Abstract
Interstitial fibrosis is a hallmark structural correlate of progressive and chronic kidney disease. There remain many uncertainties about how to best measure interstitial fibrosis both in research settings and in evaluations of renal biopsies performed for management of individual patients. Areas of uncertainty include determination of the composition of the matrix in a fibrotic parenchyma, the definition of how the interstitium is involved by fibrosing injuries, the choice of histologic stains for evaluation of renal fibrosis, and the reproducibility and robustness of measures currently employed by pathologists, both with and without the assistance of computerized imaging and assessments. In this review, we address some of these issues while citing the key studies that illustrate these difficulties. We point to future approaches that may allow a more accurate and meaningful assessment of renal interstitial fibrosis.
Keywords: collagen, fibrosis, interstitial fibrosis, morphometry, picrosirius red, trichrome stain
WHAT IS THE BEST WAY TO MEASURE RENAL FIBROSIS?: A PATHOLOGIST'S PERSPECTIVE
It is commonly accepted that interstitial fibrosis (IF) is a key, and perhaps the key, structural correlate of progressive and chronic kidney disease. It is therefore surprising that there remain many fundamental uncertainties about how to best measure fibrosis and about whether all forms of fibrosis are equally detrimental to the kidney and whether the various approaches available for measurement of fibrosis are robust and reproducible. The review will identify some of the issues underlying these uncertainties, cite some key studies that give us a basis for choosing some approaches over others, and suggest ways in which we may move forward, but regretfully will not resolve the fundamental uncertainties that we will discuss.
Chronic kidney injury is manifested by a variety of structural alterations, including the accumulation of extracellular matrix (ECM). Most of what is considered ECM is colloquially termed IF. Tubular atrophy (TA) often accompanies IF and, when occurring together, IF and TA are collectively termed IFTA.1, 2, 3, 4, 5, 6, 7, 8 Taken in isolation, IF is not necessarily a marker of the degree of intactness or function of nephron units. However, studies have shown that IF quantification can help prognosticate renal outcome in renal allografts and in such native kidney diseases as IgA nephropathy, and may be considered the best available histologic marker of chronic kidney injury.9, 10, 11, 12, 13, 14
As many investigators and practitioners ascribe a great deal of importance to the issue of IF, accurate IF measurement is often needed in a variety of applications, including research focused on the therapeutic inhibition of IF, comparison of protocol biopsies in studies of renal allografts,1, 15, 16 and for clinical prognostication as is the case with IgA nephropathy and lupus nephritis.14, 17, 18, 19, 20 However, to do this, one must understand the qualitative and quantitative issues related to the topic of IF. The qualitative issues relate to the actual composition and distribution of the IF (that is, ‘what?' and ‘where'). The quantitative issues, on the other hand, relate to the amount present (that is, ‘how much?'). In addition, one must understand the systems currently used for IFTA assessment and the implications (that is, ‘who uses this?' and ‘why').
FIBROSIS QUALITY: WHAT IS IN A SCAR?
Composition of matrix
The cortical interstitial volume normally ranges from 5 to 20% with a mean of 12%,21, 22, 23, 24 and this volume reportedly increases with age.24 The normal cortical interstitial volume is estimated at 5% in the rat.25 The renal interstitium ECM contains sulfated and non-sulfated glycosaminoglycans,21, 26 such as biglycan and decorin,27 Types I and III collagen, and fibronectin.21, 28 Type VI collagen is also present, particularly in rodents.25, 29 IF is typically considered to be an excess accumulation of fibrillar collagen, and the role of other matrix molecules such as proteoglycans and other non-collagenous proteins has not been comprehensively investigated. Knowing the composition of a fibrotic matrix is important because matrix components may determine the susceptibility of a matrix to undergo degradation by proteases and possibly undergo regression, and may determine the local tethering and/or activation of growth factors and cytokines that mediate IFTA.
Interstitial cells and their interplay with epithelial cells and vasculature
Fibroblasts constitute a large proportion of renal interstitial cells and are the major cells maintaining constituent ECM, which can be considered the kidney ‘skeleton.' Fibroblasts lack a good cell type–specific marker, making their study difficult.30 Fibroblasts and other cells may acquire a myofibroblastic phenotype, likely a crucial event in expansion of the ECM.18, 30, 31, 32, 33 Lymphocytes appear to have important roles in the development of IFTA.8, 34, 35, 36 The classes of infiltrating or resident monocyte/macrophages are heterogeneous, displaying a variety of phenotypes.37, 38, 39, 40 Some macrophages may be preferentially pro-fibrotic,38, 41 whereas other classes of monocyte/macrophages may actually attenuate fibrosis.42 Other cells also contribute to IFTA, including pericytes,19 dendritic cells,8, 36, 43, 44, 45, 46 mast cells,8, 47, 48, 49 and fibrocytes.6, 37, 40, 50, 51, 52, 53, 54 Measures of IF rarely take into account the cellularity of the fibrotic areas, and how this may reflect the age of the fibrotic process or its potential for reversibility or other biologic features of the fibrotic process.
FIBROSIS DISTRIBUTION: WHERE IS THE FIBROSIS?
Patterns of IF vary and likely do not have identical causes or consequences. For example, the patchy, ‘striped' pattern of IF with corresponding TA has been described with calcineurin inhibitor use. It has been proposed that this is because of the apparent preferential involvement of the medullary rays; however, IF also might be the result of toxic injury to discrete segments of small arteries and arterioles with consequent diminished blood supply to those portions of the cortical parenchyma supplied by the injured vessels. Despite the use of this association as a way to identify calcineurin inhibitor effect, this pattern may also be seen with hypertensive kidney disease. This ‘striped' fibrosis occurs in addition to the other changes of chronic calcineurin-induced nephrotoxicity, including hyaline arteriopathy, and nonspecific glomerulosclerosis.55
Broad scars with the loss of tubules are the sequelae of severe focal injury and destruction of parenchyma, such as in pyelonephritis and infarcts.8 Chronic obstruction extrinsic to the ureter can lead to IF/TA with relative glomerular sparing, atubular glomeruli, dilated tubules, and intratubular Tamm–Horsfall protein casts with extravasation into the interstitium.56, 57 The IF resulting from the metabolic injuries of diabetic nephropathy is both diffused and more homogeneous in distribution, although modification of the homogeneous distribution may occur as a result of concurrent vascular disease that may be of irregular severity. As kidneys age, there is often a pattern of subcapsular fibrosis, usually attributed to a marginal blood supply that is not replicated in less superficial portions of the renal cortex. Despite these associations, there is often an essentially nonspecific pattern of fibrosis in renal biopsies of patients with chronic kidney disease, including diffuse or patchy fine IF surrounding tubules, which can be either normal or atrophic. This is associated with either diffuse or focal disease of glomeruli, tubules, or vessels.7, 57 Although assessment of cortical IF is often stressed, medullary IF likely parallels cortical IF and epithelial loss, as stressed in studies by Farris et al.58
What about the interstitial microvasculature?
In allografts, loss of peritubular capillaries (PTCs) occurs following transplantation.59 One study has shown that PTCs decrease with time in allografts and are inversely related to renal function; decreased PTC density at 3 months predicts later loss of function at 1 year.59 Loss of PTC presumably results in a diminished supply of nutrients to the tubulointerstitium, and these PTC changes are often thought to parallel the presence of IF. However, it remains unclear whether loss of PTCs is causal in the development of IF and, conversely, whether restoration of the PTC density can lead to reversal of IF. Despite the obvious importance of PTC for a healthy tubulointerstitium, PTC density is rarely measured in preclinical studies of fibrosing injuries and is virtually never measured in clinical practice.
QUANTITATION METHODOLOGY: WHAT DO OUR HISTOLOGIC STAINS STAIN?
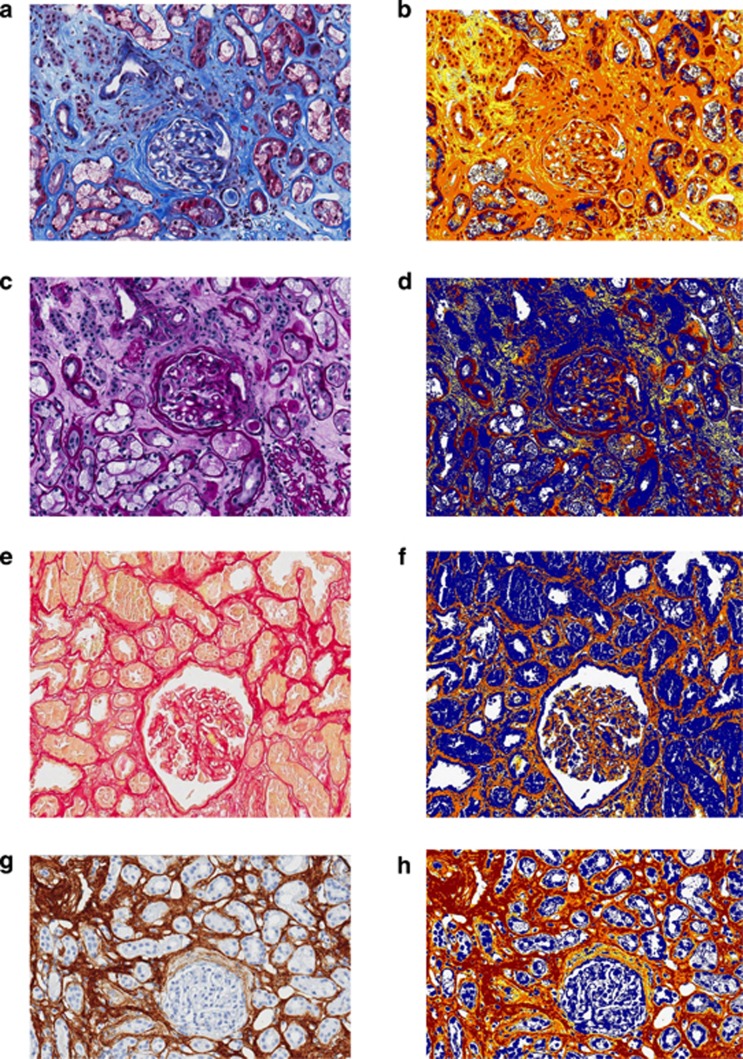
Trichrome staining (Figure 1) is often used in addition to other conventional histologic stains (hematoxylin and eosin, PAS, Silver Methenamine) to assess collagen content in the interstitium. Trichrome staining is quite practical for both clinical management of individual patients and for research studies, as it is widely available and inexpensive. For quantitation, visual assessment of trichrome-stained slides is the standard practice at many institutions;60 however, studies have shown that this approach may have poor reproducibility.61, 62 Part of the reproducibility issue arises from uncertainty as to whether the definition of IF employed is based on total area occupied by the stainable collagen or based on areas containing any amount of stainable collagen (that is, ‘fine fibrosis') as discussed further below and illustrated in studies by Furness et al.63 and Farris et al.64 Trichrome stains may not be sensitive at milder levels of fibrosis. Trichrome dyes are sensitive to length of formalin fixation, which introduces an important variable in studies of renal biopsies, which are not handled uniformly in multi-institutional studies.
Figure 1.
Commonly used stains for the histologic assessment of renal fibrosis. Examples of stains used in the assessment of interstitial fibrosis include (a) trichrome in conjunction with (c) periodic acid–Schiff, (e) Sirius Red, and (g) collagen III immunohistochemistry. In the corresponding ‘mark-up' images (b, d, f, and h) generated by a computer-assisted positive pixel count algorithm applied to the stains, tissue considered ‘positive' is ‘marked up' either yellow, orange, or red, in that order, with increasing positivity of match to the algorithm parameters; and tissue considered ‘negative,' including tubules and blood vessels, is blue. The quantitation algorithm can be used to detect the (b) ‘blue' of trichrome, the (d) ‘pink' of the periodic acid–Schiff–stained basement membranes, the (f) ‘red' of an unpolarized Sirius Red, and the (h) brown of the collagen III immunohistochemistry chromogen. (All images are at an original magnification of × 200.)
Picrosirius Red (also referred to as simply ‘Sirius Red') is examined under both polarized and unpolarized light. Sirius Red is thought to be specific for collagen types I and III under polarized light.65, 66, 67 Because of the high specificity for binding to collagen fibers, this stain has a high signal-to-noise ratio and lends itself to computerized image analysis. However, Sirius Red is not widely used and is subject to discrepancies between polarized and unpolarized measurements. Technical considerations have a large effect on performance, and it will likely be difficult to standardize Sirius Red among different laboratories. Furthermore, studies to test the reproducibility of this methodology across institutions are currently lacking. An important consideration hindering the use of Sirius Red as a standard in measuring IF is that it is more time-consuming and expensive to perform and analyze than a trichrome stain.
Collagen III immunohistochemistry is probably the least widely used fibrosis stain and thus has little clinical validation. As it also discriminates among collagen molecules, and therefore provides a very discrete signal, it has the advantage of lending itself to computerized image analysis. Technical considerations make it difficult to standardize between laboratories and even intralaboratory assays.
Measuring fibrosis
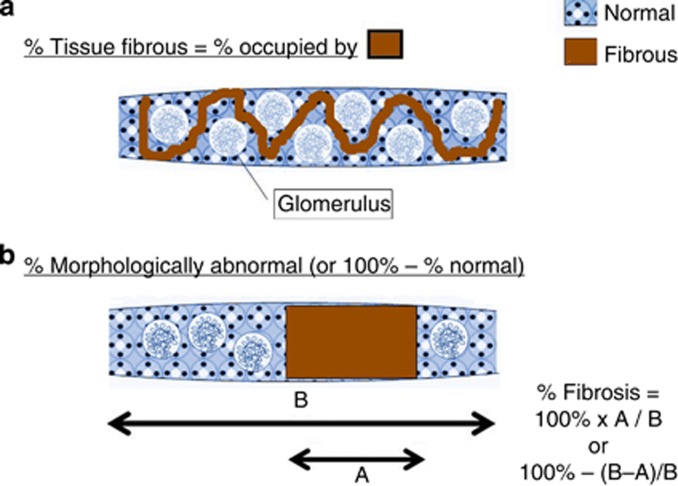
Sufficient data to enable us to decide how to characterize fibrosis of the tubulointerstitium are lacking for human assessment of histology slides. If we cannot agree on definitions, reproducibility will be a problem. Some people consider % IF to be the percent of overall tissue occupied by fibrous tissue, whereas others consider the percent of fibrous tissue to be the % of tissue that is abnormal (Figure 2). These are very different conceptual ways of considering % IF, and these perceived differences can lead to differences in pathologic interpretation and quantitation. For example, in a study that is both insightful and disappointing in its outcome, Furness et al.63 performed an interobserver variability study among 21 pathologists in 15 countries. As a first step, they circulated glass slides from 55 renal allograft biopsies and scored them. A second circulation of the same slides accompanied by feedback on how the individual pathologist deviated from the norm led to a second round of scoring. In a third step, photographs of very selected areas of the slides depicting specific pathologies were circulated. They found that international variation in histologic grading was large (that is, good reproducibility was not achieved), and persistent feedback did not improve reproducibility. For example, the kappa for IF was stated to be 0.295 for all cases circulated (compared with the highest kappa, 0.378, for intimal arteritis); moreover after feedback, the kappa actually went down from 0.306 before feedback to 0.249 after feedback. The kappa for scoring fibrosis was actually less with the use of the highly focused photographs (0.259) compared with the assessments obtained from the glass slides (0.295). In this study, it was pointed out that there is a problem in assessing the ‘area affected' by a progressive process. Therefore, it is clear that definitions have many caveats, and many key definitions are unresolved (these include definitions that encompass the usual forms of IFTA vs. parenchymal contraction in which intervening tubulointerstitial parenchyma between obsolescent glomeruli have been lost; the difference between kidney parenchymal area occupied by fibrotic matrix vs. areas containing both fibrotic matrix and intact glomeruli and tubular structures as discussed above and illustrated in Figure 2; defining the threshold for how much matrix needs to be present to identify a region of the kidney as being involved by fibrosis; and a consideration whether our definition of fibrosis should be a ‘one size fits all' approach (that is, are matrix accumulations of type III collagen equivalent to matrix accumulations of proteoglycans or other matrix proteins?)). Current analytic approaches, in either clinical or preclinical studies, generally avoid rigorous assessment of these issues.
Figure 2.
Characterization of patterns of renal fibrosis. Percent interstitial fibrosis (% IF) can be conceptually thought of in at least two ways: (a) percent of tissue occupied by fibrous tissue and (b) percent of tissue morphologically abnormal. The cartoon depicts a collagen III immunohistochemistry stain in which the chromogen stains fibrosis.
Computer-based morphometry techniques have been used to assess IF, partly because of the interobserver variability that has been shown in the past (Figure 1). These computer-based methods include morphometry of slides stained with trichrome,68, 69 Sirius Red,65, 66, 67 and collagen III immunohistochemistry.70, 71, 72 Analysis in some of these studies has shown correlation with glomerular filtration rate;64, 65, 66, 67, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80 however, as shown in the studies by Farris et al.,64 this may not improve upon assessment made by the unaided human eye.64
Other methodologies could be employed in the future. Other histologic stains that may improve upon our assessments include the Movat's pentachrome stain that allows the assessment of collagen content, proteoglycan content, and elastic tissue content with a single staining procedure.81 Stains to allow measurement of PTC density may also enhance our ability to measure clinically relevant changes in the tubulointerstitium linked to chronic kidney disease. Other sophisticated methods that could show promise in the future could include transcriptomics82, 83 and mass spectrometry.84
Fibrosis-scoring systems currently in clinical practice
Several diagnostic schema include fibrosis as an integral component. The National Institute of Health (NIH) lupus nephritis activity/chronicity indices include a provision for scoring fibrosis,17, 85 and the International Society of Nephrology and Renal Pathology Society Working Group on the Classification of Lupus Nephritis specifies that extent of IF be specified in the pathology report, although a formal score is not provided.86 The Banff Classification for renal allograft rejection includes a provision for IF. It is termed the ‘ci' score.7, 57, 87, 88, 89, 90, 91, 92, 93, 94 However, current Banff working group studies show variability in the way that pathologists score fibrosis.80 The International Study Group of Fabry Nephropathy established a scoring system that included IF.95 The ‘MEST' score developed in the Oxford Classification of IgA nephropathy includes a ‘T' component for a visual estimate of the extent of IF.14, 96 The RPS Classification of Diabetic Nephropathy does not have a formal IF score, although the system does imply that IF parallels the glomerular changes that it emphasizes in the classification.97 Specifying the extent of IF is important in these diseases, as longitudinal studies will use this information to evaluate disease prognosis and the effect of drugs on the disease. Having definitions and standardized approaches that are agreed upon will hopefully help to make the data more meaningful.
Conclusions and recommendations
We have touched on only some of the outstanding issues that confront investigators and clinicians alike in assessing renal fibrosis from pathology specimens. Among the issues critical to these endeavors, but not considered in this review, include sampling variation and artifacts, differences in fibrosis composition between animal models and human diseases, and the dynamics of IF and how features of fibrosis may change over time. In considering the immediate charge of how to best measure fibrosis, we are guided by the findings from prior studies, including our own studies,64 which have the caveat of being performed on a small number of cases with a small number of pathologist participants. Our studies did have an important advantage over many previous studies of reproducibility by utilizing clear definitions of what constitutes a fibrotic parenchyma that were made available to all study pathologists. On the basis of this study, and in conjunction with the studies of others, we conclude that for studies of human renal biopsies, trichrome staining is the cheapest and easiest approach, and, at least when considering the experience of the dedicated participants in the study by Farris et al.,64 can provide good reproducibility. In our opinion, for very fine levels of fibrosis, Sirius Red and collagen III with computerized morphometric assessment may be more robust measures.
Human-based scoring is still quite useful on a routine basis. Although slide scanners are becoming more widespread in their availability, it is still impractical to scan all of the clinical slides that pass through most renal biopsy services and still provide real-time information for clinicians and patients. In this regard, computerized morphometry often is not a practical option. For research-based studies, such as human drug studies and rodent studies, we recommend a morphometry-based analytic system that can provide objective data that are quantifiable on a continuous scale and are sensitive to lower ranges of fibrosis. In the near future, whole-slide scanners will likely become more ubiquitous in diagnostic pathology practices. When that occurs, it is likely that this degree of quantifiable data will be more readily available on human biopsies obtained for clinical management and not as part of study protocols, and possibly provide IF assessments routinely that will be useful in the care of patients with kidney disease.
Acknowledgments
This work was supported in part by grant DK83391 from the National Institutes of Health to CEA. This article is published in a supplement partially supported by the Major State Basic Research Development Program of China (no. 2012CB517700) and the Guangdong Medical Association.
All the authors declared no competing interests.
References
- Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- Zeisberg EM, Potenta SE, Sugimoto H, et al. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg M, Kalluri R. Fibroblasts emerge via epithelial-mesenchymal transition in chronic kidney fibrosis. Front Biosci. 2008;13:6991–6998. doi: 10.2741/3204. [DOI] [PubMed] [Google Scholar]
- Kaissling B, Le Hir M. The renal cortical interstitium: morphological and functional aspects. Histochem Cell Biol. 2008;130:247–262. doi: 10.1007/s00418-008-0452-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen EI, Verroust PJ. Interstitial fibrosis: tubular hypothesis versus glomerular hypothesis. Kidney Int. 2008;74:1233–1236. doi: 10.1038/ki.2008.421. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racusen LC, Solez K, Colvin R. Fibrosis and atrophy in the renal allograft: interim report and new directions. Am J Transplant. 2002;2:203–206. doi: 10.1034/j.1600-6143.2002.20303.x. [DOI] [PubMed] [Google Scholar]
- Farris AB, Colvin RB. Renal interstitial fibrosis: mechanisms and evaluation. Curr Opin Nephrol Hypertens. 2012;21:289–300. doi: 10.1097/MNH.0b013e3283521cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm PC, Nickerson P, Gough J, et al. Quantitation of allograft fibrosis and chronic allograft nephropathy. Pediatr Transplant. 1999;3:257–270. doi: 10.1034/j.1399-3046.1999.00044.x. [DOI] [PubMed] [Google Scholar]
- Risdon RA, Sloper JC, De Wardener HE. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet. 1968;2:363–366. doi: 10.1016/s0140-6736(68)90589-8. [DOI] [PubMed] [Google Scholar]
- Choi BS, Shin MJ, Shin SJ, et al. Clinical significance of an early protocol biopsy in living-donor renal transplantation: ten-year experience at a single center. Am J Transplant. 2005;5:1354–1360. doi: 10.1111/j.1600-6143.2005.00830.x. [DOI] [PubMed] [Google Scholar]
- Colvin RB, Nickeleit V.Renal transplant pathologyIn: Jennette JC, Olson JL, Schwartz MM, Silva FG (eds)Heptinstall's Pathology of the Kidney6th edn, vol. 2Lippincott Williams and Wilkins: Philadelphia, PA, USA; 20061347–1490. [Google Scholar]
- Cosio FG, Grande JP, Wadei H, et al. Predicting subsequent decline in kidney allograft function from early surveillance biopsies. Am J Transplant. 2005;5:2464–2472. doi: 10.1111/j.1600-6143.2005.01050.x. [DOI] [PubMed] [Google Scholar]
- Roberts IS, Cook HT, Troyanov S, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546–556. doi: 10.1038/ki.2009.168. [DOI] [PubMed] [Google Scholar]
- Vilayur E, Harris DC. Emerging therapies for chronic kidney disease: what is their role. Nat Rev Nephrol. 2009;5:375–383. doi: 10.1038/nrneph.2009.76. [DOI] [PubMed] [Google Scholar]
- Boor P, Sebekova K, Ostendorf T, et al. Treatment targets in renal fibrosis. Nephrol Dial Transplant. 2007;22:3391–3407. doi: 10.1093/ndt/gfm393. [DOI] [PubMed] [Google Scholar]
- Austin HA, 3rd, Muenz LR, Joyce KM, et al. Prognostic factors in lupus nephritis. Contribution of renal histologic data. Am J Med. 1983;75:382–391. doi: 10.1016/0002-9343(83)90338-8. [DOI] [PubMed] [Google Scholar]
- Alpers CE, Hudkins KL, Floege J, et al. Human renal cortical interstitial cells with some features of smooth muscle cells participate in tubulointerstitial and crescentic glomerular injury. J Am Soc Nephrol. 1994;5:201–209. doi: 10.1681/ASN.V52201. [DOI] [PubMed] [Google Scholar]
- Humphreys BD, Lin SL, Kobayashi A, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter MG, Hurwitz S, Bellamy CO, et al. Quantitative morphometry of lupus nephritis: the significance of collagen, tubular space, and inflammatory infiltrate. Kidney Int. 2005;67:94–102. doi: 10.1111/j.1523-1755.2005.00059.x. [DOI] [PubMed] [Google Scholar]
- Clapp WL, Croker BP.KidneyIn: Mills SE (ed)Histology for Pathologists3rd edn,Lippincott Williams & Wilkins: Philadelphia, PA, USA; 2007 [Google Scholar]
- Hestbech J, Hansen HE, Amdisen A, et al. Chronic renal lesions following long-term treatment with lithium. Kidney Int. 1977;12:205–213. doi: 10.1038/ki.1977.102. [DOI] [PubMed] [Google Scholar]
- Bohle A, Grund KE, Mackensen S, et al. Correlations between renal interstitium and level of serum creatinine. Morphometric investigations of biopsies in perimembranous glomerulonephritis. Virchows Arch A Pathol Anat Histol. 1977;373:15–22. doi: 10.1007/BF00432465. [DOI] [PubMed] [Google Scholar]
- Kappel B, Olsen S. Cortical interstitial tissue and sclerosed glomeruli in the normal human kidney, related to age and sex. A quantitative study. Virchows Arch A Pathol Anat Histol. 1980;387:271–277. doi: 10.1007/BF00454830. [DOI] [PubMed] [Google Scholar]
- Bonsib SM.Renal anatomy and histologyIn: Jennette JC, Olson JL, Schwartz MM, Silva FG (eds)Heptinstall's Pathology of the Kidney6th edn,Lippincott Williams & Wilkins: Philadelphia, PA, USA; 2007 [Google Scholar]
- Wall SM, Truong AV, DuBose TD., Jr H(+)-K(+)-ATPase mediates net acid secretion in rat terminal inner medullary collecting duct. Am J Physiol. 1996;271:F1037–F1044. doi: 10.1152/ajprenal.1996.271.5.F1037. [DOI] [PubMed] [Google Scholar]
- Stokes MB, Holler S, Cui Y, et al. Expression of decorin, biglycan, and collagen type I in human renal fibrosing disease. Kidney Int. 2000;57:487–498. doi: 10.1046/j.1523-1755.2000.00868.x. [DOI] [PubMed] [Google Scholar]
- Mounier F, Foidart JM, Gubler MC. Distribution of extracellular matrix glycoproteins during normal development of human kidney. An immunohistochemical study. Lab Invest. 1986;54:394–401. [PubMed] [Google Scholar]
- Karkavelas G, Kefalides NA, Amenta PS, et al. Comparative ultrastructural localization of collagen types III, IV, VI and laminin in rat uterus and kidney. J Ultrastruct Mol Struct Res. 1988;100:137–155. doi: 10.1016/0889-1605(88)90021-3. [DOI] [PubMed] [Google Scholar]
- Boor P, Ostendorf T, Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol. 2010;6:643–656. doi: 10.1038/nrneph.2010.120. [DOI] [PubMed] [Google Scholar]
- Dussaule JC, Guerrot D, Huby AC, et al. The role of cell plasticity in progression and reversal of renal fibrosis. Int J Exp Pathol. 2011;92:151–157. doi: 10.1111/j.1365-2613.2011.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meran S, Steadman R. Fibroblasts and myofibroblasts in renal fibrosis. Int J Exp Pathol. 2011;92:158–167. doi: 10.1111/j.1365-2613.2011.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ru Y, Eyden B. The ultrastructure of human tubulo-interstitial fibrosis. J Submicrosc Cytol Pathol. 2003;35:147–160. [PubMed] [Google Scholar]
- Tapmeier TT, Fearn A, Brown K, et al. Pivotal role of CD4+ T cells in renal fibrosis following ureteric obstruction. Kidney Int. 2010;78:351–362. doi: 10.1038/ki.2010.177. [DOI] [PubMed] [Google Scholar]
- Nikolic-Paterson DJ. CD4+ T cells: a potential player in renal fibrosis. Kidney Int. 2010;78:333–335. doi: 10.1038/ki.2010.182. [DOI] [PubMed] [Google Scholar]
- Snelgrove SL, Kausman JY, Lo C, et al. Renal dendritic cells adopt a pro-inflammatory phenotype in obstructive uropathy to activate T cells, but do not directly contribute to fibrosis. Am J Pathol. 2011;180:91–103. doi: 10.1016/j.ajpath.2011.09.039. [DOI] [PubMed] [Google Scholar]
- Lin SL, Castano AP, Nowlin BT, et al. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol. 2009;183:6733–6743. doi: 10.4049/jimmunol.0901473. [DOI] [PubMed] [Google Scholar]
- Anders HJ, Ryu M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney Int. 2011;80:915–925. doi: 10.1038/ki.2011.217. [DOI] [PubMed] [Google Scholar]
- Machida Y, Kitamoto K, Izumi Y, et al. Renal fibrosis in murine obstructive nephropathy is attenuated by depletion of monocyte lineage, not dendritic cells. J Pharmacol Sci. 2010;114:464–473. doi: 10.1254/jphs.10246fp. [DOI] [PubMed] [Google Scholar]
- Pilling D, Fan T, Huang D, et al. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS ONE. 2009;4:e7475. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon MA, Mylonas KJ, Hughes J. Macrophages and renal fibrosis. Semin Nephrol. 2010;30:302–317. doi: 10.1016/j.semnephrol.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Semedo P, Donizetti-Oliveira C, Burgos-Silva M, et al. Bone marrow mononuclear cells attenuate fibrosis development after severe acute kidney injury. Lab Invest. 2010;90:685–695. doi: 10.1038/labinvest.2010.45. [DOI] [PubMed] [Google Scholar]
- Olagne J, Caillard S, Gaub MP, et al. Post-transplant lymphoproliferative disorders: determination of donor/recipient origin in a large cohort of kidney recipients. Am J Transplant. 2011;11:1260–1269. doi: 10.1111/j.1600-6143.2011.03544.x. [DOI] [PubMed] [Google Scholar]
- Kurts C, Heymann F, Lukacs-Kornek V, et al. Role of T cells and dendritic cells in glomerular immunopathology. Semin Immunopathol. 2007;29:317–335. doi: 10.1007/s00281-007-0096-x. [DOI] [PubMed] [Google Scholar]
- Macconi D, Chiabrando C, Schiarea S, et al. Proteasomal processing of albumin by renal dendritic cells generates antigenic peptides. J Am Soc Nephrol. 2009;20:123–130. doi: 10.1681/ASN.2007111233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann F, Meyer-Schwesinger C, Hamilton-Williams EE, et al. Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J Clin Invest. 2009;119:1286–1297. doi: 10.1172/JCI38399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts IS, Brenchley PE. Mast cells: the forgotten cells of renal fibrosis. J Clin Pathol. 2000;53:858–862. doi: 10.1136/jcp.53.11.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamaru Y, Scandiuzzi L, Essig M, et al. Mast cell-mediated remodeling and fibrinolytic activity protect against fatal glomerulonephritis. J Immunol. 2006;176:5607–5615. doi: 10.4049/jimmunol.176.9.5607. [DOI] [PubMed] [Google Scholar]
- Veerappan A, Reid AC, O'Connor N, et al. Mast cells are required for the development of renal fibrosis in the rodent unilateral ureteral obstruction model. Am J Physiol Renal Physiol. 2012;302:F192–F204. doi: 10.1152/ajprenal.00562.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedermeier M, Reich B, Rodriguez Gomez M, et al. CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc Natl Acad Sci USA. 2009;106:17892–17897. doi: 10.1073/pnas.0906070106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai N, Furuichi K, Shinozaki Y, et al. Fibrocytes are involved in the pathogenesis of human chronic kidney disease. Hum Pathol. 2010;41:672–678. doi: 10.1016/j.humpath.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Shao DD, Suresh R, Vakil V, et al. Pivotal Advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol. 2008;83:1323–1333. doi: 10.1189/jlb.1107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekema M, Harmsen MC, van Luyn MJ, et al. Bone marrow-derived myofibroblasts contribute to the renal interstitial myofibroblast population and produce procollagen I after ischemia/reperfusion in rats. J Am Soc Nephrol. 2007;18:165–175. doi: 10.1681/ASN.2005070730. [DOI] [PubMed] [Google Scholar]
- Lin SL, Kisseleva T, Brenner DA, et al. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liptak P, Ivanyi B. Primer: Histopathology of calcineurin-inhibitor toxicity in renal allografts. Nat Clin Pract Nephrol. 2006;2:398–404. doi: 10.1038/ncpneph0225. [DOI] [PubMed] [Google Scholar]
- Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis: the role of bone morphogenic protein-7 and hepatocyte growth factor. Kidney Int Suppl. 2003. pp. S105–S112. [DOI] [PubMed]
- Solez K, Colvin RB, Racusen LC, et al. Banff '05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy ('CAN') Am J Transplant. 2007;7:518–526. doi: 10.1111/j.1600-6143.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- Farris AB, Lawson D, Cohen C, et al. Medullary injury in the human renal biopsy: fibrosis assessment. Mod Pathol. 2013;26:387A–388A. [Google Scholar]
- Steegh FM, Gelens MA, Nieman FH, et al. Early loss of peritubular capillaries after kidney transplantation. J Am Soc Nephrol. 2011;22:1024–1029. doi: 10.1681/ASN.2010050531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreso F, Lopez M, Vallejos A, et al. Serial protocol biopsies to quantify the progression of chronic transplant nephropathy in stable renal allografts. Am J Transplant. 2001;1:82–88. doi: 10.1034/j.1600-6143.2001.010115.x. [DOI] [PubMed] [Google Scholar]
- Marcussen N, Olsen TS, Benediktsson H, et al. Reproducibility of the Banff classification of renal allograft pathology. Inter- and intraobserver variation. Transplantation. 1995;60:1083–1089. doi: 10.1097/00007890-199511270-00004. [DOI] [PubMed] [Google Scholar]
- Furness PN, Taub N. International variation in the interpretation of renal transplant biopsies: report of the CERTPAP Project. Kidney Int. 2001;60:1998–2012. doi: 10.1046/j.1523-1755.2001.00030.x. [DOI] [PubMed] [Google Scholar]
- Furness PN, Taub N, Assmann KJ, et al. International variation in histologic grading is large, and persistent feedback does not improve reproducibility. Am J Surg Pathol. 2003;27:805–810. doi: 10.1097/00000478-200306000-00012. [DOI] [PubMed] [Google Scholar]
- Farris AB, Adams CD, Brousaides N, et al. Morphometric and visual evaluation of fibrosis in renal biopsies. J Am Soc Nephrol. 2011;22:176–186. doi: 10.1681/ASN.2009091005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- Grimm PC, Nickerson P, Gough J, et al. Computerized image analysis of Sirius Red-stained renal allograft biopsies as a surrogate marker to predict long-term allograft function. J Am Soc Nephrol. 2003;14:1662–1668. doi: 10.1097/01.asn.0000066143.02832.5e. [DOI] [PubMed] [Google Scholar]
- Sund S, Grimm P, Reisaeter AV, et al. Computerized image analysis vs semiquantitative scoring in evaluation of kidney allograft fibrosis and prognosis. Nephrol Dial Transplant. 2004;19:2838–2845. doi: 10.1093/ndt/gfh490. [DOI] [PubMed] [Google Scholar]
- Servais A, Meas-Yedid V, Buchler M, et al. Quantification of interstitial fibrosis by image analysis on routine renal biopsy in patients receiving cyclosporine. Transplantation. 2007;84:1595–1601. doi: 10.1097/01.tp.0000295749.50525.bd. [DOI] [PubMed] [Google Scholar]
- Servais A, Meas-Yedid V, Toupance O, et al. Interstitial fibrosis quantification in renal transplant recipients randomized to continue cyclosporine or convert to sirolimus. Am J Transplant. 2009;9:2552–2560. doi: 10.1111/j.1600-6143.2009.02803.x. [DOI] [PubMed] [Google Scholar]
- Feldman DL, Mogelesky TC, Chou M, et al. Enhanced expression of renal endothelin-converting enzyme-1 and endothelin-A-receptor mRNA in rats with interstitial fibrosis following ureter ligation. J Cardiovasc Pharmacol. 2000;36:S255–S259. doi: 10.1097/00005344-200036051-00075. [DOI] [PubMed] [Google Scholar]
- Satoh M, Kashihara N, Yamasaki Y, et al. Renal interstitial fibrosis is reduced in angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol. 2001;12:317–325. doi: 10.1681/ASN.V122317. [DOI] [PubMed] [Google Scholar]
- Nicholson ML, Bailey E, Williams S, et al. Computerized histomorphometric assessment of protocol renal transplant biopsy specimens for surrogate markers of chronic rejection. Transplantation. 1999;68:236–241. doi: 10.1097/00007890-199907270-00013. [DOI] [PubMed] [Google Scholar]
- Moreso F, Seron D, Vitria J, et al. Quantification of interstitial chronic renal damage by means of texture analysis. Kidney Int. 1994;46:1721–1727. doi: 10.1038/ki.1994.474. [DOI] [PubMed] [Google Scholar]
- De Heer E, Sijpkens YW, Verkade M, et al. Morphometry of interstitial fibrosis. Nephrol Dial Transplant. 2000;15 (Suppl 6:72–73. doi: 10.1093/ndt/15.suppl_6.72. [DOI] [PubMed] [Google Scholar]
- Celik B, Randhawa PS. Glomerular changes in BK virus nephropathy. Hum Pathol. 2004;35:367–370. doi: 10.1016/j.humpath.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Meas-Yedid V, Servais A, Noel LH, et al. New computerized color image analysis for the quantification of interstitial fibrosis in renal transplantation. Transplantation. 2011;92:890–899. doi: 10.1097/TP.0b013e31822d879a. [DOI] [PubMed] [Google Scholar]
- Miura Y, Satoh S, Saito M, et al. Factors increasing quantitative interstitial fibrosis from 0 hr to 1 year in living kidney transplant patients receiving tacrolimus. Transplantation. 2011;91:78–85. doi: 10.1097/tp.0b013e3181ff4f7f. [DOI] [PubMed] [Google Scholar]
- Pape L, Mengel M, Offner G, et al. Renal arterial resistance index and computerized quantification of fibrosis as a combined predictive tool in chronic allograft nephropathy. Pediatr Transplant. 2004;8:565–570. doi: 10.1111/j.1399-3046.2004.00229.x. [DOI] [PubMed] [Google Scholar]
- Pape L, Henne T, Offner G, et al. Computer-assisted quantification of fibrosis in chronic allograft nephropaty by picosirius red-staining: a new tool for predicting long-term graft function. Transplantation. 2003;76:955–958. doi: 10.1097/01.TP.0000078899.62040.E5. [DOI] [PubMed] [Google Scholar]
- Farris AB, Chan S, Climenhaga J, et al. Banff fibrosis study: multicenter visual assessment and computerized analysis of interstitial fibrosis in kidney biopsies. Am J Transplant. 2014;14:897–907. doi: 10.1111/ajt.12641. [DOI] [PubMed] [Google Scholar]
- Sigdel S, Gemind JT, Tomashefski JF., Jr The Movat pentachrome stain as a means of identifying microcrystalline cellulose among other particulates found in lung tissue. Arch Pathol Lab Med. 2011;135:249–254. doi: 10.5858/135.2.249. [DOI] [PubMed] [Google Scholar]
- Maluf DG, Mas VR, Archer KJ, et al. Molecular pathways involved in loss of kidney graft function with tubular atrophy and interstitial fibrosis. Mol Med. 2008;14:276–285. doi: 10.2119/2007-00111.Maluf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnag S, Einecke G, Reeve J, et al. Molecular correlates of renal function in kidney transplant biopsies. J Am Soc Nephrol. 2009;20:1149–1160. doi: 10.1681/ASN.2008080863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S, Vrana JA, Theis JD, et al. Mass spectrometry based proteomics in the diagnosis of kidney disease. Curr Opin Nephrol Hypertens. 2013;22:273–280. doi: 10.1097/MNH.0b013e32835fe37c. [DOI] [PubMed] [Google Scholar]
- Austin HA, 3rd, Boumpas DT, Vaughan EM, et al. Predicting renal outcomes in severe lupus nephritis: contributions of clinical and histologic data. Kidney Int. 1994;45:544–550. doi: 10.1038/ki.1994.70. [DOI] [PubMed] [Google Scholar]
- Weening JJ, D'Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15:241–250. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- Mengel M, Sis B, Haas M, et al. Banff 2011 Meeting report: new concepts in antibody-mediated rejection. Am J Transplant. 2012;12:563–570. doi: 10.1111/j.1600-6143.2011.03926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racusen LC, Colvin RB, Solez K, et al. Antibody-mediated rejection criteria - an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3:708–714. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- Racusen LC, Halloran PF, Solez K. Banff 2003 meeting report: new diagnostic insights and standards. Am J Transplant. 2004;4:1562–1566. doi: 10.1111/j.1600-6143.2004.00585.x. [DOI] [PubMed] [Google Scholar]
- Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- Sis B, Mengel M, Haas M, et al. Banff '09 Meeting Report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10:464–471. doi: 10.1111/j.1600-6143.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- Solez K. History of the Banff classification of allograft pathology as it approaches its 20th year. Curr Opin Organ Transplant. 2010;15:49–51. doi: 10.1097/MOT.0b013e328334fedb. [DOI] [PubMed] [Google Scholar]
- Solez K, Axelsen RA, Benediktsson H, et al. International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney Int. 1993;44:411–422. doi: 10.1038/ki.1993.259. [DOI] [PubMed] [Google Scholar]
- Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753–760. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- Fogo AB, Bostad L, Svarstad E, et al. Scoring system for renal pathology in Fabry disease: report of the International Study Group of Fabry Nephropathy (ISGFN) Nephrol Dial Transplant. 2010;25:2168–2177. doi: 10.1093/ndt/gfp528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattran DC, Coppo R, Cook HT, et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76:534–545. doi: 10.1038/ki.2009.243. [DOI] [PubMed] [Google Scholar]
- Tervaert TW, Mooyaart AL, Amann K, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21:556–563. doi: 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]