Abstract
Staphylococcus aureus is an important pathogen manifesting virulence through diverse disease forms, ranging from acute skin infections to life-threatening bacteremia or systemic toxic shock syndromes. In the latter case, the prototypical superantigen is TSST-1 (Toxic Shock Syndrome Toxin 1), encoded by tst(H), and carried on a mobile genetic element that is not present in all S. aureus strains. Transcriptional regulation of tst is only partially understood. In this study, we dissected the role of sarA, sarS (sarH1), RNAIII, rot, and the alternative stress sigma factor sigB (σB). By examining tst promoter regulation predominantly in the context of its native sequence within the SaPI1 pathogenicity island of strain RN4282, we discovered that σB emerged as a particularly important tst regulator. We did not detect a consensus σB site within the tst promoter, and thus the effect of σB is likely indirect. We found that σB strongly repressed the expression of the toxin via at least two distinct regulatory pathways dependent upon sarA and agr. Furthermore rot, a member of SarA family, was shown to repress tst expression when overexpressed, although its deletion had no consistent measurable effect. We could not find any detectable effect of sarS, either by deletion or overexpression, suggesting that this regulator plays a minimal role in TSST-1 expression except when combined with disruption of sarA. Collectively, our results extend our understanding of complex multifactorial regulation of tst, revealing several layers of negative regulation. In addition to environmental stimuli thought to impact TSST-1 production, these findings support a model whereby sporadic mutation in a few key negative regulators can profoundly affect and enhance TSST-1 expression.
Introduction
Staphylococcus aureus is a versatile commensal human pathogen capable of causing a large spectrum of diseases ranging from skin infections such as furuncles and impetigo to severe systemic illness including bacteriemia, endocarditis, osteomyelitis, and deep tissue abscesses [1]. S. aureus can transiently colonize the anterior nares, axillae, perineum or the vagina in an estimated 30% of the world’s population without provoking any disease in the vast majority of cases [2]. Under certain circumstances, however, S. aureus can gain access to underlying tissue and potentially spread throughout the body, thus becoming a particularly dangerous opportunistic pathogen [1].
In order to provoke the wide range of disease pathology, S. aureus uses an arsenal of core-chromosomally encoded virulence factors (VF) including surface associated proteins, exoenzymes, and capsular polysaccharides, allowing adhesion, immune response evasion and tissue destruction [3]. The acquisition of mobile genetic elements often encoding one or more toxins, additional immune evasion factors, or antibiotic resistance determinants augments the VF repertoire and the potential disease spectrum [4–7].
Toxic shock syndrome (TSS) is a potentially fatal illness, characterized by fever, skin rash, desquamation, hypotension, and hemodynamic shock. The TSS Toxin-1 (TSST-1) is the causative toxin for the majority of menstrual-related and half of surgical-related TSS cases while the remaining cases are triggered by enterotoxins with superantigenic properties [8, 9].
The TSST-1 superantigen toxin, encoded by tst (tstH), is not ubiquitous. It is found on various mobile pathogenicity islands (SaPI): SaPI1 (in strain RN4282), the closely related SaPIn1 (in strain N315), SaPIm1 (in strain Mu50), SaPI2 (in strain RN3984) and SaPIbov1 (in strain RF122) [10–13]. Estimates of the prevalence of strains encoding tst and sampled from healthy carriers ranges from 13–25%, indicating a large disease potential, yet the case incidence is relatively low (1-4/100,000) [14–18]. Recent studies demonstrate that SaPIs, including those encoding tst, can be packaged and efficiently disseminated by bacteriophage thus aiding their dissemination [10, 19]. Remarkably, tst is absent from most S. aureus model strains widely used for virulence regulation studies, such as NCTC8325 derivatives (RN6390, 8325–4, RN4220, SH1000, HG003), Newman, COL, and USA300, and thus the molecular pathways influencing this major superantigenic toxin remain largely unexplored [20, 21].
Various environmental triggers that influence the expression of TSST-1 have been described, such as glucose (via the ccpA catabolite repressor), O2 (possibly via the srrAB two-component system), magnesium ions, the α and β chains of hemoglobin, growth rate, pH, and TSST-1 itself [22–30]. Sub-inhibitory concentrations of nafcillin were found to induce TSST-1 expression at the transcriptional level whereas clindamycin, linezolid, and tigecycline were found to reduce TSST-1 expression [31]. A diverse number of chemical compounds displaying surfactant, membrane-active, or metabolic inhibitors also reduced TSST-1 expression [32]. The relatively high percentage of S. aureus circulating strains carrying tst coupled with the relatively low incidence of TSS argues strongly that tst expression sufficient to evoke disease occurs sporadically only with the proper combination of environmental and genetic regulation. Notably, this includes toxin susceptibility correlated with certain HLA Class II haplotypes, attenuated quorum sensing by probiotic strains within the vaginal mucosal microenvironment, circulating antibody titers sufficient to contain low level expression, as well as unexplored factors that lead to a reported 170-fold variation of TSST-1 levels detected in clinical tst samples [14, 33–35].
The complexity of S. aureus virulence regulation arises from the large number of global regulators involved in the process [28, 36, 37]. Two prominent factors thought to control TSST-1 expression are agr and sarA. The agr system, which responds to the quorum sensing auto-inducing peptide (AIP) and its effector trans-acting RNA termed RNAIII up-regulates TSST-1 [28, 38–40]. Whether the agr system effect depends upon direct interaction of RNAIII with tst transcript or alternatively by RNAIII-mediated Rot (repressor of toxins) inactivation has not been established [41–43]. The global regulator SarA has been shown to influence tst expression directly via binding of SarA to sarA cis-responding elements present on the tst promoter [39, 40]. The CcpA repressor responding to glucose binds to a cognate cre element overlapping the tst translation start site [25, 40]. Recent data show that CcpA DNA binding can also be regulated by phosphorylation mediated by HprK/HPr and metabolic cues as well as via the Stk1/Stp1 serine-threonine kinase implicated in cell wall stress sensing and antibiotic resistance [44, 45].
Previous knowledge regarding tst transcriptional regulation is derived from different strains and genetic strategies generating difficulties with the elaboration of a unified regulation pattern for this toxin. The various models include a Ptst::luxAB transcriptional fusion reporter stably inserted in various tst- strains, overexpressing TSST-1 using tst cloned on multicopy plasmids, antisense knockdown, or clinical tst+ strains harboring SaPIs such as RN4282 and MN8 [20, 22, 25, 26, 28, 30, 40, 46].
For the study reported herein, we primarily focused on RN4282, a prototypical strain bearing SaPI1, since this strain was used in the context of TSST-1 gene discovery and description of TSST-1 auto-regulatory properties and is amenable to genetic manipulation [11, 13, 26]. We discovered that the alternative stress sigma factor, sigB was required to exert strong repression of tst and TSST-1 expression. We propose that at least two different pathways mediate this effect through regulation of both sarA and agr/RNAIII. In addition, we found that sarS, a member of the SarA superfamily, imparts an additional level of negative regulation over tst expression but only consistently when combined with disruption of sarA. Collectively, these results provide additional insight into tst expression by defining negative regulators and thus exposing the potential for strongly enhanced TSST-1 expression in the event of sporadic mutation of these systems in circulating S. aureus tst + strains.
Materials and Methods
Bacterial strains
Strains and plasmids used in this study are listed in Table 1. Escherichia coli strains were grown in Luria-Bertani broth (LB) and Staphylococcus aureus strains were grown in Muller-Hinton broth (MHB). Media were supplemented with ampicillin (100 μg/ml), kanamycin (40 μg/ml), tetracycline (1–3 μg/ml), erythromycin (5 μg/ml) or chloramphenicol (15 μg/ml) when appropriate. Recombinant lysostaphin was obtained from AMBI Products LLC (Lawrence, New York). Derivatives of RN4282 containing the various indicated mutations were obtained by bacteriophage-mediated transduction using phage 80α and standard genetic procedures. All strain constructions were verified by PCR assay and appropriate primers.
Table 1. Bacterial strains and plasmids used in this study.
| Strain/plasmid | Revelant genotype or characteristic | Source/reference |
|---|---|---|
| E.coli | ||
| DH5α | restriction deficient DNA cloning strain | Gibco/BRL |
| S. aureus | ||
| RN4220 | restriction defective strain which accepts foreign DNA | [13] |
| N315P | MRSA strain N315 lacking penicillinase plasmid | [108] |
| RN4282 | clinical strain harboring SaPI1 with tst | [11] |
| ALC1001 | sigB mutant (Tn917) of RN6390 | [99] |
| ALC2057 | RN6390 sarA::kan | [63] |
| SH1000 | 8325–4 derivative with rsbU deletion repared | [21] |
| PC1072 | 8325–4 ptst -luxAB::geh Tcr | [22] |
| DA101 | PC1072 sarA::kan | [40] |
| KT201 | 8325–4 sarH1(sarS)::pKT200 ermr | [59] |
| PM466 | RN6390 agr-null, rot::tet Tcr | [64] |
| HI2672 | rot::ery identical to WA525 | [49] |
| WA400 | 8325–4 ΔRNAIII-hld region::cat | [109] |
| DA140 | RN4282 sigB- eryr, 80α transductant of ALC1001 | This study |
| DA141 | DA140 + pDA205 | This study |
| DA142 | RN4282 sarA::kan, 80α transductant of ALC2057 | This study |
| DA143 | RN4282 sarA::kan, 80α 2nd transductant of ALC2057 | This study |
| DA150 | SH1000 ptst-luxAB::geh Tcr, 80α transductant from PC1072 | This study |
| DA155 | RN4282 sarS::pKT200, 80α transductant from KT201 | This study |
| DA156 | DA142 sarS::pKT200, 80α transductant from KT201 | This study |
| DA158 | RN4282 ΔRNAIII-hld region::cat,80α transductant from WA400 | This study |
| DA160 | DA158 rot::tet, 80α transductant from PM466Δrot | This study |
| AJ1049 | RN4282 rot::ery, 80α transductant of HI2672 | This study |
| AJ1055 | AJ1049 + pWA163 | This study |
| AJ1060 | DA142 + pMK4 (empty) | This study |
| AJ1062 | DA142 + pAJ973 | This study |
| AJ1056 | DA155 + pMK4 (empty) | This study |
| AJ1058 | DA155 + pAM1865 | This study |
| Plasmids | ||
| pMK4 | E.coli-S.aureus shuttle plasmid, ampr camr | [110] |
| pWA163 | pAS1 containing rot under control of the xylA promoter Tcr | [49] |
| pDA200 | pMK4 containing Not1-Kpn Hu promoter region camr | [40] |
| pDA205 | pDA200 containing a KpnI-PstI sigB fragment camr | This study |
| pAJ973 | pMK4 containing sarA under control of its native promoter camr | This study |
| pAM1865 | pDA200 containing a KpnI-PstI sarS fragment camr | This study |
Construction of a sigB expression vector
Expression of sigB gene under the control of the nucleoid protein pHu promoter was constructed as follows. Briefly, a polymerase chain reaction (PCR) amplification of the sigB gene was performed by using N315 genomic DNA as template and primers sigBKpnRBSF and sigBPstR2 (see Table 2). After digestion, the PCR fragment was cloned into KpnI and PstI restriction sites of pDA200, a pMK4 derivative containing S. aureus HU promoter sequence [40, 47]. The resulting plasmid, pDA205, was sequence verified and electroporated into non-restrictive S. aureus strain RN4220 prior to transfer to DA140 strain (ΔsigB). Restoration of a functional σB in the resulting complemented strain, DA141, was confirmed by detection of yellow pigmentation and by transcriptional analysis of the exclusively SigB-dependent gene asp23 [48].
Table 2. Primers and probes used in this study.
| Name | Primer sequence (5'-3') |
|---|---|
| sigBKpnRBSF | GGGGTACCAGGAGGTGAATGTCTAATGGCGAAAGAGTCGAAATCAGC |
| sigBPstR2 | AACTGCAGCTATTTATGTGCTGCTTCTTGTAATTTCTTAA |
| sarAXbaBamHI | TGGTCTAGAGGATCCGTGCCATTAGTGCAAAACCTCTTAACA |
| sarAHindIIIPst | TATAACGTTCTGCAGGCGTTGATTTGGGTAGTATGCTTTGAC |
| sarSKpnRBSF | CGGGGTACCAGGAGGTGAATGTCTAATGAAATATAATAACCATGACAAAATTAGAGA |
| sarSPstR | AAAACTGCAGTTATTCAAAAACAAGATGTAAATGATCTTTATCTG |
| tst-39F | CCCTTTGTTGCTTGCGACA |
| tst-119R | GCTTTTGCAGTTTTGATTATTTGATT |
| tst-59T | TCGCTACAGATTTTACCCCTGTTCCCTTATCA |
| lux-1578F | CCGTTAACCCACACGCG |
| lux-1637R | TGCTCGTCGCATTCACAAAT |
| lux-1596T | TCACTGAAGGCGGTCCTGCGC |
Underlined regions represent restriction enzyme sequences
Construction of a sarA expression vector
Expression of sarA under the control of its entire native promoter containing its three known transcription start sites was constructed as follows. Briefly, PCR amplification of a region encompassing P3-P2-P1sarA sequence was performed by using N315 genomic DNA as template and primers sarAXbaBamHI and sarAHindIIIPst (see Table 2). After digestion, the PCR fragment was cloned into BamHI and PstI restriction sites of pMK4. The resulting plasmid, pAJ973, was sequence verified and electroporated into non-restrictive S. aureus strain RN4220 prior to transfer to DA142 strain, resulting in sarA restored strain AJ1062.
Generation and complementation of rot mutation
Whereas certain experiments used rot::tet (PM466),we engineered an alternative rot::ery mutation in RN4282 by transduction from strain HI2672 (identical to WA525, kindly provided by D. Frees, Copenhagen, Denmark), generating AJ1049 strain (see Table 1) and compatible with the tetracycline resistant and xylose-inducible rot expression vector pWA163. pWA163 was electroporated into AJ1049, resulting in the conditional rot restored AJ1055 strain. Induction of Rot expression was obtained following previously published procedures [49].
Total RNA extraction and real-time qRT-PCR assays
Overnight bacterial cultures were diluted in MHB (1/100) and grown at 37°C with vigorous agitation (210 rpm) and aerobic conditions (culture volume to tube ratio never exceeded 1:5 and with loosened screw caps) until OD600 = 1.5 to 2. Pilot studies determined by growth curves showed that sampling conditions corresponded with late exponential/post-exponential phase unless otherwise noted. Bacteria were harvested and RNA extracted as previously described [50]. The absence of contaminating DNA was always verified for every experiment by PCR using qRT-PCR probes in the absence of reverse transcription, as described [51]. Tst, lux and sarS qRT-PCR primers and probes were designed using Primer Express software and are indicated in Table 2. The sarA transcript levels were monitored using probe sets sarA 17F, 167R, and 45T, and RNAIII transcript levels were monitored with RNAIII 367F, 436R, and 388T as previously described [52]. The raw mRNA levels determined from the midpoint cycle threshold (c t) from the various strains were normalized to 16S rRNA levels, which were assayed in each round of qRT-PCR as internal controls. Data were collected for a minimum of three independent determinations. The statistical significance of strain-specific differences in normalized cycle threshold (c t) values of each transcript probe was evaluated by Student’s paired t test, and data were considered significant when P was < 0.05. For convenience in figure presentation, data were plotted to reflect fold change or %mRNA levels. Nevertheless, the reported p values always correspond to calculations with the normalized cycle thresholds. Normalized cycle threshold values for three independent experiments for the data displayed in each figure are provided in supplementary materials (S2 Fig and S1 Table).
Immunoblot analysis
Culture supernatants of RN4282 strain and derivatives were collected at OD600 1.5 to 2, and normalized based on OD600 values. Two concentration methods were found comparable. Equal amounts (20μg) of purified carbonic anhydrase gel chromatography standard (29kD) protein (Sigma) were added to each normalized supernatant as an internal control for sample recovery from spin microconcentrators (Millipore 10K MWCO), or trichloroacetic acid precipitation. The carbonic anhydrase spike also served as a marker for western transfer (followed by Ponceau Red staining of PVDF membranes as a loading control) since secreted proteins are often invisible by Coomassie staining, or can vary with strains. Alternatively after centrifugation, supernatants were precipitated with 10% (v/v) trichloroacetic acid (TCA), followed by one cycle of -20°C freezing and thawing, followed by centrifugation at 13000 rpm for 15 min. The final pellet was washed twice with 10% TCA and 80% acetone and resuspended in 40 μl of Tris-EDTA buffer, pH 8.0. Samples were spin concentrated according to the manufacturer’s recommendations. Aliquots of total exoproteins (8 μl) were loaded with an equal volume of Laemmli buffer on 12% SDS-PAGE gels and subsequently transferred onto a polyvinylidene difluoride membrane (PVDF, Bio-Rad). Uniformity of protein loading was confirmed post-transfer by Ponceau-red staining (S1 Fig). After blocking the membranes with 5% low fat milk in phosphate buffered saline, TSST-1 was probed with a 1:10,000 dilution of polyclonal anti-TSST-1 antibody (Thermo Scientific, Illinois, USA) followed by incubation with a secondary HRP-conjugated goat anti-rabbit antibody (1:10,000 BioRad). Chemiluminescence was detected using the Western Pico Super Signal reagent and the manufacturer’s recommendations (Pierce).
For time course experiments, cultures were diluted and grown as above and aliquots were removed at the indicated times and concentrated as above. For meaningful comparison, OD600 normalizations were performed for each time point, so that time points are comparable for each strain tested.
Luciferase assay
Bacterial cultures of PC1072 and DA150 luxAB reporter strains were first grown overnight in MHB containing the appropriate antibiotic and then diluted in 5ml of antibiotic-free MHB, to a final OD600 of 0.01. Cultures were then grown in aerobic conditions as described above. For the assay of luciferase activity, bacterial cultures were first normalized to an OD600 of 0.5 in a total volume of 1 ml and immediately measured with a Glomax luminometer (Promega) by addition of 20 μl of 1% decanal solution (Sigma, freshly prepared (v/v) in absolute ethanol). The statistical significance of strain-specific differences in light emission (expressed in arbitrary units) was evaluated by Student’s paired t test, and data were considered significant when P was <0.05.
Results
Many strains derived from NCTC 8325 are now known to harbor an 11 bp deletion in the gene encoding rsbU [53]. Consequently, the alternative stress sigma factor σB response remains greatly attenuated because σB remains complexed with its anti-sigma factor RsbW. This finding has prompted a re-evaluation of virulence factor regulation using strains possessing a functionally restored rsbU (for example, SH1000 or HG003) [21, 54]. In order to examine the effects of sigB disruption on tst transcription we made use of two distinct model systems: a Ptst::luxAB transcriptional fusion using an approximately 400 bp region harboring the presumptive integrality of the Ptst promoter as well as direct examination of tst transcription using strain RN4282 containing SaPI1 and tst in a native pathogenicity island genomic context [22, 40].
SigB helps repress tst expression
Previous studies performed in our laboratory showed strong activation of the tst promoter in post-exponential growth phase using a Ptst::luxAB transcriptional fusion present in the rsbU- strain PC1072 [39, 40]. Since σB activity in rsbU- strains is markedly lower than rsbU+ strains, we hypothesized that σB activity could conceivably affect tst promoter activity [55]. To address this point, we first analyzed the tst promoter activity in rsbU- and rsbU+ strain backgrounds (8325–4 and SH1000 derivatives, respectively). The results are shown in Fig 1A.
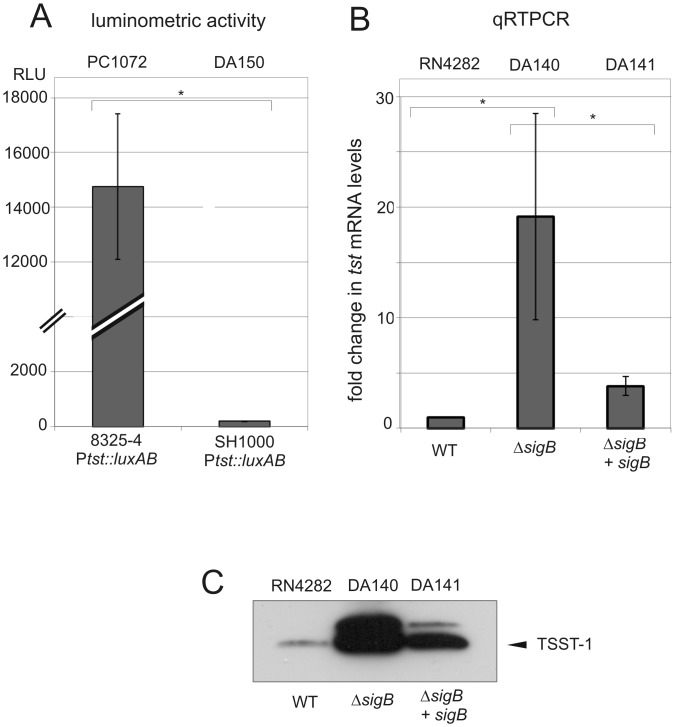
Fig 1. The effect of rsbU or sigB disruption on tst expression.
A. Luciferase reporter assay for the tst promoter. The histogram shows measured luminometric activity of the indicated strains in post-exponential growth phase (Materials and Methods). RLU: relative light units. B. Quantitative qRT-PCR measurements of tst transcripts levels in sigB wt, sigB mutant, and sigB complemented strains, in post-exponential growth phase, setting reference RN4282 as 1. Bars show +/- standard deviations. All data were compiled from three independent experiments. Statistical significance was evaluated by Student’s paired t test, and data were considered significant when P was <0.05 C. Western blot of TSST-1, using anti-TSST-1 polyclonal antibody after precipitation from supernatants of the indicated strains. Note the appearance of a strong band corresponding to unprocessed precursor TSST-1 detected in the absence of sigB and diminished upon reintroduction of multicopy sigB (upper band). The experiment shown is representative of several independent experiments.
We observed that luciferase activity levels in the rsbU+ strain (DA150) were significantly lower compared to the luciferase activity recorded from the rsbU- PC1072 strain. Several independent isolates of DA150 arising from the bacteriophage-mediated transduction transfer of the ptst-luxAB reporter showed similar results suggesting that the observed reduction of the luciferase reporter activity was linked with the restoration of a functional σB pathway and did not arise from a trivial spurious mutation. To further explore the role of sigB on tst expression, we next examined tst transcription directly by performing qRT-PCR and using strain RN4282 and its corresponding isogenic ΔsigB derivative DA140. In post-exponential growth phase, the steady-state tst transcripts levels were strongly and significantly increased by at least an order of magnitude in the ΔsigB strain DA140 compared to the parent strain RN4282 (Fig 1B). A similar induction (approximately 10-fold) was observed in exponential growth phase prior to the onset of agr-mediated quorum sensing regulation of tst via RNAIII production [40]. Reintroduction of sigB+ cloned on a multicopy plasmid, DA141, significantly reduced tst transcript levels compared to the ΔsigB mutant (Fig 1B). Taken together, we conclude from these studies that disruption of the alternative stress sigma factor σB pathway by either of two methods, sigB deletion, or by use of defective rsbU, results in significantly enhanced tst transcription.
In order to extend these findings, we next examined the impact of sigB disruption on secreted extracellular TSST-1 protein levels. Culture supernatants of RN4282 and its mutant derivatives were sampled in the post-exponential growth phase under the same conditions as for RNA analysis and examined by western blot analysis using anti-TSST-1 antibody. The results are shown in Fig 1C.
Strikingly, we observed higher amounts of TSST-1 in supernatants prepared from the ΔsigB strain compared to supernatants obtained from either RN4282 or to the sigB+ restored strain DA141. Re-introduction of sigB+ on a multicopy plasmid DA141 led to a significant diminution of TSST-1 in culture supernatants as judged by western blot analysis. The results showed, however, that σB-dependent repression was not restored entirely to wild type levels observed in RN4282 and this may be explained by experimental system employed and use of a multicopy plasmid. Nevertheless, these results point to a strong concordance between the tst transcriptional profile and the secreted TSST-1 profile (Fig 1B and 1C) with regard to sigB. Collectively, we conclude from these results that the presence of a functional sigB exerts a strong repressive effect on tst and subsequent TSST-1 expression in the RN4282 genetic background.
Expression of the global virulence regulators sarA, RNAIII and sarS in RN4282 and its ΔsigB derivative
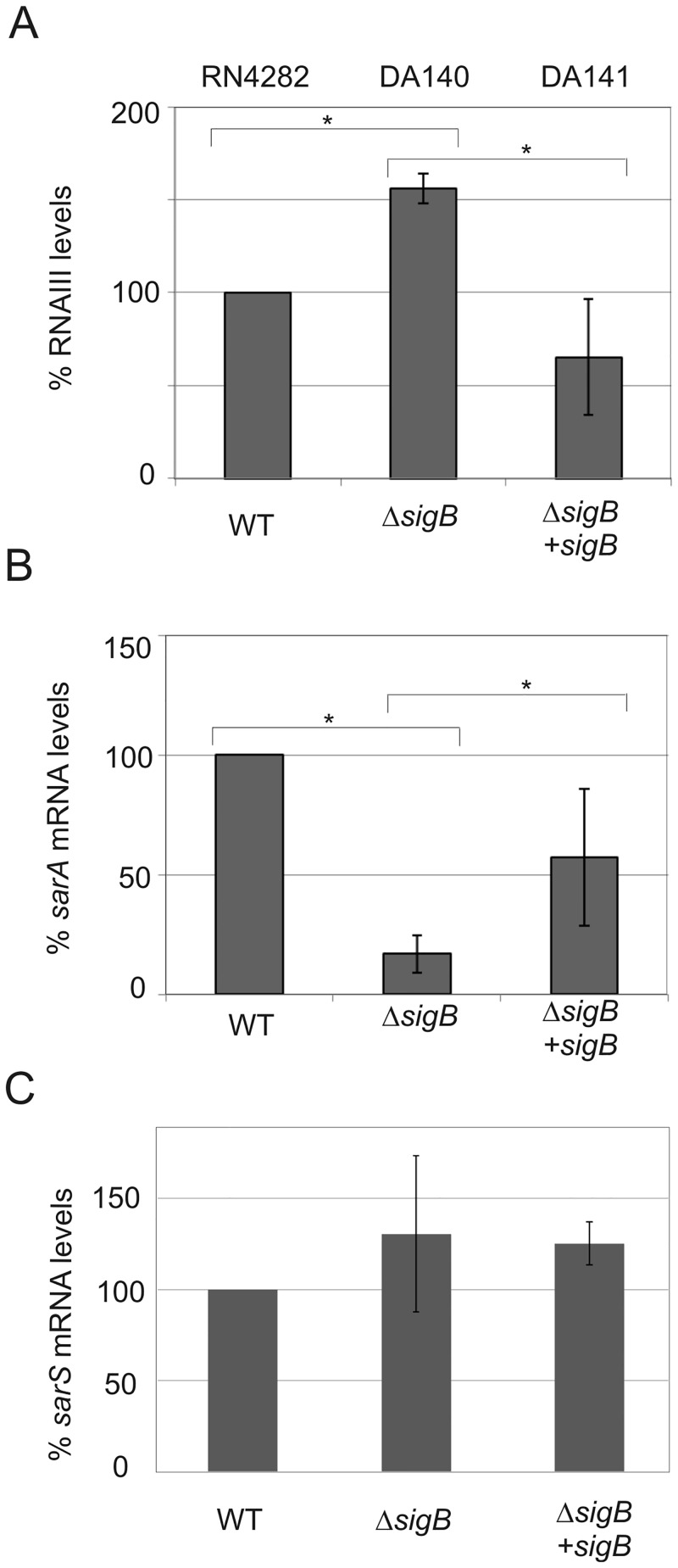
Inspection of the tst promoter sequence failed to reveal the presence of a canonical σB consensus recognition sequence (GTTTWWN 12-15 GGGWAW), previously established in Bacillus subtilis and refined following S. aureus transcriptomic analysis of a ΔsigB mutant [48, 56, 57]. Our previous work had detected only a single tst transcription start site by 5’-RACE analysis in RN4282 [40]. Since we found no σB consensus sequence within the tst promoter, we examined the possibility that an indirect regulatory mechanism could account for the sigB-mediated effect on TSST-1 expression. Since sigB has been previously shown to have an effect on sarS, sarA and RNAIII transcription, we measured steady state transcript levels in RN4282 and its isogenic ΔsigB derivative strain DA140 by qRT-PCR using probes specific for sarS, sarA and RNAIII (Fig 2) [48, 58, 59]. Interestingly, we found that the sigB disruption had no significant effect on sarS mRNA levels in this strain background (Fig 2C), while in contrast, a significant effect of the sigB disruption was observed on sarA (Fig 2B) and a modest effect on RNAIII (Fig 2A). RNAIII steady state levels in post-exponential phase revealed a moderate, yet statistically significant, increase in RNAIII level (150%, p<0.05) in the ΔsigB mutant compared to the parental RN4282 strain, or the sigB+ restored strain DA141 (Fig 2A). However, whether this slight, but statistically significant, change in RNAIII levels is physiologically relevant is difficult to ascertain. We observed a five-fold reduction (20% of the wild type level) of sarA mRNA in the ΔsigB mutant strain compared to the parental RN4282 strain (Fig 2B). Restoration of sigB on the multicopy vector (strain DA141) did significantly increase sarA mRNA levels almost three-fold compared to sigB mutant, reaching 57% of wild-type levels. Interestingly, this incomplete rescue of sarA levels, observed in the sigB restored strain DA141, mirrors the incomplete restoration of tst repression in the same DA141 strain (Fig 1B). Taken together, these results show that sigB-dependent modulation of tst transcription most likely involves contribution including, but perhaps not restricted to, a molecular pathway involving SarA regulator and RNAIII.
Fig 2. The effect of sigB disruption on RNAIII (A), sarA (B) and sarS (C) transcript levels in RN4282 and its derivatives using quantitative qRT-PCR measurements of RNA expression.
Relative changes are shown in each panel using RN4282 as 100%. Bars show +/- standard deviations and all data were compiled from three independent experiments. Statistical significance was evaluated by Student’s paired t test, and data were considered significant when P was <0.05.
The consequences of disruption of sigB for virulence factor regulation have consistently noted reciprocal regulation; notably, deletion of sigB leads to enhanced RNAIII transcription and decreased SarA transcription [48]. The regulation of tst was not reported in that study, but our results above further confirm this reciprocal regulation. To explore regulatory circuitry further, we next examined the roles of RNAIII, rot, sarA, and sarS.
Effect of the virulence regulators RNAIII and rot on TSST-1 expression in RN4282
The quorum sensing two component system agr modulates the expression of RNAIII, which in turn can affect gene expression by various mechanisms [43]. One of the genes translationally regulated by RNAIII is rot (repressor of toxins), which encodes a DNA binding protein thought to act as a global repressor of virulence genes including, for example, hemolytic toxins [41, 60, 61]. Rot is thought to act primarily as a repressor, although there are reported exceptions and recent work suggests that Rot could also cooperate positively with the two-component system SaeRS to activate expression of superantigen-like exoproteins [42, 62]. To address the question whether rot positively or negatively regulated tst expression, we constructed isogenic derivatives of RN4282 lacking RNAIII (DA158), rot (AJ1049) and both RNAIII and rot (DA160); complementation of the rot deletion by its expression on a xylose-inducible vector, was also analyzed (AJ1055). The results are shown in Fig 3.
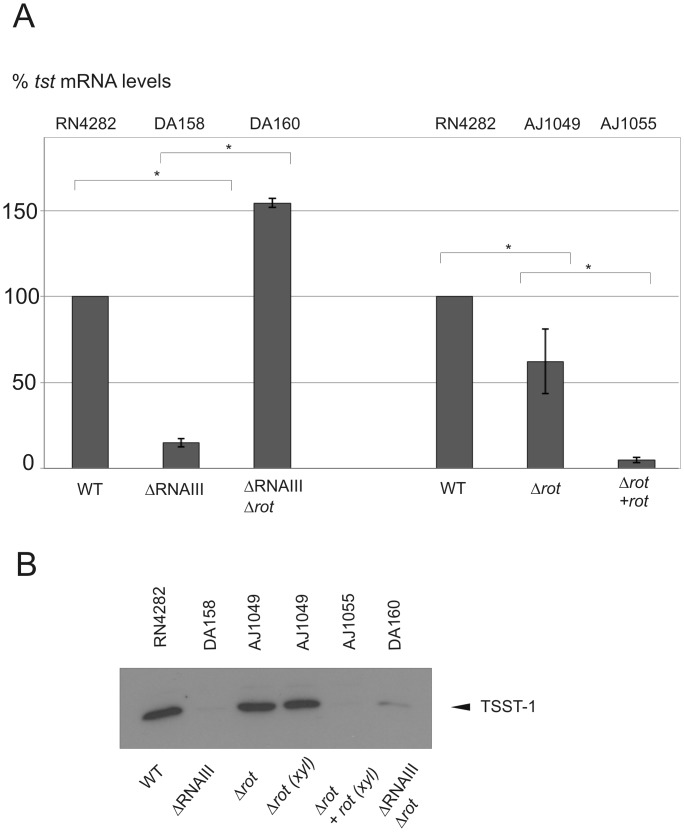
Fig 3. The effect of RNAIII and rot disruption or overexpression on tst and TSST-1 in RN4282.
A. Quantitative qRT-PCR measurements of tst expression and setting RN4282 as 100%. Bars show +/- standard deviations. All data were compiled from three independent experiments. Statistical significance was evaluated by Student’s paired t test, and data were considered significant when P was <0.05. B. Western blot of TSST-1, using anti-TSST-1 polyclonal antibody after precipitation from supernatants of the indicated strains. In lane 4 and 5 both samples include xylose to discard effect of the latter on tst expression. The experiment shown is representative of several independent experiments.
We observed that loss of RNAIII (strain DA158) resulted in a significant decrease of tst transcription (>5-fold) and expression, consistent with our observations that tst transcripts are virtually undetectable in exponential growth prior to the onset of agr-mediated sensing system. Disruption of rot provoked a slight, but significant diminution of tst transcripts levels (Fig 3A), without a consistently visible change on TSST-1 secreted levels (Fig 3B). The restoration of Rot expression from a multi-copy complementing plasmid (AJ1055) strongly reduced both tst transcripts levels and TSST-1 secreted levels compared to the Δ rot strain (AJ1049); xylose alone in the absence of the complementing plasmid had no effect on tst expression. Taken together, these results suggest that RNAIII enhances TSST-1 expression in post-exponential phase as expected; whereas rot disruption has, under our experimental conditions, no consistently measurable effect at the toxin protein expression level, but when overexpressed strongly represses at both transcriptional and expression level. These results are consistent with the model whereby RNAIII/agr activates tst transcription. Of note, no Rot binding site consensus has been identified in S. aureus to date. Finally, combination of both RNAIII and rot mutations only slightly increased tst transcription (1.5-fold), but consistently did not affect TSST-1 expression, as judged by western blot analysis (Fig 3).
Effect of the global virulence regulators sarA and sarS on TSST-1 expression in RN4282
We next examined the consequences of the disruption of the global regulator sarA, as well as the related SarA-family member sarS on tst expression in RN4282. Despite our findings above showing a lack of evidence for sigB-dependent modulation of sarS, we nevertheless chose to examine the consequence of sarS disruption on tst expression because previous study had demonstrated a key role for sarS in the regulation of various surface and secreted virulence factors (including alpha-toxin), together with the finding that sarA controls the expression of sarS through an intermediate step via another sarA family member, sarT [59, 63–65]. Sar-family members are thought to interact in a subtle and complex network to control the expression of both surface and secreted virulence factors [66]. Importantly, not all S. aureus strains encode sarT and sarU, which together are found on a small islet not considered part of the core S. aureus genome [67]. As a first step, we performed direct sequence analysis of RN4282 and confirmed the presence of the sarT/sarU encoding islet (data not shown).
We observed that tst transcription was significantly increased (approximately 3.5-fold) in the sarA disruption strain DA142 compared to its isogenic parental strain RN4282 (Fig 4A). Importantly, this enhanced transcription was mirrored at the protein level, since western blot analysis detected markedly increased TSST-1 in normalized culture supernatants of DA142 compared with RN4282 supernatants (Fig 4B). Similar results were obtained using an independent sarA disruption, DA143. It is possible that western blots using supernatants from sarA disruption strains have enhanced proteolytic activity that influences detection of TSST-1 (leading to underestimation) since we detected reductions in the carbonic anhydrase control protein added to monitor supernatants only in sarA deletion strains (S1 Fig) [68].
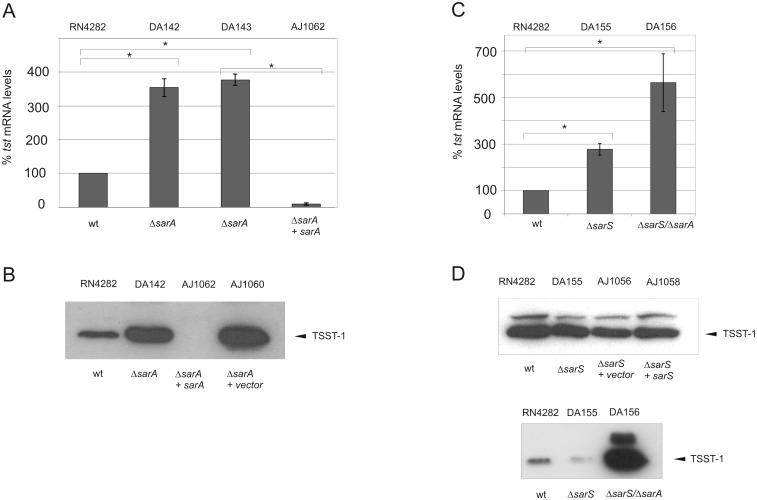
Fig 4. The effect of sarA and sarS on tst and TSST-1 toxin expression in RN4282.
A and C. Quantitative qRT-PCR measurements of tst expression in the indicated strains, setting RN4282 as 100%. Bars show +/- standard deviations. All data were compiled from three independent experiments. Statistical significance was evaluated by Student’s paired t test, and data were considered significant when P was <0.05. B and D. Western blot of TSST-1, using anti-TSST-1 polyclonal antibody after precipitation from OD-normalized supernatants of the indicated strains (Materials and Methods). The experiment shown is representative of several independent experiments.
In order to further confirm this repressive role of SarA we analyzed the effect of sarA complementation with a multi-copy pMK4 plasmid encoding sarA under control of its entire native P1/2/3 promoter upstream sequence. Complementation resulted in a strong repression of both tst transcripts and TSST-1 secreted toxin levels (Fig 4 panels A and B).
Collectively, we conclude that sarA functions as a negative regulator of tst in the RN4282 strain background. Moreover, these findings suggest that in the absence of σB, a combination of higher stimulatory RNAIII and reduced SarA levels could conceivably account for the strong induction of tst.
We next prepared isogenic strains containing disruption of either sarS (DA155), both sarA/sarS (DA156), and complementation of the sarS deletion. Tst expression was compared between each strain set by both qRT-PCR and western blot analysis of TSST-1 in normalized culture supernatants. The results revealed that deletion of sarS gene (DA155) significantly increased tst transcript levels nearly 3-fold compared to RN4282 (Fig 4C), but this stimulatory effect was not mirrored by increased TSST-1 secreted toxin (Fig 4D). Indeed, we observed in multiple trials that TSST-1 levels obtained from DA155 supernatants were equivalent, or slightly reduced compared with RN4282, but not increased compared with RN4282. Finally, we also consistently observed that complementation of DA155 with plasmid-encoded sarS did not detectably influence TSST-1 levels (Fig 4D).
In contrast with the relatively minor role we detected for sarS disruption alone, the disruption of both sarA/sarS resulted in a significant 6-fold increase in tst expression compared with RN4282. This result is in contrast with the observed 3.5-fold increase in tst transcripts detected with the sarA disruption alone (Fig 4A) indicating that sarS disruption, nevertheless can, under certain conditions, influence tst expression. Western blot analysis showed that corresponding supernatants from the strain DA156 sarA/sarS double mutant resulted in strong production of TSST-1 compared to RN4282 (Fig 4D, lower panel). Western blots of DA156 supernatants also showed the appearance of a strong band corresponding most likely to pre-TSST-1 as we had observed with supernatants obtained from DA140 containing the sigB disruption (Fig 1C), but not from DA142 sarA disruption supernatants alone (Fig 4B). Collectively, these results led us to conclude that sarS exerts little, or no significant regulation upon tst transcription in RN4282 strain, at least in our experimental conditions. In addition a sarA-dependant negative regulation can be detected independently of the presence of sarS. SarS would thus not appear to play a significant role in governing repression of TSST-1 expression in this strain background alone, but sarS can exert a synergistic effect on TSST-1 expression when combined with disruption of sarA.
Time course expression of TSST-1
Results presented above revealed that several of our mutants resulted in enhanced production of TSST-1 in post-exponential phase. We next wished to examine the possibility that various mutations resulted in detectable changes in the production of TSST-1 throughout the progression of exponential phase and early post-exponential phase as well. Time course experiments were performed using three deletion mutant strains ΔsigB, ΔsarA and Δrot. TSST-1 protein levels were assessed by western blot analysis at two-hour time intervals following dilution of washed overnight cultures to remove residual TSST-1 (Materials and Methods). The results are shown in Fig 5.
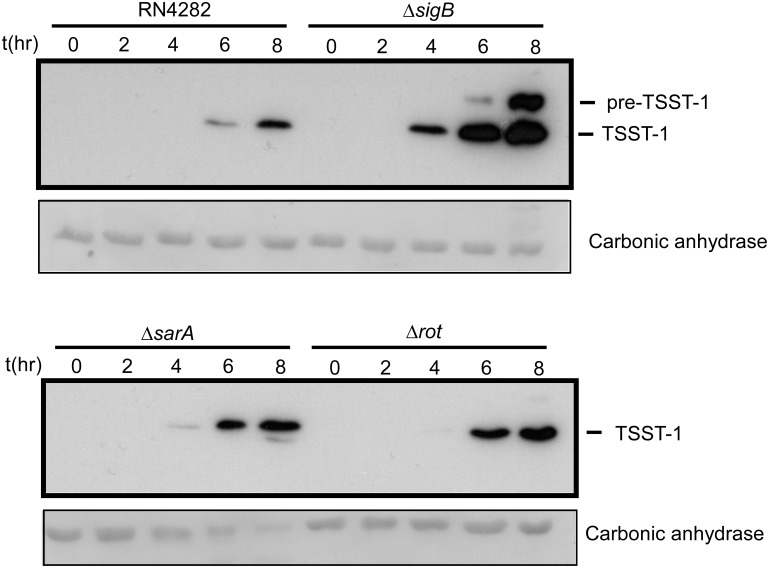
Fig 5. Western blot showing the time course production of TSST-1 in supernatants of the indicated wild type or mutant strains.
Samples, at each time point, were OD600 normalized to each other. Prior to supernatant concentration, samples were spiked with a fixed amount of pure carbonic anhydrase as an internal preparation loading control, and is shown as the Ponceau stained band from the PVDF membrane (Materials and Methods). Note the strong production of TSST-1 from the ΔsigB strain (DA140).
Consistent with data presented above, the sigB deletion strain profoundly enhanced TSST-1 expression at 4, 6 and 8 hours of growth compared with wild type RN4282, and TSST-1 was clearly detected at 4 hours, whereas no TSST-1 was detected in supernatants from RN4282. A similar effect was observed with the sarA deletion, but TSST-1 levels were only moderately enhanced compared with RN4282; TSST-1 was clearly detected at the four hour time point, a condition where no TSST-1 was detected in RN4282 supernatants. Finally, the effect of rot deletion alone indicated a detectable enhancement of TSST-1 at 6 hours compared with RN4282, and a slight enhancement at 8 hours compared with RN4282. It is important to point out that culture supernatants in these time course experiments were OD normalized to each other at the indicated time points for the purposes of interstrain comparison.
Discussion
In this study, we examined multiple regulators controlling tst transcription and TSST-1 expression. There is only scant knowledge about the regulation of this major virulence factor situated on a mobile genetic element and absent from most strains used for virulence regulation studies. Our present study now uncovers evidence for the negative regulation of tst expression by several factors including sigB, sarA, and probably rot, at least when significantly overexpressed. The remarkably strong production of TSST-1 detected in culture supernatants arising from disruption of either sigB, sarA, or disruption of sarA in combination with disruption of sarS underscores the multifactorial nature of tst regulation. We do not at this time fully understand the impact of various possible regulatory protein combinations upon the tst promoter.
Our study findings lead us to propose that in addition to environmental cues that impact sensory systems governing tst expression, sporadic mutation leading to disruption of one or several regulators could conceivably have a profound impact on TSST-1 expression and perhaps shift production sufficiently to influence progression to overt toxin-mediated disease. Given that other factors such as catabolite control protein CcpA are known to negatively regulate tst, we integrate a number of these results in a model of tst regulation depicted in Fig 6 [25].
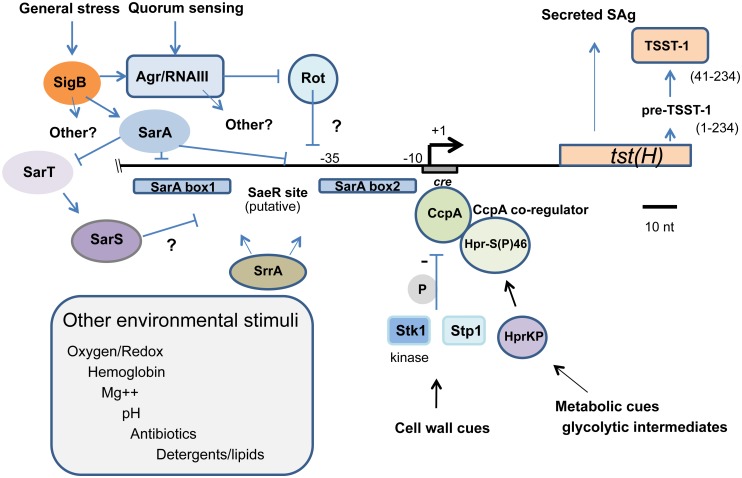
Fig 6. Model depicting the regulation of the TSST-1 superantigen in S. aureus.
The horizontal line shows the position of the transcriptional start site (+1) determined in RN4282 and 37 nucleotides upstream of the translation start site [40]. The positions of SarA boxes 1 and 2 are shown and correspond to their positions determined by both SarA consensus and direct DNA binding assay [40]. Negative regulators determined in the study reported herein include σ B, SarA, and Rot. SarA modulation of SarS is thought to occur via SarA negative regulation of the SarS activator SarT [65]. Catabolite control protein a (CcpA) binding to its cognate cis- acting cre site mediates additional tst repression by integrating signals from glycolytic intermediates via phosphorylation of the CcpA co-regulator as well as direct phosphorylation (grey P) via the Stk1 kinase which affects its DNA binding affinity [25, 44, 69]. The Stk1 S/T kinase and its cognate phosphatase, Stp1, may impart additional levels of control via the phosphorylation of SarA and the nucleoid protein, Hu, for example [45, 70, 71]. SrrA, the response regulator of the SrrAB two-component sensor, is thought to control tst regulation in response to oxygen and coenzyme Q [29, 30, 69, 100]. SrrA specific binding has been detected in the tst promoter region (Andrey, manuscript in preparation). DNA sequence with strong similarity to the consensus DNA binding site for the response regulator SaeR is shown. Although the precise involvement of SaeRS in tst regulation is unknown, it may help coordinate response to pH together with sigB [34, 101, 102]. Additional environmental stimuli known to affect TSST-1 expression are boxed although the precise genetic factors mediating these effects have yet to be defined [32, 102–107].
The model depicts the arrangement of known features of the tst promoter region previously described by our own work that precisely mapped two SarA binding sites, together with mapping of the cis-acting catabolite response element (cre) site mediating DNA-protein interaction with CcpA [25, 40]. Presumptive DNA binding sites for Rot and other regulators are presently unknown. Our model also depicts several additional levels of regulation, notably the modulation of CcpA DNA binding by Stk1 kinase dependent phosphorylation and association with its co-regulator that integrates metabolic cues by sensing glycolytic intermediates [44, 69]. Stk1 and its cognate phosphatase Stp1 constitute a serine/threonine kinase sensor system linked to virulence regulation and phosphorylation of other global regulators such as SarA, the nucleoid protein Hu, and MgrA [45, 70–72]. There are a number of environmental factors that have been shown or are suspected to impact tst expression for which there is presently no genetic explanation of the underlying sensory system and regulatory circuitry. The model provides a framework for further understanding the nature of environmental inputs known to modulate tst expression and the underlying genetic elements that integrate this regulation. Some of these points will be discussed in more detail below.
Our results showed that sigB helps to impart particularly strong repression of tst expression in both RN4282 and 8325–4 genetic backgrounds. Sequence inspection failed to reveal evidence for a σB consensus in the tst promoter, and thus σB most likely exerts its repressive effect indirectly. We presented evidence to support that indirect repression of TSST-1 expression by σB is mediated, in part, by the modulation of sarA and agr/RNAIII systems. To support this hypothesis we confirmed in RN4282 strain that sigB positively regulates sarA transcription and represses agr/RNAIII, in accordance with existing models where one of the three sarA promoters, sarP3, is controlled by σB [28, 73]. We also established that agr/RNAIII enhances TSST-1 expression and that sarA strongly represses it in RN4282. The additional effects of both upregulated sarA levels and low RNAIII levels thus likely participate in TSST-1 repression.
Previous detailed study of the sigB regulon has shown that it controls a vast network of more than 250 genes in S. aureus and that diverse environmental stress stimuli such as ethanol, and acid shock lead to σB-dependent gene expression [48]. Despite a demonstrated role for sigB-dependent regulation of agr/RNAIII and sarA described above, we cannot, of course, exclude that other sigB-dependent factors are also involved in tst regulation. Inspection of the various fold-changes in transcription measured in our study indicated that loss of sigB was by far the most consequential compared with disruption of RNAIII, rot, or sarA. This finding strongly suggests that additional sigB-dependent regulators of tst await discovery. Recent studies have explored the indirect regulation of certain genes lacking a σB consensus via SpoVG and/or the two-component system ArlRS, for example [74–77]. In addition, small non-coding RNAs have been described that are controlled by sigB, for example, RcsA, RcsD, and RcsF, but to date, their precise regulatory role is unknown [43, 78, 79]. Recent work also suggests that pigment-deficient strains, possibly related to defects in sigB, arise during the course of S. aureus community development and strong bacterial competition [80]. Future studies will certainly shed new light on sigB-dependent virulence factor regulation.
SarA is clearly important for the transcriptional regulation of tst, and here we show that sarA is a potent repressor of TSST-1. These results are in contrast with previous published studies, however, where sarA was shown by ourselves and others to enhance tst promoter expression in an alternative strain background namely, 8325–4 using a tst promoter reporter (Ptst::luxAB PC1072) strain; to our knowledge this effect was observed only in the S. aureus NCTC8325 genetic background and this may arise because of the rsbU and sarS defect in this strain [89] leading to defective sigB levels and concomitant alterations in regulatory protein combinations acting on the tst promoter [39, 40]. The disparate phenotypes of sarA mutation, depending upon the studied strain, has been previously reported regarding the control of several virulence factors, and particularly hla encoding hemolysin alpha exotoxin [28, 64, 81–84]. While sarA first appeared to be a repressor of exoproteins and an activator of membrane-bound proteins, when originally identified in a transposon Tn917LTV1 mutagenesis, several subsequent studies, primarily performed with NCTC8325 derivatives, showed sarA to be necessary for full production of secreted exoproteins, including α-hemolysin (hla) [81, 82, 85, 86]. Further studies showed that sarA repressed exoprotein synthesis in strains unrelated to NCTC8325 such as Newman and UAMS-1, confirming important differences in regulatory patterns among S. aureus strains [83]. The enhancing effect of SarA on virulence factors could not be found in other genetic backgrounds than NCTC8325. Notably little information was available regarding TSST-1 regulation in NCTC8325 related strains.
In this report we also have shown that rot can modulate tst transcription, conferring in RN4282 strain a potential additional layer of negative regulation on TSST-1. The absence of clear effect of rot disruption combined with the strong repression observed in multi-copy plasmid complementation conditions, suggests a role for gene dosage for this regulator and possibly depending upon access to the cis-acting tst promoter sequences and competition between regulators. Our data do not contradict the model that entry into post-exponential phase and activation of agr quorum sensing results in RNAIII-mediated inhibition of rot transcription and relief of Rot-dependent repression [61]. Recent work suggests, however, the possibility that by analogy with SarS, inter-strain differences and variation in Rot levels during growth phase may nevertheless contribute unpredictable levels of variation in Rot-dependent virulence factor regulation [87].
SarS is not a major regulator of tst in RN4282, at least in our experimental conditions. SarS was reported to be controlled positively by SarT; while SarT itself was found to be negatively regulated by SarA [65]. SarS-mediated repression of tst may be subject to strain-dependent variation for several reasons: sarT and sarU genes are not found on all S. aureus strains, sarS transcript levels are reduced in the widely used laboratory strain 8325 lineage, and sarS transcription and steady state protein levels may be growth phase dependent [67, 88–90].
How could SarA contribute to the regulation of tst? Specific SarA binding to the tst promoter has been determined and we have detected at least two sites by in vitro assay in our previous study [40]. Specific SarS binding has not been explored and a consensus site has not been defined for this regulator in S. aureus. Notably, however, recent work showed that SarA could bind and bend DNA at the agr P2 promoter, thereby promoting topological changes that allow additional regulatory protein-protein interactions at this promoter during exponential phase [91]. Interestingly, an additional Sar-family member, SarR, was shown in this study to bind to regions overlapping the SarA site and with higher affinity, but without inducing significant DNA bending. The consequence of this is thought to lead to a post-exponential reduction in agrP2 promoter activity arising from displacement of SarA and loss of SarA-induced DNA bending. Viewed in this context, it is tempting to speculate that Sar-family proteins may regulate additional promoters by a variety of mechanisms including positive and negative influence on transcription arising from DNA bending, facilitating protein-DNA interaction of other factors, and perhaps combinatoric occupancy of Sar family binding sites. By downregulating SarS transcription via SarT, SarA may fine tune SarS levels which contribute to tst promoter regulation together with SarA [65]. Additional detailed studies will be required to resolve the details of this mechanism.
TSST-1 possesses an approximately 40 amino acid signal sequence and mature TSST-1 superantigen is predicted to be the 41–234 amino acid polypeptide. Interestingly, our western analysis detected strong induction of both the processed and unprocessed TSST-1 in DA140 compared with RN4282, where notably unprocessed TSST-1 precursor polypeptide was low or undetectable. Several scenarios might explain this observation. The loss of sigB could affect: 1) the proper expression of spsB encoding the Type II signal peptidase; 2) facilitate enhanced secretion via the sec-pathway; 3) impair extracellular proteolysis, or alternatively, 4) facilitate TSST-1 export via for example, non-sec dependent routes. The superantigenicity of the unprocessed TSST-1 polypeptide has to our knowledge, not been explored. We did not pursue the underlying cause of these observations further in this study.
Collectively, our results reported herein and integrated with additional published findings in Fig 6 show a complex network of regulation over tst and TSST-1 expression. Notably, the tst gene is not part of the core genome and resides embedded within one of several pathogenicity islands and we are unaware of exceptions to this observation [4, 12]. Recent work has shown these pathogenicity islands to be particularly efficient parasitic sequences dedicated to a lifestyle as mobilizable defective prophage [92]. Accessory genes, such as tst and other VF, may have been incorporated in them over the course of evolution. In this perspective, it is worthwhile considering the possibility that were tst not particularly well repressed by whatever means, its expression could interfere with pathogenicity island regulation. Prophage gene expression is strongly repressed by the islet-encoded Stl [93]. Derepression of Stl repressor would predictably lead to excision and the generation of a replicative form of the pathogenicity island, which could correspondingly rapidly increase the gene copy number and disrupting regulatory protein-DNA interaction stoichiometry. This would of course predict that factors such as bacteriophage infection, or genotoxic stress evoking an SOS response, including antibiotic stress such as that described for the induction of SOS by ciprofloxacin, could theoretically modulate tst production under these circumstances by transient gene amplification [93–97].
There are a number of physiological conditions that have been described that modulate tst expression and which do not yet have adequate underlying genetic regulatory explanation (Fig 6). This includes mild acid, magnesium, membrane-active agents, growth rate, pH, and oxygen. Aerobic conditions have been long thought to stimulate TSST-1 production, yet the nature of the oxygen sensor remains elusive. One candidate sensor is the SrrAB two-component system thought to modulate gene expression changes upon aerobic/anaerobic shift, although additional redox sensors controlling tst expression cannot be excluded [29, 30, 69].
Although further studies remain necessary to fully characterize and understand the regulatory patterns of TSST-1, current knowledge suggests that sporadic mutation in a few key negative regulators can profoundly affect and enhance tst expression. Mutations in rsbU or sigB were observed in laboratory strains, as in NCTC8325, but also in clinical isolates (K26, V8, Wood46) and were shown to strongly enhance exoprotein expression (notably Hla and SspA) [54, 98, 99].
Supporting Information
Ponceau and Coomassie staining of strains used in the experiment of fig 3 (A and B) and Coomassie staining the various strains used in all other experiments (C), in post-exponential growth phase (OD600 1.5 to 2). Carbonic anhydrase was added in each supernatant as a concentration and loading control. Carbonic anhydrase digestion in DA142 and DA156 lanes of panel C can be observed and is probably due to increased secretion of proteases in sarA mutants.
(TIF)
Normalized cycle thresholds for n = 3 independent determinations for all figures presented in the text showing qRT-PCR data and prior to conversion to fold-change as described in Materials and Methods. The data are based upon the ct values presented in S1 Table. Each graphic displays Figure and corresponding probe used.
(DOCX)
(DOCX)
Acknowledgments
We thank Ambrose Cheung for his helpful comments and encouragement, and Hanne Ingmer, Dorte Frees, Jan Oscarsson, Simon J. Foster, Steffan Arvidson, and Peter McNamara for their generous gifts of strains.
Funding Statement
This work was supported by grants from the Swiss National Science Foundation 3100A0-120428 and 310030-146540 (to WLK), an MD-PhD thesis doctoral training grant from the Swiss Academy of Medical Sciences via the F. Hoffmann-La Roche Research Foundation (to DOA), a fellowship grant from the Swiss Society of Infections Diseases (to NR), the University Hospital and Medical School of Geneva, and the Canton of Geneva.
References
- 1. Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339(8):520–32. Epub 1998/08/26. . [DOI] [PubMed] [Google Scholar]
- 2. Gorwitz RJ, Kruszon-Moran D, McAllister SK, McQuillan G, McDougal LK, Fosheim GE, et al. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J Infect Dis. 2008;197(9):1226–34. Epub 2008/04/22. 10.1086/533494 . [DOI] [PubMed] [Google Scholar]
- 3. Foster TJ. Colonization and infection of the human host by staphylococci: adhesion, survival and immune evasion. Vet Dermatol. 2009;20(5–6):456–70. Epub 2010/02/25. doi: VDE825 [pii] 10.1111/j.1365-3164.2009.00825.x . [DOI] [PubMed] [Google Scholar]
- 4. Novick RP. Mobile genetic elements and bacterial toxinoses: the superantigen-encoding pathogenicity islands of Staphylococcus aureus. Plasmid. 2003;49(2):93–105. Epub 2003/05/03. doi: S0147619X02001579 [pii]. . [DOI] [PubMed] [Google Scholar]
- 5. Rooijakkers SH, van Kessel KP, van Strijp JA. Staphylococcal innate immune evasion. Trends Microbiol. 2005;13(12):596–601. Epub 2005/10/26. doi: S0966-842X(05)00280-5 [pii] 10.1016/j.tim.2005.10.002 . [DOI] [PubMed] [Google Scholar]
- 6. Spaan AN, Surewaard BG, Nijland R, van Strijp JA. Neutrophils versus Staphylococcus aureus: a biological tug of war. Annu Rev Microbiol. 2013;67:629–50. Epub 2013/07/10. 10.1146/annurev-micro-092412-155746 . [DOI] [PubMed] [Google Scholar]
- 7. van Wamel WJ, Rooijakkers SH, Ruyken M, van Kessel KP, van Strijp JA. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on beta-hemolysin-converting bacteriophages. J Bacteriol. 2006;188(4):1310–5. Epub 2006/02/03. doi: 188/4/1310 [pii] 10.1128/JB.188.4.1310-1315.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lappin E, Ferguson AJ. Gram-positive toxic shock syndromes. Lancet Infect Dis. 2009;9(5):281–90. Epub 2009/04/28. doi: S1473-3099(09)70066-0 [pii] 10.1016/S1473-3099(09)70066-0 . [DOI] [PubMed] [Google Scholar]
- 9. McCormick JK, Yarwood JM, Schlievert PM. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol. 2001;55:77–104. Epub 2001/09/07. doi: 10.1146/annurev.micro.55.1.77 55/1/77 [pii]. . [DOI] [PubMed] [Google Scholar]
- 10. Subedi A, Ubeda C, Adhikari RP, Penades JR, Novick RP. Sequence analysis reveals genetic exchanges and intraspecific spread of SaPI2, a pathogenicity island involved in menstrual toxic shock. Microbiology. 2007;153(Pt 10):3235–45. Epub 2007/10/02. doi: 153/10/3235 [pii] 10.1099/mic.0.2007/006932-0 . [DOI] [PubMed] [Google Scholar]
- 11. Kreiswirth BN, O'Reilly M, Novick RP. Genetic characterization and cloning of the toxic shock syndrome exotoxin. Surv Synth Pathol Res. 1984;3(1):73–82. Epub 1984/01/01. . [PubMed] [Google Scholar]
- 12. Novick RP, Christie GE, Penades JR. The phage-related chromosomal islands of Gram-positive bacteria. Nat Rev Microbiol. 2010;8(8):541–51. Epub 2010/07/17. doi: nrmicro2393 [pii] 10.1038/nrmicro2393 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kreiswirth BN, Lofdahl S, Betley MJ, O'Reilly M, Schlievert PM, Bergdoll MS, et al. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983;305(5936):709–12. Epub 1983/10/20. . [DOI] [PubMed] [Google Scholar]
- 14. Parsonnet J, Hansmann MA, Delaney ML, Modern PA, Dubois AM, Wieland-Alter W, et al. Prevalence of toxic shock syndrome toxin 1-producing Staphylococcus aureus and the presence of antibodies to this superantigen in menstruating women. J Clin Microbiol. 2005;43(9):4628–34. Epub 2005/09/08. doi: 43/9/4628 [pii] 10.1128/JCM.43.9.4628-4634.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Megevand C, Gervaix A, Heininger U, Berger C, Aebi C, Vaudaux B, et al. Molecular epidemiology of the nasal colonization by methicillin-susceptible Staphylococcus aureus in Swiss children. Clin Microbiol Infect. 2010;16(9):1414–20. Epub 2009/10/23. 10.1111/j.1469-0691.2009.03090.x CLM3090 [pii]. . [DOI] [PubMed] [Google Scholar]
- 16. Lamy B, Laurent F, Gallon O, Doucet-Populaire F, Etienne J, Decousser JW. Antibacterial resistance, genes encoding toxins and genetic background among Staphylococcus aureus isolated from community-acquired skin and soft tissue infections in France: a national prospective survey. Eur J Clin Microbiol Infect Dis. 2012;31(6):1279–84. Epub 2011/10/15. 10.1007/s10096-011-1441-5 . [DOI] [PubMed] [Google Scholar]
- 17. Gaventa S, Reingold AL, Hightower AW, Broome CV, Schwartz B, Hoppe C, et al. Active surveillance for toxic shock syndrome in the United States, 1986. Rev Infect Dis. 1989;11 Suppl 1:S28–34. Epub 1989/01/01. . [DOI] [PubMed] [Google Scholar]
- 18. Kreiswirth BN, Projan SJ, Schlievert PM, Novick RP. Toxic shock syndrome toxin 1 is encoded by a variable genetic element. Rev Infect Dis. 1989;11 Suppl 1:S83–8; discussion S8-9. Epub 1989/01/01. . [DOI] [PubMed] [Google Scholar]
- 19. Mir-Sanchis I, Martinez-Rubio R, Marti M, Chen J, Lasa I, Novick RP, et al. Control of Staphylococcus aureus pathogenicity island excision. Mol Microbiol. 2012;85(5):833–45. Epub 2012/06/30. 10.1111/j.1365-2958.2012.08145.x . [DOI] [PubMed] [Google Scholar]
- 20. Lindsay JA, Ruzin A, Ross HF, Kurepina N, Novick RP. The gene for toxic shock toxin is carried by a family of mobile pathogenicity islands in Staphylococcus aureus. Mol Microbiol. 1998;29(2):527–43. Epub 1998/08/28. . [DOI] [PubMed] [Google Scholar]
- 21. Horsburgh MJ, Aish JL, White IJ, Shaw L, Lithgow JK, Foster SJ. sigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325–4. J Bacteriol. 2002;184(19):5457–67. Epub 2002/09/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chan PF, Foster SJ. The role of environmental factors in the regulation of virulence-determinant expression in Staphylococcus aureus 8325–4. Microbiology. 1998;144 (Pt 9):2469–79. Epub 1998/10/23. . [DOI] [PubMed] [Google Scholar]
- 23. Schlievert PM, Case LC, Nemeth KA, Davis CC, Sun Y, Qin W, et al. Alpha and beta chains of hemoglobin inhibit production of Staphylococcus aureus exotoxins. Biochemistry. 2007;46(50):14349–58. Epub 2007/11/21. 10.1021/bi701202w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kass EH. Magnesium and the pathogenesis of toxic shock syndrome. Rev Infect Dis. 1989;11 Suppl 1:S167–73; discussion S73-5. Epub 1989/01/01. . [DOI] [PubMed] [Google Scholar]
- 25. Seidl K, Bischoff M, Berger-Bachi B. CcpA mediates the catabolite repression of tst in Staphylococcus aureus. Infect Immun. 2008;76(11):5093–9. Epub 2008/08/20. doi: IAI.00724-08 [pii] 10.1128/IAI.00724-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vojtov N, Ross HF, Novick RP. Global repression of exotoxin synthesis by staphylococcal superantigens. Proc Natl Acad Sci U S A. 2002;99(15):10102–7. Epub 2002/07/12. 10.1073/pnas.152152499 152152499 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yarwood JM, Schlievert PM. Oxygen and carbon dioxide regulation of toxic shock syndrome toxin 1 production by Staphylococcus aureus MN8. J Clin Microbiol. 2000;38(5):1797–803. Epub 2000/05/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Novick RP. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol. 2003;48(6):1429–49. Epub 2003/06/07. doi: 3526 [pii]. . [DOI] [PubMed] [Google Scholar]
- 29. Pragman AA, Yarwood JM, Tripp TJ, Schlievert PM. Characterization of virulence factor regulation by SrrAB, a two-component system in Staphylococcus aureus. J Bacteriol. 2004;186(8):2430–8. Epub 2004/04/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pragman AA, Ji Y, Schlievert PM. Repression of Staphylococcus aureus SrrAB using inducible antisense srrA alters growth and virulence factor transcript levels. Biochemistry. 2007;46(1):314–21. Epub 2007/01/03. 10.1021/bi0603266 . [DOI] [PubMed] [Google Scholar]
- 31. Stevens DL, Ma Y, Salmi DB, McIndoo E, Wallace RJ, Bryant AE. Impact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureus. J Infect Dis. 2007;195(2):202–11. Epub 2006/12/28. doi: JID36388 [pii] 10.1086/510396 . [DOI] [PubMed] [Google Scholar]
- 32. McNamara PJ, Syverson RE, Milligan-Myhre K, Frolova O, Schroeder S, Kidder J, et al. Surfactants, aromatic and isoprenoid compounds, and fatty acid biosynthesis inhibitors suppress Staphylococcus aureus production of toxic shock syndrome toxin 1. Antimicrob Agents Chemother. 2009;53(5):1898–906. Epub 2009/02/19. 10.1128/AAC.01293-08 AAC.01293-08 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kimber I, Nookala S, Davis CC, Gerberick GF, Tucker H, Foertsch LM, et al. Toxic shock syndrome: characterization of human immune responses to TSST-1 and evidence for sensitivity thresholds. Toxicol Sci. 2013;134(1):49–63. Epub 2013/05/04. 10.1093/toxsci/kft099 kft099 [pii]. . [DOI] [PubMed] [Google Scholar]
- 34. Li J, Wang W, Xu SX, Magarvey NA, McCormick JK. Lactobacillus reuteri-produced cyclic dipeptides quench agr-mediated expression of toxic shock syndrome toxin-1 in staphylococci. Proc Natl Acad Sci U S A. 2011;108(8):3360–5. Epub 2011/02/02. doi: 1017431108 [pii] 10.1073/pnas.1017431108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nagao M, Okamoto A, Yamada K, Hasegawa T, Hasegawa Y, Ohta M. Variations in amount of TSST-1 produced by clinical methicillin resistant Staphylococcus aureus (MRSA) isolates and allelic variation in accessory gene regulator (agr) locus. BMC Microbiol. 2009;9:52 Epub 2009/03/11. 10.1186/1471-2180-9-52 1471-2180-9-52 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bronner S, Monteil H, Prevost G. Regulation of virulence determinants in Staphylococcus aureus: complexity and applications. FEMS Microbiol Rev. 2004;28(2):183–200. Epub 2004/04/28. 10.1016/j.femsre.2003.09.003 S0168644503000925 [pii]. . [DOI] [PubMed] [Google Scholar]
- 37. Cheung AL, Bayer AS, Zhang G, Gresham H, Xiong YQ. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol Med Microbiol. 2004;40(1):1–9. Epub 2004/01/22. doi: S0928824403003092 [pii]. . [DOI] [PubMed] [Google Scholar]
- 38. Recsei P, Kreiswirth B, O'Reilly M, Schlievert P, Gruss A, Novick RP. Regulation of exoprotein gene expression in Staphylococcus aureus by agar. Mol Gen Genet. 1986;202(1):58–61. Epub 1986/01/01. . [DOI] [PubMed] [Google Scholar]
- 39. Chan PF, Foster SJ. Role of SarA in virulence determinant production and environmental signal transduction in Staphylococcus aureus. J Bacteriol. 1998;180(23):6232–41. Epub 1998/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Andrey DO, Renzoni A, Monod A, Lew DP, Cheung AL, Kelley WL. Control of the Staphylococcus aureus toxic shock tst promoter by the global regulator SarA. J Bacteriol. 2010;192(22):6077–85. Epub 2010/09/28. doi: JB.00146-10 [pii] 10.1128/JB.00146-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McNamara PJ, Milligan-Monroe KC, Khalili S, Proctor RA. Identification, cloning, and initial characterization of rot, a locus encoding a regulator of virulence factor expression in Staphylococcus aureus. J Bacteriol. 2000;182(11):3197–203. Epub 2000/05/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Said-Salim B, Dunman PM, McAleese FM, Macapagal D, Murphy E, McNamara PJ, et al. Global regulation of Staphylococcus aureus genes by Rot. J Bacteriol. 2003;185(2):610–9. Epub 2003/01/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Felden B, Vandenesch F, Bouloc P, Romby P. The Staphylococcus aureus RNome and its commitment to virulence. PLoS Pathog. 2011;7(3):e1002006 Epub 2011/03/23. 10.1371/journal.ppat.1002006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leiba J, Hartmann T, Cluzel ME, Cohen-Gonsaud M, Delolme F, Bischoff M, et al. A novel mode of regulation of the Staphylococcus aureus catabolite control protein A (CcpA) mediated by Stk1 protein phosphorylation. J Biol Chem. 2012;287(52):43607–19. Epub 2012/11/08. 10.1074/jbc.M112.418913 M112.418913 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ohlsen K, Donat S. The impact of serine/threonine phosphorylation in Staphylococcus aureus. Int J Med Microbiol. 2010;300(2–3):137–41. Epub 2009/09/29. doi: S1438-4221(09)00109-X [pii] 10.1016/j.ijmm.2009.08.016 . [DOI] [PubMed] [Google Scholar]
- 46. Yarwood JM, McCormick JK, Schlievert PM. Identification of a novel two-component regulatory system that acts in global regulation of virulence factors of Staphylococcus aureus. J Bacteriol. 2001;183(4):1113–23. Epub 2001/02/07. 10.1128/JB.183.4.1113-1123.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tu Quoc PH, Genevaux P, Pajunen M, Savilahti H, Georgopoulos C, Schrenzel J, et al. Isolation and characterization of biofilm formation-defective mutants of Staphylococcus aureus. Infect Immun. 2007;75(3):1079–88. Epub 2006/12/13. doi: IAI.01143-06 [pii] 10.1128/IAI.01143-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bischoff M, Dunman P, Kormanec J, Macapagal D, Murphy E, Mounts W, et al. Microarray-based analysis of the Staphylococcus aureus sigmaB regulon. J Bacteriol. 2004;186(13):4085–99. Epub 2004/06/19. 10.1128/JB.186.13.4085-4099.2004 186/13/4085 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Oscarsson J, Harlos C, Arvidson S. Regulatory role of proteins binding to the spa (protein A) and sarS (staphylococcal accessory regulator) promoter regions in Staphylococcus aureus NTCC 8325–4. Int J Med Microbiol. 2005;295(4):253–66. Epub 2005/09/01. . [DOI] [PubMed] [Google Scholar]
- 50. Renzoni A, Kelley WL, Barras C, Monod A, Huggler E, Francois P, et al. Identification by genomic and genetic analysis of two new genes playing a key role in intermediate glycopeptide resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2009;53(3):903–11. Epub 2008/12/24. doi: AAC.01287-08 [pii] 10.1128/AAC.01287-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Renzoni A, Francois P, Li D, Kelley WL, Lew DP, Vaudaux P, et al. Modulation of fibronectin adhesins and other virulence factors in a teicoplanin-resistant derivative of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2004;48(8):2958–65. Epub 2004/07/27. 10.1128/AAC.48.8.2958-2965.2004 48/8/2958 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vaudaux P, Francois P, Bisognano C, Kelley WL, Lew DP, Schrenzel J, et al. Increased expression of clumping factor and fibronectin-binding proteins by hemB mutants of Staphylococcus aureus expressing small colony variant phenotypes. Infect Immun. 2002;70(10):5428–37. Epub 2002/09/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kullik II, Giachino P. The alternative sigma factor sigmaB in Staphylococcus aureus: regulation of the sigB operon in response to growth phase and heat shock. Arch Microbiol. 1997;167(2/3):151–9. Epub 1997/03/07. doi: 71670151.203 [pii]. . [DOI] [PubMed] [Google Scholar]
- 54. Herbert S, Ziebandt AK, Ohlsen K, Schafer T, Hecker M, Albrecht D, et al. Repair of global regulators in Staphylococcus aureus 8325 and comparative analysis with other clinical isolates. Infect Immun. 2010;78(6):2877–89. Epub 2010/03/10. doi: IAI.00088-10 [pii] 10.1128/IAI.00088-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Giachino P, Engelmann S, Bischoff M. Sigma(B) activity depends on RsbU in Staphylococcus aureus. J Bacteriol. 2001;183(6):1843–52. Epub 2001/02/27. 10.1128/JB.183.6.1843-1852.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Haldenwang WG. The sigma factors of Bacillus subtilis. Microbiol Rev. 1995;59(1):1–30. Epub 1995/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gertz S, Engelmann S, Schmid R, Ziebandt AK, Tischer K, Scharf C, et al. Characterization of the sigma(B) regulon in Staphylococcus aureus. J Bacteriol. 2000;182(24):6983–91. Epub 2000/11/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bischoff M, Entenza JM, Giachino P. Influence of a functional sigB operon on the global regulators sar and agr in Staphylococcus aureus. J Bacteriol. 2001;183(17):5171–9. Epub 2001/08/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tegmark K, Karlsson A, Arvidson S. Identification and characterization of SarH1, a new global regulator of virulence gene expression in Staphylococcus aureus. Mol Microbiol. 2000;37(2):398–409. Epub 2000/08/10. doi: mmi2003 [pii]. . [DOI] [PubMed] [Google Scholar]
- 60. Geisinger E, Adhikari RP, Jin R, Ross HF, Novick RP. Inhibition of rot translation by RNAIII, a key feature of agr function. Mol Microbiol. 2006;61(4):1038–48. Epub 2006/08/02. doi: MMI5292 [pii] 10.1111/j.1365-2958.2006.05292.x . [DOI] [PubMed] [Google Scholar]
- 61. Boisset S, Geissmann T, Huntzinger E, Fechter P, Bendridi N, Possedko M, et al. Staphylococcus aureus RNAIII coordinately represses the synthesis of virulence factors and the transcription regulator Rot by an antisense mechanism. Genes Dev. 2007;21(11):1353–66. Epub 2007/06/05. doi: 21/11/1353 [pii] 10.1101/gad.423507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Benson MA, Lilo S, Nygaard T, Voyich JM, Torres VJ. Rot and SaeRS cooperate to activate expression of the staphylococcal superantigen-like exoproteins. J Bacteriol. 2012;194(16):4355–65. Epub 2012/06/12. 10.1128/JB.00706-12JB.00706-12 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cheung AL, Schmidt K, Bateman B, Manna AC. SarS, a SarA homolog repressible by agr, is an activator of protein A synthesis in Staphylococcus aureus. Infect Immun. 2001;69(4):2448–55. Epub 2001/03/20. 10.1128/IAI.69.4.2448-2455.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Oscarsson J, Kanth A, Tegmark-Wisell K, Arvidson S. SarA is a repressor of hla (alpha-hemolysin) transcription in Staphylococcus aureus: its apparent role as an activator of hla in the prototype strain NCTC 8325 depends on reduced expression of sarS. J Bacteriol. 2006;188(24):8526–33. Epub 2006/10/03. doi: JB.00866-06 [pii] 10.1128/JB.00866-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schmidt KA, Manna AC, Cheung AL. SarT influences sarS expression in Staphylococcus aureus. Infect Immun. 2003;71(9):5139–48. Epub 2003/08/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cheung AL, Nishina KA, Trotonda MP, Tamber S. The SarA protein family of Staphylococcus aureus. Int J Biochem Cell Biol. 2008;40(3):355–61. Epub 2007/12/18. doi: S1357-2725(07)00359-7 [pii] 10.1016/j.biocel.2007.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lindsay JA, Moore CE, Day NP, Peacock SJ, Witney AA, Stabler RA, et al. Microarrays reveal that each of the ten dominant lineages of Staphylococcus aureus has a unique combination of surface-associated and regulatory genes. J Bacteriol. 2006;188(2):669–76. Epub 2005/12/31. doi: 188/2/669 [pii] 10.1128/JB.188.2.669-676.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Karlsson A, Saravia-Otten P, Tegmark K, Morfeldt E, Arvidson S. Decreased amounts of cell wall-associated protein A and fibronectin-binding proteins in Staphylococcus aureus sarA mutants due to up-regulation of extracellular proteases. Infect Immun. 2001;69(8):4742–8. Epub 2001/07/12. 10.1128/IAI.69.8.4742-4748.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Somerville GA, Proctor RA. At the crossroads of bacterial metabolism and virulence factor synthesis in Staphylococci. Microbiol Mol Biol Rev. 2009;73(2):233–48. Epub 2009/06/03. doi: 73/2/233 [pii] 10.1128/MMBR.00005-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Burnside K, Lembo A, de Los Reyes M, Iliuk A, Binhtran NT, Connelly JE, et al. Regulation of hemolysin expression and virulence of Staphylococcus aureus by a serine/threonine kinase and phosphatase. PLoS One. 2010;5(6):e11071 Epub 2010/06/17. 10.1371/journal.pone.0011071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chen PR, Nishida S, Poor CB, Cheng A, Bae T, Kuechenmeister L, et al. A new oxidative sensing and regulation pathway mediated by the MgrA homologue SarZ in Staphylococcus aureus. Mol Microbiol. 2009;71(1):198–211. Epub 2008/11/15. doi: MMI6518 [pii] 10.1111/j.1365-2958.2008.06518.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Didier JP, Cozzone AJ, Duclos B. Phosphorylation of the virulence regulator SarA modulates its ability to bind DNA in Staphylococcus aureus. FEMS Microbiol Lett. 2010;306(1):30–6. Epub 2010/03/27. doi: FML1930 [pii] 10.1111/j.1574-6968.2010.01930.x . [DOI] [PubMed] [Google Scholar]
- 73. Manna AC, Bayer MG, Cheung AL. Transcriptional analysis of different promoters in the sar locus in Staphylococcus aureus. J Bacteriol. 1998;180(15):3828–36. Epub 1998/07/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Meier S, Goerke C, Wolz C, Seidl K, Homerova D, Schulthess B, et al. sigmaB and the sigmaB-dependent arlRS and yabJ-spoVG loci affect capsule formation in Staphylococcus aureus. Infect Immun. 2007;75(9):4562–71. Epub 2007/07/20. doi: IAI.00392-07 [pii] 10.1128/IAI.00392-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Schulthess B, Bloes DA, Francois P, Girard M, Schrenzel J, Bischoff M, et al. The sigmaB-dependent yabJ-spoVG operon is involved in the regulation of extracellular nuclease, lipase, and protease expression in Staphylococcus aureus. J Bacteriol. 2011;193(18):4954–62. Epub 2011/07/05. 10.1128/JB.05362-11 JB.05362-11 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liang X, Zheng L, Landwehr C, Lunsford D, Holmes D, Ji Y. Global regulation of gene expression by ArlRS, a two-component signal transduction regulatory system of Staphylococcus aureus. J Bacteriol. 2005;187(15):5486–92. Epub 2005/07/21. doi: 187/15/5486-a [pii] 10.1128/JB.187.15.5486-5492.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schulthess B, Bloes DA, Berger-Bachi B. Opposing roles of sigmaB and sigmaB-controlled SpoVG in the global regulation of esxA in Staphylococcus aureus. BMC Microbiol. 2012;12:17 Epub 2012/01/26. [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Geissmann T, Chevalier C, Cros MJ, Boisset S, Fechter P, Noirot C, et al. A search for small noncoding RNAs in Staphylococcus aureus reveals a conserved sequence motif for regulation. Nucleic Acids Res. 2009;37(21):7239–57. Epub 2009/09/30. doi: gkp668 [pii] 10.1093/nar/gkp668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nielsen JS, Christiansen MH, Bonde M, Gottschalk S, Frees D, Thomsen LE, et al. Searching for small sigmaB-regulated genes in Staphylococcus aureus. Arch Microbiol. 2011;193(1):23–34. Epub 2010/10/28. 10.1007/s00203-010-0641-1 . [DOI] [PubMed] [Google Scholar]
- 80. Koch G, Yepes A, Forstner KU, Wermser C, Stengel ST, Modamio J, et al. Evolution of resistance to a last-resort antibiotic in Staphylococcus aureus via bacterial competition. Cell. 2014;158(5):1060–71. 10.1016/j.cell.2014.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cheung AL, Koomey JM, Butler CA, Projan SJ, Fischetti VA. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc Natl Acad Sci U S A. 1992;89(14):6462–6. Epub 1992/07/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Heinrichs JH, Bayer MG, Cheung AL. Characterization of the sar locus and its interaction with agr in Staphylococcus aureus. J Bacteriol. 1996;178(2):418–23. Epub 1996/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Blevins JS, Beenken KE, Elasri MO, Hurlburt BK, Smeltzer MS. Strain-dependent differences in the regulatory roles of sarA and agr in Staphylococcus aureus. Infect Immun. 2002;70(2):470–80. Epub 2002/01/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zielinska AK, Beenken KE, Joo HS, Mrak LN, Griffin LM, Luong TT, et al. Defining the Strain-Dependent Impact of the Staphylococcal Accessory Regulator (sarA) on the Alpha-Toxin Phenotype of Staphylococcus aureus. J Bacteriol. 2011;193(12):2948–58. Epub 2011/04/12. doi: JB.01517-10 [pii] 10.1128/JB.01517-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cheung AL, Ying P. Regulation of alpha- and beta-hemolysins by the sar locus of Staphylococcus aureus. J Bacteriol. 1994;176(3):580–5. Epub 1994/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chien Y, Manna AC, Projan SJ, Cheung AL. SarA, a global regulator of virulence determinants in Staphylococcus aureus, binds to a conserved motif essential for sar-dependent gene regulation. J Biol Chem. 1999;274(52):37169–76. Epub 1999/12/22. . [DOI] [PubMed] [Google Scholar]
- 87. Jelsbak L, Hemmingsen L, Donat S, Ohlsen K, Boye K, Westh H, et al. Growth phase-dependent regulation of the global virulence regulator Rot in clinical isolates of Staphylococcus aureus. Int J Med Microbiol. 2010;300(4):229–36. Epub 2009/08/12. doi: S1438-4221(09)00058-7 [pii] 10.1016/j.ijmm.2009.07.003 . [DOI] [PubMed] [Google Scholar]
- 88. McCallum N, Bischoff M, Maki H, Wada A, Berger-Bachi B. TcaR, a putative MarR-like regulator of sarS expression. J Bacteriol. 2004;186(10):2966–72. Epub 2004/05/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Baek KT, Frees D, Renzoni A, Barras C, Rodriguez N, Manzano C, et al. Genetic variation in the Staphylococcus aureus 8325 strain lineage revealed by whole-genome sequencing. PLoS One. 2013;8(9):e77122 10.1371/journal.pone.0077122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ballal A, Manna AC. Expression of the sarA family of genes in different strains of Staphylococcus aureus. Microbiology. 2009;155(Pt 7):2342–52. Epub 2009/04/25. doi: mic.0.027417–0 [pii] 10.1099/mic.0.027417-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Reyes D, Andrey DO, Monod A, Kelley WL, Zhang G, Cheung AL. Coordinated regulation by AgrA, SarA, and SarR to control agr expression in Staphylococcus aureus. J Bacteriol. 2011;193(21):6020–31. Epub 2011/09/13. 10.1128/JB.05436-11 JB.05436-11 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ram G, Chen J, Kumar K, Ross HF, Ubeda C, Damle PK, et al. Staphylococcal pathogenicity island interference with helper phage reproduction is a paradigm of molecular parasitism. Proc Natl Acad Sci U S A. 2012;109(40):16300–5. Epub 2012/09/20. 10.1073/pnas.1204615109 1204615109 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ubeda C, Maiques E, Barry P, Matthews A, Tormo MA, Lasa I, et al. SaPI mutations affecting replication and transfer and enabling autonomous replication in the absence of helper phage. Mol Microbiol. 2008;67(3):493–503. Epub 2007/12/19. doi: MMI6027 [pii] 10.1111/j.1365-2958.2007.06027.x . [DOI] [PubMed] [Google Scholar]
- 94. Bisognano C, Kelley WL, Estoppey T, Francois P, Schrenzel J, Li D, et al. A recA-LexA-dependent pathway mediates ciprofloxacin-induced fibronectin binding in Staphylococcus aureus. J Biol Chem. 2004;279(10):9064–71. Epub 2003/12/31. 10.1074/jbc.M309836200 M309836200 [pii]. . [DOI] [PubMed] [Google Scholar]
- 95. Ubeda C, Maiques E, Knecht E, Lasa I, Novick RP, Penades JR. Antibiotic-induced SOS response promotes horizontal dissemination of pathogenicity island-encoded virulence factors in staphylococci. Mol Microbiol. 2005;56(3):836–44. Epub 2005/04/12. doi: MMI4584 [pii] 10.1111/j.1365-2958.2005.04584.x . [DOI] [PubMed] [Google Scholar]
- 96. Maiques E, Ubeda C, Campoy S, Salvador N, Lasa I, Novick RP, et al. beta-lactam antibiotics induce the SOS response and horizontal transfer of virulence factors in Staphylococcus aureus. J Bacteriol. 2006;188(7):2726–9. Epub 2006/03/21. doi: 188/7/2726 [pii] 10.1128/JB.188.7.2726-2729.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tormo-Mas MA, Mir I, Shrestha A, Tallent SM, Campoy S, Lasa I, et al. Moonlighting bacteriophage proteins derepress staphylococcal pathogenicity islands. Nature. 2010;465(7299):779–82. Epub 2010/05/18. doi: nature09065 [pii] 10.1038/nature09065 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Karlsson-Kanth A, Tegmark-Wisell K, Arvidson S, Oscarsson J. Natural human isolates of Staphylococcus aureus selected for high production of proteases and alpha-hemolysin are sigmaB deficient. Int J Med Microbiol. 2006;296(4–5):229–36. Epub 2006/03/15. doi: S1438-4221(06)00075-0 [pii] 10.1016/j.ijmm.2006.01.067 . [DOI] [PubMed] [Google Scholar]
- 99. Cheung AL, Chien YT, Bayer AS. Hyperproduction of alpha-hemolysin in a sigB mutant is associated with elevated SarA expression in Staphylococcus aureus. Infect Immun. 1999;67(3):1331–7. Epub 1999/02/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Schlievert PM, Merriman JA, Salgado-Pabon W, Mueller EA, Spaulding AR, Vu BG, et al. Menaquinone analogs inhibit growth of bacterial pathogens. Antimicrob Agents Chemother. 2013;57(11):5432–7. Epub 2013/08/21. 10.1128/AAC.01279-13 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sun F, Li C, Jeong D, Sohn C, He C, Bae T. In the Staphylococcus aureus two-component system sae, the response regulator SaeR binds to a direct repeat sequence and DNA binding requires phosphorylation by the sensor kinase SaeS. J Bacteriol. 2010;192(8):2111–27. Epub 2010/02/23. doi: JB.01524-09 [pii] 10.1128/JB.01524-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Weinrick B, Dunman PM, McAleese F, Murphy E, Projan SJ, Fang Y, et al. Effect of mild acid on gene expression in Staphylococcus aureus. J Bacteriol. 2004;186(24):8407–23. Epub 2004/12/04. doi: 186/24/8407 [pii] 10.1128/JB.186.24.8407-8423.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sarafian SK, Morse SA. Environmental factors affecting toxic shock syndrome toxin-1 (TSST-1) synthesis. J Med Microbiol. 1987;24(1):75–81. Epub 1987/08/01. . [DOI] [PubMed] [Google Scholar]
- 104. Schlievert PM, Kelly JA. Clindamycin-induced suppression of toxic-shock syndrome—associated exotoxin production. J Infect Dis. 1984;149(3):471 Epub 1984/03/01. . [DOI] [PubMed] [Google Scholar]
- 105. Kiyota H, Kendrick MI, Kass EH. Influence of magnesium concentration on production of exoprotein and beta-lactamase by Staphylococcus aureus and Staphylococcus hemolyticus. J Infect Dis. 1989;160(6):1061–3. Epub 1989/12/01. . [DOI] [PubMed] [Google Scholar]
- 106. Taylor D, Holland KT. Production of toxic shock syndrome toxin 1 by Staphylococcus aureus under aerobic and anaerobic conditions and the effect of magnesium ion limitation. Rev Infect Dis. 1989;11 Suppl 1:S151–6. Epub 1989/01/01. . [DOI] [PubMed] [Google Scholar]
- 107. Mills JT, Dodel AW, Kass EH. Regulation of staphylococcal toxic shock syndrome toxin-1 and total exoprotein production by magnesium ion. Infect Immun. 1986;53(3):663–70. Epub 1986/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ponceau and Coomassie staining of strains used in the experiment of fig 3 (A and B) and Coomassie staining the various strains used in all other experiments (C), in post-exponential growth phase (OD600 1.5 to 2). Carbonic anhydrase was added in each supernatant as a concentration and loading control. Carbonic anhydrase digestion in DA142 and DA156 lanes of panel C can be observed and is probably due to increased secretion of proteases in sarA mutants.
(TIF)
Normalized cycle thresholds for n = 3 independent determinations for all figures presented in the text showing qRT-PCR data and prior to conversion to fold-change as described in Materials and Methods. The data are based upon the ct values presented in S1 Table. Each graphic displays Figure and corresponding probe used.
(DOCX)
(DOCX)