed] [Google Scholar]
- Satterlie RA, Case JF. Gap junctions suggest epithelial conduction within the comb plates of the ctenophore Pleurobrachia bachei. Cell and Tissue Research. 1978;193(1):87–91. doi: 10.1007/BF00221603. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Hannah MJ, Huttner WB. Synaptic-like microvesicles of neuroendocrine cells originate from a novel compartment that is continuous with the plasma membrane and devoid of transferrin receptor. Journal of Cell Biology. 1997;137(2):445–458. doi: 10.1083/jcb.137.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz K, Janssen-Bienhold U, Gundelfinger ED, Kreutz MR, Weiler R. Calcium-binding protein Caldendrin and CaMKII are localized in spinules of the carp retina. Journal of Comparative Neurology. 2004;479(1):84–93. doi: 10.1002/cne.20314. doi:10.1002/cne.20314. [DOI] [PubMed] [Google Scholar]
- Schuster T, Krug M, Wenzel J. Spinules in axospinous synapses of the rat dentate gyrus: Changes in density following long-term potentiation. Brain Research. 1990;523(1):171–174. doi: 10.1016/0006-8993(90)91654-y. [DOI] [PubMed] [Google Scholar]
- Sebé-Pedrós A, Burkhardt P, Sanchez-Pons N, Fairclough SR, Lang BF, King N, et al. Insights into the origin of metazoan filopodia and microvilli. Molecular Biology and Evolution. 2013;30(9):2013–2023. doi: 10.1093/molbev/mst110. doi:10.1093/molbev/mst110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SR, Meinertzhagen IA. Evolutionary progression at synaptic connections made by identified homologous neurones. Proceedings of the National Academy of Sciences of the USA. 1986;83(20):7961–7965. doi: 10.1073/pnas.83.20.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer NM, Lehmann MJ, Jimenez-Soto LF, Horensavitz C, Pypaert M, Mothes W. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nature Cell Biology. 2007;9(3):310–315. doi: 10.1038/ncb1544. doi:10.1038/ncb1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer NM, Mothes W. Cytonemes and tunneling nanotubules in cell-cell communication and viral pathogenesis. Trends in Cell Biology. 2008;18(9):414–420. doi: 10.1016/j.tcb.2008.07.003. doi:10.1016/j.tcb.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shupliakov O, Low P, Grabs D, Gad H, Chen H, David C, et al. Synaptic vesicle endocytosis impaired by disruption of dynamin-SH3 domain interactions. Science. 1997;276(5310):259–263. doi: 10.1126/science.276.5310.259. [DOI] [PubMed] [Google Scholar]
- Smith JE, Clark AW, Kuster TA. Suppression by elevated calcium of black widow spider venom activity at frog neuromuscular junctions. Journal of Neurocytology. 1977;6(5):519–539. doi: 10.1007/BF01205217. [DOI] [PubMed] [Google Scholar]
- Smith CL, Varoqueaux F, Kittelmann M, Azzam RN, Cooper B, Winters CA, et al. Novel cell types, neurosecretory cells, and body plan of the early-diverging metazoan Trichoplax adhaerens. Current Biology. 2014;24(14):1565–1572. doi: 10.1016/j.cub.2014.05.046. doi:10.1016/j.cub.2014.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobkowicz HM, August BK, Slapnick SM, Luthy DF. Terminal dendritic sprouting and reactive synaptogenesis in the postnatal organ of Corti in culture. Journal of Comparative Neurology. 1998;397(2):213–230. doi: 10.1002/(sici)1096-9861(19980727)397:2<213::aid-cne5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, Rose JE, Scott GL, Levenick CV. Distribution of synaptic ribbons in the developing organ of Corti. Journal of Neurocytology. 1986;15(6):693–714. doi: 10.1007/BF01625188. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, Slapnick SM, August BK. Apoptosis of inner hair cells caused by laser ablation of their spiral ganglion neurons in cultures of the mouse organ of Corti. Journal of Neurocytology. 1999;28(10–11):939–954. doi: 10.1023/a:1007086525385. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, Slapnick SM, August BK. Differentiation of spinous synapses in the mouse organ of corti. Synapse. 2002;45(1):10–24. doi: 10.1002/syn.10080. doi:10.1002/syn.10080. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, Slapnick SM, August BK. Reciprocal synapses between inner hair cell spines and afferent dendrites in the organ of corti of the mouse. Synapse. 2003;50(1):53–66. doi: 10.1002/syn.10241. doi:10.1002/syn.10241. [DOI] [PubMed] [Google Scholar]
- Sorra KE, Fiala JC, Harris KM. Critical assessment of the involvement of perforations, spinules, and spine branching in hippocampal synapse formation. Journal of Comparative Neurology. 1998;398(2):225–240. [PubMed] [Google Scholar]
- Spacek J, Harris KM. Trans-endocytosis via spinules in adult rat hippocampus. Journal of Neuroscience. 2004;24(17):4233–4241. doi: 10.1523/JNEUROSCI.0287-04.2004. doi:10.1523/JNEUROSCI.0287-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark WS, Carlson SD. Ultrastructure of capitate projections in the optic neuropil of Diptera. Cell and Tissue Research. 1986;246(3):481–486. doi: 10.1007/BF00215187. [DOI] [PubMed] [Google Scholar]
- Stark WS, Sapp R, Carlson SD. Ultrastructure of the ocellar visual system in normal and mutant Drosophila melanogaster. Journal of Neurogenetics. 1989;5:127–153. doi: 10.3109/01677068909066203. [DOI] [PubMed] [Google Scholar]
- Stewart MG, Davies HA, Sandi C, Kraev IV, Rogachevsky VV, Peddie CJ, et al. Stress suppresses and learning induces plasticity in CA3 of rat hippocampus: A three-dimensional ultrastructural study of thorny excrescences and their postsynaptic densities. Neuroscience. 2005a;131(1):43–54. doi: 10.1016/j.neuroscience.2004.10.031. doi:10.1016/j.neuroscience.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Stewart MG, Medvedev NI, Popov VI, Schoepfer R, Davies HA, Murphy K, et al. Chemically induced long-term potentiation increases the number of perforated and complex postsynaptic densities but does not alter dendritic spine volume in CA1 of adult mouse hippocampal slices. European Journal of Neuroscience. 2005b;21(12):3368–3378. doi: 10.1111/j.1460-9568.2005.04174.x. doi:10.1111/j.1460-9568.2005.04174.x. [DOI] [PubMed] [Google Scholar]
- Sukhdeo SC, Sukhdeo MVK. Mesenchyme cells in Fasciola hepatica (Platyhelminthes): Primitive glia? Tissue and Cell. 1994;26(1):123–131. doi: 10.1016/0040-8166(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Tao-Cheng JH, Dosemeci A, Gallant PE, Miller S, Galbraith JA, Winters CA, et al. Rapid turnover of spinules at synaptic terminals. Neuroscience. 2009;160(1):42–50. doi: 10.1016/j.neuroscience.2009.02.031. doi:10.1016/j.neuroscience.2009.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrant SB, Routtenberg A. The synaptic spinule in the dendritic spine: Electron microscopic study of the hippocampal dentate gyrus. Tissue and Cell. 1977;9(3):461–473. doi: 10.1016/0040-8166(77)90006-4. [DOI] [PubMed] [Google Scholar]
- Tarrant SB, Routtenberg A. Postsynaptic membrane and spine apparatus: Proximity in dendritic spines. Neuroscience Letters. 1979;11(3):289–294. doi: 10.1016/0304-3940(79)90010-7. [DOI] [PubMed] [Google Scholar]
- Toh Y, Kuwabara M. Fine structure of the dorsal ocellus of the worker honeybee. Journal of Morphology. 1974;143:285–306. doi: 10.1002/jmor.1051430304. [DOI] [PubMed] [Google Scholar]
- Toh Y, Kuwabara M. Synaptic organization of the fleshfly ocellus. Journal of Neurocytology. 1975;4(3):271–287. doi: 10.1007/BF01102113. [DOI] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402(6760):421–425. doi: 10.1038/46574. doi:10.1038/46574. [DOI] [PubMed] [Google Scholar]
- Trujillo-Cenóz O. Some aspects of the structural organization of the intermediate retina of dipterans. Journal of Ultrastructure Research. 1965;13(1):1–33. doi: 10.1016/s0022-5320(65)80086-7. [DOI] [PubMed] [Google Scholar]
- Ueda Y. The role of phosphoinositides in synapse function. Molecular Neurobiology. 2014;50(3):821–838. doi: 10.1007/s12035-014-8768-8. doi:10.1007/s12035-014-8768-8. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Hayashi Y. PIP(3) regulates spinule formation in dendritic spines during structural long-term potentiation. Journal of Neuroscience. 2013;33(27):11040–11047. doi: 10.1523/JNEUROSCI.3122-12.2013. doi:10.1523/JNEUROSCI.3122-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn JE. Fine structure of synaptogenesis in the vertebrate central nervous system. Synapse. 1989;3(3):255–285. doi: 10.1002/syn.890030312. doi:10.1002/syn.890030312. [DOI] [PubMed] [Google Scholar]
- Wagner HJ. Light-dependent plasticity of the morphology of horizontal cell terminals in cone pedicles of fish retinas. Journal of Neurocytology. 1980;9(5):573–590. doi: 10.1007/BF01205026. [DOI] [PubMed] [Google Scholar]
- Wagner HJ, Djamgoz MB. Spinules: a case for retinal synaptic plasticity. Trends in Neurosciences. 1993;16(6):201–206. doi: 10.1016/0166-2236(93)90155-f. [DOI] [PubMed] [Google Scholar]
- Wanner G, Vogl K, Overmann J. Ultrastructural characterization of the prokaryotic symbiosis in “Chlorochromatium aggregatum”. Journal of Bacteriology. 2008;190(10):3721–3730. doi: 10.1128/JB.00027-08. doi:10.1128/JB.00027-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG, Waxman M, Pappas GD. Coordinated micropinocytotic activity of adjacent neuronal membranes in mammalian central nervous system. Neuroscience Letters. 1980;20(2):141–146. doi: 10.1016/0304-3940(80)90136-6. [DOI] [PubMed] [Google Scholar]
- Weedman DL, Pongstaporn T, Ryugo DK. Ultrastructural study of the granule cell domain of the cochlear nucleus in rats: Mossy fiber endings and their targets. The Journal of Comparative Neurology. 1996;369(3):345–360. doi: 10.1002/(SICI)1096-9861(19960603)369:3<345::AID-CNE2>3.0.CO;2-5. doi:10.1002/(SICI)1096-9861(19960603)369:3<345:AID-CNE2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Weiler R, Schultz K. Ionotropic non-N-methyl-D-aspartate agonists induce retraction of dendritic spinules from retinal horizontal cells. Proceedings of the National Academy of Sciences of the USA. 1993;90(14):6533–6537. doi: 10.1073/pnas.90.14.6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler R, Schultz K, Janssen-Bienhold U. Ca(2+)-dependency of spinule plasticity at dendrites of retinal horizontal cells and its possible implication for the functional role of spinules. Vision Research. 1996;36(24):3891–3900. doi: 10.1016/s0042-6989(96)00148-4. [DOI] [PubMed] [Google Scholar]
- Westfall JA. Ultrastructure of synapses in a primitive coelenterate. Journal of Ultrastructure Research. 1970;32(3):237–246. doi: 10.1016/s0022-5320(70)80004-1. [DOI] [PubMed] [Google Scholar]
- Westrum LE, Blackstad TW. An electron microscopic study of the stratum radiatum of the rat hippocampus (regio superior, CA 1) with particular emphasis on synaptology. Journal of Comparative Neurology. 1962;119:281–309. doi: 10.1002/cne.901190303. [DOI] [PubMed] [Google Scholar]
- Wierenga CJ, Becker N, Bonhoeffer T. GABAergic synapses are formed without the involvement of dendritic protrusions. Nature Neuroscience. 2008;11(9):1044–1052. doi: 10.1038/nn.2180. doi:10.1038/nn.2180. [DOI] [PubMed] [Google Scholar]
- Williams JB. Unicellular adhesive secretion glands and other cells in the parenchyma of Temnocephala novaezealandiae (Platyhelminthes, Temnocephaloidea): Intercell relationships and nuclear pockets. New Zealand Journal of Zoology. 1994;21(2):167–178. [Google Scholar]
- Wood CR, Rosenbaum JL. Ciliary ectosomes: Transmissions from the cell’s antenna. Trends in Cell Biology. 2015;25(5):276–285. doi: 10.1016/j.tcb.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KA. Peripheral sensilla of some lower invertebrates: The Platyhelminthes and Nematoda. Microscopy Research and Technique. 1992;22(3):285–297. doi: 10.1002/jemt.1070220306. doi:10.1002/jemt.1070220306. [DOI] [PubMed] [Google Scholar]
- Wright KA, Hui N. Post-labial sensory structures on the cecal worm, Heterakis gallinarum. Journal of Parasitology. 1976;62(4):579–584. [PubMed] [Google Scholar]
- Yao PJ, Petralia RS, Bushlin I, Wang Y, Furukawa K. Synaptic distribution of the endocytic accessory proteins AP180 and CALM. Journal of Comparative Neurology. 2005;481(1):58–69. doi: 10.1002/cne.20362. doi:10.1002/cne.20362. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Uchigashima M, Yamasaki M, Katona I, Yamazaki M, Sakimura K, et al. Unique inhibitory synapse with particularly rich endocannabinoid signaling machinery on pyramidal neurons in basal amygdaloid nucleus. Proceedings of the National Academy of Sciences of the USA. 2011;108(7):3059–3064. doi: 10.1073/pnas.1012875108. doi:10.1073/pnas.1012875108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao HM, Wenthold RJ, Petralia RS. Glutamate receptor targeting to synaptic populations on Purkinje cells is developmentally regulated. Journal of Neuroscience. 1998;18(14):5517–5528. doi: 10.1523/JNEUROSCI.18-14-05517.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer J, Lawrence J, Raisman G. A quantitative electron microscopic study of synaptic reorganization in the rat medial habenular nucleus after transection of the stria medullaris. Neuroscience. 1982;7(8):1905–1928. doi: 10.1016/0306-4522(82)90006-9. [DOI] [PubMed] [Google Scholar]