Abstract
Chronic progressive renal fibrosis leads to end-stage renal failure many patients with chronic kidney disease (CKD). Loss of the rich peritubular capillary network is a prominent feature, and seems independent of the specific underlying disease. The mechanisms that contribute to peritubular capillary regression include the loss of glomerular perfusion, as flow-dependent shear forces are required to provide the survival signal for endothelial cells. Also, reduced endothelial cell survival signals from sclerotic glomeruli and atrophic or injured tubule epithelial cells contribute to peritubular capillary regression. In response to direct tubular epithelial cell injury, and the inflammatory reaction that ensues, capillary pericytes dissociate from their blood vessels, also reducing endothelial cell survival. In addition, direct inflammatory injury of capillary endothelial cells, for instance in chronic allograft nephropathy, also contributes to capillary dropout. Chronic tissue hypoxia, which ensues from the rarefaction of the peritubular capillary network, can generate both an angiogenic and a fibrogenic response. However, in CKD, the balance is strongly tipped toward fibrogenesis. Understanding the underlying mechanisms for failed angiogenesis in CKD and harnessing endothelial-specific survival and pro-angiogenic mechanisms for therapy should be our goal if we are to reduce the disease burden from CKD.
Keywords: angiogenesis, capillary dropout, fibrosis, hypoxia, rarefaction, shear stress
In adult humans, renal glomerular capillaries produce a nearly protein-free ultrafiltrate of plasma at the prodigious rate of 120–180 l per day. The postglomerular microvasculature of the kidney is similarly specialized, providing for the return of massive amounts of reabsorbed glomerular filtrate back into the vasculature. Peritubular capillaries also deliver sufficient oxygen to support the ion transport work of renal tubular epithelial cells. Failure of the glomerular capillary bed, whether mediated by inflammation as in glomerulonephritis,1 disordered remodeling in diabetic nephropathy,2 or underlying genetic faults that produce glomerular malfunction,3 not only alters permselective filtration, but also leads to regression and rarefaction of the postglomerular peritubular microvasculature. Loss of perfusion, toxicity of filtered proteins, and inflammation all contribute to tubular and peritubular microvascular atrophy.2 In the setting of transplantation, ischemic acute kidney injury in combination with direct inflammatory damage to the peritubular capillary endothelium also lead to capillary dropout.4 This rarefaction of the peritubular capillary network, in turn, results in tissue hypoxia and is a central component of chronic progressive renal fibrosis. It has been suggested that an inadequate angiogenic response to hypoxia may underlie microvascular rarefaction in chronic kidney disease (CKD), but the mechanisms that lead to the apparent imbalance between angiogenesis and fibrosis in CKD are as yet poorly understood. Although the contributions of tubular epithelial cell damage, activation of myofibroblast proliferation, and the role of pericytes in renal fibrosis have been studied in some detail, microvascular repair and angiogenesis mechanisms in CKD need urgent attention.
PERITUBULAR CAPILLARY REGRESSION
In developing embryos and during wound healing, immature capillaries are regularly removed by spatially and temporally controlled mechanisms that have evolved to match blood supply to tissue oxygen demand. Similarly, in adult tissues, balanced angiogenesis and capillary regression (Figure 1) maintain the appropriate relationship between capillary density and oxygen requirements.5, 6 A major stimulus for capillary regression is the absence of blood flow. Blood flow produces mechanical shear forces at the apical surface of endothelial cells, facing the vessel lumen, that elicit powerful survival signals. Laminar shear activates growth factor–independent phosphorylation of the pro-survival signaling kinase Akt and is one of the strongest inhibitors of endothelial cell apoptosis. Shear stimulates pro-angiogenic nitric oxide7 as well as vascular endothelial growth factor (VEGF) and VEGFR2 expression in capillary endothelial cells, and activates NADPH oxidase-dependent physiological signals that are disturbed by cessation of flow.8 When shear forces cease, as in peritubular capillaries when glomerular perfusion fails,9 execution of endothelial cell apoptosis programs, in part through activation of the Fas-FasL signaling pathway, loss of appropriate signals from the extracellular matrix, and supporting pericytes as well as macrophage-mediated processes remove the unperfused capillaries. The mechanisms that lead to capillary regression have been studied in detail in the wound-healing response.10, 11 However, in the kidney, peritubular capillary regression, when flow in the postglomerular capillary system fails, has received little attention.
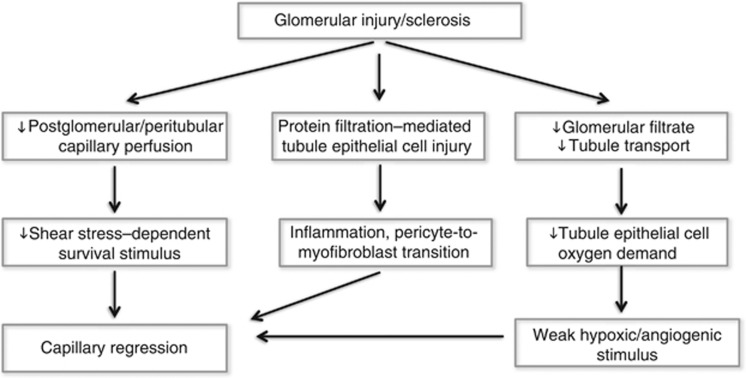
Figure 1.
Mechanisms that contribute to the regression of the postglomerular capillary network when the glomerular circulation fails.
Direct injury of peritubular capillaries by inflammatory mechanisms also leads to capillary rarefaction in humans, particularly in the setting of transplantation.12 The peritubular capillary endothelium is the main target for chronic antibody-mediated rejection, resulting in microvascular rarefaction and chronic progressive fibrosis.4 Antibody–antigen-mediated endothelial injury in this setting is complement dependent, and results in activation of endothelial cells, which become pro-thrombotic, facilitate platelet and leucocyte adhesion, and undergo complex intracellular mechanisms that can lead to apoptosis.13
Peritubular capillary regression and interstitial fibrosis are also observed in response to direct tubular epithelial cell injury. For instance, chronic ureteral obstruction results in tubular atrophy, tubulointerstitial fibrosis, and peritubular capillary rarefaction14, although glomerular perfusion is reduced only late in the disease. In infants with the congenital nephrotic syndrome of the Finnish type, the primary lesion in podocytes leads to massive proteinuria and is associated with renal tubular toxicity, progressive peritubular capillary rarefaction, and interstitial fibrosis.3 Proximal tubule epithelial cells in vitro produce inflammatory chemokines when exposed to plasma proteins, and excess protein in the glomerular filtrate is associated with the production of inflammatory mediators including monocyte chemoattractant protein-1 by proximal tubule epithelial cells.2, 15 Finally, direct, specific sub-lethal injury of proximal tubule epithelial cells with genetically targeted diphtheria toxin is sufficient to elicit a strong peritubular inflammatory response with secondary interstitial fibrosis, peritubular capillary dropout, and glomerulosclerosis.16 As expression of inflammatory mediators by tubule epithelial cells in this model precedes the interstitial inflammatory and fibrosis responses, it is evident that primary tubular epithelial cell injury can lead to capillary regression and tubulointerstitial fibrosis.2 However, it also needs to be recognized that inflammatory responses that involve monocyte chemoattractant protein-1-mediated monocyte–macrophage recruitment are generally associated with an increase in tissue perfusion and collateral blood vessel formation.5 It is not clear why inflammatory cells in the renal interstitium do not evoke a similar angiogenic response.
The response to continuous or repeated proximal tubule injury includes a substantial expansion of the number of interstitial myofibroblasts, which in turn lay down the peritubular fibrotic matrix. Some of these cells arise from the tubular epithelium itself through the process of epithelial to mesenchymal transition; some are marrow derived,17 and even endothelial to mesenchymal transition has been reported.18 However, there now is strong evidence that myofibroblast accumulation in the renal interstitium during renal fibrosis largely represents the mobilization of vascular pericytes.19 These cells normally surround peritubular microvessels, but in response to tubular injury, they detach and migrate into the interstitium where they dedifferentiate and proliferate. It has been postulated that the loss of pericytes in association with proteolytic remodeling of capillary basement membrane may destabilize peritubular capillaries and contribute to microvascular regression.20
CAPILLARY RAREFACTION RESULTS IN TUBULOINTERSTITIAL HYPOXIA
That rarefaction of the renal microcirculation is associated with tissue hypoxia has been shown indirectly using nuclear hypoxia-inducible factor-1α (HIF-1α) as a marker, or more directly by demonstrating increased binding of pimonidazole, a widely used maker of hypoxic tissues. In rats, hypoxia and increased HIF-1α accumulation in areas of peritubular capillary rarefaction has been described in the progressive anti-Thy1.121 and the remnant kidney model.22, 23 In mice, folic acid nephropathy is associated with hypoxia without increase in HIF-1α abundance,24 whereas in progressive adriamycin-induced renal injury, an increase of active, nuclear HIF-1α is observed in renal tubular and interstitial inflammatory cells.25 In aging rats, HIF-1α abundance also increases in areas of peritubular capillary rarefaction.26 Similarly, in human patients, increased HIF-1α abundance in renal tubular epithelial cells has been described in progressive immunoglobulin A nephrophathy27 and in progressive proteinuric glomerular diseases.28
HIF-1 is a dimeric transcription factor consisting of HIF-1α/HIF-1β. The HIF-1 dimer activates a number of hypoxia-inducible genes. Under normoxic conditions, hydroxylation of HIF-1α by oxygen-sensitive prolyl hydroxylases enhances HIF-1α binding to the von Hippel-Lindau gene product, targeting HIF-1α for proteasomal destruction. Prolyl hydroxylase activity is reduced in hypoxic cells, leading to HIF-1α stabilization and HIF-1 activation. The HIF-1-induced genes include erythropoietin, VEGF, placenta-derived growth factor, angiopoietins 1 and 2, as well as platelet-derived growth factor-β.29 It is therefore reasonable to expect that hypoxia, via HIF-1 activation, would induce VEGF expression when renal perfusion is reduced by microvascular rarefaction, and that increased VEGF expression would stimulate new blood vessel ingrowth from still functioning vessels. However, although hypoxia and HIF-1 activation are observed, there is essentially no angiogenic response in chronic renal fibrosis.
Renal tubular epithelial cells express VEGF under basal conditions, with a gradient of expression that is lowest in proximal tubule epithelium and highest in medullary collecting duct cells. Hypoxia strongly induces VEGF expression in cultured proximal tubule epithelial cells30, 31 and in human renal tubular epithelial cells in vivo.32 However, although microvascular rarefaction may induce hypoxia and activate HIF-1, direct proximal tubule injury with folic acid in mice is associated with increased von Hippel-Lindau gene expression, reduced HIF-1α action, and reduced VEGF expression.24 Also, despite induction of HIF-1α in the remnant kidney model and in adriamycin-induced chronic renal disease in mice, proximal tubule epithelial cell VEGFA expression is reduced.25, 33 Similarly, in human kidney with tubulointerstitial fibrosis, VEGF expression is observed in the interstitium but not in atrophic tubular epithelial cells.32 Thus, although hypoxia is observed in the tubulointerstitial compartment of kidneys, in which the peritubular microvasculature has regressed, atrophic tubular epithelial cells no longer express VEGF. Inadequate VEGF synthesis by renal tubular epithelial cells therefore seems to be a component of the poor angiogenic response in renal fibrosis.
Transforming growth factor-beta 1 (TGF-β1), a major pro-fibrotic cytokine in progressive renal fibrosis,34 can also stimulate angiogenesis indirectly by inducing VEGFA expresssion35, 36 and by acting synergistically in the hypoxia response. In kidney fibroblasts, hypoxia stimulates TGF-β1 gene expression,37 and conversely, in normoxic mesangial cells, TGF-β1 directly induces HIF-1α and HIF-2 protein expression.38 Furthermore, HIF-1α is stabilized by TGF-β1,39 and hypoxia and TGF-β1 synergistically induce VEGFA expression.30, 40 In endothelial cells, HIF-1α interacts with Smad3 to induce VEGF and endoglin expression,41 and hypoxia induces TGF-β1 gene expression in endothelial cells through HIF-1α.
If TGF-β1 only promoted angiogenesis, synergy between HIF-1 and TGF-β1 could serve to ameliorate capillary rarefaction in progressive renal fibrosis. However, the endothelial cell response to TGF-β1 is complex and can also result in apoptosis and capillary pruning. In endothelial cells, two distinct signaling pathways are activated by TGF-β1 as reviewed in detail elsewhere.42 The ubiquitous TβRII/ALK5 pathway leads to Smad2/3 phosphorylation, inhibition of endothelial cell proliferation, and activation of apoptosis. By contrast, activation of the endoglin/TβRII/ALK1 pathway leads to Smads1/5/8 phosphorylation, enhancing endothelial cell proliferation and survival. Through dual effects on endothelial cells and by regulating pericyte recruitment, TGF-β1 controls blood vessel pattern and stability.42 As the TGF-β1 is produced in abundance in the renal fibrosis response to injury, and as TGF-β1 acting via Alk5/Smad3 promotes capillary regression and endothelial cell apoptosis, it seems plausible that capillary regression reflects the anti-proliferative, anti-survival TGF-β1 action in endothelial cells. Which of the TGF-β1 pathways is activated in peritubular capillary endothelial cells during renal fibrosis and capillary regression is as yet unclear.
THE ANGIOGENIC RESPONSE
Mechanisms that lead to new blood vessel formation have received massive attention since Folkman43 first suggested that tumor growth can be limited when angiogenesis is inhibited. Angiogenesis is the formation of new blood vessels by sprouting and elongation from existing vessels.44 Central to the angiogenic process is the rapid activation of hypoxia-inducible factors HIFs in tissues in response to decreased oxygen levels, leading to the production of angiogenic growth factors29 and inhibition of TGF-β1 signaling in endothelial cells.45 Angiogenic sprouts then form in the direction of the hypoxic stimulus and a gradient of VEGF and other angiogenic growth factors. Sprouting and elongation of the new vessel is followed by stabilization and vessel specification.46
Endothelial cells at the tip of the angiogenic sprout, responding to growth factor gradients, determine the direction and pattern of new blood vessel growth. Elongation of the new vessel depends on proliferation of endothelial cells in the stalk of the developing vessel.47 In the absence of controlled endothelial cell proliferation, a normal vasculature cannot be established, and pathological angiogenesis cannot proceed.48 A complex array of growth factors and their intracellular signaling systems regulate proliferation of stalk endothelial cells, including VEGFs,49 fibroblast growth factors,50 and the angiopoietins.51 The TGF-β family of proteins are involved in vessel stabilization and the establishment of perivascular cells.42, 52
The regulation of endothelial cell proliferation during sprouting angiogenesis depends on the activity of the intracellular PI3K/Akt/PTEN axis.53 Akt is a Ser/Thr kinase that promotes cell survival, proliferation, migration, and angiogenesis by phosphorylating a number of downstream proteins. Constitutively, active Akt induces pathological angiogenesis,54 and fetal vascularization is impaired in Akt-deficient mice.55 Akt is phosphorylated on T308 by phosphoinositide-dependent kinase-1 and on S473 by the mTOR complex 2.56, 57 For phosphorylation to occur, Akt must be recruited to the plasma membrane where it and phosphoinositide-dependent kinase-1 bind phosphatidylinositol (3,4,5) trisphosphate.58 In turn, phosphatidylinositol (3,4,5) trisphosphate is generated by phosphatidylinositol 3-kinase-mediated phosphorylation of phosphatidylinositol 4,5-bisphosphate in response to activation of receptor tyrosine kinases that include VEGFR2 and fibroblast growth factor receptors.59, 60 Activation of Akt is opposed by the phosphatase and tensin homolog deleted on chromosome 10 (PTEN), which dephosphorylates phosphatidylinositol (3,4,5) trisphosphate.61, 62 PTEN is a major tumor suppressor deleted in many tumors. Less well known is the fact that PTEN also regulates angiogenesis.63 Deletion of PTEN causes hyperproliferation of endothelial cells and disordered angiogenesis in mice,63, 64 and PTEN activation inhibits angiogenesis, favoring the establishment of a stable, differentiated endothelium, or, if excessive, endothelial cell apoptosis.65 Which of these intracellular mechanisms control capillary endothelial cell sprouting and proliferation during progressive renal fibrosis has not been critically explored so far.
ALTERNATIVE MECHANISMS OF BLOOD VESSEL FORMATION
In the embryo, the first blood vessels form through the process of vasculogenesis, which refers to the homotypic association of hemagioblast cells producing blood islands that then coalesce to form the dorsal aorta and the early cardiac tube. Similarly, in the kidney, the glomerular microvasculature initially develops through the in situ assembly of angioblasts66 into precapillary cords devoid of vascular lumens.67 Migration of angioblasts into the capillary cleft of the developing nephron depends on the elaboration of VEGF by immature podocytes and is guided by neuropilin, a semaphorin III receptor that binds VEGF68, and by podocyte-derived Ephrin-B2.69
The typical sprouting angiogenesis observed in ischemic tissues or in the hypoxic tumor environment is not observed in renal glomeruli. Instead, the increase in glomerular size observed in diabetic nephropathy70 and in response to other stimuli that result in an increase in glomerular capillary loops71 proceeds through intussusceptive growth. Intussusceptive angiogenesis is defined as the splitting of existing capillaries, which is initiated by the formation of transcapillary cellular pillars.72 Intussusceptive glomerular capillary repair is observed in the rat model of anti-Thy1.1 glomerululonephritis.73 Proliferating glomerular endothelial cells tend to remain confined within the existing glomerular capillary basement membrane, and angiogenic sprouts do not appear to penetrate the mesangium. Finally, although podocytes continually produce vastly more VEGF than other differentiated cells in vivo,74 podocyte-derived VEGF does not serve as a stimulus for sprouting angiogenesis in glomeruli, but instead is required for the formation and maintenance of a properly differentiated, fenestrated endothelium.75 Although glomeruli usually are not hypoxic, progressive sclerosis with obliteration of capillary lumens is nonetheless observed in most forms of chronic glomerulonephritis and late in diabetic nephropathy. Thus, in glomerular sclerosis, fibrosis is also favored over angiogenesis.
VASCULAR LUMEN FORMATION
In order to survive, new capillaries, whether formed by sprouting angiogenesis or splitting of existing capillaries, must form and maintain vascular lumens, as a failure of blood flow results in regression of capillaries. The formation of vascular lumens progresses through several steps that involve the polarization of endothelial cells through the establishment of vascular endothelial-cadherin–dependent cell–cell junctions,76 the synthesis and sorting of apical vs. basolateral proteins, organization and anchoring of apical plasma membrane-spanning proteins to cortical actin,77 and the consequent repulsion of adjacent apical surfaces due to the expression of anti-adhesive sialoglycoproteins, including podocalyxin and CD34 on the apical surface.76, 78 In glomerular capillaries, the formation of capillary lumens, furthermore, requires the removal of redundant cells through TGF-β1-dependent apoptosis.67
A critical step in the assembly of the apical endothelial cell structure during vascular lumen formation is the coupling of the cytosolic tail of sialoglycoproteins and other signaling molecules to cortical actin via ERM proteins.78 ERM proteins generally regulate cell-surface architecture by coupling key transmembrane proteins to cortical actin, serving both structural and signaling functions.77 In endothelial cells, the main ERM is moesin.79 Moesin associates with the apical plasma membrane of endothelial cells during the development of vascular lumens,78, 80 and moesin is critically required for vascular lumen formation in Zebrafish.80 Recently, a role for chloride intracellular channel (CLIC) proteins in the morphogenesis of endothelial cell tubes and in regulating ERM proteins has been described. CLIC4 is required for hollowing of capillaries in vitro,81 and in endothelial cells derived from CLIC4-deficient mice, lumen formation is impaired.82 CLIC1 has a role in branching morphogenesis during vascular development.83 Furthermore, Chalothorn et al.84 found that the vascular density is reduced in CLIC4-deficient mice. Although the evidence is still incomplete, the data so far suggest that phosphorylation of moesin is supported by CLIC proteins, and that CLIC4-dependent activation of moesin is required for lumen formation in endothelial cells. The role of adequate vascular lumen formation in the angiogenic response to renal hypoxia or collapse of vascular lumens during microvascular rarefaction in renal fibrosis has not been addressed so far.
A FAILED ANGIOGENIC RESPONSE IN CKD?
It has been postulated that failed angiogenesis may be central to progressive renal fibrosis, whether due to ischemic acute kidney injury,85, 86 diabetic nephropathy,87, 88 progressive allograft nephropathy,4 large-vessel renovascular disease,89 the nephrotic syndrome,3 glomerulonephritis,9 chronic ureteral obstruction,14 loss of renal mass as in the remnant kidney,22 or aging.26, 90 This seemingly ubiquitous failure of a robust angiogenic response to hypoxia raises the question whether powerful anti-angiogeneic mechanisms prevent renal angiogenesis in general. However, a robust angiogenic response with remodeling is observed in patients with adult autosomal polycystic kidney disease, as renal cysts expand and render parts of the renal interstitium hypoxic,91 and also in patients with renal cell carcinoma where HIF-1α is stabilized due to loss of the von Hippel-Lindau gene product.92 Also, in the early stages of diabetic nephropathy, glomerular capillary expansion is well described,88 peri-glomerular angiogenic vessels have been reported,93 excess VEGF-driven endothelial cell proliferation is observed,94 and anti-angiogenic therapy has shown some promise in reducing glomerular hypertrophy.95 Also, therapies directed at increasing blood flow or endothelial cell proliferation and repair in chronic progressive renal fibrosis89, 96 have met with some success at preserving the renal microvasculature. Therefore, renal glomerular and peritubular microvascular endothelial cells clearly can respond to angiogenic stimuli, but these mechanisms are inhibited in progressive renal fibrosis (Figure 2). Although the process of angiogenesis and vascular growth and remodeling is extremely well studied, the endothelial cell–specific mechanisms that lead to a failed angiogenesis response during renal fibrosis have received relatively little attention to date.
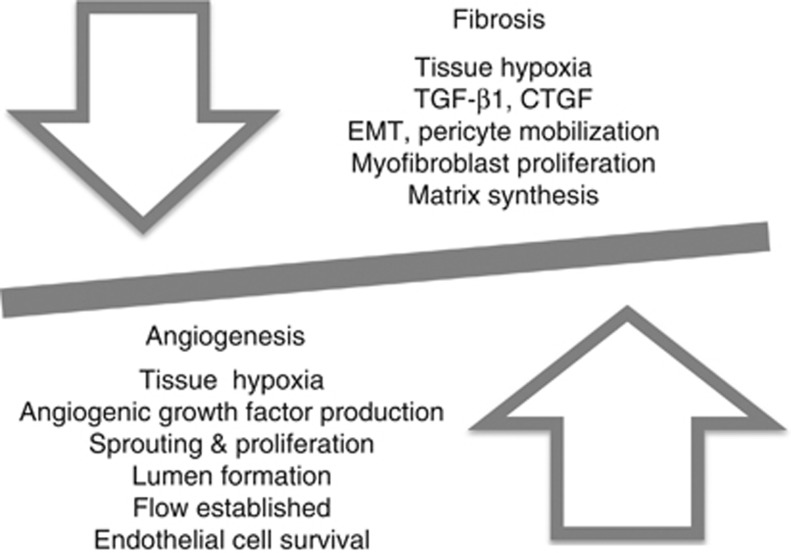
Figure 2.
Tissue hypoxia generates both angiogenic and fibrogenic signals. In chronic kidney disease, the balance is strongly shifted toward tissue fibrosis. CTGF, connective tissue growth factor; EMT, epithelial-to-mesenchymal transition; TGF-β1, transforming growth factor-beta 1.
In most tissues, an initial robust angiogenic response to acute hypoxia is followed by progressive fibrosis and vessel regression if hypoxia persists.11 Hence, the fibrotic response to chronic hypoxia is not unique to kidney. Indeed, that peritubular capillary loss may contribute to progressive renal fibrosis due to persistent, chronic hypoxia, was already suggested in 1975.97 The fibrosis response to chronic hypoxia is driven, at least in part, by the HIFs, which, in cooperation with TGF-β1, strongly activate remodeling and fibrosis pathways, including epithelial to mesenchymal transition98 and matrix production by renal tubules99 and interstitial myofibroblasts.100 Hence, the same processes that can stimulate angiogenesis also drive the fibrosis response to hypoxia.
We are therefore left with three major potential mechanisms that would favor fibrosis over angiogenesis in CKD (Figure 2). First, atrophy or destruction of renal tubular epithelial cells results in reduced angiogenic mediator expression despite HIF-1 activation, blunting the angiogenic response and favoring fibrosis. The work suggesting that pericytes dissociate from peritubular capillaries in response to renal epithelial cell injury, furthermore, suggests that epithelial cells maintain ‘their' microvasculture by indirect, pericyte-dependent signals. Similar mechanisms may operate in the glomerulus where loss or destruction of podocytes leads to reduced VEGF availability, and a fibrogenic response by the mesangial ‘pericytes.' Second, it is attractive to postulate that excess TGF-β1 in the tubulointerstitial or mesangial compartment triggers endothelial cell apoptosis via the ALK5/Smad3 pathway, resulting in capillary regression. Although TGF-β1 can activate angiogenesis indirectly through VEGF synthesis, and can support endothelial cell survival and proliferation by activating the endothelium-specific ALK1/Smad1/5/8 pathway, persistently high concentrations of TGF-β1 activate ALK5/Smad3 in endothelial cells, which results in VEGF receptor downregulation and the induction of endothelial cell apoptosis. This process is a part of normal blood vessel pruning and is very likely operating in the fibrotic tubulointerstitium. Third, blood vessel perfusion is a critical determinant of endothelial cell survival. Loss of glomerular perfusion secondary to glomerulonephritis or glomerulosclerosis leads to coordinate regression of the associated peritubular capillary and atrophy of epithelial cells in the accompanying tubule. Loss of glomerular perfusion therefore sets up a vicious cycle of postglomerular microvascular regression, chronic hypoxia, and fibrosis.
SUMMARY
Renal microvascular rarefaction is a central phenomenon observed in all forms of progressive renal fibrosis. In part, involution of peritubular capillaries results from the loss of perfusion when glomeruli become sclerotic, and inflammatory mechanisms participate in the destruction of peritubular capillaries. Reduced microvascular blood flow, in turn, results in chronic hypoxia within the renal interstitum. Whereas hypoxia can activate both angiogenesis and fibrotic tissue remodeling, this balance is strongly tipped toward fibrosis in CKD. If it were possible to harness the endothelial cell–specific mechanisms that lead to endothelial cell survival, new vessel formation, and preservation of capillary lumens, it might be possible to halt or even reverse the relentlessly progressive renal fibrosis that so often leads to renal failure.
Acknowledgments
Our work was supported by Canadian Institutes of Health Research MOP 641814, by a grant from the Kidney Foundation of Canada, and by a Pfizer Cardiovascular Program peer-reviewed grant (BJB, principal investigator). The Division of Nephrology at the University of Alberta supported the tuition fees of the graduate student MO. This article is published in a supplement partially supported by the Major State Basic Research Development Program of China (no. 2012CB517700) and the Guangdong Medical Association. BJB holds a patent for a prosthetic vascular device but receives no royalties.
The authors declare no competing interests.
References
- Eddy AA. Experimental insights into the tubulointerstitial disease accompanying primary glomerular lesions. J Am Soc Nephrol. 1994;5:1273–1287. doi: 10.1681/ASN.V561273. [DOI] [PubMed] [Google Scholar]
- Bonventre JV. Can we target tubular damage to prevent renal function decline in diabetes. Semin Nephrol. 2012;32:452–462. doi: 10.1016/j.semnephrol.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaukinen A, Lautenschlager I, Helin H, et al. Peritubular capillaries are rarefied in congenital nephrotic syndrome of the Finnish type. Kidney Int. 2009;75:1099–1108. doi: 10.1038/ki.2009.41. [DOI] [PubMed] [Google Scholar]
- Sis B. Endothelial molecules decipher the mechanisms and functional pathways in antibody-mediated rejection. Hum Immunol. 2012;73:1218–1225. doi: 10.1016/j.humimm.2012.07.332. [DOI] [PubMed] [Google Scholar]
- Schaper W. Collateral circulation: past and present. Basic Res Cardiol. 2009;104:5–21. doi: 10.1007/s00395-008-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landazuri N, Joseph G, Guldberg RE, et al. Growth and regression of vasculature in healthy and diabetic mice after hindlimb ischemia. Am J Physiol Regul Integr Comp Physiol. 2012;303:R48–R56. doi: 10.1152/ajpregu.00002.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Zeiher AM. Endothelial cell apoptosis in angiogenesis and vessel regression. Circ Res. 2000;87:434–439. doi: 10.1161/01.res.87.6.434. [DOI] [PubMed] [Google Scholar]
- Browning EA, Chatterjee S, Fisher AB. Stop the flow: a paradigm for cell signaling mediated by reactive oxygen species in the pulmonary endothelium. Annu Rev Physiol. 2012;74:403–424. doi: 10.1146/annurev-physiol-020911-153324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MG, Suzuki Y, Tanifuji C, et al. Peritubular ischemia contributes more to tubular damage than proteinuria in immune-mediated glomerulonephritis. J Am Soc Nephrol. 2008;19:290–297. doi: 10.1681/ASN.2007020226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietecha MS, Cerny WL, DiPietro LA. Mechanisms of vessel regression: toward an understanding of the resolution of angiogenesis. Curr Top Microbiol Immunol. 2013;367:3–32. doi: 10.1007/82_2012_287. [DOI] [PubMed] [Google Scholar]
- Johnson A, DiPietro LA. Apoptosis and angiogenesis: an evolving mechanism for fibrosis. FASEB J. 2013;27:3893–3901. doi: 10.1096/fj.12-214189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einecke G, Sis B, Reeve J, et al. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant. 2009;9:2520–2531. doi: 10.1111/j.1600-6143.2009.02799.x. [DOI] [PubMed] [Google Scholar]
- Pober JS, Min W, Bradley JR. Mechanisms of endothelial dysfunction, injury, and death. Annu Rev Pathol. 2009;4:71–95. doi: 10.1146/annurev.pathol.4.110807.092155. [DOI] [PubMed] [Google Scholar]
- Ohashi R, Shimizu A, Masuda Y, et al. Peritubular capillary regression during the progression of experimental obstructive nephropathy. J Am Soc Nephrol. 2002;13:1795–1805. doi: 10.1097/01.asn.0000018408.51388.57. [DOI] [PubMed] [Google Scholar]
- Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest. 2006;116:288–296. doi: 10.1172/JCI27699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grgic I, Campanholle G, Bijol V, et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82:172–183. doi: 10.1038/ki.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninichuk V, Anders HJ. Bone marrow-derived progenitor cells and renal fibrosis. Front Biosci. 2008;13:5163–5173. doi: 10.2741/3072. [DOI] [PubMed] [Google Scholar]
- He J, Xu Y, Koya D, et al. Role of the endothelial-to-mesenchymal transition in renal fibrosis of chronic kidney disease. Clin Exp Nephrol. 2013;17:488–497. doi: 10.1007/s10157-013-0781-0. [DOI] [PubMed] [Google Scholar]
- Duffield JS, Humphreys BD. Origin of new cells in the adult kidney: results from genetic labeling techniques. Kidney Int. 2011;79:494–501. doi: 10.1038/ki.2010.338. [DOI] [PubMed] [Google Scholar]
- Campanholle G, Ligresti G, Gharib SA, et al. Cellular mechanisms of tissue fibrosis. 3. Novel mechanisms of kidney fibrosis. Am J Physiol Cell Physiol. 2013;304:C591–C603. doi: 10.1152/ajpcell.00414.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Tanaka T, Yamamoto T, et al. Hypoperfusion of peritubular capillaries induces chronic hypoxia before progression of tubulointerstitial injury in a progressive model of rat glomerulonephritis. J Am Soc Nephrol. 2004;15:1574–1581. doi: 10.1097/01.asn.0000128047.13396.48. [DOI] [PubMed] [Google Scholar]
- Zhang B, Liang X, Shi W, et al. Role of impaired peritubular capillary and hypoxia in progressive interstitial fibrosis after 56 subtotal nephrectomy of rats. Nephrology (Carlton) 2005;10:351–357. doi: 10.1111/j.1440-1797.2005.00412.x. [DOI] [PubMed] [Google Scholar]
- Manotham K, Tanaka T, Matsumoto M, et al. Evidence of tubular hypoxia in the early phase in the remnant kidney model. J Am Soc Nephrol. 2004;15:1277–1288. doi: 10.1097/01.asn.0000125614.35046.10. [DOI] [PubMed] [Google Scholar]
- Yuan HT, Li XZ, Pitera JE, et al. Peritubular capillary loss after mouse acute nephrotoxicity correlates with down-regulation of vascular endothelial growth factor-A and hypoxia-inducible factor-1 alpha. Am J Pathol. 2003;163:2289–2301. doi: 10.1016/s0002-9440(10)63586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kairaitis LK, Wang Y, Gassmann M, et al. HIF-1alpha expression follows microvascular loss in advanced murine adriamycin nephrosis. Am J Physiol Renal Physiol. 2005;288:F198–F206. doi: 10.1152/ajprenal.00244.2003. [DOI] [PubMed] [Google Scholar]
- Kang DH, Anderson S, Kim YG, et al. Impaired angiogenesis in the aging kidney: vascular endothelial growth factor and thrombospondin-1 in renal disease. Am J Kidney Dis. 2001;37:601–611. doi: 10.1053/ajkd.2001.22087. [DOI] [PubMed] [Google Scholar]
- Namikoshi T, Satoh M, Horike H, et al. Implication of peritubular capillary loss and altered expression of vascular endothelial growth factor in IgA nephropathy. Nephron Physiol. 2006;102:p9–16. doi: 10.1159/000088405. [DOI] [PubMed] [Google Scholar]
- Rudnicki M, Perco P, Enrich J, et al. Hypoxia response and VEGF-A expression in human proximal tubular epithelial cells in stable and progressive renal disease. Lab Invest. 2009;89:337–346. doi: 10.1038/labinvest.2008.158. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33:207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Lan HY, Zhu HJ, et al. Differential regulation of VEGF by TGF-beta and hypoxia in rat proximal tubular cells. Am J Physiol Renal Physiol. 2004;287:F658–F664. doi: 10.1152/ajprenal.00040.2004. [DOI] [PubMed] [Google Scholar]
- Kim BS, Chen J, Weinstein T, et al. VEGF expression in hypoxia and hyperglycemia: reciprocal effect on branching angiogenesis in epithelial-endothelial co-cultures. J Am Soc Nephrol. 2002;13:2027–2036. doi: 10.1097/01.asn.0000024436.00520.d8. [DOI] [PubMed] [Google Scholar]
- Grone HJ, Simon M, Grone EF. Expression of vascular endothelial growth factor in renal vascular disease and renal allografts. J Pathol. 1995;177:259–267. doi: 10.1002/path.1711770308. [DOI] [PubMed] [Google Scholar]
- Kang DH, Joly AH, Oh SW, et al. Impaired angiogenesis in the remnant kidney model: I. Potential role of vascular endothelial growth factor and thrombospondin-1. J Am Soc Nephrol. 2001;12:1434–1447. doi: 10.1681/ASN.V1271434. [DOI] [PubMed] [Google Scholar]
- Eddy AA, Neilson EG. Chronic kidney disease progression. J Am Soc Nephrol. 2006;17:2964–2966. doi: 10.1681/ASN.2006070704. [DOI] [PubMed] [Google Scholar]
- Pertovaara L, Kaipainen A, Mustonen T, et al. Vascular endothelial growth factor is induced in response to transforming growth factor-beta in fibroblastic and epithelial cells. J Biol Chem. 1994;269:6271–6274. [PubMed] [Google Scholar]
- Kitamura S, Maeshima Y, Sugaya T, et al. Transforming growth factor-beta 1 induces vascular endothelial growth factor expression in murine proximal tubular epithelial cells. Nephron Exp Nephrol. 2003;95:e79–e86. doi: 10.1159/000073675. [DOI] [PubMed] [Google Scholar]
- Norman JT, Clark IM, Garcia PL. Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int. 2000;58:2351–2366. doi: 10.1046/j.1523-1755.2000.00419.x. [DOI] [PubMed] [Google Scholar]
- Hanna C, Hubchak SC, Liang X, et al. Hypoxia-inducible factor-2alpha and TGF-beta signaling interact to promote normoxic glomerular fibrogenesis. Am J Physiol Renal Physiol. 2013;305:F1323–F1331. doi: 10.1152/ajprenal.00155.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon S, Charbonneau M, Grandmont S, et al. Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J Biol Chem. 2006;281:24171–24181. doi: 10.1074/jbc.M604507200. [DOI] [PubMed] [Google Scholar]
- Sanchez-Elsner T, Botella LM, Velasco B, et al. Synergistic cooperation between hypoxia and transforming growth factor-beta pathways on human vascular endothelial growth factor gene expression. J Biol Chem. 2001;276:38527–38535. doi: 10.1074/jbc.M104536200. [DOI] [PubMed] [Google Scholar]
- Sanchez-Elsner T, Botella LM, Velasco B, et al. Endoglin expression is regulated by transcriptional cooperation between the hypoxia and transforming growth factor-beta pathways. J Biol Chem. 2002;277:43799–43808. doi: 10.1074/jbc.M207160200. [DOI] [PubMed] [Google Scholar]
- Pardali E, Goumans MJ, ten Dijke P. Signaling by members of the TGF-beta family in vascular morphogenesis and disease. Trends Cell Biol. 2010;20:556–567. doi: 10.1016/j.tcb.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Crivellato E. "Sprouting angiogenesis", a reappraisal. Dev Biol. 2012;372:157–165. doi: 10.1016/j.ydbio.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Wei H, Bedja D, Koitabashi N, et al. Endothelial expression of hypoxia-inducible factor 1 protects the murine heart and aorta from pressure overload by suppression of TGF-beta signaling. Proc Natl Acad Sci USA. 2012;109:E841–E850. doi: 10.1073/pnas.1202081109. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Jeltsch M, Leppanen VM, Saharinen P, et al. Receptor tyrosine kinase-mediated angiogenesis. Cold Spring Harb Perspect Biol. 2013;5:1–21. doi: 10.1101/cshperspect.a009183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol. 2010;22:617–625. doi: 10.1016/j.ceb.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Jiang BH, Liu LZ. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv Cancer Res. 2009;102:19–65. doi: 10.1016/S0065-230X(09)02002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly DT, Heuvelman DM, Nelson R, et al. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84:1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D, Brown KD, Birdwell CR, et al. Control of proliferation of human vascular endothelial cells. Characterization of the response of human umbilical vein endothelial cells to fibroblast growth factor, epidermal growth factor, and thrombin. J Cell Biol. 1978;77:774–788. doi: 10.1083/jcb.77.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Jakobsson L, van Meeteren LA. Transforming growth factor beta family members in regulation of vascular function: in the light of vascular conditional knockouts. Exp Cell Res. 2013;319:1264–1270. doi: 10.1016/j.yexcr.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Varma S, Lal BK, Zheng R, et al. Hyperglycemia alters PI3k and Akt signaling and leads to endothelial cell proliferative dysfunction. Am J Physiol Heart Circ Physiol. 2005;289:H1744–H1751. doi: 10.1152/ajpheart.01088.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry B, Banyard J, McLaughlin ER, et al. AKT1 overexpression in endothelial cells leads to the development of cutaneous vascular malformations in vivo. Arch Dermatol. 2007;143:504–506. doi: 10.1001/archderm.143.4.504. [DOI] [PubMed] [Google Scholar]
- Yang ZZ, Tschopp O, Hemmings-Mieszczak M, et al. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278:32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Wick MJ, Dong LQ, Riojas RA, et al. Mechanism of phosphorylation of protein kinase B/Akt by a constitutively active 3-phosphoinositide-dependent protein kinase-1. J Biol Chem. 2000;275:40400–40406. doi: 10.1074/jbc.M003937200. [DOI] [PubMed] [Google Scholar]
- Andjelkovic M, Alessi DR, Meier R, et al. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- Alessi DR, Andjelkovic M, Caudwell B, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Franke TF, Yang SI, Chan TO, et al. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gericke A, Leslie NR, Losche M, et al. PtdIns(4,5)P2-mediated cell signaling: emerging principles and PTEN as a paradigm for regulatory mechanism. Adv Exp Med Biol. 2013;991:85–104. doi: 10.1007/978-94-007-6331-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Hamada K, Sasaki T, et al. Role of PTEN/PI3K pathway in endothelial cells. Biochem Soc Trans. 2007;35:172–176. doi: 10.1042/BST0350172. [DOI] [PubMed] [Google Scholar]
- Hamada K, Sasaki T, Koni PA, et al. The PTEN/PI3K pathway governs normal vascular development and tumor angiogenesis. Genes Dev. 2005;19:2054–2065. doi: 10.1101/gad.1308805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y, Nishimura K, Sumpio BE. Phosphatase PTEN is inactivated in bovine aortic endothelial cells exposed to cyclic strain. J Cell Biochem. 2007;100:515–526. doi: 10.1002/jcb.21085. [DOI] [PubMed] [Google Scholar]
- Robert B, St, John PL, Hyink DP, et al. Evidence that embryonic kidney cells expressing flk-1 are intrinsic, vasculogenic angioblasts. Am J Physiol. 1996;271:F744–F753. doi: 10.1152/ajprenal.1996.271.3.F744. [DOI] [PubMed] [Google Scholar]
- Fierlbeck W, Liu A, Coyle R, et al. Endothelial cell apoptosis during glomerular capillary lumen formation in vivo. J Am Soc Nephrol. 2003;14:1349–1354. doi: 10.1097/01.asn.0000061779.70530.06. [DOI] [PubMed] [Google Scholar]
- Robert B, Zhao X, Abrahamson DR. Coexpression of neuropilin-1, Flk1, and VEGF(164) in developing and mature mouse kidney glomeruli. Am J Physiol Renal Physiol. 2000;279:F275–F282. doi: 10.1152/ajprenal.2000.279.2.F275. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Takahashi K, Gerety S, et al. Temporally compartmentalized expression of ephrin-B2 during renal glomerular development. J Am Soc Nephrol. 2001;12:2673–2682. doi: 10.1681/ASN.V12122673. [DOI] [PubMed] [Google Scholar]
- Dei Cas A, Gnudi L. VEGF and angiopoietins in diabetic glomerulopathy: how far for a new treatment. Metabolism. 2012;61:1666–1673. doi: 10.1016/j.metabol.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Scruggs BS, Zuo Y, Donnert E, et al. Increased capillary branching contributes to angiotensin type 1 receptor blocker (ARB)-induced regression of sclerosis. Am J Pathol. 2011;178:1891–1898. doi: 10.1016/j.ajpath.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burri PH, Hlushchuk R, Djonov V. Intussusceptive angiogenesis: its emergence, its characteristics, and its significance. Dev Dyn. 2004;231:474–488. doi: 10.1002/dvdy.20184. [DOI] [PubMed] [Google Scholar]
- Notoya M, Shinosaki T, Kobayashi T, et al. Intussusceptive capillary growth is required for glomerular repair in rat Thy-1.1 nephritis. Kidney Int. 2003;63:1365–1373. doi: 10.1046/j.1523-1755.2003.00876.x. [DOI] [PubMed] [Google Scholar]
- Simon M, Gröne HJ, Jöhren O, et al. Expression of vascular endothelial growth factor and its receptors in human renal ontogenesis and in adult kidney. Am J Physiol. 1995;268:F240–F250. doi: 10.1152/ajprenal.1995.268.2.F240. [DOI] [PubMed] [Google Scholar]
- Eremina V, Quaggin SE. The role of VEGF-A in glomerular development and function. Curr Opin Nephrol Hypertens. 2004;13:9–15. doi: 10.1097/00041552-200401000-00002. [DOI] [PubMed] [Google Scholar]
- Lammert E, Axnick J. Vascular lumen formation. Cold Spring Harb Perspect Med. 2012;2:a006619. doi: 10.1101/cshperspect.a006619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. 2010;11:276–287. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strilic B, Kucera T, Eglinger J, et al. The molecular basis of vascular lumen formation in the developing mouse aorta. Dev Cell. 2009;17:505–515. doi: 10.1016/j.devcel.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Berryman M, Franck Z, Bretscher A. Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J Cell Sci. 1993;105 (Pt 4:1025–1043. doi: 10.1242/jcs.105.4.1025. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kaiser MS, Larson JD, et al. Moesin1 and Ve-cadherin are required in endothelial cells during in vivo tubulogenesis. Development. 2010;137:3119–3128. doi: 10.1242/dev.048785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung JJ, Hobert O, Berryman M, et al. Chloride intracellular channel 4 is involved in endothelial proliferation and morphogenesis in vitro. Angiogenesis. 2009;12:209–220. doi: 10.1007/s10456-009-9139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov B, Bruno J, Gordon N, et al. Chloride intracellular channel protein-4 functions in angiogenesis by supporting acidification of vacuoles along the intracellular tubulogenic pathway. Am J Pathol. 2009;174:1084–1096. doi: 10.2353/ajpath.2009.080625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung JJ, Kitajewski J. Chloride intracellular channel 1 functions in endothelial cell growth and migration. J Angiogenes Res. 2010;2:23. doi: 10.1186/2040-2384-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalothorn D, Zhang H, Smith JE, et al. Chloride intracellular channel-4 is a determinant of native collateral formation in skeletal muscle and brain. Circ Res. 2009;105:89–98. doi: 10.1161/CIRCRESAHA.109.197145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int. 2007;72:151–156. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- Kwon O, Hong SM, Sutton TA, et al. Preservation of peritubular capillary endothelial integrity and increasing pericytes may be critical to recovery from postischemic acute kidney injury. Am J Physiol Renal Physiol. 2008;295:F351–F359. doi: 10.1152/ajprenal.90276.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenmeyer MT, Kretzler M, Boucherot A, et al. Interstitial vascular rarefaction and reduced VEGF-A expression in human diabetic nephropathy. J Am Soc Nephrol. 2007;18:1765–1776. doi: 10.1681/ASN.2006121304. [DOI] [PubMed] [Google Scholar]
- Advani A, Gilbert RE. The endothelium in diabetic nephropathy. Semin Nephrol. 2012;32:199–207. doi: 10.1016/j.semnephrol.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Chade AR. Renovascular disease, microcirculation, and the progression of renal injury: role of angiogenesis. Am J Physiol Regul Integr Comp Physiol. 2011;300:R783–R790. doi: 10.1152/ajpregu.00657.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Kato H, Kojima I, et al. Hypoxia and expression of hypoxia-inducible factor in the aging kidney. J Gerontol A Biol Sci Med Sci. 2006;61:795–805. doi: 10.1093/gerona/61.8.795. [DOI] [PubMed] [Google Scholar]
- Wei W, Popov V, Walocha JA, et al. Evidence of angiogenesis and microvascular regression in autosomal-dominant polycystic kidney disease kidneys: a corrosion cast study. Kidney Int. 2006;70:1261–1268. doi: 10.1038/sj.ki.5001725. [DOI] [PubMed] [Google Scholar]
- Richard S, Gardie B, Couve S, et al. Von Hippel-Lindau: how a rare disease illuminates cancer biology. Semin Cancer Biol. 2013;23:26–37. doi: 10.1016/j.semcancer.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Stout LC, Whorton EB. Pathogenesis of extra efferent vessel development in diabetic glomeruli. Hum Pathol. 2007;38:1167–1177. doi: 10.1016/j.humpath.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004;65:2003–2017. doi: 10.1111/j.1523-1755.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- Zent R, Pozzi A. Antiangiogenic therapy in diabetic nephropathy. J Am Soc Nephrol. 2006;17:325–327. doi: 10.1681/ASN.2005121290. [DOI] [PubMed] [Google Scholar]
- Kim YG, Suga SI, Kang DH, et al. Vascular endothelial growth factor accelerates renal recovery in experimental thrombotic microangiopathy. Kidney Int. 2000;58:2390–2399. doi: 10.1046/j.1523-1755.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- Matsubara O, Takemura T. Renal microvasculature in chronic renal failure. Bull Tokyo Med Dent Univ. 1975;22:193–205. [PubMed] [Google Scholar]
- Higgins DF, Kimura K, Bernhardt WM, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117:3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orphanides C, Fine LG, Norman JT. Hypoxia stimulates proximal tubular cell matrix production via a TGF-beta1-independent mechanism. Kidney Int. 1997;52:637–647. doi: 10.1038/ki.1997.377. [DOI] [PubMed] [Google Scholar]
- Haase VH. Pathophysiological consequences of HIF activation: HIF as a modulator of fibrosis. Ann NY Acad Sci. 2009;1177:57–65. doi: 10.1111/j.1749-6632.2009.05030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]