Abstract
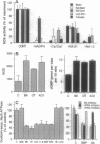
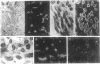
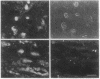
The inter- and intracellular regulator nitric oxide (NO) has been suggested to play a role in the modulation of cellular excitability, but the mechanism(s) by which this occurs remain unclear. Using the kidney as a model system, we report here evidence that NO, produced in response to various hormones and cytokines, can effect long-term alterations in the activity of the membrane sodium pump. This regulation of Na, K-ATPase, which occurs in a system of NO-containing renal tubules, involves cGMP and cGMP-dependent protein kinase. Na, K-ATPase can also be regulated by alterations of cGMP initiated through NO-independent factors, such as atriopeptin, and in nonrenal tissues, such as cerebellum. Regulation of the membrane sodium pump by NO and cGMP, therefore, represents a mechanism for hormonal modulation of ion gradients, not only in kidney but also in other organs, including brain, where NO and cGMP play a prominent role in cellular function.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aperia A., Bertorello A., Seri I. Dopamine causes inhibition of Na+-K+-ATPase activity in rat proximal convoluted tubule segments. Am J Physiol. 1987 Jan;252(1 Pt 2):F39–F45. doi: 10.1152/ajprenal.1987.252.1.F39. [DOI] [PubMed] [Google Scholar]
- Balment R. J., Brimble M. J., Forsling M. L. Oxytocin release and renal actions in normal and Brattleboro rats. Ann N Y Acad Sci. 1982;394:241–253. doi: 10.1111/j.1749-6632.1982.tb37432.x. [DOI] [PubMed] [Google Scholar]
- Cantiello H. F., Ausiello D. A. Atrial natriuretic factor and cGMP inhibit amiloride-sensitive Na+ transport in the cultured renal epithelial cell line, LLC-PK1. Biochem Biophys Res Commun. 1986 Jan 29;134(2):852–860. doi: 10.1016/s0006-291x(86)80498-3. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Bredt D. S., Fotuhi M., Hwang P. M., Snyder S. H. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7797–7801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner F. Factors influencing the onset of ouabain inhibition of Na,K-ATPase from guinea-pig myocardium. Br J Pharmacol. 1990 Oct;101(2):337–343. doi: 10.1111/j.1476-5381.1990.tb12711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein F. H. Hypoxia of the renal medulla. Q J Med. 1985 Dec;57(224):807–810. [PubMed] [Google Scholar]
- Felsenfeld D. P., Sweadner K. J. Fine specificity mapping and topography of an isozyme-specific epitope of the Na,K-ATPase catalytic subunit. J Biol Chem. 1988 Aug 5;263(22):10932–10942. [PubMed] [Google Scholar]
- Goldring S. R., Dayer J. M., Ausiello D. A., Krane S. M. A cell strain cultured from porcine kidney increases cyclic AMP content upon exposure to calcitonin or vasopressin. Biochem Biophys Res Commun. 1978 Jul 28;83(2):434–440. doi: 10.1016/0006-291x(78)91009-4. [DOI] [PubMed] [Google Scholar]
- Hull R. N., Cherry W. R., Weaver G. W. The origin and characteristics of a pig kidney cell strain, LLC-PK. In Vitro. 1976 Oct;12(10):670–677. doi: 10.1007/BF02797469. [DOI] [PubMed] [Google Scholar]
- Ibarra F., Aperia A., Svensson L. B., Eklöf A. C., Greengard P. Bidirectional regulation of Na+,K(+)-ATPase activity by dopamine and an alpha-adrenergic agonist. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):21–24. doi: 10.1073/pnas.90.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K., Chang B., Kerwin J. F., Jr, Wagenaar F. L., Huang Z. J., Murad F. Formation of endothelium-derived relaxing factor in porcine kidney epithelial LLC-PK1 cells: an intra- and intercellular messenger for activation of soluble guanylate cyclase. J Pharmacol Exp Ther. 1991 Jan;256(1):38–43. [PubMed] [Google Scholar]
- Joyce N. C., DeCamilli P., Lohmann S. M., Walter U. cGMP-dependent protein kinase is present in high concentrations in contractile cells of the kidney vasculature. J Cyclic Nucleotide Protein Phosphor Res. 1986;11(3):191–198. [PubMed] [Google Scholar]
- Kase H., Iwahashi K., Nakanishi S., Matsuda Y., Yamada K., Takahashi M., Murakata C., Sato A., Kaneko M. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem Biophys Res Commun. 1987 Jan 30;142(2):436–440. doi: 10.1016/0006-291x(87)90293-2. [DOI] [PubMed] [Google Scholar]
- Katz A. I., Doucet A., Morel F. Na-K-ATPase activity along the rabbit, rat, and mouse nephron. Am J Physiol. 1979 Aug;237(2):F114–F120. doi: 10.1152/ajprenal.1979.237.2.F114. [DOI] [PubMed] [Google Scholar]
- Light D. B., Corbin J. D., Stanton B. A. Dual ion-channel regulation by cyclic GMP and cyclic GMP-dependent protein kinase. Nature. 1990 Mar 22;344(6264):336–339. doi: 10.1038/344336a0. [DOI] [PubMed] [Google Scholar]
- Light D. B., Schwiebert E. M., Karlson K. H., Stanton B. A. Atrial natriuretic peptide inhibits a cation channel in renal inner medullary collecting duct cells. Science. 1989 Jan 20;243(4889):383–385. doi: 10.1126/science.2463673. [DOI] [PubMed] [Google Scholar]
- Lincoln T. M., Cornwell T. L. Intracellular cyclic GMP receptor proteins. FASEB J. 1993 Feb 1;7(2):328–338. doi: 10.1096/fasebj.7.2.7680013. [DOI] [PubMed] [Google Scholar]
- Markewitz B. A., Michael J. R., Kohan D. E. Cytokine-induced expression of a nitric oxide synthase in rat renal tubule cells. J Clin Invest. 1993 May;91(5):2138–2143. doi: 10.1172/JCI116439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., Villani G. M., Jothianandan D., Furchgott R. F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):708–716. [PubMed] [Google Scholar]
- Meister B., Fryckstedt J., Schalling M., Cortés R., Hökfelt T., Aperia A., Hemmings H. C., Jr, Nairn A. C., Ehrlich M., Greengard P. Dopamine- and cAMP-regulated phosphoprotein (DARPP-32) and dopamine DA1 agonist-sensitive Na+,K+-ATPase in renal tubule cells. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8068–8072. doi: 10.1073/pnas.86.20.8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrmann M., Cantiello H. F., Ausiello D. A. Inhibition of epithelial Na+ transport by atriopeptin, protein kinase c, and pertussis toxin. Am J Physiol. 1987 Aug;253(2 Pt 2):F372–F376. doi: 10.1152/ajprenal.1987.253.2.F372. [DOI] [PubMed] [Google Scholar]
- Mundel P., Bachmann S., Bader M., Fischer A., Kummer W., Mayer B., Kriz W. Expression of nitric oxide synthase in kidney macula densa cells. Kidney Int. 1992 Oct;42(4):1017–1019. doi: 10.1038/ki.1992.382. [DOI] [PubMed] [Google Scholar]
- Nakane M., Mitchell J., Förstermann U., Murad F. Phosphorylation by calcium calmodulin-dependent protein kinase II and protein kinase C modulates the activity of nitric oxide synthase. Biochem Biophys Res Commun. 1991 Nov 14;180(3):1396–1402. doi: 10.1016/s0006-291x(05)81351-8. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Romero J. C., Lahera V., Salom M. G., Biondi M. L. Role of the endothelium-dependent relaxing factor nitric oxide on renal function. J Am Soc Nephrol. 1992 Mar;2(9):1371–1387. doi: 10.1681/ASN.V291371. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Gagne G. D., Nakane M., Pollock J. S., Miller M. F., Murad F. Mapping of neural nitric oxide synthase in the rat suggests frequent co-localization with NADPH diaphorase but not with soluble guanylyl cyclase, and novel paraneural functions for nitrinergic signal transduction. J Histochem Cytochem. 1992 Oct;40(10):1439–1456. doi: 10.1177/40.10.1382087. [DOI] [PubMed] [Google Scholar]
- Shahedi M., Laborde K., Bussières L., Dechaux M., Sachs C. Protein kinase C activation causes inhibition of Na/K-ATPase activity in Madin-Darby canine kidney epithelial (MDCK) cells. Pflugers Arch. 1992 Mar;420(3-4):269–274. doi: 10.1007/BF00374458. [DOI] [PubMed] [Google Scholar]
- Snyder G. L., Girault J. A., Chen J. Y., Czernik A. J., Kebabian J. W., Nathanson J. A., Greengard P. Phosphorylation of DARPP-32 and protein phosphatase inhibitor-1 in rat choroid plexus: regulation by factors other than dopamine. J Neurosci. 1992 Aug;12(8):3071–3083. doi: 10.1523/JNEUROSCI.12-08-03071.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl W. L., Baskin D. G. Histochemistry of ATPases. J Histochem Cytochem. 1990 Aug;38(8):1099–1122. doi: 10.1177/38.8.2164057. [DOI] [PubMed] [Google Scholar]
- Steardo L., Nathanson J. A. Brain barrier tissues: end organs for atriopeptins. Science. 1987 Jan 23;235(4787):470–473. doi: 10.1126/science.2879355. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Cho H. J., Kwon N. S., Weise M. F., Nathan C. F. Purification and characterization of the cytokine-induced macrophage nitric oxide synthase: an FAD- and FMN-containing flavoprotein. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7773–7777. doi: 10.1073/pnas.88.17.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K., Snyder G. L., Greengard P. Nitric oxide/cGMP pathway stimulates phosphorylation of DARPP-32, a dopamine- and cAMP-regulated phosphoprotein, in the substantia nigra. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3462–3465. doi: 10.1073/pnas.90.8.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhler M. D. Cloning and expression of a novel cyclic GMP-dependent protein kinase from mouse brain. J Biol Chem. 1993 Jun 25;268(18):13586–13591. [PubMed] [Google Scholar]
- Vanderwinden J. M., Mailleux P., Schiffmann S. N., Vanderhaeghen J. J., De Laet M. H. Nitric oxide synthase activity in infantile hypertrophic pyloric stenosis. N Engl J Med. 1992 Aug 20;327(8):511–515. doi: 10.1056/NEJM199208203270802. [DOI] [PubMed] [Google Scholar]
- Wilcox C. S., Welch W. J., Murad F., Gross S. S., Taylor G., Levi R., Schmidt H. H. Nitric oxide synthase in macula densa regulates glomerular capillary pressure. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11993–11997. doi: 10.1073/pnas.89.24.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanase M., Handler J. S. Activators of protein kinase C inhibit sodium transport in A6 epithelia. Am J Physiol. 1986 Mar;250(3 Pt 1):C517–C522. doi: 10.1152/ajpcell.1986.250.3.C517. [DOI] [PubMed] [Google Scholar]