Abstract
Hypertension in pediatric kidney transplant recipients contributes to long-term graft loss, yet treatment options—including angiotensin-converting enzyme inhibitors—are poorly characterized in this vulnerable population. We conducted a multicenter, open-label pharmacokinetic (PK) study of daily oral lisinopril in 22 children (ages 7–17 years) with stable kidney transplant function. Standard non-compartmental PK analyses were performed at steady state. Effects on blood pressure were examined in lisinopril-naïve patients (n=13). Oral clearance declined in proportion to underlying kidney function; however, in patients with low estimated glomerular filtration rate (30–59 ml/min per 1.73m2), exposure (standardized to 0.1 mg/kg/day dose) was within the range reported previously in children without a kidney transplant. In lisinopril-naïve patients, 85% and 77% had a ≥6 mmHg reduction in systolic and diastolic blood pressure, respectively. Lisinopril was well tolerated. Our study provides initial insight on lisinopril use in children with a kidney transplant, including starting dose considerations.
Keywords: hypertension, kidney transplant, lisinopril, pharmacokinetics
The incidence of untreated or inadequately treated hypertension in pediatric kidney transplant recipients is nearly 60%, a figure that has been steady over the last 2 decades.1 Uncontrolled hypertension, beginning within the first year post-transplant, represents a modifiable risk factor that contributes to long-term graft loss.1,2 The requirement for treatment with antihypertensive medications at any time from 6 months to 3 years post-transplant is associated with graft dysfunction and a lower glomerular filtration rate (GFR).3 Calcium channel blockers are considered the preferred first-line agent in this circumstance,4 and they are the most commonly used drug class to control post-transplant hypertension in children.5 However, compared with calcium channel blockers, angiotensin-converting enzyme inhibitors (ACEIs) may have additional beneficial effects for prolonging allograft survival in kidney transplant recipients by lowering intraglomerular hypertension and reducing the activity of profibrotic molecules such as TGF-β and inflammatory mediators.6 Recent reports in adults document an increased use of ACEI in the 2-year period after kidney transplantation between 1990 and 2002. By the end of this period, 45% of patients were receiving these agents, and their use was associated with a significantly higher graft survival.7
Despite greater potential benefits, there is a reluctance to use ACEIs in pediatric kidney transplant recipients who develop hypertension because this class of drugs may compromise the angiotensin II-dependent component of GFR in patients with a single transplanted kidney. In addition, they may cause clinically significant hyperkalemia.8 The low use of ACEI in pediatric kidney transplant patients is noteworthy because these drugs lower urinary protein excretion and are reno-protective in pediatric and adult patients with reduced kidney mass and/or diminished renal function.9
Lisinopril, a long-acting ACEI, is approved by the U.S. Food and Drug Administration (FDA) for the treatment of adult and pediatric patients with essential hypertension. Based on recovery of the drug in the urine, the apparent extent of absorption of lisinopril is ~25% with large inter-patient variability. In adults, peak serum concentrations of lisinopril are seen approximately 7 hours after oral dosing.10 The disposition of lisinopril in children is similar to adults, with peak concentrations observed within 6 hours after dosing and the extent of drug absorption ~28%.11,12 The drug is not appreciably bound to plasma proteins and thus, its disposition appears to be unaltered by low serum albumin levels.13 Lisinopril is not appreciably metabolized, and it is excreted largely unchanged in the urine via glomerular filtration with no apparent contribution via active tubular secretion.14 Impaired renal function decreases the elimination of lisinopril, but this only becomes clinically relevant at a GFR below 30 mL/min/1.73m2.
Compared to healthy children with essential hypertension, the disposition of and response to antihypertensive medications may be altered in pediatric patients with a renal transplant consequent to the presence of a solitary kidney, potential alterations in renal hemodynamics, renal effects of drugs used to prevent acute rejection, and extra-renal organ dysfunction secondary to chronic kidney disease (CKD) prior to receipt of the kidney allograft. Thus, it is important to understand if lisinopril disposition is altered in pediatric patients who receive a renal transplant. Given the widespread use of this drug in pediatrics, we conducted a clinical trial to evaluate the pharmacokinetics (PK) and safety/tolerability profile of lisinopril in children and adolescents with hypertension after kidney transplantation. The impact of lisinopril on blood pressure was also examined.
RESULTS
Patient characteristics
A total of 26 patients were enrolled and dosed (Figure 1). Three patients did not complete the PK study day due to inability to establish intravenous access (n=1), withdrawal of consent (n=1), and an AE requiring study termination (n=1). One additional lisinopril-naïve patient completed PK sampling, but all blood samples were lost after shipment to an off-site storage facility in the wake of Hurricane Sandy. Therefore, a total of 22 patients were available for PK analysis (Table 1).
Figure 1.
CONSORT diagram.
Table 1.
Demographics of PK population patients by dose group
| Parameter | Lisinopril dose group
|
All (n=22) | ||
|---|---|---|---|---|
| Low dose 0.1 mg/kg (n=12) | Middle dose 0.2 mg/kg (n=8) | High dose 0.4 mg/kg (n=2) | ||
| Age (y) | 14.9 ± 2.3 | 13 ± 3 | 9.5 ± 3.5 | 13.8 ± 3.0 |
| Weight (kg) | 56.8 ± 19.4 | 50.2 ± 28.7 | 23.1 ± 3 | 51.3 ± 23.8 |
| Female | 4 (42%) | 2 (25%) | 1 (50%) | 7 (32%) |
| Race/ethnicity | ||||
| White | 7 (58%) | 2 (25%) | 2 (100%) | 11 (50%) |
| Black | 3 (25%) | 4 (50%) | 0 | 7 (32%) |
| American Indian or Alaska Native | 0 (0) | 1 (13%) | 0 | 1 (5%) |
| Not reported | 2 (17%) | 1 (13%) | 0 | 3 (14%) |
| Ethnicity | ||||
| Hispanic/Latino | 2 (17%) | 2 (25%) | 0 | 4 (18%) |
| eGFR at baseline (ml/min per 1.73m2) | 72.5 ± 25.7 (29.6, 111.2) | 62 ± 16.7 (29.2, 79.8) | 89.3 ± 44.4 (57.8, 120.6) | 70.2 ± 24.4 |
| eGFR at PK visit (ml/min per 1.73m2) | 73.1 ± 30.7 (36.7, 139.0) | 63.2 ± 18.4 (30.3, 86.0) | 100.2 ± 56.6 (60.0, 140.2) | 72.0 ± 29.4 |
| Time since transplant (years) | 4.8 ± 4.7 | 4.9 ± 3.4 | 0.9 ± 0.4 | 4.5 ± 4.1 |
Data are mean ± SD (range) or counts (%); PK, pharmacokinetic; eGFR, estimated glomerular filtration rate calculated by modified Schwartz formula (= 0.413 x length [cm]/serum creatinine [mg/dl]).
Twelve patients in the PK analysis population were enrolled in the lisinopril-naïve group and received a drug dose according to the study protocol. The highest dose enrolled in the lisinopril-naïve group in the low estimated glomerular filtration rate (eGFR) group was 0.2 mg/kg daily (1 patient) and in the high eGFR group was 0.4 mg/kg (2 patients). Ten patients were receiving lisinopril as standard of care (SoC). The median dose in the lisinopril SoC group was 0.12 mg/kg/day (range, 0.03–0.21 mg/kg). All patients in the lisinopril SoC group received lisinopril once daily, except 1 who received lisinopril twice daily. Patients were similar between the lisinopril-naïve and lisinopril SoC groups in terms of age, weight, and eGFR (Supplementary Table S1). There was a trend to a shorter time since transplant in the lisinopril-naïve vs. lisinopril SoC patients (3.1 ± 3.0 years vs. 6.1 ± 4.7 years; p=0.08) although the difference between the mean values was not significant.
Concomitant antihypertensive medications in the PK analysis population included amlodipine (n=15), atenolol (n=2), clonidine (n=2), isradipine (n=2), and carvedilol (n=1). Concomitant immunosuppressive medications included mycophenolate (n=19), prednisone (n=18), tacrolimus (n=16), sirolimus (n=7), and azathioprine (n=1). Antihypertensive and immunosuppressive medication doses remained stable during the study period. No new oral concomitant medications were started during the study period except for the following: odansetron (n=1; 0.2 mg/kg dose group), esomeprazole (n=1, 0.2 mg/kg dose group), mycophenolate mofetil (n=1, 0.1 mg/kg dose group), and vitamin D (n=1, 0.4 mg/kg dose). No patient in the study had more than 1 transplant before enrollment.
Pharmacokinetics
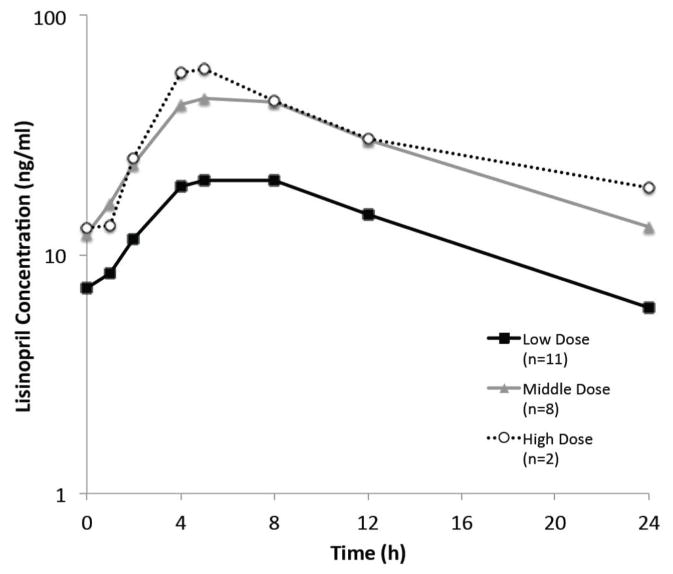
The mean steady-state concentration-time profiles of lisinopril once daily varied by dose level (Figure 2 and Supplementary Figure S1). Lisinopril PK exhibited dose proportionality with area-under-the-concentration-time-curve over 24 hours (AUC0–24) 2-fold higher in the 0.2 mg/kg dose group compared with the 0.1 mg/kg dose group (Table 2; geometric mean 640 vs. 298 ng/ml*h respectively; p <0.001). The geometric mean AUC0–24 across all patients dose-adjusted to a 0.1 mg/kg dose was 328 ng*h/ml (coefficient of variation [CV] 60%). Dose-adjusted AUC0–24, maximum concentration (Cmax), and concentration at 24 hours (C24) were not significantly different between the 0.1 and 0.2 mg/kg dose groups (Table 3). Similarly, apparent oral clearance was similar between the 0.1 mg/kg and 0.2 mg/kg dose groups (geometric mean 17.9 vs. 18.6 L/h/70 kg, respectively; p=0.84). The extent of lisinopril absorption based on urinary recovery (fe in urine), time of maximum concentration (Tmax), and the apparent terminal elimination half-life (t1/2) were also comparable across dose groups. Given the small sample size in the high-dose group (n=2), interpretation of the PK results in this group is very limited.
Figure 2.
Mean lisinopril concentration versus time after multiple dosing following once-daily dosing in patients who have received a kidney transplant. Patients were stratified by dose group: low dose = 0.1 mg/kg; middle dose = 0.2 mg/kg; and high dose = 0.4 mg/kg. One patient received twice-daily dosing and was not included.
Table 2.
Lisinopril pharmacokinetic parameters in patients with a kidney transplant
| PK parameter | Lisinopril dose
|
||
|---|---|---|---|
| Low dose 0.1 mg/kg (n=12) | Middle dose 0.2 mg/kg (n=8) | High dose 0.4 mg/kg (n=2) | |
| Dose (mg/kg/day) | |||
| Median | 0.10 | 0.19 | 0.44 |
| Range | (0.03, 0.14) | (0.17, 0.23) | (0.40, 0.48) |
| AUC0–24 (ng·h/ml) | |||
| GeoMean | 298 | 640 | 702 |
| CV% | (46.5) | (28.6) | (66.4) |
| CL/F per 70 kg (L/h/70 kg) | |||
| GeoMean | 17.9 | 18.6 | 32.8 |
| CV% | (61.2) | (34.4) | (54.1) |
| Cmax (ng/ml) | |||
| GeoMean | 20.9 | 47.7 | 58.0 |
| CV% | (41.2) | (25.1) | (41.2) |
| Tmax (h) | |||
| Median | 5.0 | 5.0 | 4.5 |
| Range | (4.0, 8.1) | (4.0, 8.0) | (4.0, 5.0) |
| C24 (ng/ml) | |||
| GeoMean | 5.4 | 11.2 | 13.1 |
| CV% | (51.7) | (64.1) | (212) |
| t1/2, terminal (h) | |||
| GeoMean | 9.4 | 9.0 | 12.4 |
| CV% | (30.1) | (46.1) | (131) |
| fe in urine | |||
| GeoMean | 0.19 | 0.18 | 0.21 |
| CV% | (23.7) | (14.8) | (27.8) |
| Renal CL per 70 kg (L/h/70 kg) | |||
| GeoMean | 3.4 | 3.4 | 6.8 |
| CV% | (60.4) | (46.0) | (94.4) |
PK, pharmacokinetic; AUC, area under the plasma concentration-time curve; CL/F, oral clearance; Cmax, maximum observed concentration; Tmax, time of observed maximal concentration; C24, concentration at 24 hours; t1/2, terminal half-life; fe, fraction of dose excreted in urine during dosing interval; GeoMean, geometric mean; CV%, percent coefficient of variation.
Table 3.
Lisinopril PK parameters dose adjusted to a 0.1 mg/kg by dose group
| PK parameter | Low dose 0.1 mg/kg (n=12) | Middle dose 0.2 mg/kg (n=8) | p-value |
|---|---|---|---|
| AUC0–24 per 0.1 mg/kg | |||
| GeoMean | 365 | 333 | 0.71 |
| 95% CI | (243, 549) | (265, 419) | |
| Cmax per 0.1 mg/kg | |||
| GeoMean | 26.9 | 24.8 | 0.70 |
| 95% CI | (18.4, 39.5) | (20.9, 29.5) | |
| C24 per 0.1 mg/kg | |||
| GeoMean | 6.9 | 5.8 | 0.61 |
| 95% CI | (4.1, 11.7) | (3.5, 9.7) | |
PK, pharmacokinetic; AUC, area under the plasma concentration-time curve; GeoMean, geometric mean; CI, confidence interval; Cmax, maximum observed concentration; C24, concentration at 24 hours.
The mean eGFR at the PK visit in the low and high GFR groups was 44.4 ± 9.6 ml/min per 1.73m2 and 84.8 ± 26.5 ml/min per 1.73m2, respectively. Lisinopril clearance was affected by renal function. Apparent oral clearance was 11.9 (95% confidence interval [CI] 8.4, 7.0) L/h/70 kg in the low GFR group versus 24.0 (95% CI 19.4, 29.5) L/h/70 kg in the high GFR group (p<0.001). Accordingly, AUC0–24 dose adjusted to 0.1 mg/kg was 2-fold higher for the low GFR group compared with the high GFR group (553 vs. 256 ng·h/ml; p<0.001). When plotting allometrically scaled oral clearance (CL/F) versus eGFR (Figure 3A), lisinopril clearance increased in proportion to eGFR. In addition, the geometric mean ratio of renal CL/eGFR was 0.84 (95% CI 0.71, 1.0), which suggests, as expected, that renal CL of lisinopril is similar to GFR. Consequently, there is a trend toward higher AUC0–24 per 0.1 mg/kg as eGFR decreases (Figure 3B). For comparison, the geometric mean AUC0–24 per 0.1 mg/kg (=437 ng*h/ml) in a historical cohort of 16 children of similar age (age range, 12–15 years) were also included.15,16 The mean GFR in the historical cohort was not reported; however, only 1 patient had a GFR <60 ml/min per 1.73m2. The extent of lisinopril absorption based on urinary recovery was not affected by eGFR (low eGFR 0.18 vs. high eGFR 0.19; p=0.42). There was no indication of a signal for a specific concomitant immunosuppressant affecting fe in urine, and patients were generally on similar immunosuppressant regimens.
Figure 3.

Impact of eGFR on (A) oral clearance allometrically scaled to a 70 kg adult and (B) AUC0–24 dose adjusted to 0.1 mg/kg/day. The geometric mean AUC0–24 dose adjusted to 0.1 mg/kg in historical hypertensive children is shown for comparison.
Lasso regression analysis identified enrollment type (lisinopril-naïve vs. SoC) as a significant predictor of clearance in addition to eGFR. Apparent oral clearance was higher in treatment-naïve patients compared with SoC patients (CL/F/70 kg 23.9 vs. 14.7 L/h/70 kg, respectively). No other significant predictors of clearance were identified.
BP effects in lisinopril-naïve patients
Thirteen lisinopril-naïve patients had blood pressure (BP) data available at baseline and while on lisinopril treatment. Across all 13 patients, the mean reduction in systolic BP from baseline at the lisinopril trough time-point was 9.0 ± 6.9 mmHg, with 11 (85%) having had at least a 6 mmHg reduction. The mean reduction in diastolic BP from baseline at the time of trough (Cmin) plasma lisinopril concentrations was 6.2 ± 9.9 mmHg, with 10 (77%) having had at least a 6 mmHg reduction. Table 4 shows the change in BP from baseline by dose group. At the lisinopril Cmin, change in systolic BP from baseline in the 0.2 mg/kg dose group estimated from a linear mixed effects model was significant and clinically relevant (−12 [95% CI −19.6, −4.4] mmHg). The reduction in systolic BP was smaller in the 0.1 mg/kg dose group and did not reach statistical significance (−5.8 [95% CI −13.9, 2.2] mmHg). Differences between dose groups were not statistically significant. In the 12 patients with both PK and BP data available, no strong relationships between change in systolic or diastolic BP and pre-dose lisinopril concentration or AUC0–24 was observed (R2 ≤0.15 for all 4 analyses; Supplementary Figure S2). Changes in BP at other time-points during the dosing interval on the PK visit were not examined due to the confounding effect of the iohexol administration with a lowering of BP (data not shown).
Table 4.
Change in clinic blood pressure by dose group in lisinopril-naïve patients
| Baseline BP (mmHg) | Lisinopril BP (mmHg) | Change in BP from baseline
|
||
|---|---|---|---|---|
| Mean | 95% CI | |||
| Trough (pre-dose) | ||||
| Systolic | ||||
| 0.1 mg/kg (n=6) | 121.2 ± 3.9 | 115.3 ± 7.6 | −5.8 | (−13.9, 2.2) |
| 0.2 mg/kg (n=5) | 129.6 ± 6.7 | 117.6 ± 6.5 | −12 | (−19.6, −4.4) |
| 0.4 mg/kg (n=2) | 124.5 ± 9.2 | 113.5 ± 6.4 | −11 | N/A |
| Diastolic | ||||
| 0.1 mg/kg (n=6) | 75.7 ± 12.8 | 69.2 ± 6.6 | −6.5 | (−21, 8) |
| 0.2 mg/kg (n=5) | 73.8 ± 7.5 | 68.2 ± 11.8 | −5.6 | (−15, 3.8) |
| 0.4 mg/kg (n=2) | 76.5 ± 16.3 | 69.5 ± 14.8 | −7 | N/A |
Data are mean ± SD. BP, blood pressure; CI, confidence interval.
Of patients with systolic BP or diastolic BP >90th percentile at enrollment, 5 (56%) of the 9 patients achieved a systolic BP <90th percentile, and 3 (60%) of these 5 patients also achieved a diastolic BP <90th percentile at the lisinopril Cmin time-point. No patient required additional antihypertensive medications for BP control after lisinopril initiation.
Safety
Treatment-emergent adverse events (AEs) were reported in 11 of the 26 enrolled patients (6/15 lisinopril-naïve and 5/11 lisinopril SoC). One serious AE was reported—an episode of gastroenteritis that required hospitalization—but was considered unrelated to lisinopril. No AEs related to the use of lisinopril were reported in SoC patients. In lisinopril-naïve patients, AE rates by dose group were 2/6 in 0.1 mg/kg/day, 2/6 in 2 mg/kg/day, and 2/3 in the 0.4 mg/kg/day group, with 1 patient in each dose group reporting AEs related to study drug. The following AEs deemed as potentially related to study drug were reported: dizziness, nausea (2 patients), stomach ache, and eGFR decrease (see below).
One patient in the high-dose group was withdrawn from the study per protocol after a >20% decline in eGFR (99.7 to 78.4 mL/min per 1.73m2) was detected at the interim visit while the patient was receiving lisinopril 0.2 mg/kg per day. The eGFR returned to the baseline level after lisinopril termination. No other patient had a decrease in eGFR ≥16%. The Bland-Altman plot to compare iohexol GFR (iGFR) and eGFR calculated using the Schwartz bedside formula showed modest correlation (concordance correlation coefficient = 0.69) but with some variation across GFR averages of the 2 measures (Supplementary Figure S3). The median change from baseline in eGFR and serum potassium was −2 (range −21–13) mL/min per 1.73m2 and 0.1 (range −1.3–0.7) mEq/L, respectively, in lisinopril-naïve patients (Supplementary Table S2). During follow-up, 1 lisinopril-naïve patient who continued on lisinopril (0.16 mg/kg/day) experienced dizziness and nausea considered related to lisinopril. Lisinopril was subsequently discontinued.
DISCUSSION
In this report, we describe the PK, short-term safety/tolerability, and preliminary efficacy data for use of lisinopril to treat hypertension in pediatric kidney transplant recipients—an essential step in demonstrating lisinopril as a safe and effective treatment option for this population. The PK of lisinopril in patients with a kidney transplant was comparable to a historical cohort of children who did not have a kidney transplant and who were given the drug for management of hypertension.11 In addition, lisinopril was generally well tolerated and was accompanied by a lowering of BP at approved pediatric doses in nearly all patients with a kidney transplant.
There are several additional key findings. First, the clearance of lisinopril was not affected by age after scaling allometrically for size across the age range studied. This is consistent with our current understanding of renal maturation where, by 1 year of age, GFR scaled for size is >90% of adult levels.17–19 Second, lisinopril clearance was dose-proportional, and a doubling of the lisinopril dose can be expected to lead to a 2-fold higher systemic drug exposure. Lastly, lisinopril clearance increased in proportion to eGFR; a finding that corroborates prior knowledge with regard to the renal excretion of the drug in adult patients.12,20 Therefore, for a given lisinopril dose, systemic drug exposure in those with renal impairment will be higher compared with those with no renal impairment. The size of our study cohort and duration of treatment were not sufficient to detect whether increased systemic exposure to the drug in patients with renal compromise had an effect on the AE profile.
The disposition of lisinopril in pediatric patients with a kidney transplant was generally similar to that in those without a kidney transplant.11,15,16 As expected, GFR was the major determinant of drug clearance, and none of the factors associated with transplantation, including concomitant administration of immunosuppressive agents, appeared to affect lisinopril clearance. The time to maximum concentration after an oral dose and terminal elimination half-life were also similar between the 2 populations (Tmax 5h vs. 6h and t1/2 9.5h vs. 8.8h in those with and without a kidney transplant, respectively). However, AUC0–24 and Cmax dose adjusted to a 0.1 mg/kg daily dose were lower in patients with a kidney transplant compared to those without a kidney transplant. The lower lisinopril exposure in patients with a kidney transplant may be due, in part, to reduced oral bioavailability of lisinopril in the kidney transplant population. This is suggested by the lower fraction of drug excreted in urine, which was 0.19 in the current study vs. 0.27 in pediatric patients without a kidney transplant.20 The reason for this reduced absorption may relate to the large number of concomitant medications (including immunosuppressive medications) resulting in potential drug-drug interactions that affect lisinopril absorption. Alternatively, the underlying disease state in pediatric patients with a kidney transplant may alter intestinal function, which, in turn, might be expected to potentially affect either the rate and/or extent of absorption.
When considering the effect of renal impairment on lisinopril clearance in pediatric patients with a kidney transplant, the changes in exposure are likely not clinically relevant at typical lisinopril starting doses in those with eGFR ≥30 ml/min per 1.73m2. For example, the AUC0–24 dose adjusted to a 0.1 mg/kg daily dose in pediatric patients with a kidney transplant and moderate renal impairment was on average 553 ng*h/ml, which is comparable to values reported in those without a kidney transplant receiving lisinopril (AUC0–24 dose adjusted to a 0.1 mg/kg daily dose 437 ng*h/ml) and in adults receiving 5 mg daily (AUC0–24 450 ng*h/ml).15,16,21 Therefore, no dose adjustment is likely to be needed in pediatric patients with a kidney transplant who have moderate renal impairment (eGFR 30–59 ml/min per 1.73m2), consistent with current dose recommendations in children and adults without a kidney transplant in whom the starting dose is not adjusted until eGFR <30 ml/min per 1.73m2.20
The current study provides insight into the effect of lisinopril on BP in pediatric patients with a kidney transplant. In the 13 lisinopril-naïve patients with clinical BP data before and after lisinopril treatment, more than 75% had a reduction of ≥6 mmHg in systolic and/or diastolic pressure. No apparent exposure-response relationship was evident in our study cohort. Our inability to detect such a relationship may be attributed to the small sample size of our cohort and the fact that only 2 patients were enrolled in the highest-dose group. Nonetheless, the BP response at 0.1 and 0.2 mg/kg per day was clinically relevant as it corroborates the available anecdotal evidence that lisinopril at these doses is effective in normalizing BP in this population as has been the experience when using it to treat hypertension in children without a kidney transplant.22 Use of the highest dose (0.4 mg/kg) may not be necessary to achieve a clinically relevant reduction in blood pressure and may increase the risk of adverse events. Whether nighttime dosing to improve nocturnal BP control would achieve better overall BP levels and greater preservation of kidney function in pediatric kidney transplant recipients will require further study, including ambulatory blood pressure monitoring (ABPM) assessment of morning versus evening dosing.23
Lisinopril was well tolerated at the doses used in this study. Only 1 patient had a decline in GFR that required discontinuation of the study drug. In this patient, the 22% decline in eGFR was based on a 0.1 mg/dL increase in the serum creatinine concentration from 0.3 to 0.4 mg/dL. In the interests of safety, we applied a very strict 20% limit to the allowable decline in GFR following initiation of lisinopril. Generally, up to a 30% decline in GFR is considered an acceptable change if an ACEI is used as a reno-protective agent in patients with CKD. Our data are encouraging, but close monitoring of GFR will be necessary in future trials of lisinopril in this patient population. We relied upon eGFR to assess kidney function in this study because it was more feasible for the serial determinations required by the protocol. Iohexol clearance measurements confirmed that the estimated GFRs were reasonably representative of the underlying kidney function in the transplant population. More work is needed to clarify the relationship between eGFR and iGFR to determine the optimal method to assess the effect of long-term used of ACEI in kidney function in renal transplant recipients. This is underscored by studies suggesting that specific formulas to estimate GFR are required in pediatric patients who have a kidney allograft.24 No cases of hyperkalemia occurred in our cohort of pediatric renal transplant recipients who were treated with an ACEI.
Despite the relatively small sample size of our study cohort and the narrow range of daily doses, both the PK and pharmacodynamic (PD) results observed corroborate and expand on what is known regarding the disposition and action of this drug in pediatric patients. Our decision to include standard-of-care patients, in retrospect, was important in expanding our study cohort so as to enable us to obtain meaningful, generalizable PK data obtained in a “target” population (i.e., those in whom the drug was being used to treat hypertension). The clinically relevant reduction in BP at the 0.1 and 0.2 mg/kg/day doses suggests that the higher dose may not be necessary to achieve BP control. However, we did not evaluate the basal activity of the renin angiotensin system in these patients, which may have been informative in understanding variation in BP response. In addition, long-term treatment studies will be required to determine if intensified BP control using an ACEI as part of the treatment regimen will achieve reno-protection in pediatric patients with a kidney transplant as was the case in ESCAPE trial of children with CKD who maintained native kidney function.25,26 The same caveat applies to children <6 years of age as controlled clinical trials characterizing the PK and PD of lisinopril in younger children remain to be performed. It should be noted, however, that if lisinopril is used during the first year of life at a time where the expected normal GFR is considerably lower than adult values, an increase in systemic drug exposure and its attendant PD effects can be expected. Finally, trials involving long-term lisinopril exposure will be required to demonstrate the sustained safety and efficacy of ACEIs such as lisinopril in the treatment of pediatric kidney transplant recipients. The results from the current study can be used to inform such future clinical trials.
Conclusion
The PK of lisinopril in pediatric patients with a kidney transplant was consistent with previous data in those without a kidney transplant, and lisinopril clearance was predictably related to underlying kidney function. The drug was well tolerated and associated with a clinically meaningful reduction in BP. The currently labeled lisinopril starting dose of 0.07 mg/kg once daily (up to 5 mg total) in patients with hypertension is likely appropriate based on PK considerations for hypertensive children with a kidney transplant and eGFR ≥ 30 ml/min per 1.73m2.
METHODS
Study design
This was a prospective, open-label, multi-center safety and PK study of lisinopril in pediatric kidney transplant recipients. It was conducted across 7 sites within the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Pediatric Trials Network (PTN). The study protocol was approved by the institutional review board at each participating site. Informed parental consent and patient assent, where appropriate, were obtained for each patient.
Patients
Patients were kidney transplant recipients 7–17 years of age. Two eligible populations were enrolled. The first population comprised lisinopril-naïve patients who were required to have systolic BP ≥75th percentile for age, sex, and height; an eGFR ≥30 ml/min per 1.73m2; stable allograft function (<20% change in serum creatinine during the prior 30 days); and stable immunosuppressive regimen (<10% dose change during the prior 14 days). The second population enrolled included lisinopril SoC patients for whom lisinopril was already prescribed as part of an anti-hypertensive regimen. Because recruitment of lisinopril-naïve patients was slower than projected, the lisinopril SoC patient population was added mid-study, and the systolic BP criterion was expanded from the original study criterion of >90th percentile in the naïve cohort. Patients in either enrollment population were excluded if they had: 1) received an ACEI other than lisinopril, an angiotensin receptor blocker (ARB), or renin antagonist within 30 days prior to enrollment; 2) known allergy or hypersensitivity to ACEI, iohexol, or iodine; 3) stage 2 hypertension; 4) serum potassium >6 mEq/L; 5) ongoing plasmapheresis treatment; 6) history of angioedema; or 7) positive pregnancy test.
Lisinopril dose and formulation
Lisinopril-naïve patients received oral lisinopril once daily at doses that approximated 0.1 mg/kg, 0.2 mg/kg, or 0.4 mg/kg until the PK visit. The PK visit occurred 10–16 days after the start of lisinopril treatment. Those who received 0.4 mg/kg daily were first started on 0.2 mg/kg daily. They returned after 3–7 days for an interim visit, and, if no adverse events (hyperkalemia or decline in eGFR ≥20%) were experienced, then the dose was increased to 0.4 mg/kg daily and continued until the PK visit. Patients were assigned to a dose level based on eGFR in a dose-escalation strategy. Patients were enrolled in either a low eGFR (30–59 ml/min per 1.73m2) or high eGFR (≥60 ml/min per 1.73m2) group. Within each eGFR group, the planned enrollment strategy was for the first 3 patients to receive lisinopril 0.1 mg/kg daily, the next 4 patients to receive 0.2 mg/kg daily, and the final 4 patients to receive 0.4 mg/kg daily.
Lisinopril SoC patients received the dose of lisinopril already prescribed as part of their ongoing management. The PK visit occurred 11–41 days after enrollment. For analysis, patients were assigned to a dose-level group (0.1, 0.2, or 0.4 mg/kg) and eGFR group (low or high) as defined above.
Lisinopril was given orally as a tablet (2.5 mg, 5 mg, 10 mg, or 20 mg dose strength). For lisinopril-naïve patients, the dose prescribed was within 2.5 mg (the lowest dose strength available in tablet formulation) of the assigned weight-based dose level. Patients were instructed to take the medication at the same time and in the same manner each day. Medication adherence was assessed by use of a medication diary. In addition, on each of the 2 days prior to the PK visit, the patient was contacted via phone by a study nurse and instructed to record the date and time of study medication administration. On the day of the PK visit, patients fasted for 2 hours prior to study drug administration.
Pharmacokinetic sampling
Venous blood samples (1 mL) for quantitation of lisinopril were collected pre-dose and at 1, 2, 4, 5, 8, 12, and 24 hours post-lisinopril dose. The blood samples were collected in EDTA-K2 tubes and centrifuged within 30 minutes at 1500–2000 g for 10 minutes at 4° C. Plasma was removed and stored within 8 hours at <−70° C until analysis, which did not exceed 18 months for any sample. In addition, a 24-hour quantitative urine collection was performed for drug level measurement following lisinopril dosing. The 24-hour urine collection was started after the patient voided just prior to receiving the dose of lisinopril. All urine excreted was pooled over the following time intervals: 0–4, 4–8, 8–12, and 12–24 hours. Urine was kept refrigerated at 4° C during a collection time period. Samples were then gently mixed by inversion, the volume recorded, and two 8 ml aliquots were stored at < −70° C until analysis. On the PK study day, iohexol clearance was concurrently performed to evaluate the correlation between the modified Schwartz formula and iohexol clearance-based determination of GFR. A 4–time-point blood sampling procedure over 5 hours (0, 2, 4, and 5 hours) was used in accordance with procedures established in the CKiD cohort study.27 The iohexol determinations were performed in the laboratory of George J. Schwartz, MD, University of Rochester Medical Center.
Blood pressure measurement
BP was measured at the start of study (prior to first dose of lisinopril in lisinopril-naïve patients) and at preselected study time points on the PK study day (pre-dose, 10 minutes post-iohexol infusion, and 4, 8, 12, and 24 hours post lisinopril dose). At each time point, 2–3 BP readings in the sitting position were obtained using the appropriate cuff size, and the values were averaged.
Safety
All patients were monitored for AEs throughout study enrollment and were followed for at least 30 days following the PK study day. Only treatment-emergent events are summarized. All new events that occurred or pre-existing conditions that worsened in frequency or intensity were recorded as AEs. Laboratory tests were performed at the baseline and PK visits.
Analytical methods
The concentration of lisinopril in plasma and urine was measured using high-pressure liquid chromatography–tandem mass spectrometry (HPLC-MS/MS) by OpAns, LLC (Durham, NC). Methods were validated according to FDA guidance. For plasma, the lower limit of quantification was 0.5 ng/ml, and the calibration curve ranged from 0.5–500 ng/mL. The within-run and between-run coefficients of variation ranged from 2.3–10.9% and 6.1–9.6%, respectively, across the concentrations spanning the range of linearity. For urine, the lower limit of quantification was 50 ng/ml, and the calibration curve ranged from 50–15,000 ng/mL. The within-run and between-run coefficients of variation ranged from 2.4–6.1% and 3.6–6.3%, respectively, across the concentrations spanning the range of linearity.
Pharmacokinetic analyses
Steady-state PK parameters for lisinopril were estimated from plasma and urine concentration data via noncompartmental analysis using Phoenix™ WinNonlin® (version 6.3; Certara, St. Louis, MO). The Cmax and Tmax were obtained directly from the observed data. Clast was defined as the last observed quantifiable concentration during the dosage interval and was equivalent to the C24 for patients on once-daily dosing and the concentration at 12 hours (C12) for the single patient on twice-daily dosing. The AUC0–24 was calculated using the linear/logarithmic trapezoidal method. AUC0–24 was calculated as AUC0–12 x 2 for the 1 patient on twice-daily dosing. To allow direct comparisons across dose groups, Cmax, C24, and AUC0–24 were also dose-adjusted to a 0.1 mg/kg daily dose based on patient dose and weight. CL/F was calculated as daily dose/AUC0–24. CL/F was then scaled for size to a 70 kg adult (CL/F/70 kg) using an allometric exponent of 0.75.17,18 The terminal rate constant (λz) was determined by linear regression analysis of the terminal portion of the log plasma concentration–time curve. The t1/2 was calculated as ln 2/λz. It is worth noting that due to the PK sampling strategy and slower absorption rate of lisinopril, the robustness of the estimate of t1/2 for patients may have been limited. Renal clearance (renal CL) was calculated as the total amount of lisinopril excreted in urine over 24 hours divided by the AUC0–24, except for the 1 patient who was taking twice-daily dosing for whom renal CL was calculated as the amount of lisinopril excreted in urine over 12 hours divided by AUC0–12. Renal CL was normalized to ml/min per 1.73m2 to allow direct comparison to eGFR. The fraction of lisinopril dose excreted in urine over a dosing interval (fe in urine) was calculated from the cumulative measured urinary recovery over the dosing interval divided by the dose.
Statistical analyses
Summary demographic data are presented as count (%) or mean ± standard deviation (SD). Summary PK data are presented as the geometric mean (GeoMean) and CV%, except daily dose and Tmax, which are presented as the median and range. PK parameters were compared by 0.1 vs. 0.2 mg/kg/day dose groups and low vs. high eGFR groups. The 0.4 mg/kg/day dose group was not compared due to the limited sample size (n=2). Differences between groups were compared using the student t-test or the Mann-Whitney U test for continuous data and the Fisher’s exact test for categorical data. Log transformation of all PK parameters except Tmax was performed prior to statistical analysis. The impact of potentially clinically relevant covariates on log-transformed CL/F/70 kg was examined by a Lasso regression analysis using 5-fold cross-validation with the predicted residual sums of squares (PRESS) selection criterion in SAS version 9.3. The following covariates were considered: age, sex, time since transplant, tacrolimus use, sirolimus use, prednisone use, mycophenolate use, enrollment type (lisinopril-naïve vs. SoC), daily lisinopril dose, eGFR, urine protein/creatinine ratio, and microalbuminuria.
To examine the effect of lisinopril on BP, change in sitting systolic and diastolic BP from baseline (i.e., prior to lisinopril initiation) was calculated in lisinopril-naïve patients using the measured BP immediately before lisinopril administration (i.e., trough BP) on the PK visit. Mean BP change was estimated separately for the 0.1 and 0.2 mg/kg/day dose groups using 95% confidence intervals based on the t-distribution. For safety assessments, an intent-to-treat approach was used. In addition, the highest grade clinical laboratory abnormality while on lisinopril was reported.
All tests of significance were 2-sided, and the significance limit accepted for all statistical analyses was α = 0.05. Adjustments for multiplicity were not performed for these exploratory, descriptive comparisons. All statistical analyses were performed in SAS version 9.3.
Supplementary Material
STUDY HIGHLIGHTS.
What is the current knowledge on the topic?
Lisinopril is approved to treat essential hypertension in children and adolescents; however, little is known about its use in pediatric kidney transplant recipients.
What question did this study address?
This study sought to characterize lisinopril PK, short-term safety, and preliminary efficacy in children with a kidney transplant.
What this study adds to our knowledge
The PK of lisinopril in children with a kidney transplant was similar to historical hypertensive children without a kidney transplant. Lisinopril clearance increased in proportion to kidney function. Lisinopril was generally well tolerated and was accompanied by a lowering of BP at approved pediatric doses in the study population.
How this might change clinical pharmacology and therapeutics
The current approved lisinopril starting dose of 0.07 mg/kg once daily (up to 5 mg total) in children with hypertension is likely appropriate based on PK considerations for hypertensive children with a kidney transplant and eGFR ≥30 ml/min per 1.73m2.
Acknowledgments
The authors would also like to thank Karen Pendergast, MPH, and Parisa Jabbarzadegan, BS, MT, both formerly of the Duke Clinical Research Institute, as well as Ashley Wegel, MPH, and Julie Debski, BS, at the EMMES Corporation, Rockville, MD, for their outstanding management of the study. The concentration of lisinopril in plasma and urine was measured using high-pressure liquid chromatography–tandem mass spectrometry performed at OpAns Laboratory (Durham, NC, USA).
SOURCE OF FUNDING
This work was funded under NICHD contract HHSN2752010000031 for the Pediatric Trials Network.
The Pediatric Trials Network Administrative Core Committee
Daniel K. Benjamin Jr., Duke Clinical Research Institute, Durham, NC; Katherine Y. Berezny, Duke Clinical Research Institute, Durham, NC; Edmund Capparelli, University of California–San Diego, San Diego, CA; Michael Cohen-Wolkowiez, Duke Clinical Research Institute, Durham, NC; Gregory L. Kearns, Children’s Mercy Hospital, Kansas City, MO; Matthew Laughon, University of North Carolina at Chapel Hill, Chapel Hill, NC; Andre Muelenaer, Virginia Tech Carilion School of Medicine, Roanoke, VA; T. Michael O’Shea, Wake Forest Baptist Medical Center, Winston Salem, NC; Ian M. Paul, Penn State College of Medicine, Hershey, PA; P. Brian Smith, Duke Clinical Research Institute Durham, NC; John van den Anker, George Washington University School of Medicine and Health, Washington, DC; Kelly Wade, Children’s Hospital of Philadelphia, Philadelphia, PA; Thomas J. Walsh, Weill Cornell Medical College of Cornell University, New York, NY.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development: David Siegel, Perdita Taylor-Zapata, Anne Zajicek, Zhaoxia Ren, Katerina Tsilou, Alice Pagan
The EMMES Corporation (Data Coordinating Center): Gina Simone
Footnotes
ClinicalTrials.gov identifier: NCT01491919
AUTHOR CONTRIBUTIONS
Wrote manuscript: Trachtman, Frymoyer, Lewandowski, Greenbaum, Feig, Gipson, Warady, Goebel, Schwartz, Lewis, Anand, Patel
Designed research: Trachtman, Frymoyer, Feig, Gipson, Warady, Patel
Performed research: Trachtman, Frymoyer, Greenbaum, Feig, Gipson, Warady, Goebel, Schwartz, Lewis, Patel
Analyzed data: Trachtman, Frymoyer, Lewandowski, Anand, Patel
Contributed new reagents/analytical tools: Schwartz, Lewis
CONFLICTS OF INTEREST/DISCLOSURES
Dr. Frymoyer received support from the National Institute of Child Health and Human Development (NICHD; 1K23HD079557 and HSSN275000002). Drs. Anand and Lewandowski receive support from Government Contract HHSN267200700051C (PI: Benjamin). Dr. Patel received support for research from NICHD contract HHSN27500007 and from industry for drug development in adults and children with kidney disease (www.dcri.duke.edu/research/coi.jsp). The remaining authors have no conflicts of interest to disclose.
References
- 1.Mitsnefes MM, Khoury PR, McEnery PT. Early post-transplantation hypertension and poor long-term renal allograft survival in pediatric patients. J Pediatr. 2003;143:98–103. doi: 10.1016/S0022-3476(03)00209-9. [DOI] [PubMed] [Google Scholar]
- 2.Silverstein DM, LeBlanc P, Hempe JM, Ramcharan T, Boudreaux JP. Tracking of blood pressure and its impact on graft function in pediatric renal transplant recipients. J Pediatr Transplant. 2007;11:860–867. doi: 10.1111/j.1399-3046.2007.00753.x. [DOI] [PubMed] [Google Scholar]
- 3.Mitsnefes MM, Omoloja A, McEnery PT. Short-term pediatric renal transplant survival: blood pressure and allograft function. Pediatr Transplant. 2001;5:160–165. doi: 10.1034/j.1399-3046.2001.t01-1-00051.x. [DOI] [PubMed] [Google Scholar]
- 4.Cross NB, Webster AC, Masson P, O’Connell PJ, Craig JC. Antihypertensives for kidney transplant recipients: systematic review and meta-analysis of randomized controlled trials. Transplantation. 2009;88:7–18. doi: 10.1097/TP.0b013e3181a9e960. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen SE, et al. Neutrophil gelatinase-associated lipocalcin (NGAL) and kidney injury molecule 1 (KIM1) in patients with diabetic nephropathy: a cross-sectional study and the effects of lisinopril. Diabet Med. 2010;27:1144–1150. doi: 10.1111/j.1464-5491.2010.03083.x. [DOI] [PubMed] [Google Scholar]
- 6.Inigo P, et al. Effects of losartan and amlodipine on intrarenal hemodynamics and TGF-β(1) plasma levels in a crossover trial in renal transplant recipients. J Am Soc Nephrol. 2001;12:822–827. doi: 10.1681/ASN.V124822. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez AA, et al. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in renal transplantation between 1990 and 2002 in Spain. NDT Plus. 2010;3(Suppl 2):ii21–25. doi: 10.1093/ndtplus/sfq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorof JM, Goldstein SL, Brewer ED, Steiger HM, Portman RJ. Use of anti-hypertensive medications and post-transplant renal allograft function in children. Pediatr Transplant. 2000;4:21–27. doi: 10.1034/j.1399-3046.2000.00082.x. [DOI] [PubMed] [Google Scholar]
- 9.Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest. 2006;116:288–296. doi: 10.1172/JCI27699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knutter I, et al. Transport of angiotensin-converting enzyme inhibitors by H+/peptide transporters revisited. J Pharmacol Exp Therap. 2008;327:432–441. doi: 10.1124/jpet.108.143339. [DOI] [PubMed] [Google Scholar]
- 11.Hogg RJ, et al. A multicenter study of the pharmacokinetics of lisinopril in pediatric patients with hypertension. Pediatr Nephrol. 2007;22:695–701. doi: 10.1007/s00467-006-0399-5. [DOI] [PubMed] [Google Scholar]
- 12.Prinivil® (lisinopril tablets) package insert. Whitehouse Station, NJ: Merck & Co., Inc; 2003. [Google Scholar]
- 13.Qin F, Wang D, Yang S, Jing L, Xiong Z, Li F. Quantitative determination of lisinopril in human plasma by high performance liquid chromatography-tandem mass spectrometry and its application in a pharmacokinetic study. Biomed Chromatogr. 2012;26:691–696. doi: 10.1002/bmc.1715. [DOI] [PubMed] [Google Scholar]
- 14.Lin JH, Chen IW, Ulm EH, Duggan DE. Differential handling of angiotensin-converting enzyme inhibitors enalaprilat and lisinopril in rats. Drug Metab Dispos. 1988;16:392–396. [PubMed] [Google Scholar]
- 15.U.S. Food and Drug Administration. Clinical Pharmacology and Biopharmaceutics Review. [Accessed July 22, 2014];CDER Approval Package for Prinivil®: Application Number 19-558/S-043. 2003 Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/19-558S043_Prinivil.cfm.
- 16.Heads of Medicines Agencies. [Accessed July 22, 2014];Lisinopril Paediatric Public Assessment Report: SE/W/002/pdWS/001. 2009 Available at: http://www.hma.eu/fileadmin/dateien/Human_Medicines/CMD_h_/Paediatric_Regulation/Assessment_Reports/Article_45_work-sharing/Lisinopril_2009_11b_45PaedPAR.pdf.
- 17.Anderson BJ, Holford NHG. Mechanism-based concepts of size and maturity in pharmacokinetics. Ann Rev Pharmacol Toxicol. 2008;48:303–332. doi: 10.1146/annurev.pharmtox.48.113006.094708. [DOI] [PubMed] [Google Scholar]
- 18.Holford N, Heo YA, Anderson B. A pharmacokinetic standard for babies and adults. J Pharm Sci. 2013;102:2941–2952. doi: 10.1002/jps.23574. [DOI] [PubMed] [Google Scholar]
- 19.Rhodin MM, et al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol. 2009;24:67–76. doi: 10.1007/s00467-008-0997-5. [DOI] [PubMed] [Google Scholar]
- 20.Zestril® (lisinopril tablets) package insert. Vol. 2009 Wilmington, DE: AstraZeneca; 2009. [Google Scholar]
- 21.van Schaik BA, Geyskes GG, van der Wouw PA, van Rooij HH, Porsius AJ. Pharmacokinetics of lisinopril in hypertensive patients with normal and impaired renal function. Eur J Clin Pharmacol. 1988;34:61–65. doi: 10.1007/BF01061419. [DOI] [PubMed] [Google Scholar]
- 22.Soffer B, Zhang Z, Miller K, Vogt BA, Shahinfar S. A double-blind, placebo-controlled, dose-response study of the effectiveness and safety of lisinopril for children with hypertension. Am J Hypertens. 2003;16:795–800. doi: 10.1016/s0895-7061(03)00900-2. [DOI] [PubMed] [Google Scholar]
- 23.Hermida RC, et al. Chronotherapeutics of conventional blood pressure-lowering medications: a simple, low-cost means of improving management and treatment outcomes of hypertensive-related disorders. Curr Hypertens Rep. 2014;16:412. doi: 10.1007/s11906-013-0412-x. [DOI] [PubMed] [Google Scholar]
- 24.Siddique K, Leonard D, Borders L, Seikaly MG. Validation of the CKiD formulae to estimate GFR in children post renal transplant. Pediatr Nephrol. 2014;29:445–451. doi: 10.1007/s00467-013-2660-z. [DOI] [PubMed] [Google Scholar]
- 25.Knoll GA, et al. The Canadian ACE-inhibitor trial to improve renal outcomes and patient survival in kidney transplantation—study design. Nephrol Dial Transplant. 2008;23:354–335. doi: 10.1093/ndt/gfm574. [DOI] [PubMed] [Google Scholar]
- 26.ESCAPE Trial Group. Wühl E, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639–1650. doi: 10.1056/NEJMoa0902066. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz GJ, et al. New equations to estimate GFR in children. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.