Abstract
Depression during pregnancy has been linked to in utero stress and is associated with long-lasting symptoms in offspring, including anxiety, helplessness, attentional deficits, and social withdrawal. Depression is diagnosed in 10-20% of expectant mothers, but the impact of antidepressant treatment on offspring development is not well documented, particularly for females. Here, we used a prenatal stress model of maternal depression to test the hypothesis that in utero antidepressant treatment could mitigate the effects of prenatal stress. We also investigated the effects of prenatal stress and antidepressant treatment on gene expression related to GABAergic and serotonergic neurotransmission in the amygdala, which may underlie behavioral effects of prenatal stress. Nulliparous female rats were implanted with osmotic minipumps delivering clinically-relevant concentrations of escitalopram and mated. Pregnant dams were exposed to 12 days of mixed-modality stressors, and offspring were behaviorally assessed in adolescence (postnatal day 28) and adulthood (beyond day 90) to determine the extent of behavioral change. We found that in utero stress exposure, regardless of escitalopram treatment, increased anxiety-like behavior in adolescent females and profoundly influenced amygdala expression of the chloride transporters KCC2 and NKCC1, which regulate GABAergic function. In contrast, prenatal escitalopram exposure alone elevated amygdala expression of 5-HT1A receptors. In adulthood, anxiety-like behavior returned to baseline and gene expression effects in the amygdala abated, whereas deficits emerged in novel object recognition for rats exposed to stress during gestation. These findings suggest prenatal stress causes age-dependent deficits in anxiety-like behavior and amygdala function in female offspring, regardless of antidepressant exposure.
Keywords: prenatal stress, female, GABA, anxiety, antidepressant, serotonin
2. Introduction
The impact of maternal depression and its treatment on offspring neurodevelopment is of great consequence to human health. Depression affects approximately 11% of expectant mothers (Gaynes et al., 2005) and a disproportionate percentage of women in the general population (Mitchell et al., 2011; Parker and Brotchie, 2010). Despite these findings, few preclinical studies have documented the impact of clinically relevant in utero antidepressant exposure on offspring, and fewer have studied the impact on female offspring (Bourke et al., 2014).
In utero exposure to antidepressants may mitigate the effects of depression-related gestational stress or may directly influence offspring development. Prenatal stress is associated with later-life symptoms of anxiety, helplessness, attentional deficits, and social withdrawal in both humans and animal models (Bourke et al., 2013a; Frye and Wawrzycki, 2003; Mueller and Bale, 2007; Newport et al., 2002). Emotional deficits caused by prenatal stress are often sex-dependent (Mueller and Bale, 2007; Tibu et al., 2014; Van den Hove et al., 2013), and sex may also modulate the effects of in utero antidepressant exposure.
The impact of prenatal stress on offspring behavior is mediated in part by changes to the basolateral nucleus of the amygdala (BLA), a brain structure involved in emotional perception and behavior (Ledoux, 2004; Pape and Pare, 2010; Walker and Davis, 2008). A wealth of evidence has correlated prenatal stress effects in the BLA with elevated anxiety-like behavior (Buss et al., 2012; Cratty et al., 1995; Kraszpulski et al., 2006; Sadler et al., 2011). Furthermore, prenatal stress alters amygdala neuron excitability (Ehrlich et al., 2015) and gene expression related to GABA (Laloux et al., 2012; Sarro et al., 2014), an inhibitory neurotransmitter that tightly regulates amygdala function and affective state (Ehrlich et al., 2009; Quirk and Gehlert, 2003; Rainnie et al., 2004).
Signaling by the fast GABA receptor, GABAA, is modulated the by the intracellular concentration of the receptor's main permeant ion, chloride. The concentration gradient of chloride across neuronal membranes, which dictates the strength and sign of GABAergic synaptic currents, is established by chloride transporters that accumulate or extrude chloride ions. During postnatal development, BLA neurophysiology is highly dynamic (Ehrlich et al., 2012; Thompson et al., 2008), as neurons reduce expression of the chloride accumulator, NKCC1 (Na-K-Cl cotransporter 1), in favor of the chloride extruder, KCC2 (K-Cl cotransporter 2), switching GABAA receptors from excitatory to inhibitory (Ehrlich et al., 2013). In adults, stress influences GABAergic transmission by regulating the balance NKCC1 and KCC2 expression (Maguire, 2014; Sarkar et al., 2011). Despite the stress sensitivity of these transporters and their role in neuronal maturation, it is unknown whether prenatal stress or escitalopram influences their expression in the developing amygdala.
Here, we examined effects of prenatal antidepressant exposure and its interaction with prenatal stress on developing female offspring. We used a rodent prenatal stress model of maternal depression (Bourke et al., 2013a; Bourke et al., 2013b) in which dams are continuously exposed to clinically-relevant doses of escitalopram, a selective serotonin reuptake inhibitor, throughout gestation concurrent with chronic, unpredictable mild stress. The effects of this paradigm on maternal behavior (Bourke et al., 2013a) and male offspring (Bourke et al., 2013b) have previously been reported. Here we investigated the age-dependent effects of prenatal stress and/or chronic in utero antidepressant exposure on behavior and chloride transporter expression in the BLA during adolescence and adulthood on female offspring. We hypothesized that anxiogenic effects of prenatal stress would reflect dampened GABAergic function in the BLA, and such effects would be mitigated by prenatal antidepressant exposure. Given that escitalopram may help treat depression via regulation of serotonergic transmission in the amygdala (Arnone et al., 2012; Bigos et al., 2008; Lanzenberger et al., 2012; Rosenblau et al., 2012), we also measured offspring expression of two amygdala serotonin receptors linked to the treatment of depression, 5-Ht1a and 5-Ht7 (Bosker et al., 2001; Hahn et al., 2010; Naumenko et al., 2014; Takeda et al., 2005). The collected data indicate that in utero exposure to chronic stress, regardless of escitalopram treatment, caused temporary behavioral and gene expression deficits during adolescence that resolved by adulthood.
3. Methods
3.1 Prenatal Stress and Escitalopram Exposure
Rats used in this experiment were bred in-house from male Sprague-Dawley retired breeders and nulliparous females weighing 200-225 grams, purchased from Charles River Laboratories (Charles River, Wilmington, MA). Both the stress paradigm and drug administration paradigms have been previously characterized (Bourke et al., 2013a; Bourke et al., 2013b). Nulliparous female rats were implanted with Alzet 28-day osmotic minipumps (model 2ML4, Alzet, Cupertino, CA) delivering either 0.9% saline or escitalopram oxalate in 0.9% saline based upon the expected weight of the pregnant dam on gestational day (G) 21 (Bourke et al., 2013a; Bourke et al., 2013b). The estimated expected weight was based on assessment of G21 weights from 4 previous studies (n=36). For these studies, the actual dose on G21 was 12.2 mg/kg/day. This results in steady-state serum drug concentrations that are always within the clinically observed range even though the dose is slightly higher early in the experiment prior to the weight gain associated with pregnancy (Bourke et al., 2013a). Escitalopram oxalate was generously provided by Lundbeck USA (Paramus NJ). Three days after minipump implantation, females were bred with retired breeder males. Gestational day 0 was established as the day when a sperm plug was noted. On G9, the chronic unpredictable mild stress model of depression began and consisted of restraint, cage tilt, damp bedding, cage changes, noise, and overnight illumination (Bourke et al., 2013a). Prenatal stress began on G9 because it corresponds with development of the fetal central nervous system (Clancy et al., 2001) and minimizes premature termination of the pregnancy as a result of excessive stress. The final prenatal stress exposure occurred on G20.
3.2 Animals
All offspring were kept on a 12:12 light:dark cycle (lights on at 7:00 AM) in a humidity (60%) and temperature (20°C-23°C) controlled facili ty with their natural mother. We (Bourke et al., 2013a) have reported that neither this stressor paradigm nor escitalopram treatment modifies gestational length, litter size, or maternal care of the offspring. Rodent diet 5001 chow (Purina Mills, Richmond, IN) and water were available ad libitum throughout the study. Three days after birth, rat pups were sexed, and litters were culled to six male and two female pups per litter. Only female rats were used for this study; male offspring were allocated to a parallel study following weaning (Bourke et al., 2013b). Animals were weaned on post-natal day (PND) 21 and kept in same-sex pairs. Only one pup from a litter was assigned to an endpoint in order to prevent litter effects (Holson and Pearce, 1992). Each group was assigned between 8 and 12 pups. Experimental groups included non-stress/saline (Control), non-stress/escitalopram (Escit), stress/saline (Stress), and stress/escitalopram (Stress + Escit) with assessment occurring either during adolescence or in adulthood. All experiments were performed in accordance with the Institutional Animal Care and Use Committee of Emory University and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
3.3 Behavior
Although rodents cannot be queried regarding their affective states, behavioral tests have been established which probe circuitry implicated in the pathogenesis of depression and anxiety (Crawley, 2007; Whishaw and Kolb, 2004). Behavioral testing was conducted between PND 28 and 35 for the adolescent endpoints with the administration of one test per day with the exception of the sucrose consumption test which lasted 48 hours in the animals' home cage. An additional cohort of rats with identical rearing was used to assess behavior in adulthood (Control n = 12; Escit n = 11; Stress n = 15; Stress + Escit n = 10). For the adult endpoint, rats were maintained in the colony as described until at least PND 90, and then, adult behavior was assessed. Behaviors were recorded and scored using Cleversys Top Scan/Forced Swim software (Reston, VA). All behavioral tests and scoring were conducted by an experimenter blind to treatment groups. Behavioral equipment was cleaned with 70% ethanol between animals.
3.3.1 Open Field
Rats were placed in the center of a 70 × 70 cm2 arena and were allowed to explore for ten minutes. Overall distance traveled as well as the time spent in the center of the arena versus the periphery was used as a metric of anxiety-like behaviors (Prut and Belzung, 2003). This test was conducted in both the light and dark cycles in order to assess behavior both during the dormant and active phases, respectively, of the rat. In the light cycle condition, testing was conducted under bright overhead illumination by fluorescent lighting. In the dark cycle condition, testing was conducted under dim red light
3.3.2 Social Interaction
Rats were again placed in the center of a 70 × 70 cm2 arena for assessment of social interaction. In this particular test, the arena contained an age-matched novel female stimulus conspecific. Experimental animals were allowed to explore the arena for ten minutes and latency to interact with the novel animal as well as total time interacting with the novel animal were measured. Testing took place during the light cycle under bright light.
3.3.3 Novel Object Recognition
Rats were placed in the center of a 70 × 70 cm2 arena containing two identical objects. Following a one hour delay, rats were again placed in the arena with one familiar object (from previous exposure) and a novel object. Time spent investigating the novel object was measured as an index of object recognition memory (Ennaceur and Delacour, 1988). The initial object exposure lasted fifteen minutes, and the recorded object recognition session lasted five minutes and took place during the light cycle.
3.3.4 Elevated Plus Maze
The elevated plus maze consists of a “+” shaped maze with two open arms and two closed arms elevated 1 m off the ground. Total arm entries as well as time spent in the open versus closed arms were recorded with the latter used as a metric of anxiety-like behavior (Walf and Frye, 2007). In addition, risk assessment behaviors were tabulated as an additional metric of anxiety-like behavior (Whishaw and Kolb, 2004). The test lasted for five minutes and was conducted during the animals' dark cycle.
3.4 Gene Expression
Animals were killed by decapitation under isoflurane anesthesia on PND 42 (adolescents) or PND 90-120 (adults), and the brains were quickly removed. To quantify the expression of chloride transporter and serotonin receptor transcripts in isolated basolateral amygdala (BLA) tissue from adolescents, 350 μm coronal slices containing the BLA were prepared as previously described (Ryan et al., 2012). The BLA from each slice was excised immediately by microdissection. For adult rats, following decapitation brains were immediately frozen on dry ice and stored at -80°C until dissect ion. The BLA was dissected using a 1mm micropunch tool and subsequently stored until RNA extraction. For adolescent samples, BLA samples were homogenized in Trizol (Invitrogen, Carlsbad, CA), and isolated RNA was reverse transcribed with a cocktail containing 5 μl of 10× RT buffer, 10 mM dNTP mix, 10× random hexanucleotides, Multiscribe RT 5 U/μl, and RNAase free water. For adult samples, BLA samples were homogenized in Trizol (Invitrogen, Carlsbad, CA), and isolated RNA was reverse transcribed with a cocktail containing 2 μl of 10× RT buffer, 8 mM dNTP mix, 10× random hexanucleotides, Multiscribe RT 5 U/μl, and RNAase free water. cDNA was stored at −20°C. All reagents were obtained from Applied Biosystems (Foster City, CA). All reactions were prepared in triplicate using a 40 cycle thermal cycling program. Measured primers include Nkcc1 (Taqman ID: Rn00582505_m1), Kcc2 (Rn00592624_m1), and 5Ht1a (Rn00561409_s1). Realtime PCR reactions were performed using an Applied Biosystems 7500 Fast-Real Time PCR system (Applied Biosystems, Foster City, CA). All mRNA measurements were normalized to 18S (Hs99999901_s1) rRNA expression. We calculated fold change using the 2-ΔΔCt method (Livak and Schmittgen, 2001) and presented fold changes for each experimental group normalized to average values for the control group.
3.5 Hormone Assessments
A separate cohort of rats with identical rearing was used to measure serum corticosterone and estradiol concentrations. For corticosterone measures, tail snip blood was collected after a restraint stress challenge (30-minute session in an acrylic rat restrainer; BrainTree Scientific, Braintree, MA) on PND 38. Plasma corticosterone was assessed using the ImmunoChem 125I Corticosterone RIA kit with a sensitivity of 1 ng/mg (MP Biomedicals, Orangeburg, NY). On PND 42, rats were rapidly decapitated, and trunk blood was collected for estradiol assessment (DSL-4400 RIA Kit with a sensitivity of 4.7 pg/mL; DSL, Webster, TX). All samples were run in duplicate.
3.6 Data Analysis
Behavior and hormone assay data were compared using two-way ANOVA with the factors of stress (control vs stress) and drug (vehicle vs escitalopram) following assessment of equal variance and normality. Gene expression data were analyzed as described above, and compared using two-way ANOVA. Data were considered significant when p < 0.05. All data are expressed as mean ± the standard error of the mean (S.E.M.).
4. Results
4.1 Prenatal escitalopram treatment did not rescue the anxiogenic effect of prenatal stress during adolescence
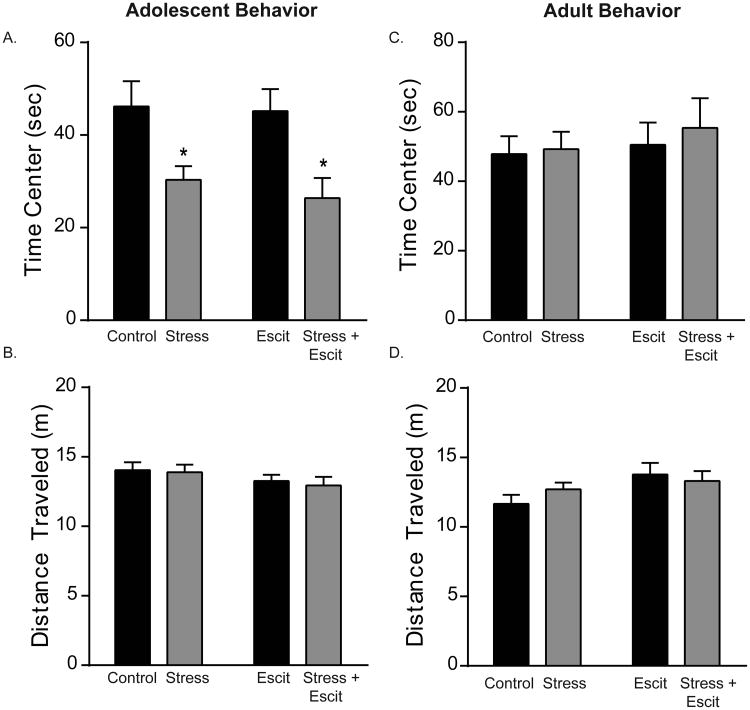
We first determined whether prenatal escitalopram could modulate the behavioral effects of prenatal stress (Figure 1). When testing occurred during the animals' dark cycle, prenatal stress reduced the time adolescent female offspring spent in the center of the open field (main effect of prenatal stress, F1,28 = 14.91, p < 0.05; Figure 1A). In contrast, prenatal escitalopram had no effect on open field behavior (p > 0.05 for main effect of drug and interaction between stress and drug). Overall mobility (distance traveled) did not differ among groups (p > 0.05; Figure 1B). When testing occurred during the animals' light cycle, no differences in open field behavior were detected (p > 0.05; Supplemental Figure S1). Importantly, these effects of prenatal stress were limited to adolescence, because no differences in time spent in field center or total distance traveled were observed in adulthood (p > 0.05; Figure 1C & D).
Figure 1. Anxiety-like behavior of adolescent, female offspring exposed in utero to stress and/or escitalopram.
A, B) Rats were allowed to explore an open field for ten minutes. Prenatal stress reduces central tendency regardless of escitalopram treatment (A). No differences in mobility were detected as evidenced by equivalent total distance traveled (B). No differences were observed in time spent in the center (C) or mobility (D) in adults. For all, * indicates p < 0.05 and error bars indicate standard error of the mean (S.E.M.).
Elevated plus maze activity revealed similar effects. Although no differences were detected in the total number of elevated plus arm entries over the five minute period (p > 0.05; Supplemental Figure S3), offspring exposed to prenatal stress exhibited more risk-assessment behavior. Rats exposed to stress in utero showed a higher degree of risk-assessment behavior as evidenced by a greater number of stretch attend postures (main effect of stress: F1,27 = 9.95, p < 0.05) relative to non-stressed animals (10.50 ± 1.8 and 5.62 ± 1.39 postures, respectively). In addition, in utero escitalopram exposure also increased stretch attend postures (Escit: 8.57 ± 1.01; Stress + Escit: 15.13 ± 2.47 postures), producing a main effect of drug treatment (F1,27 = 4.37, p < 0.05) when compared to vehicle-exposed rats.
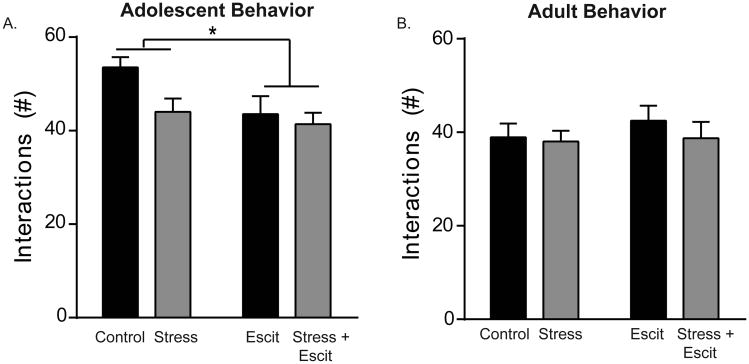
4.2 Prenatal escitalopram reduced social interaction in adolescent females
We also tested the effects of prenatal stress and escitalopram on social interaction in adolescent females (Figure 2A). Exposure to escitalopram in utero reduced social interaction in adolescence as evidenced by a main effect of drug on social behavior (F1,28 = 4.61, p < 0.05; 43.50 ± 3.90 interactions for Escit vs. 53.50 ± 2.24 interactions for Controls). The impact of prenatal stress exposure on social interaction was less robust; although the effect was directionally similar, the difference did not reach statistical significance (F1,28 = 3.91, p = 0.06). However, it is important to note that the power of this particular comparison was below the desired level of 0.8; therefore, although the null cannot be rejected in this particular case, it is possible that a significant effect of prenatal stress would be documented with a larger sample size. There was no interaction between the two conditions (p > 0.05). Consistent with the resolution of the anxiogenic effects of prenatal stress by adulthood, no effects of prenatal stress or escitalopram on social interaction were detected in adulthood (Figure 2B; p > 0.05 in all cases).
Figure 2. Effects of prenatal stress and/or escitalopram on social interaction in adolescent and adult female offspring.
A) Prenatal escitalopram reduced social interaction with a novel conspecific of the same age and sex regardless of prenatal stress exposure. Prenatal stress did not have an independent main effect on this behavior (p = 0.06). B) These behavioral effects were transient and were not documented in adult offspring. For all, * indicates p < 0.05 and error bars indicate standard error of the mean (S.E.M.).
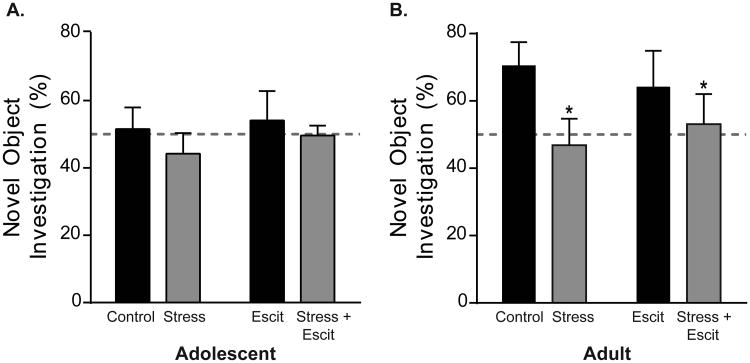
4.3 Prenatal escitalopram did not rescue prenatal stress-induced deficits in novel object recognition in adult females
We subsequently tested the effects of prenatal stress and escitalopram on memory for familiar objects following a 1 hour delay after initial exposure (Figure 3A). Consistent with previous literature, control females did not exhibit a preference for novel objects during adolescence. The adolescent control females investigated the novel object 51.59 ± 6.36 % of the time, and therefore, were operating at chance (Figure 3A). Prenatal stress and escitalopram treatment did not affect performance in adolescence as revealed by a two-way ANOVA (p > 0.05 in all cases).
Figure 3. Effects of prenatal stress and/or escitalopram on novel object recognition in adolescent and adult female offspring.
A) One hour following pre-exposure, adolescent females showed no preference for the novel object, regardless of in utero condition. Rats investigated the novel object around chance levels (dashed line) in each group. B) When identically tested in adulthood, control female offspring now exhibit a preference for the novel object. Prenatal stress exposure, in the absence or presence of concurrent prenatal escitalopram exposure, prevented novel object recognition, causing investigation at chance levels. For all, * indicates p < 0.05 and error bars indicate standard error of the mean (S.E.M.).
The deficit in novel object recognition was overcome with age as control females investigated the novel object well above chance (70.58 ± 6.84 %) in adulthood (Figure 3B). Importantly, prenatal stress prevented the emergence of novel object recognition because adult female offspring previously exposed to prenatal stress still investigated around chance levels (46.85 ± 7.87 %; main effect of stress: F1,42 = 4.12, p < 0.05). In contrast, prenatal escitalopram exposure did not impair novel object recognition in adulthood (p > 0.05) and prenatal stress and escitalopram did not interact to differentially alter investigation beyond the main effect of stress alone (p > 0.05).
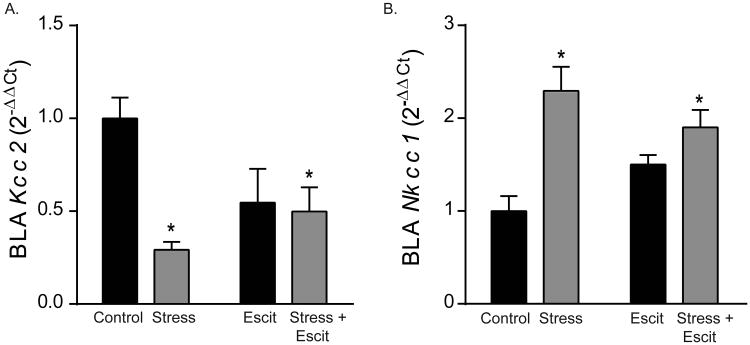
4.4 Prenatal stress decreased expression of Kcc2 and increased expression of Nkcc1 inamygdala of adolescent offspring
Next, we investigated changes to amygdala gene expression that could account for the observed behavioral effects of prenatal stress and escitalopram. BLA excitability is regulated by GABAergic tone and chloride transporter function, and we found that prenatal stress decreased Kcc2 expression and increased Nkcc1 expression during adolescence. Prenatal stress exposure decreased Kcc2 mRNA levels by 70% in the BLA (Figure 4A; main effect of stress: F1,28 = 8.80, p < 0.05). Prenatal escitalopram did not independently alter expression of Kcc2 (p > 0.05 for main effect of drug); however, prenatal stress interacted with escitalopram (F1,28 = 6.71, p < 0.05). Expression of Nkcc1 mRNA increased by 2.2-fold in response to prenatal stress (Figure 4B, main effect of stress: F1,28 = 20.40, p < 0.05;), but was not independently impacted by prenatal escitalopram (main effect of drug: p > 0.05). The combination of prenatal stress and escitalopram again had a similar effect to prenatal stress alone in adolescence, increasing Nkcc1 mRNA 2-fold (interaction: F1,28 = 5.69, p < 0.05).
Figure 4. Effects of prenatal stress and escitalopram on gene expression of chloride transporters in the BLA of adolescent female offspring.
A) Prenatal stress exposure decreased gene expression of Kcc2, normalized to adolescent Controls. This effect was not mitigated by escitalopram. B) Prenatal exposure to stress or the combination of stress and escitalopram increased gene expression of Nkcc1, normalized to Controls, in the BLA. For all, * indicates p < 0.05 and error bars indicate standard error of the mean (S.E.M.).
By adulthood, the effects of prenatal stress on chloride transporter expression had abated. No effects of prenatal stress, escitalopram, or the combination of the two were observed on Kcc2 (p > 0.05 in all cases) or Nkcc1 expression (p > 0.05 in all cases) in the BLA of adult offspring (Table 1).
Table 1. Effects of prenatal stress and escitalopram on gene expression of chloride transporters and serotonin receptors in the BLA of adult female offspring.
| Kcc2 | Nkcc1 | 5-Ht1a | 5-Ht7 | ||
|---|---|---|---|---|---|
| Saline | No Stress | 1 ± 0.227 | 1 ± 0.248 | 1 ± 0.240 | 1 ± 0.235 |
| Stress | 0.810 ± 0.186 | 1.011 ± 0.236 | 0.771 ± 0.223 | 0.472 ± 0.122 | |
| Escitalopram | No Stress | 0.870 ± 0.273 | 1.209 ± 0.403 | 0.752 ± 0.318 | 0.847 ± 0.281 |
| Stress | 0.960 ± 0.276 | 1.002 ± 0.301 | 1.173± 0.312 | 0.954 ± 0.192 |
Values expressed are mean fold change (2-ΔΔCt) ± SEM
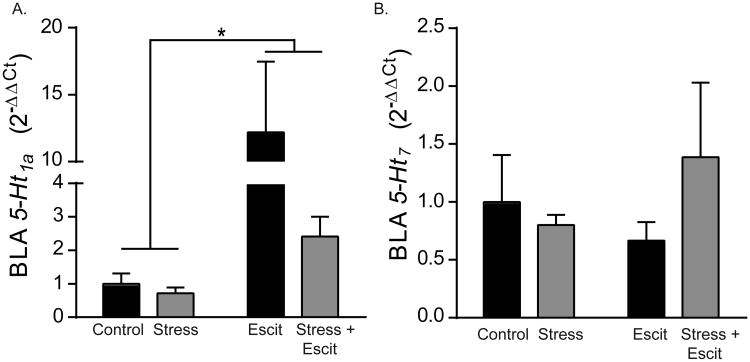
4.5 Prenatal escitalopram increases expression of inhibitory 5-Ht1a in adolescent offspringamygdala
We next tested the effects of prenatal stress and escitalopram on serotonin receptor expression in offspring BLA (Figure 5A). Exposure to prenatal escitalopram, but not stress, led to a pronounced, approximately 12-fold increase during adolescence in mRNA expression for the inhibitory, Gi-coupled serotonin receptor, 5-Ht1a (Figure 5A; F1,28 = 5.89, p < 0.05; n = 8). There was no effect of prenatal stress (p >0.05) nor an interaction of prenatal stress and escitalopram on expression of 5-Ht1a (p > 0.05). In contrast to the effect on 5-Ht1a, there was no effect of prenatal stress or escitalopram on BLA expression of the excitatory, Gs-coupled serotonin receptor, 5-Ht7 (Figure 5B; p > 0.05 in all cases).
Figure 5. Effects of prenatal stress and escitalopram on gene expression of serotonin receptors in the BLA of adolescent female offspring.
A) Gene expression, normalized to Controls, of 5Ht1a in the BLA of adolescent rats was increased by prenatal exposure to escitalopram. B) Expression of 5HT7 was not altered by either prenatal stress or prenatal escitalopram. For all, * indicates p < 0.05 and error bars indicate standard error of the mean (S.E.M.).
Similar to the observed changes to chloride transporter expression, by adulthood all effects on 5-Ht1a expression in adolescence had abated. No effects of prenatal stress, escitalopram, or the combination of the two were observed on 5-Ht1a in the BLA of adult offspring (Table 1; p > 0.05 in all cases). Similarly, no significant effects of any treatment were observed on adult BLA expression of 5-Ht7 (Table 1; p > 0.05 in all cases).
4.6 Neither prenatal stress nor escitalopram altered serum corticosterone or estradiol in adolescence
In contrast to gene expression and behavior, physiological measures of the stress response were not altered by prenatal stress or escitalopram (Supplemental Figure S4). Serum corticosterone concentrations, measured after a 30 minute restraint stressor in a separate cohort of animals, were not altered by prenatal exposure to either stress or escitalopram. Control (432.30 ± 57.76 ng/mL), Stress (427.61 ± 32.73 ng/mL), Escit (404.03 ± 42.87 ng/mL), and Stress + Escit (454.81 ± 22.51 ng/mL) groups all had comparable concentrations of serum corticosterone as measured by a two-way ANOVA (p > 0.05 in all cases). Similarly, estradiol concentrations did not differ by group (Control: 33.10 ± 1.88 pg/mL; Stress: 28.30 ± 2.27 pg/mL; Escit: 29.28 ± 1.0 pg/mL; and Stress + Escit: 30.88 ± 1.21 pg/mL; p > 0.05).
5. Discussion
Collectively, these data demonstrate that prenatal exposure to chronic, unpredictable mild stress influenced behavior of female offspring and their expression of genes regulating neurotransmission in the BLA. Our behavioral studies have shown that prenatal escitalopram did not mitigate the anxiogenic effect of prenatal stress in adolescent female offspring. Similarly, escitalopram did not mitigate the adverse impact of prenatal stress on memory in adulthood. We followed up these findings by investigating changes in neurotransmitter-related gene expression in the amygdala that could contribute to the behavioral alterations caused by prenatal stress and escitalopram. Exposure to prenatal stress shifted expression during adolescence away from the mature chloride transporter, Kcc2, and towards the immature chloride transporter, Nkcc1. This change in chloride transporter expression may disinhibit the BLA, potentially accounting for the anxiogenic effects observed in adolescence.
5.1 Behavioral effects of prenatal stress and escitalopram
Behavioral effects of prenatal stress and sex differences within the manifestation of these effects are prevalent across stress models and strains of rats (Mueller and Bale, 2007; Tibu et al., 2014; Veru et al., 2014); however, prenatal chronic stress in conjunction with in utero antidepressant exposure is less well understood. In our hands, prenatal stress increased anxiety-like behavior in adolescent female offspring. Prenatal escitalopram did not mitigate the effects of prenatal stress because females exposed to both manipulations exhibited altered central tendency in the open field and elevated risk-assessment behavior in the EPM. The demonstration of prenatal stress-induced increases in anxiety-like behavior is consistent with a precedent for anxiogenic effects of prenatal stress in adolescent female offspring (Baker et al., 2008) and chronic stress during other phases of development has also been shown to produce longstanding changes in female anxiety-like behaviors (Bourke et al., 2011).
All adolescent females, regardless of prenatal treatment or lack thereof, exhibited deficits in novel object recognition. However, prenatal stress prevented the typical emergence of this capacity in adulthood. There is a precedent for deficits in novel object recognition in adolescence, and studies of prenatal stress have found no effects on object recognition at this age (Markham et al., 2010). Our results confirm previous findings that prenatal stress exposure alters cognitive performance in adult rodents (Abdul Aziz et al., 2012; Markham et al., 2010; Schulz et al., 2011). These data suggest that prenatal exposure to chronic stress has long-lasting, detrimental effects to the offspring that are not mitigated by escitalopram. The behavioral effects of prenatal stress were observed despite no change in serum corticosterone concentrations, suggesting that organizational changes to neural signaling are triggered early in development which persist and mature through adolescence and into adulthood.
5.2 Prenatal stress shifts expression of chloride transporters to a more excitatory configuration in the adolescent BLA
Altered chloride transporter expression has been observed following neuronal stress (Wake et al., 2007), acute stress in rodents (Maguire, 2014; Sarkar et al., 2011), and seizures (Toole et al., 2014), suggesting GABA physiology is susceptible to environmental influence. We provide evidence that prenatal stress shifts mRNA expression in the BLA away from Kcc2 and towards Nkcc1. By adulthood, we observed the mature configuration of chloride transporter expression, regardless of prenatal condition, suggesting prenatal stress may delay until adolescence the typical developmental switch in transporter expression (Ehrlich et al., 2013).
Around two weeks of age in the rat BLA, expression of the chloride accumulator, Nkcc1, typically declines and that of the chloride extruder, Kcc2, emerges, driving a reduction in intracellular chloride levels and a switch from excitatory to inhibitory GABAA receptors (Ehrlich et al., 2013). These receptors mediate all of the fast synaptic inhibition in the adult BLA and their function is classically enhanced by anxiolytic drugs including benzodiazepines and barbiturates (Ehrlich et al., 2009; Rainnie et al., 1991). Therefore, the reduction in the ratio of Kcc2:Nkcc1 should decrease inhibitory tone in the amygdala by rendering GABAA receptors less inhibitory.
Diminished amygdala inhibition has been argued to drive anxiety disorders in humans (Quirk and Gehlert, 2003; Rainnie et al., 2004), and we propose the anxiogenic effect of prenatal stress is due to a reduction in relative expression of Kcc2 to Nkcc1 in the BLA of adolescent females. Furthermore, altered chloride transporter expression in the developing amygdala may confer susceptibility for developmental psychiatric disorders, and a similar shift towards Nkcc1 expression was recently implicated in autism spectrum disorders (Tyzio et al., 2014).
5.3 Prenatal escitalopram increases 5-Ht1a expression in the adolescent BLA and alters social behavior
Gene expression of 5-Ht1a was increased in the adolescent BLA more than 10-fold following in utero exposure to chronic escitalopram. Alterations to 5-HT systems following prenatal exposure to antidepressants have been reported previously (Cabrera-Vera and Battaglia, 1998; Bourke et al., 2014). In the current study, the effect of escitalopram was specific to the inhibitory, Gi-coupled 5-Ht1a receptor, as no effect of prenatal escitalopram was observed on expression of the excitatory, Gs-coupled 5-Ht7. Previous studies have reported decreased expression of 5Ht1a in the brain following early life stress (Franklin et al., 2011) and unpredictable stress in adulthood (Hazra et al., 2012), but neither of these previous studies nor the present study have identified an effect on serotonergic function due to prenatal stress (Abdul Aziz et al., 2012). 5-HT1a receptors can influence amygdala function via action at autoreceptors (Fisher et al., 2006), but the observed effects on mRNA expression in the BLA likely reflect postsynaptic changes that should directly regulate amygdala neuron excitability rather than local serotonin release. Interestingly, females typically express higher postsynaptic 5-HT1A binding than males (Schiller et al., 2006), so effects of prenatal escitalopram on BLA receptor expression and emotional behavior may be sex-specific. The effects of escitalopram exposure on gene expression in adolescence were not observed in adulthood, and we previously reported a comparable lack of effects for adult male offspring exposed to identical procedures in utero (Bourke et al., 2013b).
While prenatal exposure to antidepressants has been investigated for possible links to cardiovascular malformations (Malm, 2012), hypertension (Grigoriadis et al., 2014), and autism spectrum disorders (Sørensen et al., 2013), little risk has been documented (Bourke et al., 2014). The changes reported here in terms of serotonin receptor expression are transient as they were not noted in adulthood. We also report a minor change in social behavior for offspring that had been exposed to escitalopram in utero (Figure 2A), but this attenuation in social behavior is transient (Figure 2B) and does not appear to be coupled to deficits in the other behavioral endpoints examined (Figures 1, S1, S2, 3).
Together, these data suggest that prenatal stress has both significant and long-lasting effects on neurodevelopment and behavior of female offspring. Effects of prenatal chronic stress on behavior, including emotional behavior and learning and memory, were observed regardless of concurrent prenatal exposure to escitalopram. These findings suggest prenatal stress causes transient, age-dependent deficits in anxiety-like behavior and amygdala function in female offspring, regardless of antidepressant exposure.
Supplementary Material
S1. No effect of prenatal stress or escitalopram on behavior in the open field test during the light cycle. Adolescent female rats were assessed for anxiety-like behaviors in the open field during the light cycle. In contrast to dark cycle testing (Figure 1), no group differences were detected in terms of time in center (left) or total distance travelled, suggesting no locomotor deficits (right). For all, error bars indicate standard error of the mean (S.E.M.); in all cases, p > 0.05 for main effects and interaction.
S2. Locomotor activity in the elevated plus maze. Open arm entries during a five minute elevated plus maze test were not affected by prenatal stress or escitalopram (p > 0.05 for main effects and interaction).
S3. No effect of prenatal stress or escitalopram on hormones. Serum concentrations of corticosterone (CORT) (left, measured on PND 38 immediately following a restraint stressor) and estradiol (right, measured on PND 42) were measured in a separate cohort of identically reared, adolescent female offspring. Prenatal stress and escitalopram had no effect on hormone levels as compared to control rats.
Highlights.
- Prenatal stress enhances anxiety-like behavior of female rats temporarily during adolescence.
- Prenatal stress promotes an immature configuration of chloride transporters in the adolescent amygdala.
- Prenatal stress causes memory impairment in adult females.
- Concurrent treatment with Escitalopram in utero does not prevent the effects of prenatal stress on behavior or amygdala gene expression.
Acknowledgments
Funding: This work was funded by the following grants from the National Institutes of Health: MH-077928 to ZNS, MH-069852 to DGR, RR-00165 to the Yerkes National Primate Research Center, and MH-090729 to DEE.
Footnotes
MJO serves as a consultant to H. Lundbeck and receives compensation for these services. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdul Aziz NHK, Kendall Da, Pardon MC. Prenatal exposure to chronic mild stress increases corticosterone levels in the amniotic fluid and induces cognitive deficits in female offspring, improved by treatment with the antidepressant drug amitriptyline. Behav Brain Res. 2012;231:29–39. doi: 10.1016/j.bbr.2012.02.040. [DOI] [PubMed] [Google Scholar]
- Arnone D, Mckie S, Elliott R, Thomas EJ, Juhasz G, Williams SR, Deakin J, Anderson IM. Increased amygdala responses to sad but not fearful faces in major depression: relation to mood state and pharmacological treatment. Am J Psychiatry. 2012;169:841–850. doi: 10.1176/appi.ajp.2012.11121774. [DOI] [PubMed] [Google Scholar]
- Baker S, Chebli M, Rees S, Lemarec N, Godbout R, Bielajew C. Effects of gestational stress: 1. Evaluation of maternal and juvenile offspring behavior. Brain Res. 2008;1213:98–110. doi: 10.1016/j.brainres.2008.03.035. [DOI] [PubMed] [Google Scholar]
- Bigos KL, Pollock BG, Aizenstein HJ, Fisher PM, Bies RR, Hariri AR. Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharmacol. 2008;33:3221–5. doi: 10.1038/npp.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosker FJ, Cremers TI, Jongsma ME, Westerink BH, Wikstrom HV, den Boer JA. Acute and chronic effects of citalopram on postsynaptic 5-hydroxytryptamine(1A) receptor-mediated feedback: a microdialysis study in the amygdala. J Neurochem. 2001;76:1645–1653. doi: 10.1046/j.1471-4159.2001.00194.x. [DOI] [PubMed] [Google Scholar]
- Bourke CH, Capello CF, Rogers SM, Yu ML, Boss-Williams KA, Weiss JM, Stowe ZN, Owens MJ. Prenatal exposure to escitalopram and/or stress in rats: A prenatal stress model of maternal depression and its treatment. Psychopharmacol. 2013a;228:231–41. doi: 10.1007/s00213-013-3030-z. Erratum http://www.ncbi.nlm.nih.gov/pubmed/25106390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Neigh GN. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm Behav. 2011;60:112–20. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Stowe ZN, Neigh GN, Olson DE, Owens MJ. Prenatal exposure to escitalopram and/or stress in rats produces limited effects on endocrine, behavioral, or gene expression measures in adult male rats. Neurotoxicol Teratol. 2013b;39:100–9. doi: 10.1016/j.ntt.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Stowe ZN, Owens MJ. Prenatal antidepressant exposure: clinical and preclinical findings. Pharmacol Rev. 2014;66:435–65. doi: 10.1124/pr.111.005207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Poggi E, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci USA. 2012;109:E1312–19. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Vera TM, Battaglia G. Prenatal exposure to fluoxetine (Prozac) produces site-specific and age-dependent alterations in brain serotonin transporters in rat progeny: evidence from autoradiographic studies. J Pharm Exp Ther. 1998;286:1474–1481. [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Cratty MS, Ward HE, Johnson A, Azzaro J. Prenatal stress increases corticotropin-releasing factor (CRF) content and release in rat amygdala minces. Brain Res. 1995;675:297–302. doi: 10.1016/0006-8993(95)00087-7. [DOI] [PubMed] [Google Scholar]
- Crawley JN. What is wrong with my mouse? 2nd. John Wiley & Sons, Inc.; Hoboken, New Jersey: 2007. [Google Scholar]
- Ehrlich DE, Rainnie DG. Prenatal stress alters the development of socioemotional behavior and amygdala neuron excitability in rats. Neuropsychopharm. 2015 doi: 10.1038/npp.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich DE, Ryan S, Rainnie D. Postnatal development of electrophysiological properties of principal neurons in the rat basolateral amygdala. J Physiol. 2012;590:4819–38. doi: 10.1113/jphysiol.2012.237453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich DE, Ryan SJ, Hazra R, Guo JD, Rainnie DG. Postnatal maturation of GABAergic transmission in the rat basolateral amygdala. J Neurophysiol. 2013;110:926–41. doi: 10.1152/jn.01105.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Lüthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–71. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Fisher PM, Meltzer CC, Ziolko SK, Price JC, Moses-Kolko EL, Berga SL, Hariri aR. Capacity for 5-HT1A-mediated autoregulation predicts amygdala reactivity. Nat Neurosci. 2006;9:1362–3. doi: 10.1038/nn1780. [DOI] [PubMed] [Google Scholar]
- Franklin TB, Linder N, Russig H, Thöny B, Mansuy IM. Influence of early stress on social abilities and serotonergic functions across generations in mice. PLoS One. 2011;6:e21842. doi: 10.1371/journal.pone.0021842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Wawrzycki J. Effect of prenatal stress and gonadal hormone condition on depressive behaviors of female and male rats. Horm Behav. 2003;44:319–326. doi: 10.1016/s0018-506x(03)00159-4. [DOI] [PubMed] [Google Scholar]
- Gaynes B, Gavin N, Meltzer-Brody S, Lohr K, Swinson T, Gartlehner G, Brody S, Miller W. Perinatal Depression: Prevalence, screening accuracy, and screening outcomes: Summary. AHRQ Evidence Report Summaries. 2005 doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriadis S, VonderPorten EH, Mamisashvili L, Tomlinson G, Dennis CL, Koren G, Steiner M, Mousmanis P, Cheung A, Ross LE. Prenatal exposure to antidepressants and persistent pulmonary hypertension of the newborn: systematic review and meta-analysis. BMJ. 2014;348:f6932–f6932. doi: 10.1136/bmj.f6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Lanzenberger R, Wadsak W, Spindelegger C, Moser U, Mien LK, Mitterhauser M, Kasper S. Escitalopram enhances the association of serotonin-1A autoreceptors to heteroreceptors in anxiety disorders. J Neurosci. 2010;30:14482–14489. doi: 10.1523/JNEUROSCI.2409-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazra R, Guo JD, Dabrowska J, Rainnie DG. Differential distribution of serotonin receptor subtypes in BNST(ALG) neurons: modulation by unpredictable shock stress. Neuroscience. 2012;225:9–21. doi: 10.1016/j.neuroscience.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–8. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Kraszpulski M, Dickerson PA, Salm A. Prenatal stress affects the developmental trajectory of the rat amygdala. Stress. 2006;9:85–95. doi: 10.1080/10253890600798109. [DOI] [PubMed] [Google Scholar]
- Laloux C, Mairesse J, Van Camp G, Giovine A, Branchi I, Bouret S, Morley-Fletcher S, Bergonzelli G, Malagodi M, Gradini R, Nicoletti F, Darnaudéry M, Maccari S. Anxiety-like behaviour and associated neurochemical and endocrinological alterations in male pups exposed to prenatal stress. Psychoneuroendocrinol. 2012;37:1646–58. doi: 10.1016/j.psyneuen.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Lanzenberger R, Kranz GS, Haeusler D, Akimova E, Savli M, Hahn A, Mitterhauser M, Spindelegger C, Philippe C, Fink M, Wadsak W, Karanikas G, Kasper S. Prediction of SSRI treatment response in major depression based on serotonin transporter interplay between median raphe nucleus and projection areas. Neuroimage. 2012;63:874–81. doi: 10.1016/j.neuroimage.2012.07.023. [DOI] [PubMed] [Google Scholar]
- Ledoux J. The amygdala. Curr Biol. 2004;17:868–874. [Google Scholar]
- Maguire J. Stress-induced plasticity of GABAergic inhibition. Front Cell Neurosci. 2014;8:157. doi: 10.3389/fncel.2014.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malm H. Prenatal exposure to selective serotonin reuptake inhibitors and infant outcome. Ther Drug Monit. 2012;34:607–14. doi: 10.1097/FTD.0b013e31826d07ea. [DOI] [PubMed] [Google Scholar]
- Markham JA, Taylor AR, Taylor SB, Bell DB, Koenig JI. Characterization of the cognitive impairments induced by prenatal exposure to stress in the rat. Front Behav Neurosci. 2010;4:173. doi: 10.3389/fnbeh.2010.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernández-Díaz S. Medication use during pregnancy, with particular focus on prescription drugs: 1976-2008. Am J Obs Gyn. 2011;205:51.e1–8. doi: 10.1016/j.ajog.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol Behav. 2007;91:55–65. doi: 10.1016/j.physbeh.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Naumenko VS, Popova NK, Lacivita E, Leopoldo M, Ponimaskin EG. Interplay between serotonin 5-HT1A and 5-HT7 receptors in depressive disorders. CNS Neurosci Ther. 2014;20:582–590. doi: 10.1111/cns.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport J, Stowe ZN, Nemeroff CB. Parental depression: Animal models of an adverse life event. Am J Psychiatry. 2002;159:1265–1283. doi: 10.1176/appi.ajp.159.8.1265. [DOI] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G, Brotchie H. Gender differences in depression. Int Rev Psychiatry. 2010;22:429–36. doi: 10.3109/09540261.2010.492391. [DOI] [PubMed] [Google Scholar]
- Parks CL, Robinson PS, Sibille E, Shenk T, Sibillet E, Totht M. Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci USA. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Quirk G, Gehlert D. Inhibition of the amygdala: key to pathological states? Ann NY Acad Sci. 2003;985:263–272. doi: 10.1111/j.1749-6632.2003.tb07087.x. [DOI] [PubMed] [Google Scholar]
- Rainnie D, Asprodini E, Shinnick-Gallagher P. Inhibitory transmission in the basolateral amygdala. J Neurophysiol. 1991;66:999–1009. doi: 10.1152/jn.1991.66.3.999. [DOI] [PubMed] [Google Scholar]
- Rainnie D, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24:3471–9. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblau G, Sterzer P, Stoy M, Park S, Friedel E, Heinz A, Pilhatsch M, Bauer M, Ströhle A. Functional neuroanatomy of emotion processing in major depressive disorder is altered after successful antidepressant therapy. J Psychopharmacol. 2012;26:1424–33. doi: 10.1177/0269881112450779. [DOI] [PubMed] [Google Scholar]
- Ryan SJ, Ehrlich DE, Jasnow AM, Daftary S, Madsen TE, Rainnie DG. Spike-timing precision and neuronal synchrony are enhanced by an interaction between synaptic inhibition and membrane oscillations in the amygdala. PLoS One. 2012;7:e35320. doi: 10.1371/journal.pone.0035320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler TR, Nguyen PT, Yang J, Givrad TK, Mayer Ea, Maarek JMI, Hinton DR, Holschneider DP. Antenatal maternal stress alters functional brain responses in adult offspring during conditioned fear. Brain Res. 2011;1385:163–74. doi: 10.1016/j.brainres.2011.01.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar J, Wakefield S, Mackenzie G, Moss SJ, Maguire J. Neurosteroidogenesis is required for the physiological response to stress: Role of neurosteroid-sensitive GABA A receptors. J Neurosci. 2011;31:18198–18210. doi: 10.1523/JNEUROSCI.2560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarro EC, Sullivan RM, Barr G. Unpredictable neonatal stress enhances adult anxiety and alters amygdala gene expression related to serotonin and GABA. Neuroscience. 2014;258:147–61. doi: 10.1016/j.neuroscience.2013.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller L, Jähkel M, Oehler J. The influence of sex and social isolation housing on pre- and postsynaptic 5-HT1A receptors. Brain Res. 2006;1103:76–87. doi: 10.1016/j.brainres.2006.05.051. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Pearson JN, Neeley EW, Berger R, Leonard S, Adams CE, Stevens KE. Maternal stress during pregnancy causes sex-specific alterations in offspring memory performance, social interactions, indices of anxiety, and body mass. Physiol Behav. 2011;104:340–7. doi: 10.1016/j.physbeh.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen MJ, Grønborg TK, Christensen J, Parner ET, Vestergaard M, Schendel D, Pedersen LH. Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin Epidemiol. 2013;5:449–59. doi: 10.2147/CLEP.S53009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda H, Tsuji M, Ikoshi H, Yamada T, Masuya J, Iimori M, Matsumiya T. Effects of a 5-HT7 receptor antagonist DR4004 on the exploratory behavior in a novel environment and on brain monoamine dynamics in mice. Eur J Pharmacol. 2005;518:30–39. doi: 10.1016/j.ejphar.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Thompson JV, Sullivan RM, Wilson Da. Developmental emergence of fear learning corresponds with changes in amygdala synaptic plasticity. Brain Res. 2008;1200:58–65. doi: 10.1016/j.brainres.2008.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibu F, Hill J, Sharp H, Marshall K, Glover V, Pickles A. Evidence for sex differences in fetal programming of physiological stress reactivity in infancy. Dev Psychopathol. 2014:1–10. doi: 10.1017/S0954579414000194. [DOI] [PubMed] [Google Scholar]
- Toole KKO, Hooper A, Wakefield S, Maguire J. Seizure-induced disinhibition of the HPA axis increases seizure susceptibility. Epilepsy Res. 2014;108:29–43. doi: 10.1016/j.eplepsyres.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzio R, Nardou R, Ferrari DC, Tsintsadze T, Shahrokhi A, Eftekhari S, Khalilov I, Tsintsadze V, Brouchoud C, Chazal G, Lemonnier E, Lozovaya N, Burnashev N, Ben-Ari Y. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science. 2014;343:675–9. doi: 10.1126/science.1247190. 80- [DOI] [PubMed] [Google Scholar]
- Van den Hove DLa, Kenis G, Brass A, Opstelten R, Rutten BPF, Bruschettini M, Blanco CE, Lesch KP, Steinbusch HWM, Prickaerts J. Vulnerability versus resilience to prenatal stress in male and female rats; implications from gene expression profiles in the hippocampus and frontal cortex. Eur Neuropsychopharmacol. 2013;23:1226–46. doi: 10.1016/j.euroneuro.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Veru F, Laplante DP, Luheshi G, King S. Prenatal maternal stress exposure and immune function in the offspring. Stress. 2014;17:133–48. doi: 10.3109/10253890.2013.876404. [DOI] [PubMed] [Google Scholar]
- Wake H, Watanabe M, Moorhouse AJ, Kanematsu T, Horibe S, Matsukawa N, Asai K, Ojika K, Hirata M, Nabekura J. Early changes in KCC2 phosphorylation in response to neuronal stress result in functional downregulation. J Neurosci. 2007;27:1642–50. doi: 10.1523/JNEUROSCI.3104-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf Aa, Frye Ca. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–8. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Kolb B. The behavior of the laboratory rat: a handbook with tests. Oxford University Press; New York, New York: 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1. No effect of prenatal stress or escitalopram on behavior in the open field test during the light cycle. Adolescent female rats were assessed for anxiety-like behaviors in the open field during the light cycle. In contrast to dark cycle testing (Figure 1), no group differences were detected in terms of time in center (left) or total distance travelled, suggesting no locomotor deficits (right). For all, error bars indicate standard error of the mean (S.E.M.); in all cases, p > 0.05 for main effects and interaction.
S2. Locomotor activity in the elevated plus maze. Open arm entries during a five minute elevated plus maze test were not affected by prenatal stress or escitalopram (p > 0.05 for main effects and interaction).
S3. No effect of prenatal stress or escitalopram on hormones. Serum concentrations of corticosterone (CORT) (left, measured on PND 38 immediately following a restraint stressor) and estradiol (right, measured on PND 42) were measured in a separate cohort of identically reared, adolescent female offspring. Prenatal stress and escitalopram had no effect on hormone levels as compared to control rats.