Summary
Neural reflex circuits regulate cytokine release to prevent potentially damaging inflammation and maintain homeostasis. In the inflammatory reflex, sensory input elicited by infection or injury travels through the afferent vagus nerve to integrative regions in the brainstem, and efferent nerves carry outbound signals that terminate in the spleen and other tissues. Neurotransmitters from peripheral autonomic nerves subsequently promote acetylcholine-release from a subset of CD4+ T cells that relay the neural signal to other immune cells, e.g. through activation of α7 nicotinic acetylcholine receptors on macrophages. Here, we review recent progress in the understanding of the inflammatory reflex and discuss potential therapeutic implications of current findings in this evolving field.
Keywords: inflammation, neuroimmunology, spleen, vagus nerve
Neural regulation of inflammation is ancient
The immune system must prevent invasion by pathogens, but simultaneously tolerate symbionts (1, 2) and avoid excessive responses against host tissue which could cause non-resolving inflammation and autoimmune diseases (3). Under certain conditions, for example in sepsis, the immune response can be more injurious than the pathogen invasion (4). Immunity is normally strictly regulated to effectively suppress pathogens and tumors but simultaneously allows for host-microbial synergy, organism growth, and reproduction. Within the immune system, inhibitory cytokines, soluble cytokine receptors, lipoxins and resolvins, as well as glucocorticoids and other hormones, can constrain activity and promote resolution (5, 6). Recent discoveries show that neural reflexes that control inflammation are an ancient component of animal physiology (7). Caenorhabditis elegans is one of the least complex animals with a nervous system. These nematodes are equipped with a feeding system, gut, and a nervous system, but lack skeletal elements and a circulatory system. In this relatively simple organism, the innate immune response to bacterial invasion of non-neural cells is regulated by nerve signals (8). Neural reflexes regulate immunity also in higher vertebrates (3). Indeed, the nervous system has a number of features which makes it well suited for homeostatic control.
Neural reflexes
Nerves are uniquely capable signal transmitters. In contrast to endocrine factors, they deliver precisely targeted signals instantaneously. The central nervous system (CNS) communicates with practically all parts of the body through sensory and motor neurons. The complex organization of the CNS with numerous types of highly organized neuronal systems continuously process multiple external and internal inputs and interacting layers of neural networks in the CNS can integrate set points and sensory signals to fine-tune the motor response. In this way, autonomic homeostatic reflexes regulate a number of vital functions, including heart rate, vascular tone, respiration, temperature, and intestinal motility. Accumulated experimental evidence indicates that the immune system is regulated in a similar manner through the inflammatory reflex. This system of balanced integration of input signals and set points provides for sophisticated optimization and coordination of organ function.
Reflex control of immunity: the sensory arc
An important task for the nervous system is to sense and respond to threats, be it with pain and behavioral changes or reflex withdrawal from noxious stimuli. Hence, it is resonable that the CNS is involved in sensing and regulation of inflammation. Infection and injury can induce a wide range of behavioral and physiological responses, including social isolation, reduced food intake, increased sleep, fever, hyperalgesia and mobilization of energy reserves (9), which indicates that the CNS monitors inflammation and manages resources to promote homeostasis. Alert signals from the periphery reach the brain through several different routes, including via specialized cells in the brain vasculature, choroid plexus and circumventricular organs, and through TLRs and cytokine receptors in the brain (10–12). Peripheral afferent nerves transmit ‘danger signals’ and elicit neural reflexes which regulate the immune response. A prototypical neural reflex circuit of this kind is the ‘inflammatory reflex’ which includes a sensory arm in the afferent vagus and a motor arm in the efferent vagus nerve (3) (Fig. 1). The following sections focus on this neural circuit.
Fig. 1. The inflammatory reflex.
Immune responses are regulated by neural reflex circuits that sense peripheral inflammation and provide regulatory feedback through specific nervous signals and humoral factors. Sensory vagus fibers innervate a multitude of organs, for example the intestine and glomus caroticum. They are activated by cytokines induced by tissue damage or PAMPs in the periphery and transmit signals to the nucleus tractus solitarius (NTS) in the brainstem. Polysynaptic relays connect to the vagal motor neurons in the dorsal vagal motor nucleus and nucleus ambiguus and sympathoexcitatory neurons in the rostral ventrolateral medulla. Efferent vagus nerve signals travel to the celiac plexus and also directly to target organs and suppress innate immune responses. Activation of afferent vagus signals also triggers a ‘sickness response’ and activates the hypothalamic-pituitary-adrenal (HPA) axis, which promotes glucocorticoid release from the adrenal glands.
Cytokine-producing innate immune cells respond to pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs), derived from microbes or damaged host tissue. Dendritic cells, capable of releasing IL-1β and prostaglandins (13, 14), reside in close proximity of vagus nerve fibers in the periphery, for example in lymph nodes and the intestine (10). Afferent neurons in the vagus nerve express receptors for IL-1β and prostaglandins (15) and mRNA and protein for TLR4 have been found in the nodose ganglion of the afferent vagus nerve (16) and in the carotid chemoreceptor (17). Injection of intravenous or intra-peritoneal LPS activates afferent vagus neurons (18, 19) and administration of IL-1β in the periphery gives rise to nerve signals (20, 21) as well as transcription and protein synthesis (15, 19) in the afferent vagus nerve. c-fos staining, a marker of neuronal activity, was combined with retrograde tracing of vagus fibers terminating in the gut, and showed that a high percentage of these efferent neurons express c-fos 24 h after experimental abdominal surgery. Selective ablation of vagal fibers to the gut reduced the number of c-fos-positive neurons and increased leukocyte recruitment into the muscular wall of the intestine (22). Furthermore, administration of microbial species in the periphery slows heart rate, a classical sign of increased efferent vagus nerve activity. Extensive pharmacological and physiological studies have established that vagus nerve activity is associated with increased heart rate variability (HRV). Decreased HRV has been linked to sensing of increased cytokine levels, PAMPs and DAMPs and bacterial and fungal infection (16, 17, 23, 24). There is a strong correlation between decreased HRV and morbidity and mortality in a number of diseases associated with excessive inflammation, including myocardial infarction, rheumatoid arthritis, ulcerative colitis and multiple organ failure, and the anti-inflammatory effects of efferent vagus signaling likely contributes to this association (25–30).
Signals through the afferent vagus nerve reach the nucleus tractus solitarius (NTS) in the brainstem medulla oblongata, which is interconnected with the dorsal motor nucleus, where the majority of efferent vagus nerve fibers originate. Afferent signaling also activates central neurons that project to the hypothalamus and other CNS nuclei (10). These neural projections are important for the inflammatory response, e.g. regulation of the neurohormonal hypothalamus-pituitary-adrenal (HPA) axis with its associated glucocorticoid production, and for induction of fever. Furthermore, afferent vagus nerve activity in response to LPS or IL-1β-administration in the periphery causes efferent vagus nerve signaling to the thymus, as well as increased activity in the splenic nerve (20, 31, 32). Subdiaphragmatic vagotomy abolishes the effects on adrenocorticotropin of intraperitoneal administration of LPS or IL-1β (10), unless systemic IL-1β levels reach high levels (33), and blocks IL-1β- induced hyperthermia (34). Increased cytokine levels in the periphery and exogenous administration of proinflammatory cytokines also elicit sickness behavior. Reciprocally, blockade of the cytokines IL-1β, IL-6 and TNF abolishes this response (35, 36). Hence, a sensory arc for inflammation is in place.
These findings suggest that peripheral nerves alert the CNS to threats before the condition becomes severe enough to produce systemic levels of inflammatory mediators that can directly activate receptors in the brain. The processing mechanisms in the CNS of afferent nervous signals elicited by peripheral inflammation are not well understood. CNS perception of inflammation alters behavior, a range of physiological parameters, and induces nervous signals that regulate immunity in the periphery. In support of this notion, pharmacological activation of the CNS can protect from excessive cytokine production by efferent signaling through the vagus nerve (37); the tetravalent guanylhydrazone CNI-1493 (semapimod) likely functions by activating CNS centers and subsequent activation of the efferent vagus nerve-based cholinergic arm of the inflammatory reflex (38). Intrathecal CNI-1493 inhibits peripheral inflammation and this effect is abolished by vagotomy (39, 40). Pharmacological modulation of central muscarinic cholinergic activation by muscarine, the M1 receptor agonist McN-A-343, and the M2 receptor antagonist methoctramine is associated with increased efferent vagus nerve activity and reduces serum TNF levels significantly during endotoxemia (41). Treatment with galantamine, a centrally acting acetylcholine esterase inhibitor used clinically to increase central cholinergic signaling for treatment of Alzheimer¢s disease, also reduces serum levels of TNF in endotoxemia, and this effect is attenuated by vagotomy (42), corroborating the role of the vagus nerve in this system.
Although facets of the sensing and processing of immunerelated signals by the CNS have been delineated, understanding of the complete stepwise process is incomplete and yet unidentified pathways are likely to exist. Nevertheless, more has recently been learned about how cholinergic regulatory signals from the CNS are propagated to target immune cells through efferent cholinergic nerves.
Reflex control of immunity: the efferent arc
Neuroanatomy
Activation of efferent cholinergic signaling has profound effects on the systemic production of TNF in endotoxemia (6). The vagus nerve provides a key pathway for this effect. Both pharmacological and electrical stimulation of the vagus nerve substantially improve outcome and reduce pro-inflammatory cytokine levels in a wide range of experimental inflammatory and auto-immune diseases (Table 1). Reciprocally, ablation of the cervical vagus nerve prevents activation of the motor vagus from reducing systemic TNF in endotoxemia (37, 43).
Table 1.
Experimental models of inflammation in which activation of the efferent arc of the inflammatory reflex has beneficial effects
| Disease model |
Mode of efferent arc inflammatory reflex activation |
Outcome |
|---|---|---|
| Endotoxemia | Galantamine† | Improved survival. Reduced serum TNF (42) |
| Physostigmine† | Attenuated capillary leakage. Decrease interaction time between leukocytes and endothelium (148) | |
| VNS | Attenuated shock. Reduced TNF in liver and serum (56) | |
| Decreased serum and myocardial TNF (43) | ||
| Reduced liver and spleen TNF (47) | ||
| GTS-21* | Improved survival. Reduced serum TNF (155) | |
| Cecal ligation and puncture | Neostigmine† and physostigmine† | Improved survival. Reduced serum TNF, IL-1β, and IL-6. Reduced neutrophil lung infiltration (151) |
| GTS-21* | Improved survival. Reduced serum HMGB1(155) | |
| VNS | Improved survival. Reduced serum HMGB1(156) | |
| Prevented hypotension. Reduced hepatic damage. Reduced serum TNF (157) | ||
| Increased survival (158) | ||
| Colitis | VNS | Decreased body weight loss. Improved histological score (159) |
| Pancreatitis | GTS-21* | Improved histological score. Decreased serum IL-6 (160) |
| Hemorrhagic shock | VNS | Reverted hypotension and prolonged survival. Attenuated serum TNF (53) |
| Intracerebral hemorrhage | Muscarine | Increased HRV. Reduced brain edema. Reduced TNF and IL-1β in brain and spleen (161) |
| Ischemia-reperfusion | ||
| Suprarrenal aortic | VNS | Prevented hypotension. Reduced TNF in heart, liver and serum (40) |
| Myocardial | PNU-282987* | Reduced infarct size. Decreased serum TNF, HMGB1, and troponin I (136) |
| VNS | Reduced lethality. Decreased histopathological changes in heart (162) | |
| Renal | GTS-21* | Improved renal function. Attenuated TNF in kidney. Reduced tubular necrosis (163) |
| Artery occlusion shock | VNS | Reverted hypotension. Increased survival. Reduced leukocyte infiltration to lung and ileum. Decreased serum TNF (164) |
| Carrageenan-induced inflammation | VNS and GTS-21* | Inhibited expression of endothelial cell adhesion molecules (149) |
| Arthritis | AR-R17779* | Improved clinical arthritis score. Reduced synovial inflammation. Reduced plasma and synovial TNF (165) |
| Burn-induced injury | VNS | Decreased gut permeability (54) |
| Improved pulmonary histopathology (51, 55). | ||
| Hypertension-induced end organ damage | PNU-282987* | Decreased end organ damage. Decreased TNF, IL-1β, and IL-6, and inhibited NFκB activation in heart, aorta and kidney (125) |
| Hemorrhage | VNS | Reduced bleeding time independent of changes in heart rate or blood pressure. Increased thrombin / antithrombin III complex generation (166) |
| Postoperative ileus | VNS | Improved gastric emptying. Reduced TNF and IL-6 in peritoneal cavity (116) |
| AR-R17779* | Improved gastric emptying. Reduced inflammatory cell recruitment (167) | |
| Ventilator-induced lung injury | VNS and PNU-282987* | Attenuated lung injury. Decreased lung IL-6 and substance P. Decreased plasma IL-6 (134) |
| GTS-21* | Improved lung function. Decreased TNF lung and plasma levels (135) |
VNS, vagus nerve stimulation.
Selective for a7nAChR.
Acetylcholinesterase inhibitor.
The vagus nerve originates in the brainstem medulla oblongata and travels down the neck before spreading to the organs of the chest and abdomen. A series of tracing experiments using injection of fluorescent anterograde carbocyanine dyes into the dorsal motor nucleus of the rat vagus tracks efferent vagal neurons to visceral ganglia, including the celiac plexus (44, 45). Signals in the vagus nerve are subsequently propagated to the spleen via the splenic nerve (46, 47). Approximately 90% of TNF released systemically in the early phase of rodent endotoxemia originates in the spleen (46) and splenectomy protects against sepsis lethality (48). Therefore, the spleen is a primary target for signals in the efferent pathway of the inflammatory reflex. Vagus nerve stimulation (VNS) in vivo attenuates endotoxemic TNF production in splenic red pulp and marginal zone macrophages (46). The inhibitory effect of electrical VNS on splenic TNF production is abrogated by surgical ablation of the splenic nerve or by catecholamine depletion by reserpine, indicating a functional connection between vagal cholinergic and splenic catecholaminergic nerve fibers through these ganglia. Electrical stimulation of the distal stump of a transected cervical vagus nerve resulted in catecholamine release to the blood stream, but not in splenectomized animals (49). Within celiac plexus ganglia, vagus nerve efferents may transmit information through interneurons (50) or to postsynaptic, catecholaminergic neurons directly. Severing of the common celiac nerve also abolishes TNF suppression by VNS, suggesting that the efferent arc of the inflammatory reflex is functionally hardwired through the vagus nerve and celiac plexus to the spleen (47, 48).
The CNS can transmit signals to the celiac ganglion not only through the vagus nerve, but also through the sympathetic chain. However, data from experimental diseases support requirement of vagal efferent signaling for the integrity of the inflammatory reflex. After cervical vagotomy, electrical stimulation of the distal vagus stumps protects against injuries from hypovolemic shock, burns and injuries from mechanical ventilation (51–53). In two burn models, VNS protected against injury, but if vagotomy was performed below the region of stimulation, the protective effect was abolished (54, 55). Another recent study showed that increased vagal activity, as determined by the high frequency (HF) domains of HRV, inversely correlates to TNF and IL-6 levels in endotoxin-stimulated whole blood (23), suggesting that the innate immune response to PAMPs is under control of efferent signals in the vagus nerve. In line with this, treatment of cultured macrophages with agonists for nicotinic acetylcholine receptors such as nicotine or acetylcholine significantly reduces their endotoxin-induced release of TNF and other pro-inflammatory cytokines, including cytokines associated with inflammasome activation such as IL-1β and IL-18 (56). Taken together, our current understanding is that the vagus nerve acts reflexively to dampen inflammation in the periphery, specifically, but not exclusively, through neural projections to the spleen. This signal culminates in inhibition of cytokine producing immune cells by activation of their cholinergic receptors. Intriguingly, the spleen lacks cholinergic innervation and the splenic nerve is chatecoholaminergic. How does this neural signal then activate cholinergic receptors in the spleen?
Nerve to immune cell signaling in spleen
Stimulation of the splenic nerve increases splenic levels of norepinephrine (49). Anatomically, the adrenergic splenic nerve fibers terminate in T-cell-rich areas and have been observed to form synaptic-like structures with immune cells (57). This organization raises the possibility of intimate crosstalk between efferent splenic nerves and resident splenic immune cells. Blocking beta-adrenergic, but not alpha-adrenergic, receptors in the spleen ex vivo abolished the inhibition of splenic TNF production by splenic nerve activation (58). Recently, these findings were supported by observations in vivo. Vida et al. found that splenic nerve stimulation or administration of β-adrenergic agonists, respectively, indeed reduced systemic levels of TNF in endotoxemia (59). Hence, adrenergic signaling in the spleen has significant effects on TNF release.
Concomitantly, it has been shown that the integrity of the inflammatory reflex is critically dependent on expression of the α7 nicotinic acetylcholine receptor (α7nAChR), since electrical or pharmacological activation of the cholinergic anti-inflammatory pathway does not attenuate TNF release in α7nAChR-deficient mice (42, 60). Systemic levels of TNF in endotoxemia were higher in the α7nAChR-deficient animals than in controls, suggesting tonic inhibition of inflammation mediated by signaling through the α7nAChR. α7nAChR is expressed at several anatomic locations and in different cell types, including neurons, glial cells, and T cells. It is common in the brain, and present in autonomic ganglia (61, 62). Accumulating evidence indicates that the α7nAChR expression in macrophages is vital for acetylcholine-mediated suppression of TNF release: cholinergic agonists fail to inhibit TNF release in macrophages with suppressed α7nAChR expression mediated by anti-sense treatment (60) or in macrophages from α7nAChR-deficient mice (63). Because of the known wide-spread expression of α7nAChR, it was still theoretically possible that the integrity of the inflammatory reflex relied on α7nAChR not in macrophages, but in the CNS or in autonomic ganglia. This ambiguity was resolved by performing VNS in animals with chimeric expression of the α7nAChR. The chimeras were created by cross-transferring bone-marrow: Wild type mice were transplanted with bone-marrow from α7nAChR-deficient donors, effectively creating mice with a α7nAChR-deficient immune system and a wildtype nervous system. Reciprocally, α7nAChR deficient mice received bone-marrow from wildtype donors, which created mice with a wildtype immune system and a α7nAChR-deficient nervous system. In endotoxemia, VNS failed to reduce systemic TNF levels in animals with an α7nAChR deficient immune system, while α7nAChR deficiency in the nervous system did not interfere with the TNF attenuating effects of VNS (64). This observation locates the requirement for α7nAChR in the efferent arc of the inflammatory reflex to immune cells, and indicates that cholinergic ligands directly regulate immune cells in vivo. Hence, both adrenergic and cholinergic signaling in the spleen play key roles in the inflammatory reflex.
It has long been known that the spleen contains substantial amounts of acetylcholine, but its source had not been thoroughly investigated. In fact, soon after Otto Loewi in the early 20th century elucidated the chemical basis of synaptic transmission by stimulating the vagus nerve of an innervated frog heart causing it to slow, and then using conditioned media from this stimulation to slow a second, denervated heart, Henry Dale isolated the active agent, acetylcholine, from ox and horse spleen (65, 66). Today, it is axiomatic that acetylcholine is a principle neurotransmitter released under the control of action potentials in the vagus nerve. Although it has been known for a century that the spleen contains large amounts of acetylcholine, the source and role of splenic acetylcholine was not well understood.
Choline acetyltransferase-expressing T cells in the inflammatory reflex
T lymphocytes can express choline acetyltransferase (ChAT), the enzyme that catalyzes acetylcholine biosynthesis and are capable of making and releasing acetylcholine (67). These insights together with the effect of VNS on immune cells, the requirement of spleen acetylcholine, and the proximity of nerve endings and T cells in spleen, lead to the discovery of a role for splenic T cells in propagation of nerve signals. Analysis of microdialysis samples from spleen showed that activation of the vagus nerve increased splenic acetylcholine levels (68). Hence, although the spleen is devoid of cholinergic nerve fibers, signals conveyed by the vagus nerve can promote release of acetylcholine in spleen. This realization suggested that acetylcholine-producing lymphocytes link the catecholaminergic splenic nerve signal to acetylcholine-responsive target cells to attenuate release of TNF. Splenic nerve endings predominantly terminate in the splenic white pulp, and stimulation of the splenic nerve increases release of norepinephrine from the spleen (57, 69).
Adrenergic stimulation promotes acetylcholine release from T cells (68). If specialized T lymphocytes in the spleen white pulp are a functional and integral part of the efferent arc of the inflammatory reflex, VNS should fail to reduce endotoxemic levels of TNF in T-cell-deficient mice. In agreement with this prediction, VNS does not reduce systemic levels of TNF in endotoxemia in T cell deficient nude mice (68). Hence, T cells are necessary for the integrity of the inflammatory reflex. Further studies revealed that ChAT-expressing T cells capable of acetylcholine synthesis constitute approximately 10% of CD3+ CD4+ CD44hi CD62Llow cells. Adoptive transfer of these cells is sufficient to restore the integrity of the efferent arc of the inflammatory reflex in T cell deficient nude mice. In support of the notion that acetylcholine production and release is a key part of this anti-inflammatory pathway, transfer of CD3+ CD4+ CD44hi CD62Llow ChAT− cells to nude mice fails to restore function of the efferent arc of the inflammatory reflex. The finding that adoptive transfer into nude mice of CD4+ T cells in which ChAT had been knocked down failed to restore the inflammatory reflex provided further proof for the role of ChAT in the T cells in this novel link between the nervous system and innate immune cells (68). These data demonstrate that neural signals transmitted through the vagus nerve culminate in release of spleen acetylcholine from a small subset of memory T cells. Acetylcholine then acts on bone marrow derived cells via α7nAChR signaling to attenuate TNF release in spleen (Fig. 2). In effect, it can be said that CD3+ CD4+ CD44hi CD62Llow ChAT+ memory T cells, ‘ChAT+ T cells’, relay neural input via neurotransmitter release and thus fulfill a function of interneurons.
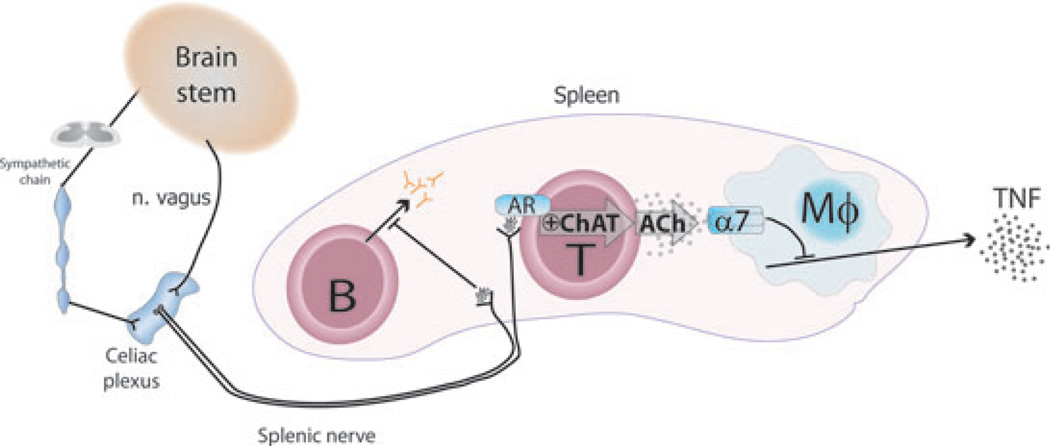
Fig. 2. Current model of the efferent arc of the inflammatory reflex.
Efferent signals from the brain stem travel through the efferent vagus nerve to the celiac plexus, which also receives input from the sympathetic trunk. The catecholaminergic splenic nerve arises in the celiac plexus and projects to the spleen. Choline acetyltransferase+ (ChAT) T cells and B cells are found in close proximity of splenic nerve fibers. Efferent, outgoing signals in the vagus nerve activate the splenic nerve, which releases its neurotransmitters, including norepinephrine, in the spleen. Activation of choline acetyltransferase-expressing T cells, possibly through adrenergic receptors (AR), promotes production and release of T cell-derived acetylcholine (ACh). This acetylcholine then acts on the α7 nicotinic acetylcholine receptors (α7) on macrophages and other immune cells and suppresses endotoxin-induced release of TNF. Activation of the splenic nerve also arrests B-cell migration and inhibits antibody production.
Properties of choline acetyltransferase-expressing T cells
The population of ChAT+ T cells is a subset which has several interesting features. Of the β-adrenergic receptors, they predominantly express the β2. The effect of activation of β2 adrenergic receptors on T cells is complex, and it has been suggested that β2 activation promotes a Th2 response (70).
The anatomical distribution of ChAT+ T cells is only partly known. They constitute about 2–3% of CD4+ T cells in spleen, around 1% of CD4+ T cells in inguinal lymph nodes, but approximately 10% of CD4+ T cells in Peyer′s patches. The cause of this differential distribution is unknown. ChAT-expression in CD4+ T cells is stimulated by T-cell receptor activation by plate-bound anti-CD3 anti-bodies or other T-cell activators (68, 71) as is acetylcholine production (72) which suggests that antigen binding may promote ChAT+ expression. It is conceivable that specific antigens derived from the gut promote the high local frequency of ChAT+ T cells in Peyer′s patches, but this remains to be investigated.
When ChAT+ T cells are activated by plate-bound anti-CD3 antibodies, they secrete a combination of IL-10, IL-17 and IFNγ at similar levels, and IL-2 at a considerable lower level (68). IL-10 is associated with inhibition of inflammation and regulatory Foxp3+ T (Tr1) cells (2) and ChAT+ T cells attenuate macrophage cytokine production in spleen. Regulatory T cells are believed to have developed to provide for tolerance of symbiotic microorganisms in the intestine. Later, or simultaneously, IL-17-producing T cells emerged as a protection against invading extracellular pathogens especially at mucosal surfaces as those in the intestine (2, 73). Hence, the phenotype of ChAT+ T cells, which reside at a relatively high frequency in Peyer′s patches in comparison with other immune organs, can be of interest in this context. It is tempting to consider the hypothesis that nerve-controlled T cells have evolved to function in both permissive/regulatory and defensive / cytotoxic roles at mucosal surfaces like the intestine.
It has not been reported whether ChAT+ T cells express Foxp3 or exhibit other hallmarks of a T-regulatory phenotype. ChAT+ T cells do express markers of CD4+ memory cells, so they are likely primed. It remains unknown if their pattern of cytokine secretion reflects the presence of yet unknown subclasses of ChAT+ T cells, or if ChAT+ T cells encompass a multitude of T-helper cell variants.
Acetylcholine biosynthesis, release and metabolism in T cells
Most aspects of acetylcholine biosynthesis and release are known from studies performed in neurons. The reaction is catalyzed by ChAT which uses choline and acetyl-coenzyme A (acetyl-CoA) as substrates. Acetylcholine release depends on the cellular location of acetylcholine storage, and the nature of stimuli. In lymphocytes, the process of acetylcholine synthesis and release has not been fully characterized. Some aspects of acetylcholine biology that may be relevant in ChAT+ T cells are discussed here.
The cholinergic genomic locus is comprised of the ChAT and vesicular acetylcholine transporter (VAChT) genes. The ChAT gene contains 15 exons. The first three are named N, R, and M, and are not translated. VChAT is localized within the first intron of ChAT, and because of the requirement of both genes for acetylcholine synthesis and release in neurons, it was proposed that their expression is coregulated (74). Several different ChAT mRNA species have been found in the nervous system of rodents and humans. These mRNA species arise from activation of different promoter regions and alternative splicing. They contain the same coding region but differ in the non-coding 5′ region, and thus are named N, R, and M types (75, 76). In vitro translation of the human ChAT mRNA types N1, N2, and R obtained from brain and spinal cord results in a 69 kDa protein product, whereas translation of the M type yields 69 kDa, and 82 kDa products (77). The protein product of another splice variant of ChAT lacking exons 6–9 with an approximate molecular weight of 50 kDa is found preferentially in the peripheral nervous system, but not in brain of mice and rats. Whether this peripheral ChAT has catalytic activity is unknown (78). Soluble and membrane-bound types of ChAT have been isolated from mouse and rat brain, and show different activity in vitro (79, 80). Human leukemic T cell lines express the N1, N2, and M mRNA types, but not the R type (81). It is not known whether they express soluble or membrane-bound ChAT or both.
Synthesis and release
In neurons, acetylcholine is stored in synaptic vesicles, a process dependent on the vesicular acetylcholine transporter (VAChT). Acetylcholine in synaptic vesicles is released in quanta upon membrane depolarization and subsequent increase in intracellular calcium levels. Current data suggest that this release mode is associated with acetylcholine synthesis by membrane-bound ChAT whose activity is coupled to acetylcholine transport into synaptic vesicles (80). Acetylcholine can also be released in a non-quantal manner independent of membrane depolarization.
Experiments with ChAT-expressing fibroblasts that lack the components for acetylcholine storage and release indicate that acetylcholine can accumulate in the cytosol and be released continuously, independent of changes in intracellular calcium levels (82). Recently, it was shown that acetylcholine release in human leukemic T cells stimulated with a phytohemaglutinin (PHA) is dependent on mediatophore, a membrane protein which can act as a pore protein and translocate acetyl-choline to the outside of the cell (83). Since T cells upregulate ChAT and release acetylcholine upon stimulation with PHA (71, 72), it has been proposed that increased intracellular calcium as a result of T-cell activation could be responsible for acetylcholine release from cytosol through mediatophore (83). However, it is still not clear whether T cells express VAChT, and the possibility of acetylcholine storage and release from vesicles remains (81, 84).
We found acetylcholine in the supernatants of resting ChAT-expressing T cells, which suggests basal, non-quantal acetylcholine release. When ChAT-expressing T cells were incubated in the presence of norepinephrine, acetylcholine levels increased in supernatants (85). The mechanism of adrenergic receptor-induced acetylcholine release is not known. Neuronal ChAT activity is regulated by phosphorylation via PKC, Ca2+/calmodulin kinase II, and PKA (80, 86). It is possible that adrenergic signaling via PKA increases acetyl-choline synthesis in T cells through enhanced ChAT activity, which together with a non-quantal leakage of acetylcholine could result in increased acetylcholine extracellular levels.
Source of choline and acetyl-CoA
Another aspect of acetylcholine synthesis with potential significance, given the anti-inflammatory role of acetylcholine from T cells, is the metabolic source of acetylcholine’s precursors: choline and acetyl coenzyme A (acetyl-CoA). The rate-limiting step for acetylcholine biosynthesis in neurons is the uptake of extracellular choline by the high affinity choline transporter 1 (CHT1) (87). Non-selective low affinity organic cation transporters can also take up extracellular choline (88). CHT1 mRNA has been detected in a leukemic human T cell line (89), but it is not known if primary T cells express it. Choline can also be synthesized de novo via phospholipid metabolism (90). Experiments in which CHT1 was transfected into a murine fibroblast cell line engineered to express ChAT and capable of synthesizing acetylcholine suggest that both extracellular choline and choline derived from membrane phospholipids can be used as substrate for synthesis of acetylcholine in non-neuronal cells (91). The extent to which choline is taken up or synthesized de novo for acetycholine production by T cells is unknown.
Acetyl-CoA can originate from the oxidative metabolism of glucose and fatty acids through glycolysis and beta-oxidation, respectively. Acetyl-CoA in mitochondria enters the tricarboxylic acid (TCA) cycle, generating NADH and FADH2 that are transferred to the electron transport chain to yield ATP through oxidative phosphorylation. Resting T cells generate ATP through oxidative metabolism in mitochondria (92). Upon activation, metabolic pathway utilization shifts so that glucose does not enter the TCA cycle, and lactate is produced. This metabolic pattern, which occurs in the presence of oxygen, is analogous to the metabolic strategy of cancer cells and favors proliferation as it frees metabolites to satisfy demand for nucleotide, lipid, and protein synthesis (93). Once the effector phase is overcome, T cells return to mitochondrial oxidative metabolism, and, in CD8+ T cells, fatty acid oxidation promotes cell proliferation and survival (94, 95).
T cells stimulated with polyclonal activators upregulate ChAT expression, and synthesize and release acetylcholine (68, 71, 72), suggesting that the metabolic pattern of activated T cells provides acetyl-CoA for acetylcholine synthesis. On the other hand, ChAT-expressing T cells have markers of a memory phenotype, which might indicate that acetyl-CoA is obtained through oxidative metabolism in mitochondria (Fig. 3). The coordination between T-cell activation status and metabolic pathway utilization culminating in acetylcholine synthesis and release remains to be determined.
Fig. 3. Putative mechanism of acetylcholine synthesis and release in lymphocytes.
Acetylcholine (ACh) synthesis is catalyzed by choline acetyltransferase (ChAT) that utilizes acetyl-coenzyme A (Acetyl-CoA) and choline as substrates. Acetyl-CoA can derive from glucose and fatty acid oxidation through glycolysis and beta-oxidation, respectively. Choline can be synthesized de novo, obtained from membrane phospholipid, or taken up through choline transporter 1 (ChT1) after hydrolysis of acetylcholine by acetylcholinesterase (AChE). In T cells, cytosolic acetylcholine is released through mediatophore; however, release of vesicular acetylcholine has not been ruled out. Acetylcholine is released by T cells through unknown mechanisms upon polyclonal stimulation or incubation with norepinephrine. An intriguing question is whether acetylcholine is released in a quantal or non-quantal fashion. Blue arrows indicate unknown processes.
The meandering anatomy of the vagus nerve makes possible a multitude of anti-inflammatory reflex circuits
Since the vagus nerve meanders and extends branches to a multitude of tissues and organs, it is likely that some of these neural connections take part in other neuro-immune reflex circuits than the prototypical inflammatory reflex.
Berthoud and Neuhuber have reviewed the anatomy of the vagus nerve with particular focus on the afferent vagal neurons (96). The supradiaphragmatic vagus nerve innervates the skin of the external acoustic meatus (auricular branch) and the dura of the posterior cranial fossa (meningeal branch), larynx, pharynx, and upper esophagus (pharyngeal branches), aortic arch (cervical cardiac branches), trachea and esophagus (recurrent laryngeal), lungs, and heart. The subdiaphragmatic vagus nerve innervates the liver, pylorus, antrum, pancreas, intestine, and stomach (96). Efferent vagus branches also reach the uterus (97), adrenal gland (98), and gallbladder (99). A recent study reports efferent vagus innervation of abdominal adipose tissue (100). Vagus nerve fibers have also been shown to innervate the thymus (32) and to influence thymic lymphocyte release (101).
The vagus interfaces with the enteric nervous system and provides central coordination of the gut. Efferent vagal neurons terminate on enteric neurons throughout the esophagus and gastrointestinal tract (102) and make direct synaptic contacts on neurons of the myenteric ganglia (103, 104). The intestine from the duodenum to the proximal colon is innervated by the vagus nerve either directly or through enteric neural ganglia. Within the small intestine, the Peyer’s patches are well situated to connect immune sensory information from the gut to a vago-vagal inflammatory reflex: Filled with immune cells capable of microbial detection and cytokine production (105), these patches are surrounded by enteric ganglia and directly innervated by enteric and parasympathetic neurons, whose fibers traverse in close proximity to immune cells (106–112).
In contrast, cholinergic neurons have not been found in the spleen. However, the efferent vagus nerve terminates in the major prevertebral ganglia (44), which communicates with the spleen and kidneys through interfacing with postganglionic neurons in the suprarenal, celiac, and superior mesenteric ganglia. Intriguingly, a portion of the vagus motor nucleus has been reported to contain catecholaminergic neurons (44, 113).
Immune-regulatory signaling through the vagus nerve can circumvent the celiac ganglion and spleen
Data accumulated by our laboratory and others have shown a requirement of an intact spleen for the integrity of the efferent arc of the inflammatory reflex in systemic endotoxemia, as well as in other experimental disease models. However, the spleen may not be required for the vagus nerve to regulate inflammation in tissues that it directly innervates.
The intestines are richly innervated by cholinergic fibers of the enteric nervous system. The latter are well known to communicate bidirectionally with the vagus nerve to coordinate digestion and sensing of nutrients (114) and microflora (115). Boeckxstaens and colleagues recently described a vagovagal inflammatory reflex in the gut. They first demonstrated that VNS reduces inflammation in intestinal macrophages and reduces hypomobility in postoperative ileus (116). This effect is likely mediated by nicotinic receptors on cells in the gut, as the effect of VNS was blocked by incubation of the intestine in the nicotinic receptor antagonist hexamethonium. To find the specific vagus motor fibers that are activated during postoperative ileus, they identified intestine- and spleen-specific vagal neurons by injecting cholera toxin-B conjugated with Alexa Fluor 555 into the ilium and spleen of mice and subsequently detected the retrograde tracer in the dorsal motor nucleus of the vagus nerve. Intestine- and spleen-specific vagal neurons activated during postoperative ileus were identified by staining for c-fos in tracer-tagged neurons. This approach revealed that following surgically induced postoperative ileus, there was a widespread activation (42%) of intestine-specific motor neurons and a relatively limited activation (7%) of spleen-specific motor neurons in the dorsal motor nucleus of the vagus nerve, indicating that most of the tonic vagal suppression of inflammation is mediated by the efferent vagus directed not to the spleen, but to the inflamed intestinal tissue. Selective denervation of the intestinal vagus input abolished c-fos staining in both the nucleus of the solitary tract and the dorsal motor nucleus of the vagus, further supporting that afferent vagal input originating in inflamed tissue is required for the activation of the efferent vagal arm of the inflammatory reflex (22). Additional support for a direct effect by the vagus nerve on innervated intestines was recently provided from a study in our own laboratory: VNS, but not splenic nerve stimulation, reduced lesion area in a standard rat model of indomethacin-induced small intestinal ulceration. In contrast to the abundant findings on endotoxemia, splenectomy prior to VNS does not abrogate the anti-inflammatory effects of VNS in the intestine. These results indicate that this particular anti-inflammatory signal is communicated by vagal efferents directly to the intestine, and is independent of signaling to the spleen (Y. A. Levine, unpublished observations).
The inflammatory reflex reaches beyond innate immunity
Interestingly, the inflammatory reflex regulates not only innate, but also adaptive immune responses. For example, the response to live and heat killed Streptococcus pneumoniae is regulated by efferent neural signals: Daily treatment with nicotine or the selective α7nAChR agonist GTS-21 reduced antibody-production by approximately 50%. Pharmacological activation of the α7nAChR or electrical activation of the vagus nerve arrested the normal splenic marignal zone B cell migration along reticular fibers to the red pulp associated with this immune response and inhibited antibody production, but not if the splenic nerve was severed (117). Extrafollicular CD138+ plasma cells were predominantly found in close proximity to splenic nerves. The arrested cells expressed CD11b and their distribution was significantly altered if splenic nerve signaling was impaired, supporting earlier studies that show that cholinergic downregulation of leukocyte CD11b+ controls leukocyte migration (118).
The inflammatory reflex through the vagus nerve is a prototypical example of a neural immune regulatory reflex. Other immunoregulatory neural circuits have also been discovered. One example is the neural control of invariant natural killer T (iNKT) cells in the context of stroke. It is known that victims of stroke suffer a considerable risk of pneumonia after onset of the disease. Wong et al. recently found that, in mice, a profoundly altered function of hepatic iNKT cells after stroke is responsible for the increased risk of infection and associated mortality and that it can be rescued by treatment with the neurotransmitter norepinephrine (119). In vivo, this effect was mediated by adrenergic neurons, since depletion of neuronal norepinephrine by treatment with 6-hydroxydopamine abolished the effect of stroke on iNKT behavior. Therefore, iNKT function directly respond to neuronal efference in a way that had direct implications on rate of pneumonia development and mortality.
A recent report by Arima et al. demonstrated that, in experimental multiple sclerosis, access to the CNS through the blood brain barrier for autoreactive T cells by local induction of CCL20 in lumbar dorsal bloodvessels is regulated by neural signals (120, 121). Hence, it is increasingly clear that neural circuits regulate a wide variety of immune system-related processes in functionally and anatomically distinct locations. It is likely that a range of different afferent and efferent nerves participate in sensing and regulation of immune responses.
Intriguingly, not only CD3+ CD4+ CD44hi CD62Llow ChAT+ leukocytes are capable of biosynthesizing and releasing acetyl-choline. The extensive work of Kawashima and colleagues has demonstrated that B cells make acetylcholine and that dendritic cells express ChAT mRNA under certain in vitro conditions (72, 122, 123). The potential role of these subsets in the inflammatory reflex and in other neuro-immune interactions remains to be elucidated.
In summary, inflammation in the periphery activates afferent peripheral nerves that stimulate centers in the CNS and trigger a concerted response that generates adaptive behavioral as well as immunological changes. In view of the accumulated data in this field, it is likely that nervous sensing and reflex regulation of inflammation occurs in a variety of tissues and organs.
Targeting the inflammatory reflex in experimental disease: emerging applications
In addition to exerting an anti-inflammatory effect in experimental models of sepsis, pancreatitis, ischemia reperfusion, and arthritis (Table 1), current research suggests that vagal activation can potentially modulate other diseases with an underlying inflammatory component. Similarly, research on the anti-inflammatory effect of cholinergic agonists, in particular selective α7nAChR agonists, has expanded into new research areas, suggesting a broader therapeutic potential than previously considered. Below, we comment on novel research areas where the vagus nerve and α7nAChR have been implicated in pathogenesis or disease progression, or where activation of the efferent arc of the inflammatory reflex through VNS or selective α7nAChR agonists improves disease outcome.
The vasculature
Inflammation and endothelial dysfunction play an important role tissue damage induced by hypertension (124). A role for α7nAChR in this process has been inferred from increased end organ damage, and higher pro-inflammatory cytokine levels in serum of α7nAChR-deficient mice subjected to hypertension. Chronic administration of PNU-282987, a selective α7nAChR agonist, reduced tissue levels of TNF, IL-1β, and IL-6 and decreased end organ damage in spontaneously hypertensive rats, highlighting the therapeutic potential of α7nAChR agonists for reducing hypertension-associated morbidity (125).
Cholesterol-laden macrophages, so called foam cells, critically participate in atherogenesis. Macrophages obtained from α7nAChR knockout mice have higher cholesterol content, increased uptake of oxidized LDL and higher production of reactive oxygen species, hinting to a possible role of cholinergic signaling in macrophages in atherosclerosis (126).
The gut
A functional connection between the vagus nerve and immune responses in the gut has been established. VNS restores gut motility after postoperative ileus, an effect dependent on attenuation of cytokine production by macrophages residing in the intestinal muscularis (116). Similarly, central activation of the vagus nerve with semapimod reduces intestinal inflammation, and improves gut motility after surgery (127). Functional vagus-to-gut connections have also been proposed to explain the protective effect of postinjury VNS on gut endothelial permeability in burn and brain trauma models (55, 128). As discussed above, a gut-to-brain-to-gut anti-inflammatory reflex mediated by the vagus nerve has been suggested (22). In a similar fashion, indirect detection of intestinal lipid through activation of cholecystochinine-receptors on vagal afferents culminates in vagus nerve-mediated reduction of serum TNF and IL-6, and attenuation of gut permeability in a model of hemorrhagic shock (129, 130). An elegant in vivo visualization of increased NFκB activation in mice subjected to subdiaphragmatic vagotomy has provided further evidence of vagus-mediated regulation of inflammation in the gut. In these experiments, vagotomy increased NFκB activity in the colon and exacerbated NFκB activation and pro-inflammatory cytokine production in a mouse model of colitis (131). Interestingly, transfer of spleen T cells from vagotomized mice to mice subjected to colitis also exacerbated inflammation, suggesting tonic vagal control of T-cell function (68, 131, 132).
The discovery of a functional connection between the brain and the immune system raises the question of whether different states of mind can cause or modify disease progression. An intriguing example of central neural modulation of gut inflammation was recently shown in a mouse model of colitis. Depression-like behavior elicited by central administration of reserpine exacerbated colitis, an effect that was attenuated by antidepressants but not by antidepressants plus vagotomy. Interestingly, the pro-inflammatory effect of depression-like behavior was transferable by macrophages, suggesting a crucial link between mental states and the peripheral immune system mediated through the vagus nerve (133). Chronic administration of the lactic acid bacteria Lacto-bacillus rhamnosus, induced alterations in GABA receptor mRNA expression, decreased stress-induced corticosterone serum levels, and reduced depression-like behavior, effects that were dependent on an intact vagus nerve (115). Together, these findings open the exciting possibility of a bidirectional brain-to-gut communication mediated by the vagus nerve that can be investigated in the context of gut mucosal immunity, and the recently established link between metabolic disorders and gut microbiota.
Use of selective α7nAChR agonists
Identification of the efferent arc of the inflammatory reflex established the possibility of regulating disease progression by targeting cholinergic receptors, both muscarinic and nicotinic, in the brain and periphery. To date, several cholinergic agonists have been shown to reduce pro-inflammatory cytokine levels and ameliorate disease in several different experimental models including endotoxemia, cecal ligation and puncture, ischemia-reperfusion, pancreatitis, and rheumatoid arthritis (Table 1). More recently, selective α7nAChR agonists have been shown to reduce lung TNF and IL-6 levels, and improve lung function in experimental models of lung injury induced by mechanical ventilation (134, 135); to attenuate serum TNF and HMGB1 levels, and reduce infarct size in a rat model of myocardial ischemia-reperfusion (136).
Selective α7nAChR nicotinic agonists are desirable over the non-selective nicotinic agonists because of their fewer side-effects. One such agonist, GTS-21, was well tolerated in clinical trials for treatment of cognitive disorders (137, 138). GTS-21 was recently tested experimentally in humans with endotoxemia. Despite an inverse correlation between GTS-21 plasma concentration and TNF, IL-6, and IL-1RA, there was no significant effect between groups receiving GTS-21 or placebo. The lack of effect was attributed to highly variable concentrations among individuals, and low plasma GTS-21 concentrations despite administration of the highest dose previously reported to be safe in humans (139).
One aspect to consider for clinical development of α7nAChR-selective agonists is a partial duplication of CHRNA7, the gene that encodes α7nAChR. This partial duplication consists of a hybrid gene named CHRFAM7A that contains exons 5–10 of CHRNA7 and 4 exons of the evolutionary novel FAM7A gene (140). Translation of the CHRFAM7A transcript, named dupa7, yields a truncated protein that lacks the binding site of acetylcholine. CHRFAM7A polymorphisms have been associated with Alzheimer’s disease and schizophrenia, and other neurologic conditions (141, 142), and its transcript has been identified in the human CNS and immune cells (143, 144). The CHRFAM7A protein product has been recently identified as a dominant negative regulator of α7nAChR activity in neurons (145), but its role in immune cells has not been fully elucidated. The CHRFAM7A transcript is downregulated in macrophages upon stimulation with LPS, IL-1β or nicotine (146, 147), suggesting involvement in regulation of inflammation via modulation of cholinergic signaling through α7nAChR. It is tempting to speculate that CHRFAM7A polymorphisms might be associated with individual differences in responsiveness to cholinergic modulation by the vagus nerve and selective α7nAChR agonists.
Use of acetylcholinesterase inhibitors
Acetylcholine is enzymatically hydrolyzed into acetate and choline in synaptic clefts in the CNS and periphery. This step of acetylcholine metabolism catalyzed by acetylcholinesterase is critical for tight temporal and spatial control of nerve impulses. In keeping with the anti-inflammatory effect of acetylcholine, administration of acetylcholinesterase inhibitors, which enhance cholinergic signaling by increasing acetylcholine levels, has been shown to attenuate disease severity and decrease pro-inflammatory cytokine levels in various experimental models of inflammation. Galantamine, a centrally acting acetylcholinesterase inhibitor in clinical use for treatment of Alzheimer′s disease, significantly attenuated serum TNF levels, and reduced mortality in endotoxemia (42). These effects were mediated through brain muscarinic receptors and require intact vagus nerve signaling (42). Physostigmine reduced capillary leakage and interaction time between leukocytes and endothelium in endotoxemia (148), a finding consistent with inhibition of endothelial activation caused by α7nAChR agonists (149, 150). Moreover, physostigmine or neostigmine increased survival, reduced TNF, IL-1β, and IL-6, and reduced neutrophil lung infiltration in the cecal ligation and puncture sepsis model (151). The anti-inflammatory efficacy of peripherally-acting acetylcholinesterase inhibitors is somewhat unclear, because administration of neostigmine failed to exert protective effects against endotoxemia-induced organ injury (152) and to suppress the inflammatory response in experimental ventilator-induced lung injury (135).
Targeting the inflammatory reflex for clinical therapy
Clinical trials
The discovery of efferent vagus nerve effects on inflammation and the key role of α7nAChR have spawned several clinical trials utilizing nerve stimulators or selective cholinergic agonists. For instance, there is an ongoing phase II trial to demonstrate safety and efficacy of an implantable electric vagus nerve stimulator in subjects with rheumatoid arthritis. Other clinical trials investigate selective α7nAChR agonists in asthma and type II diabetes mellitus. In light of the multitude of experimental inflammatory and inflammation-associated diseases that are alleviated by interventions in the inflammatory reflex (Table 1), it is plausible that treatment of a variety of inflammatory diseases can be improved by therapeutic neural regulation of immune system activity.
Future therapeutic targets and unresolved questions
The realization that T cells are essential for the vagus nerve mediated regulation of cytokine production (68) not only provides a novel conceptual understanding of neuro-immune communication, i.e. lymphocytes assuming the function of interneurons, but raises a multitude of novel therapeutic possibilities involving modulation of acetylcholine production and release from lymphocytes. However, the current understanding of ChAT+ T cells and other ChAT+ lymphocytes is limited and several questions need to be addressed. ChAT+ T cells share markers with memory cells, which suggests that they are previously activated. It is not known how antigen and neural efference interact to regulate acetylcholine release by lymphocytes. Is acetylcholine production by ChAT+ T cells antigen-specific or antigen independent and can ChAT+ T cells support specific tolerance under neural control? The work on neural immuneregulation in C. elegans (8) suggests that neural control of immunity developed before the advent of circulating immune cells, and it is tempting to speculate that animals benefitted functionally from the overarching neural control of emerging adaptive immune cells along the intestine (2).
Little is known about acetylcholine turnover in the immune system and in ChAT+ T cells. Understanding of leukocyte acetylcholine metabolism, substrates, synthesis, release and catabolism, would facilitate pharmacological interventions and possibly reveal new therapeutic targets to promote efferent signaling in the inflammatory reflex for suppression of excessive inflammation and cytokine production in inflammatory disease. A detailed understanding of the neuro-immune interface would be useful for development of targeted therapy. There are partly conflicting reports on the anatomy of the efferent arc of the inflammatory reflex to the spleen (44, 113) and the central mechanisms responsible for reflex propagation from afferent inflammatory signals to the efferent arc of the inflammatory reflex remain to be unraveled. Investigation of circuits suggested by traditional medicine such as acupuncture can potentially provide rational scientific evidence for select alternative medicine approaches and further expand our understanding of neuro-immune interactions.
Another yet unexplored field is the interaction between circulating acetylcholine-producing lymphocytes and the vasculature. Measurable levels of circulating acetylcholine are present in many animals (153). Acetylcholine is a potent vasodilator that activates receptors on vascular endothelial cells and promotes production and release of nitric oxide (NO) (154). Regional relaxation of vessels increases blood flow and can contribute to tissue swelling and redness in inflammation. The source of circulating acetylcholine is to our knowledge not conclusively determined. In light of the efficient acetylcholine-catabolizing butyrylcholinesterase present in plasma, it is unlikely that synaptic release from relatively remote cholinergic nerve endings contributes substantially to the total amount of circulating acetylcholine. Since lymphocytes have the capacity to produce acetylcholine, they are a likely source of acetyl-choline in circulating blood (68, 122). Ongoing studies in our laboratory are addressing the effects of this lymphocyte-derived acetylcholine on the vascular bed and blood pressure.
Conclusions
The discoveries of neural control of inflammation and the key functional role of the lymphocyte cholinergic system in the inflammatory reflex provide novel and unique insights into regulation of immunological homeostasis and inflammation. Although much remains to be learned and explored in the field, this knowledge provides a foundation for development and implementation of novel nerve-stimulatory devices and new drugs for treatment of a range of inflammatory diseases in the near future.
Acknowledgements
We thank Valentin A Pavlov and Eugene Golanov for critically reading the manuscript. This work was supported in part by grants from National Institute of General Medical Sciences, NIH, to K.J.T. and from the Wenner-Gren Foundations in Stockholm to P.S.O. K.J.T. is consultant to and Y.A.L. is an employee of SetPoint Medical, Inc.
Footnotes
M.R.B and P.S.O. have no conflicts of interest to declare.
References
- 1.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 2.Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–1773. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8:776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serhan CN, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 7.Tracey KJ. Cell biology. Ancient neurons regulate immunity. Science. 2011;332:673–674. doi: 10.1126/science.1206353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun J, Singh V, Kajino-Sakamoto R, Aballay A. Neuronal GPCR controls innate immunity by regulating noncanonical unfolded protein response genes. Science. 2011;332:729–732. doi: 10.1126/science.1203411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watkins LR, Maier SF. Implications of immune-to-brain communication for sickness and pain. Proc Nat Acad Sci USA. 1999;96:7710–7713. doi: 10.1073/pnas.96.14.7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goehler LE, Gaykema RP, Hansen MK, Anderson K, Maier SF, Watkins LR. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton Neurosci. 2000;85:49–59. doi: 10.1016/S1566-0702(00)00219-8. [DOI] [PubMed] [Google Scholar]
- 11.Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Nat Acad Sci USA. 2005;102:5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boettger MK, et al. Antinociceptive effects of tumor necrosis factor alpha neutralization in a rat model of antigen-induced arthritis: evidence of a neuronal target. Arthritis Rheum. 2008;58:2368–2378. doi: 10.1002/art.23608. [DOI] [PubMed] [Google Scholar]
- 13.Mills KH. TLR-dependent T cell activation in autoimmunity. Nat Rev Immunol. 2011;11:807–822. doi: 10.1038/nri3095. [DOI] [PubMed] [Google Scholar]
- 14.Fogel-Petrovic M, Long JA, Knight DA, Thompson PJ, Upham JW. Activated human dendritic cells express inducible cyclo-oxygenase and synthesize prostaglandin E2 but not prostaglandin D2. Immunol Cell Biol. 2004;82:47–54. doi: 10.1111/j.1440-1711.2004.01213.x. [DOI] [PubMed] [Google Scholar]
- 15.Ek M, Kurosawa M, Lundeberg T, Ericsson A. Activation of vagal afferents after intravenous injection of interleukin-1beta: role of endogenous prostaglandins. J Neurosci. 1998;18:9471–9479. doi: 10.1523/JNEUROSCI.18-22-09471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosoi T, Okuma Y, Matsuda T, Nomura Y. Novel pathway for LPS-induced afferent vagus nerve activation: possible role of nodose ganglion. Autonomic neuroscience: basic & clinical. 2005;120:104–107. doi: 10.1016/j.autneu.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez R, et al. Lipopolysaccharide signaling in the carotid chemoreceptor pathway of rats with sepsis syndrome. Respir Physiol Neurobiol. 2011;175:336–348. doi: 10.1016/j.resp.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Gaykema RP, et al. Bacterial endotoxin induces fos immunoreactivity in primary afferent neurons of the vagus nerve. Neuro-ImmunoModulation. 1998;5:234–240. doi: 10.1159/000026343. [DOI] [PubMed] [Google Scholar]
- 19.Goehler LE, Gaykema RP, Hammack SE, Maier SF, Watkins LR. Interleukin-1 induces c-Fos immunoreactivity in primary afferent neurons of the vagus nerve. Brain Res. 1998;804:306–310. doi: 10.1016/s0006-8993(98)00685-4. [DOI] [PubMed] [Google Scholar]
- 20.Niijima A. The afferent discharges from sensors for interleukin 1 beta in the hepatoportal system in the anesthetized rat. J Auton Nerv Syst. 1996;61:287–291. doi: 10.1016/s0165-1838(96)00098-7. [DOI] [PubMed] [Google Scholar]
- 21.Goehler LE, et al. Blockade of cytokine induced conditioned taste aversion by sub-diaphragmatic vagotomy: further evidence for vagal mediation of immune-brain communication. Neurosci Lett. 1995;185:163–166. doi: 10.1016/0304-3940(95)11251-q. [DOI] [PubMed] [Google Scholar]
- 22.Cailotto C, et al. Neuroanatomical evidence demonstrating the existence of the vagal anti-inflammatory reflex in the intestine. Neurogastroenterol Motil. 2012;24:191–e93. doi: 10.1111/j.1365-2982.2011.01824.x. [DOI] [PubMed] [Google Scholar]
- 23.Marsland AL, Gianaros PJ, Prather AA, Jennings JR, Neumann SA, Manuck SB. Stimulated production of proinflammatory cytokines covaries inversely with heart rate variability. Psychosom Med. 2007;69:709–716. doi: 10.1097/PSY.0b013e3181576118. [DOI] [PubMed] [Google Scholar]
- 24.Fairchild KD, Srinivasan V, Moorman JR, Gaykema RP, Goehler LE. Pathogen-induced heart rate changes associated with cholinergic nervous system activation. Am J Physiol Regul Integr Comp Physiol. 2011;300:R330–R339. doi: 10.1152/ajpregu.00487.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt H, et al. Autonomic dysfunction predicts mortality in patients with multiple organ dysfunction syndrome of different age groups. Crit Care Med. 2005;33:1994–2002. doi: 10.1097/01.ccm.0000178181.91250.99. [DOI] [PubMed] [Google Scholar]
- 27.Bruchfeld A, et al. Whole blood cytokine attenuation by cholinergic agonists ex vivo and relationship to vagus nerve activity in rheumatoid arthritis. J Intern Med. 2010;268:94–101. doi: 10.1111/j.1365-2796.2010.02226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldstein RS, et al. Cholinergic anti-inflammatory pathway activity and High Mobility Group Box-1 (HMGB1) serum levels in patients with rheumatoid arthritis. Mol Med. 2007;13:210–215. doi: 10.2119/2006-00108.Goldstein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aydemir M, et al. Cardiac autonomic profile in rheumatoid arthritis and systemic lupus erythematosus. Lupus. 2010;19:255–261. doi: 10.1177/0961203309351540. [DOI] [PubMed] [Google Scholar]
- 30.Sharma P, Makharia GK, Ahuja V, Dwivedi SN, Deepak KK. Autonomic dysfunctions in patients with inflammatory bowel disease in clinical remission. Dig Dis Sci. 2009;54:853–861. doi: 10.1007/s10620-008-0424-6. [DOI] [PubMed] [Google Scholar]
- 31.Niijima A, Hori T, Aou S, Oomura Y. The effects of interleukin-1 beta on the activity of adrenal, splenic and renal sympathetic nerves in the rat. J Auton Nerv Syst. 1991;36:183–192. doi: 10.1016/0165-1838(91)90042-2. [DOI] [PubMed] [Google Scholar]
- 32.Niijima A. An electrophysiological study on the vagal innervation of the thymus in the rat. Brain Res Bull. 1995;38:319–323. doi: 10.1016/0361-9230(95)00103-l. [DOI] [PubMed] [Google Scholar]
- 33.Watkins LR, Maier SF, Goehler LE. Cytokine-to-brain communication: a review & analysis of alternative mechanisms. Life Sci. 1995;57:1011–1026. doi: 10.1016/0024-3205(95)02047-m. [DOI] [PubMed] [Google Scholar]
- 34.Watkins LR, et al. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci Lett. 1995;183:27–31. doi: 10.1016/0304-3940(94)11105-r. [DOI] [PubMed] [Google Scholar]
- 35.Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- 36.Kent S, Bluthe RM, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- 37.Borovikova LV, et al. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton Neurosci. 2000;85:141–147. doi: 10.1016/S1566-0702(00)00233-2. [DOI] [PubMed] [Google Scholar]
- 38.Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med. 2003;9:125–134. [PMC free article] [PubMed] [Google Scholar]
- 39.Oke SL, Tracey KJ. From CNI-1493 to the immunological homunculus: physiology of the inflammatory reflex. J Leukoc Biol. 2008;83:512–517. doi: 10.1189/jlb.0607363. [DOI] [PubMed] [Google Scholar]
- 40.Bernik TR, et al. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J Vasc Surg. 2002;36:1231–1236. doi: 10.1067/mva.2002.129643. [DOI] [PubMed] [Google Scholar]
- 41.Pavlov VA, et al. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc Nat Acad Sci USA. 2006;103:5219–5223. doi: 10.1073/pnas.0600506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pavlov VA, et al. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun. 2009;23:41–45. doi: 10.1016/j.bbi.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernik TR, et al. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med. 2002;195:781–788. doi: 10.1084/jem.20011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berthoud HR, Powley TL. Interaction between parasympathetic and sympathetic nerves in prevertebral ganglia: morphological evidence for vagal efferent innervation of ganglion cells in the rat. Microsc Res Tech. 1996;35:80–86. doi: 10.1002/(SICI)1097-0029(19960901)35:1<80::AID-JEMT7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 45.Berthoud HR, Powley TL. Characterization of vagal innervation to the rat celiac, suprarenal and mesenteric ganglia. J Auton Nerv Syst. 1993;42:153–169. doi: 10.1016/0165-1838(93)90046-w. [DOI] [PubMed] [Google Scholar]
- 46.Rosas-Ballina M, et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Nat Acad Sci USA. 2008;105:11008–11013. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huston JM, et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huston JM, et al. Splenectomy protects against sepsis lethality and reduces serum HMGB1 levels. J Immunol. 2008;181:3535–3539. doi: 10.4049/jimmunol.181.5.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vida G, Pena G, Deitch EA, Ulloa L. alpha7-cholinergicreceptormediatesvagalinductio-nofsplenicnorepinephrine. J Immunol. 2011;186:4340–4346. doi: 10.4049/jimmunol.1003722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smolen AJ. Morphology of synapses in the autonomic nervous system. J Electron Microsc Tech. 1988;10:187–204. doi: 10.1002/jemt.1060100205. [DOI] [PubMed] [Google Scholar]
- 51.Song XM, et al. Effect of vagus nerve stimulation on thermal injury in rats. Burns. 2010;36:75–81. doi: 10.1016/j.burns.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 52.dos Santos CC, Shan Y, Akram A, Slutsky AS, Haitsma JJ. Neuroimmune regulation of ventilator-induced lung injury. Am J Respir Crit Care Med. 2011;183:471–482. doi: 10.1164/rccm.201002-0314OC. [DOI] [PubMed] [Google Scholar]
- 53.Guarini S, et al. Efferent vagal fibre stimulation blunts nuclear factor-kappaB activation and protects against hypovolemic hemorrhagic shock. Circulation. 2003;107:1189–1194. doi: 10.1161/01.cir.0000050627.90734.ed. [DOI] [PubMed] [Google Scholar]
- 54.Costantini TW, et al. Efferent vagal nerve stimulation attenuates gut barrier injury after burn: modulation of intestinal occludin expression. J Trauma. 2010;68:1349–1354. doi: 10.1097/TA.0b013e3181dccea0. discussion 54-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krzyzaniak M, et al. Postinjury vagal nerve stimulation protects against intestinal epithelial barrier breakdown. J Trauma. 2011;70:1168–1175. doi: 10.1097/TA.0b013e318216f754. discussion 75-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borovikova LV, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 57.Felten SY, Olschowka J. Noradrenergic sympathetic innervation of the spleen: II. Tyro-sine hydroxylase (TH)-positive nerve terminals form synapticlike contacts on lymphocytes in the splenic white pulp. J Neurosci Res. 1987;18:37–48. doi: 10.1002/jnr.490180108. [DOI] [PubMed] [Google Scholar]
- 58.Kees MG, Pongratz G, Kees F, Scholmerich J, Straub RH. Via beta-adrenoceptors, stimulation of extrasplenic sympathetic nerve fibers inhibits lipopolysaccharide-induced TNF secretion in perfused rat spleen. J Neuroimmunol. 2003;145:77–85. doi: 10.1016/j.jneuroim.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 59.Vida G, et al. β2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system. FASEB J. 2011;25:4476–4485. doi: 10.1096/fj.11-191007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang H, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 61.Broide RS, Leslie FM. The alpha7 nicotinic acetylcholine receptor in neuronal plasticity. Mol Neurobiol. 1999;20:1–16. doi: 10.1007/BF02741361. [DOI] [PubMed] [Google Scholar]
- 62.Lips KS, et al. Coexpression and spatial association of nicotinic acetylcholine receptor subunits alpha7 and alpha10 in rat sympathetic neurons. Journal of molecular neuroscience: MN. 2006;30:15–16. doi: 10.1385/JMN:30:1:15. [DOI] [PubMed] [Google Scholar]
- 63.Parrish WR, et al. Modulation of TNF release by choline requires alpha7 sub-unit nicotinic acetylcholine receptor-mediated signaling. Mol Med. 2008;14:567–574. doi: 10.2119/2008-00079.Parrish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olofsson P, et al. α7 Nicotinic Acetylcholine Receptor (α7nAChR) expression in bone-marrow derived non T cells is required for the inflammatory reflex. Mol Med. 2011;18:539–543. doi: 10.2119/molmed.2011.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Todman D. Henry Dale and the discovery of chemical synaptic transmission. Eur Neurol. 2008;60:162–164. doi: 10.1159/000145336. [DOI] [PubMed] [Google Scholar]
- 66.Dale HH, Dudley HW. The presence of histamine and acetylcholine in the spleen of the ox and the horse. J Physiol. 1929;68:97–123. doi: 10.1113/jphysiol.1929.sp002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fujii T, Takada-Takatori Y, Kawashima K. Basic and clinical aspects of non-neuronal acetylcholine: expression of an independent, non-neuronal cholinergic system in lymphocytes and its clinical significance in immunotherapy. J Pharmacol Sci. 2008;106:186–192. doi: 10.1254/jphs.fm0070109. [DOI] [PubMed] [Google Scholar]
- 68.Rosas-Ballina M, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bellinger DL, Felten SY, Collier TJ, Felten DL. Noradrenergic sympathetic innervation of the spleen: IV. Morphometric analysis in adult and aged F344 rats. J Neurosci Res. 1987;18:55–63. doi: 10.1002/jnr.490180110. [DOI] [PubMed] [Google Scholar]
- 70.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve-an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- 71.Fujii T, et al. Induction of choline acetyl-transferase mRNA in human mononuclear leukocytes stimulated by phytohemagglutinin, a T-cell activator. J Neuroimmunol. 1998;82:101–107. doi: 10.1016/S0165-5728(97)00195-1. [DOI] [PubMed] [Google Scholar]
- 72.Rinner I, Kawashima K, Schauenstein K. Rat lymphocytes produce and secrete acetylcholine in dependence of differentiation and activation. J Neuroimmunol. 1998;81:31–37. doi: 10.1016/s0165-5728(97)00155-0. [DOI] [PubMed] [Google Scholar]
- 73.Cooper MD, Herrin BR. How did our complex immune system evolve? Nat Rev Immunol. 2010;10:2–3. doi: 10.1038/nri2686. [DOI] [PubMed] [Google Scholar]
- 74.Mallet J, et al. The cholinergic locus: ChAT and VAChT genes. J Physiol Paris. 1998;92:145–147. doi: 10.1016/S0928-4257(98)80153-8. [DOI] [PubMed] [Google Scholar]
- 75.Misawa H, Ishii K, Deguchi T. Gene expression of mouse choline acetyltransferase. Alternative splicing and identification of a highly active promoter region. J Biol Chem. 1992;267:20392–20399. [PubMed] [Google Scholar]
- 76.Kengaku M, Misawa H, Deguchi T. Multiple mRNA species of choline acetyltransferase from rat spinal cord. Brain Res Mol Brain Res. 1993;18:71–76. doi: 10.1016/0169-328x(93)90174-n. [DOI] [PubMed] [Google Scholar]
- 77.Misawa H, Matsuura J, Oda Y, Takahashi R, Deguchi T. Human choline acetyltransferase mRNAs with different 5’-region produce a 69-kDa major translation product. Brain Res Mol Brain Res. 1997;44:323–333. doi: 10.1016/s0169-328x(96)00231-8. [DOI] [PubMed] [Google Scholar]
- 78.Tooyama I, Kimura H. A protein encoded by an alternative splice variant of choline acetyltransferase mRNA is localized preferentially in peripheral nerve cells and fibers. J Chem Neuroanat. 2000;17:217–226. doi: 10.1016/s0891-0618(99)00043-5. [DOI] [PubMed] [Google Scholar]
- 79.Benishin CG, Carroll PT. Multiple forms of choline-O-acetyltransferase in mouse and rat brain: solubilization and characterization. J Neurochem. 1983;41:1030–1039. doi: 10.1111/j.1471-4159.1983.tb09047.x. [DOI] [PubMed] [Google Scholar]
- 80.Sha D, Jin H, Kopke RD, Wu JY. Choline acetyltransferase: regulation and coupling with protein kinase and vesicular acetylcholine transporter on synaptic vesicles. Neurochem Res. 2004;29:199–207. doi: 10.1023/b:nere.0000010449.05927.f9. [DOI] [PubMed] [Google Scholar]
- 81.Ogawa H, Fujii T, Watanabe Y, Kawashima K. Expression of multiple mRNA species for choline acetyltransferase in human T-lymphocytes. Life Sci. 2003;72:2127–2130. doi: 10.1016/s0024-3205(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 82.Misawa H, Takahashi R, Deguchi T. Calcium-independent release of acetylcholine from stable cell lines expressing mouse choline acetyltransferase cDNA. J Neurochem. 1994;62:465–470. doi: 10.1046/j.1471-4159.1994.62020465.x. [DOI] [PubMed] [Google Scholar]
- 83.Fujii T, Takada-Takatori Y, Horiguchi K, Kawashima K. Mediatophore regulates acetylcholine release from T cells. J Neuroimmunol. 2012;244:16–22. doi: 10.1016/j.jneuroim.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 84.Tayebati SK, El-Assouad D, Ricci A, Amenta F. Immunochemical and immunocytochemical characterization of cholinergic markers in human peripheral blood lymphocytes. J Neuroimmunol. 2002;132:147–155. doi: 10.1016/s0165-5728(02)00325-9. [DOI] [PubMed] [Google Scholar]
- 85.Woodman I. Neuroimmunology: Nervous ChAT. Nat Rev Immunol. 2011;11:720. doi: 10.1038/nri3091. [DOI] [PubMed] [Google Scholar]
- 86.Dobransky T, Davis WL, Xiao GH, Rylett RJ. Expression, purification and characterization of recombinant human choline acetyltransferase: phosphorylation of the enzyme regulates catalytic activity. Biochem J. 2000;349:141–151. doi: 10.1042/0264-6021:3490141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Okuda T, Haga T. High-affinity choline transporter. Neurochem Res. 2003;28:483–488. doi: 10.1023/a:1022809003997. [DOI] [PubMed] [Google Scholar]
- 88.Michel V, Yuan Z, Ramsubir S, Bakovic M. Choline transport for phospholipid synthesis. Exp Biol Med (Maywood) 2006;231:490–504. doi: 10.1177/153537020623100503. [DOI] [PubMed] [Google Scholar]
- 89.Fujii T, Okuda T, Haga T, Kawashima K. Detection of the high-affinity choline transporter in the MOLT-3 human leukemic T-cell line. Life Sci. 2003;72:2131–2134. doi: 10.1016/s0024-3205(03)00073-0. [DOI] [PubMed] [Google Scholar]
- 90.Li Z, Vance DE. Phosphatidylcholine and choline homeostasis. J Lipid Res. 2008;49:1187–1194. doi: 10.1194/jlr.R700019-JLR200. [DOI] [PubMed] [Google Scholar]
- 91.Fujii T, et al. Acetylcholine synthesis and release in NIH3T3 cells coexpressing the high-affinity choline transporter and choline acetyltransferase. J Neurosci Res. 2009;87:3024–3032. doi: 10.1002/jnr.22117. [DOI] [PubMed] [Google Scholar]
- 92.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 93.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science (New York, NY) 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van der Windt GJ, et al. Mitochondrial respiratory capacity is a critical regulator of CD8 + T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pearce EL, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci: Basic Clin. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 97.Ortega-Villalobos M, Garcia-Bazan M, Solano-Flores LP, Ninomiya-Alarcon JG, Guevara-Guzman R, Wayner MJ. Vagus nerve afferent and efferent innervation of the rat uterus: an electrophysiological and HRP study. Brain Res Bull. 1990;25:365–371. doi: 10.1016/0361-9230(90)90221-k. [DOI] [PubMed] [Google Scholar]
- 98.Coupland RE, Parker TL, Kesse WK, Mohamed AA. The innervation of the adrenal gland III. Vagal innervation. J Anat. 1989;163:173–181. [PMC free article] [PubMed] [Google Scholar]
- 99.Mawe GM. Nerves and Hormones Interact to Control Gallbladder Function. News Physiol Sci. 1998;13:84–90. doi: 10.1152/physiologyonline.1998.13.2.84. [DOI] [PubMed] [Google Scholar]
- 100.Kreier F, et al. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat-functional implications. J Clin Investig. 2002;110:1243–1250. doi: 10.1172/JCI15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Antonica A, Magni F, Mearini L, Paolocci N. Vagal control of lymphocyte release from rat thymus. J Auton Nerv Syst. 1994;48:187–197. doi: 10.1016/0165-1838(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 102.Berthoud HR, Patterson LM, Zheng H. Vagal-enteric interface: vagal activation-induced expression of c-Fos and p-CREB in neurons of the upper gastrointestinal tract and pancreas. Anat Rec. 2001;262:29–40. doi: 10.1002/1097-0185(20010101)262:1<29::AID-AR1008>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 103.Hayakawa T, Kuwahara S, Maeda S, Tanaka K, Seki M. Direct synaptic contacts on the myenteric ganglia of the rat stomach from the dorsal motor nucleus of the vagus. J Comp Neurol. 2006;498:352–362. doi: 10.1002/cne.21069. [DOI] [PubMed] [Google Scholar]
- 104.Walter GC, Phillips RJ, Baronowsky EA, Powley TL. Versatile, high-resolution anterograde labeling of vagal efferent projections with dextran amines. J Neurosci Methods. 2009;178:1–9. doi: 10.1016/j.jneumeth.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jung C, Hugot JP, Barreau F. Peyer’s Patches: The Immune Sensors of the Intestine. Int J Inflam. 2010;2010:823710. doi: 10.4061/2010/823710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Krammer HJ, Kuhnel W. Topography of the enteric nervous system in Peyer’s patches of the porcine small intestine. Cell Tissue Res. 1993;272:267–272. doi: 10.1007/BF00302732. [DOI] [PubMed] [Google Scholar]
- 107.Balemba OB, et al. The organisation of the enteric nervous system in the submucous and mucous layers of the small intestine of the pig studied by VIP and neurofilament protein immunohistochemistry. J Anat. 1998;192:257–267. doi: 10.1046/j.1469-7580.1998.19220257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Balemba OB, et al. The topography, architecture and structure of the enteric nervous system in the jejunum and ileum of cattle. J Anat. 1999;195:1–9. doi: 10.1046/j.1469-7580.1999.19510001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kulkarni-Narla A, Beitz AJ, Brown DR. Catecholaminergic, cholinergic and peptidergic innervation of gut-associated lymphoid tissue in porcine jejunum and ileum. Cell Tissue Res. 1999;298:275–286. doi: 10.1007/s004419900096. [DOI] [PubMed] [Google Scholar]
- 110.Defaweux V, et al. Interfaces between dendritic cells, other immune cells, and nerve fibres in mouse Peyer’s patches: potential sites for neuroinvasion in prion diseases. Microsc Res Tech. 2005;66:1–9. doi: 10.1002/jemt.20135. [DOI] [PubMed] [Google Scholar]
- 111.Vulchanova L, Casey MA, Crabb GW, Kennedy WR, Brown DR. Anatomical evidence for enteric neuroimmune interactions in Peyer’s patches. J Neuroimmunol. 2007;185:64–74. doi: 10.1016/j.jneuroim.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chiocchetti R, et al. Anatomical evidence for ileal Peyer’s patches innervation by enteric nervous system: a potential route for prion neuroinvasion? Cell Tissue Res. 2008;332:185–194. doi: 10.1007/s00441-008-0583-y. [DOI] [PubMed] [Google Scholar]
- 113.Tayo EK, Williams RG. Catecholaminergic parasympathetic efferents within the dorsal motor nucleus of the vagus in the rat: a quantitative analysis. Neurosci Lett. 1988;90:1–5. doi: 10.1016/0304-3940(88)90776-8. [DOI] [PubMed] [Google Scholar]
- 114.Nefti W, Chaumontet C, Fromentin G, Tome D, Darcel N. A high-fat diet attenuates the central response to within-meal satiation signals and modifies the receptor expression of vagal afferents in mice. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1681–R1686. doi: 10.1152/ajpregu.90733.2008. [DOI] [PubMed] [Google Scholar]
- 115.Bravo JA, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Nat Acad Sci USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.de Jonge WJ, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 117.Mina-Osorio P, Rosas-Ballina M, Valdes-Ferrer SI, Al-Abed Y, Tracey KJ, Diamond B. Neural signaling in the spleen controls B cell responses to blood-borne antigen. Mol Med. 2012 doi: 10.2119/molmed.2012.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huston JM, et al. Cholinergic neural signals to the spleen down-regulate leukocyte trafficking via CD11b. J Immunol. 2009;183:552–559. doi: 10.4049/jimmunol.0802684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wong CH, Jenne CN, Lee WY, Leger C, Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science. 2011;334:101–105. doi: 10.1126/science.1210301. [DOI] [PubMed] [Google Scholar]
- 120.Arima Y, et al. Regional neural activation defines a gateway for autoreactive T cells to cross the blood-brain barrier. Cell. 2012;148:447–457. doi: 10.1016/j.cell.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 121.Tracey KJ. Immune cells exploit a neural circuit to enter the CNS. Cell. 2012;148:392–394. doi: 10.1016/j.cell.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kawashima K, Fujii T. Expression of non-neuronal acetylcholine in lymphocytes and its contribution to the regulation of immune function. Front Biosci. 2004;9:2063–2085. doi: 10.2741/1390. [DOI] [PubMed] [Google Scholar]
- 123.Kawashima K, Yoshikawa K, Fujii YX, Moriwaki Y, Misawa H. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci. 2007;80:2314–2319. doi: 10.1016/j.lfs.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 124.Bautista LE. Inflammation, endothelial dysfunction, and the risk of high blood pressure: epidemiologic and biological evidence. J Hum Hypertens. 2003;17:223–230. doi: 10.1038/sj.jhh.1001537. [DOI] [PubMed] [Google Scholar]
- 125.Li DJ, et al. Dysfunction of the cholinergic anti-inflammatory pathway mediates organ damage in hypertension. Hypertension. 2011;57:298–307. doi: 10.1161/HYPERTENSIONAHA.110.160077. [DOI] [PubMed] [Google Scholar]
- 126.Wilund KR, et al. Macrophages from alpha 7 nicotinic acetylcholine receptor knockout mice demonstrate increased cholesterol accumulation and decreased cellular paraoxonase expression: A possible link between the nervous system and atherosclerosis development. Biochem Biophys Res Commun. 2009;390:148–154. doi: 10.1016/j.bbrc.2009.09.088. [DOI] [PubMed] [Google Scholar]
- 127.The F, et al. Central activation of the cholinergic anti-inflammatory pathway reduces surgical inflammation in experimental postoperative ileus. Br J Pharmacol. 2011;163:1007–1016. doi: 10.1111/j.1476-5381.2011.01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bansal V, et al. Stimulating the central nervous system to prevent intestinal dysfunction after traumatic brain injury. J Trauma. 2010;68:1059–1064. doi: 10.1097/TA.0b013e3181d87373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Luyer MD, Greve JW, Hadfoune M, Jacobs JA, Dejong CH, Buurman WA. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J Exp Med. 2005;202:1023–1029. doi: 10.1084/jem.20042397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lubbers T, et al. Cholecystokinin /Cholecys-tokinin-1 receptor-mediated peripheral activation of the afferent vagus by enteral nutrients attenuates inflammation in rats. Ann Surg. 2010;252:376–382. doi: 10.1097/SLA.0b013e3181dae411. [DOI] [PubMed] [Google Scholar]
- 131.O’Mahony C, van der Kleij H, Bienenstock J, Shanahan F, O’Mahony L. Loss of vagal anti-inflammatory effect: in vivo visualization and adoptive transfer. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1118–R1126. doi: 10.1152/ajpregu.90904.2008. [DOI] [PubMed] [Google Scholar]
- 132.Karimi K, Bienenstock J, Wang L, Forsythe P. The vagus nerve modulates CD4+T cell activity. Brain Behav Immun. 2010;24:316–323. doi: 10.1016/j.bbi.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 133.Ghia JE, Park AJ, Blennerhassett P, Khan WI, Collins SM. Adoptive transfer of macrophage from mice with depressionlike behavior enhances susceptibility to colitis. Inflamm Bowel Dis. 2011;17:1474–1489. doi: 10.1002/ibd.21531. [DOI] [PubMed] [Google Scholar]
- 134.Bregeon F, et al. Activation of nicotinic cholinergic receptors prevents ventilator-induced lung injury in rats. PLoS ONE. 2011;6:e22386. doi: 10.1371/journal.pone.0022386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kox M, et al. alpha7 nicotinic acetylcholine receptor agonist GTS-21 attenuates ventilator-induced tumour necrosis factor-alpha production and lung injury. Br J Anaesth. 2011;107:559–566. doi: 10.1093/bja/aer202. [DOI] [PubMed] [Google Scholar]
- 136.Xiong J, et al. Postconditioning with alpha7nAChR agonist attenuates systemic inflammatory response to myocardial ischemia-reperfusion injury in rats. Inflammation. 2012 doi: 10.1007/s10753-012-9449-2. [DOI] [PubMed] [Google Scholar]
- 137.Kitagawa H, et al. Safety, pharmacokinetics, and effects on cognitive function of multiple doses of GTS-21 in healthy, male volunteers. Neuropsychopharmacology. 2003;28:542–551. doi: 10.1038/sj.npp.1300028. [DOI] [PubMed] [Google Scholar]
- 138.Freedman R, et al. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008;165:1040–1047. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kox M, Pompe JC, Gordinou de Gouberville MC, van der Hoeven JG, Hoedemaekers CW, Pickkers P. Effects of the alphaal-pha7nAChR agonist GTS-21 on the innate immune response in humans. Shock. 2011;36:5–11. doi: 10.1097/SHK.0b013e3182168d56. [DOI] [PubMed] [Google Scholar]
- 140.Gault J, et al. Genomic organization and partial duplication of the human alpha7 neuronal nicotinic acetylcholine receptor gene (CHRNA7) Genomics. 1998;52:173–185. doi: 10.1006/geno.1998.5363. [DOI] [PubMed] [Google Scholar]
- 141.Feher A, Juhasz A, Rimanoczy A, Csibri E, Kalman J, Janka Z. Association between a genetic variant of the alpha-7 nicotinic acetylcholine receptor subunit and four types of dementia. Dement Geriatr Cogn Disord. 2009;28:56–62. doi: 10.1159/000230036. [DOI] [PubMed] [Google Scholar]
- 142.Flomen RH, et al. Association study of CHR-FAM7A copy number and 2 bp deletion polymorphisms with schizophrenia and bipolar affective disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:571–575. doi: 10.1002/ajmg.b.30306. [DOI] [PubMed] [Google Scholar]
- 143.Villiger Y, et al. Expression of an alpha7 duplicate nicotinic acetylcholine receptor-related protein in human leukocytes. J Neuroimmunol. 2002;126:86–98. doi: 10.1016/s0165-5728(02)00057-7. [DOI] [PubMed] [Google Scholar]
- 144.Severance EG, Yolken RH. Novel alpha7 nicotinic receptor isoforms and deficient cholinergic transcription in schizophrenia. Genes Brain Behav. 2008;7:37–45. doi: 10.1111/j.1601-183X.2007.00323.x. [DOI] [PubMed] [Google Scholar]
- 145.Araud T, et al. The chimeric gene CHR-FAM7A, a partial duplication of the CHRNA7 gene, is a dominant negative regulator of alpha7*nAChR function. Biochem Pharmacol. 2011;82:904–914. doi: 10.1016/j.bcp.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.de Lucas-Cerrillo AM, et al. Function of partially duplicated human alpha77 nicotinic receptor subunit CHRFAM7A gene: potential implications for the cholinergic anti-inflammatory response. J Biol Chem. 2011;286:594–606. doi: 10.1074/jbc.M110.180067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Benfante R, et al. Expression of the alpha7 nAChR subunit duplicate form (CHR-FAM7A) is down-regulated in the monocytic cell line THP-1 on treatment with LPS. J Neuroimmunol. 2011;230:74–84. doi: 10.1016/j.jneuroim.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 148.Peter C, et al. Effects of physostigmine on microcirculatory alterations during experimental endotoxemia. Shock (Augusta, GA) 2010;33:405–411. doi: 10.1097/SHK.0b013e3181b77e82. [DOI] [PubMed] [Google Scholar]
- 149.Saeed RW, et al. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005;201:1113–1123. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chatterjee PK, Al-Abed Y, Sherry B, Metz CN. Cholinergic agonists regulate JAK2 / -STAT3 signaling to suppress endothelial cell activation. Am J Physiol Cell Physiol. 2009;297:C1294–C1306. doi: 10.1152/ajpcell.00160.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hofer S, et al. Pharmacologic cholinesterase inhibition improves survival in experimental sepsis. Crit Care Med. 2008;36:404–408. doi: 10.1097/01.CCM.0B013E31816208B3. [DOI] [PubMed] [Google Scholar]
- 152.Akinci SB, et al. Effect of neostigmine on organ injury in murine endotoxemia: missing facts about the cholinergic antiinflammatory pathway. World J Surg. 2005;29:1483–1489. doi: 10.1007/s00268-005-0073-2. [DOI] [PubMed] [Google Scholar]
- 153.Fujii T, Kawashima K. An independent non-neuronal cholinergic system in lymphocytes. Jpn J Pharmacol. 2001;85:11–15. doi: 10.1254/jjp.85.11. [DOI] [PubMed] [Google Scholar]
- 154.Furchgott RF. The role of endothelium in the responses of vascular smooth muscle to drugs. Annu Rev Pharmacol Toxicol. 1984;24:175–197. doi: 10.1146/annurev.pa.24.040184.001135. [DOI] [PubMed] [Google Scholar]
- 155.Pavlov VA, et al. Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med. 2007;35:1139–1144. doi: 10.1097/01.CCM.0000259381.56526.96. [DOI] [PubMed] [Google Scholar]
- 156.Huston JM, et al. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit Care Med. 2007;35:2762–2768. doi: 10.1097/01.CCM.0000288102.15975.BA. [DOI] [PubMed] [Google Scholar]
- 157.Song XM, et al. The protective effect of the cholinergic anti-inflammatory pathway against septic shock in rats. Shock (Augusta, GA) 2008;30:468–472. doi: 10.1097/SHK.0b013e31816d5e49. [DOI] [PubMed] [Google Scholar]
- 158.Boland C, Collet V, Laterre E, Lecuivre C, Wittebole X, Laterre PF. Electrical vagus nerve stimulation and nicotine effects in peritonitis-induced acute lung injury in rats. Inflammation. 2011;34:29–35. doi: 10.1007/s10753-010-9204-5. [DOI] [PubMed] [Google Scholar]
- 159.Meregnani J, et al. Anti-inflammatory effect of vagus nerve stimulation in a rat model of inflammatory bowel disease. Auton Neurosci: Basic Clin. 2011;160:82–89. doi: 10.1016/j.autneu.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 160.van Westerloo DJ, et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130:1822–1830. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 161.Lee ST, et al. Cholinergic anti-inflammatory pathway in intracerebral hemorrhage. Brain Res. 2010;1309:164–171. doi: 10.1016/j.brainres.2009.10.076. [DOI] [PubMed] [Google Scholar]
- 162.Mioni C, et al. Activation of an efferent cholinergic pathway produces strong protection against myocardial ischemia /reperfusion injury in rats. Crit Care Med. 2005;33:2621–2628. doi: 10.1097/01.ccm.0000186762.05301.13. [DOI] [PubMed] [Google Scholar]
- 163.Yeboah MM, et al. Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney Int. 2008;74:62–69. doi: 10.1038/ki.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Altavilla D, et al. Activation of the cholinergic anti-inflammatory pathway reduces NF-kappab activation, blunts TNF-alpha production, and protects againts splanchic artery occlusion shock. Shock. 2006;25:500–506. doi: 10.1097/01.shk.0000209539.91553.82. [DOI] [PubMed] [Google Scholar]
- 165.van Maanen MA, et al. Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum. 2009;60:114–122. doi: 10.1002/art.24177. [DOI] [PubMed] [Google Scholar]
- 166.Czura CJ, et al. Vagus nerve stimulation regulates hemostasis in Swine. Shock. 2010;33:608–613. doi: 10.1097/SHK.0b013e3181cc0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.The FO, et al. Activation of the cholinergic anti-inflammatory pathway ameliorates postoperative ileus in mice. Gastroenterology. 2007;133:1219–1228. doi: 10.1053/j.gastro.2007.07.022. [DOI] [PubMed] [Google Scholar]