Abstract
Effects of patient characteristics on rehabilitation outcomes (functional status at discharge, discharged home) were assessed in a retrospective study of Medicare beneficiaries admitted to inpatient rehabilitation facilities (IRFs) following hospitalization for hip fracture in 2009 (n=34,984). Hierarchical regression analysis showed significantly higher functional status at discharge (p<.0001) for patients with these characteristics: White or Asian; younger; female; lived alone; higher functional status at admission; fewer comorbidities; no tier comorbidities; longer IRF length of stay (LOS). Likelihood of discharged home was higher for patients with these characteristics: Hispanic (1.49 [1.32–1.68]), Asian (1.35 [1.04–1.74]), or Black (1.28 [1.12–1.47]); younger (0.96 [0.96–0.96]); female (1.14 [1.08–1.20]); lived with others (2.12 [2.01–2.23]); higher functional status at admission (1.06 [1.06–1.06]); fewer comorbidities, no tier comorbidities; longer LOS (1.61 [1.56–1.67]). Functional status at admission, tier comorbidities, and race/ethnicity contributed most to variance in functional status at discharge. Living with others contributed most to variance in discharged home.
Keywords: hip fractures, rehabilitation, racial disparities, outcomes research
Introduction
Inpatient Rehabilitation Facilities
Inpatient Rehabilitation Facilities (IRFs) are post-acute care (PAC) providers that deliver intensive rehabilitation services to individuals who experience functional loss due to an injury or worsening medical condition. IRFs provide medical supervision, an interdisciplinary team approach to treatment and evaluation, and a medical director with specialty rehabilitation training and/or experience who manages the delivery of services full-time in freestanding facilities or at least 20 hours per week in hospital-based units (Centers for Medicare & Medicaid Services [CMS], 2010). Medicare-certified IRFs (n=1,196 in 2009) deliver intensive rehabilitation services– approximately 80% in specialized hospital-based units and 20% in freestanding rehabilitation hospitals (Medicare Payment Advisory Commission [MedPAC], 2011).
Because rehabilitation services provided in IRFs are more intensive than those provided in other PAC settings, the Centers for Medicare and Medicaid Services (CMS) reimburse IRFs at higher rates. However, facilities must adhere to certain classification criteria in order to be defined as IRFs and qualify for such reimbursement (Centers for Medicare & Medicaid Services [CMS], 2009). Medicare-reimbursable IRF services must be medically necessary for the patient; requiring rehabilitation nursing care, and multiple therapies (e.g., physical therapy, occupational therapy, speech-language pathology) for at least 3 hours/day, 5 days/week (the requirements listed are based on the CMS Manual available during 2009 (year of the study) and which has since been updated. Active patient participation in therapy, realistic goals for functional independence, and coordinated multidisciplinary team approaches are also required (CMS, 2010). Furthermore, each IRF must comply with a minimum 60% threshold, meaning that 60% of its patient population must be diagnosed with at least one of 13 qualifying medical conditions: stroke, severe advanced osteoarthritis, lower limb total joint replacement, spinal cord injury, congenital deformity, major multiple trauma, hip fracture, brain injury, neurological disorders, burns, active polyarticular rheumatoid arthritis, amputations, or systematic vasculitis with joint inflammation (Centers for Medicare & Medicaid Services [CMS], 2012). The intent of this 60% rule is to ensure that the most appropriate Medicare beneficiaries can access IRF care, while those with lower acuity are served in less intensive, less costly settings.
Since 2004, when CMS began to enforce the rule, IRFs have adjusted patterns of admission to ensure compliance with the 60% rule (MedPAC, 2011). Consequences of these adjustments included changed case-mix and reduced IRF admissions (Moran Company, 2006). Between 2004 and 2009, IRF admissions dropped by 21% (455,000 to 361,000) and payments to IRFs by 6% ($6.43 to $6.07 billion) (MedPAC, 2011).
The General Accounting Office (GAO), directed by Congress to determine whether the list of 13 qualifying medical conditions was clinically appropriate, recommended retaining the list, but emphasized that condition alone was not a sufficient criterion for identifying patients for whom IRF services were most appropriate (Government Accountability Office [GAO], 2005). The GAO therefore recommended conducting research on patient characteristics in order to better describe patient subgroups within each condition which would benefit most from IRF services, with the goal of ultimately refining criteria (GAO, 2005). That report provided the impetus for this study, which examines effects of multiple patient characteristics on rehabilitation outcomes in a large sample of Medicare beneficiaries receiving IRF care for a single qualifying medical condition: hip fracture.
Hip Fracture
U.S. hip fracture incidence was 793.5/100,000 women and 369/100,000 men in 2009 (Brauer, Coca-Perraillon, Cutler, & Rosen, 2009); compared to previous years, rates improved slightly among older Whites but not among older Black, Asian, or Hispanic individuals (Wright et al., 2012). Outcomes of hip fracture in older adults include significant loss of function, disability, and excess mortality (life expectancy reduced up to 25%) (Abrahamsen, van Staa, Ariely, Olson, & Cooper, 2009; Braithwaite, Col, & Wong, 2003). Following surgical repair and stabilization in acute care hospitals, 90% of hip fracture patients are discharged to institutional post-acute settings for rehabilitation: approximately 80% to skilled nursing facilities (SNF) and 20% to IRFs (Freburger, Holmes, & Ku, 2012).
In 2009, hip fracture patients comprised 15.5% of the IRF patient mix (Medicare Payment Advisory Commission [MedPAC], 2010). Compared to SNFs, IRFs show promise for improving functional outcomes of hip fracture patients after shorter lengths of stay (LOS), but their costs are much higher (Deutsch et al., 2005; Herbold, Bonistall, & Walsh, 2011; Munin et al., 2005). Available evidence on characteristics of hip fracture patients who are most likely to benefit from IRF rehabilitation is based either on national data collected before enforcement of the 60% rule (Graham, Chang, Bergés, Granger, & Ottenbacher, 2008; Munin et al., 2005; Ottenbacher et al., 2003) or on local studies limited to single facilities (Herbold et al., 2011; Semel, Gray, Ahn, Nasr, & Chen, 2010). Building on previous research (Graham et al., 2008; Ottenbacher et al., 2003), this study contributes to the literature because it is, to our knowledge, the first to use the CMS Inpatient Rehabilitation Facility-Patient Assessment Instrument [IRF-PAI] dataset to examine relationships between multiple patient characteristics and rehabilitation outcomes in a national sample of Medicare beneficiaries with hip fractures who were admitted to IRFs for post-acute rehabilitation. Andersen’s Model for Health Services Use (Andersen, 2008) was used as a framework (Figure 1) for assessing individual characteristics known to influence rehabilitation outcomes in IRF patients (Ahmed, Graham, Karmarkar, Granger, & Ottenbacher, 2013; Deutsch et al., 2005; Graham et al., 2008; Herbold et al., 2011; Munin et al., 2005; Nguyen-Oghalai, Ottenbacher, Granger, Smith, & Goodwin, 2006; Ottenbacher et al., 2003; Reistetter et al., 2011). These included age, race, and gender (predisposing factors); social support (an enabling factor); functional status on admission, number of comorbidities, and tier comorbidities (need-related factors); and use of IRF services (a health behavior factor), operationalized as length of stay (LOS). Two rehabilitation outcomes were examined: functional status at discharge and discharge setting. Operational definitions of all variables are provided below.
Figure 1.
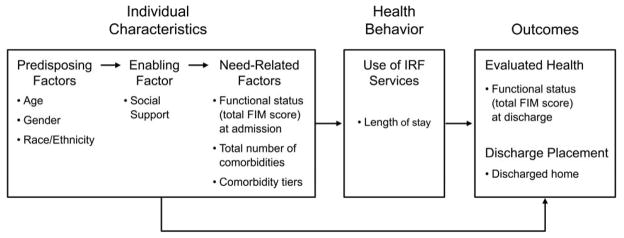
Anderson’s Model of Health Services Use (2008) as applied to individual characteristics, health behavior (service use), and outcomes in Medicare beneficiaries with hip fracture in inpatient rehabilitation facilities.
Method
Study Population
This retrospective study used Inpatient Rehabilitation Facility–Patient Assessment Instrument (IRF-PAI) data (Centers for Medicare & Medicaid Services [CMS], 2013). The 59,337 records yielded 53,120 hip fracture patients whose first admission to an IRF occurred during 2009. Exclusion criteria included (1) age<65 years on admission (n=1828); (2) discharged from acute care hospital and admitted to Medicare-certified IRF with primary diagnosis other than hip fracture (International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9CM] codes 0008.11, 0008.12, or 0008.2) (n=12,824); (3) not living at home before acute care hospital admission, as indicated by any code other than 01 for the “pre-hospital living setting” item on the IRF-PAI (n=2,438) (4) unknown discharge setting (n=5); (4) initial patient assessment >3 days after IRF admission (n=15); (5) admission to IRF>30 days after hip fracture (n=826); (6) delirious (n=143) or comatose (n=12) on IRF admission; and (7) death during rehabilitation period in the IRF (n=45). Final sample size was n=34,984. These exclusion criteria follow the criteria developed by the Centers for Medicare and Medicaid Services (CMS) reflecting patients with an atypical course of rehabilitation (Carter et al., 2002; Stineman et al., 1994). The Institutional Review Board of the authors’ university approved this research (Institutional Review Board Health Services Research [IRB-HSR] #15734) prior to commencement of the study.
Measures
Data source
CMS created the IRF-PAI (CMS, 2012) to determine payment for each Medicare-Part A fee-for-service patient admitted to an IRF, using admission and discharge data from patient assessments in the IRF. The IRF-PAI includes data on patient demographics, social support, comorbidities, functional measures, length of stay, and discharge setting.
Individual characteristics
Predisposing factors were operationalized as age at time of IRF admission; gender; and self-reported race/ethnicity as Asian, Black or African American, Hispanic, Non-Hispanic White, or Other (American Indian, Alaska Native, Native Hawaiian, Pacific Islander). One enabling factor was operationalized as a dichotomous social support variable, measured as living with someone (family/relative, friend, attendant or other) vs. living alone prior to hospitalization.
Need-related factors
These factors were operationalized as functional status at admission, number of comorbidities, and tier comorbidities.
Functional status at admission was measured with the Functional Independence Measure (FIM) which assesses both motor (13 items: self-care, sphincter control, mobility, and locomotion) and cognitive abilities (5 items: communication and social cognition). Each item is rated on a 7-point scale (range: total assistance=1 to complete independence=7); total FIM score may therefore range from 18–126. Per CMS guidelines (CMS, 2012), any FIM item scored as 0 (activity did not occur) at discharge was converted to 1 (total assistance). The FIM has been found to be reliable and valid (Ottenbacher, Hsu, Granger, & Fiedler, 1996). The IRF-PAI training manual (CMS, 2004) specifies that the admission assessment score is the lowest FIM score recorded for the patient within the first three calendar days of the IRF stay; this is the variable used in our analysis.
Comorbidities at admission were assessed as follows: 1) total number of comorbidities at admission (range 0–10); 2) presence or absence of comorbidities categorized at four levels (Tier 1-most severe, Tier 2-moderately severe, Tier 3-mild; No tier-none of the listed comorbidities). CMS uses the tier system to adjust IRF reimbursement for comorbidities that increase care burden and resource use (Carter & Totten, 2005).
Health behavior
One health behavior factor was operationalized as use of IRF services and measured as length of stay (LOS=total number of nights the patient stayed in the IRF).
Outcomes
Inpatient Rehabilitation Outcomes were: functional status at discharge, and discharge setting.
Functional status at discharge was operationalized as total FIM score (sum score of all 18 items). The IRF-PAI training manual (CMS, 2004, p.III-3) specifies that FIM at discharge reflects “the lowest score within any 24-hour period within the three calendar days comprising the discharge assessment period” (that is, the last three calendar days of the patient’s IRF stay); this variable is designated FIM score at discharge in our analysis.
Discharge setting was operationalized as the dichotomous variable discharged home. Discharged home was coded as yes for patients discharged from the IRF to “home” (their private residence in the community, as indicated by a code of 01 for the IRF-PAI “discharge to living setting” item). As indicated earlier, this study sample was limited to patients who had lived in their own homes prior to hip fracture. Discharged home was coded as no for patients discharged from the IRF to any other setting, including board and care, intermediate care, transitional living, skilled nursing facilities, assisted living residences, other rehabilitation facilities, and hospitals.
Data Analysis
Descriptive statistics were calculated for all variables. Relationships among continuous variables were assessed with Pearson correlation analysis. Hierarchical regression analyses with FIM score at discharge as outcome variable were conducted sequentially over blocks of variables in the order of the Andersen Model for Health Services Use (predisposing, enabling, need-related, and health behavior variables). Hierarchical logistic regression analyses were used to assess contributions of predisposing, enabling, need-related, and health behavior variables to the likelihood of being discharged home. Statistical significance was set at P <.05. All analyses used SAS version 9.2 (SAS Institute Inc., 2013).
Results
Sample Characteristics
Characteristics of Medicare patients who received inpatient rehabilitation for hip fracture in 2009 are reported in Table 1. Most patients were female and non-Hispanic White; mean patient age was 81.4 years. Over 60% reported living with someone prior to hospitalization. Mean FIM score at admission was 59.2 (range 18–108). Mean number of comorbidities was 8.3. Seventy-six percent of patients had no tier comorbidities; 16% had at least one Tier 3 comorbidity (mild), 6% had at least one Tier 2 (moderately severe); 2% had at least one Tier 1 (most severe). Mean length of stay for all patients was 13.3 days. Mean FIM score at discharge was 86.0; 66% of patients were discharged home.
Table 1.
Characteristics of Medicare Beneficiaries Admitted to Inpatient Rehabilitation Facilities Following Hospitalization for Hip Fracture in 2009 (N=34,984).
| Variables | % | Mean | SD |
|---|---|---|---|
| Predisposing Factors | |||
| Age | 81.4 | 7.4 | |
| Race / Ethnicity | |||
| White a | 88.3 | ||
| Hispanic | 4.5 | ||
| Black | 3.3 | ||
| Asian | 1.0 | ||
| Other | 2.7 | ||
| Gender | |||
| Male a | 28.6 | ||
| Female | 71.4 | ||
| Enabling Factor | |||
| Social Support | |||
| Living Alone a | 37.8 | ||
| Living with Someone | 62.2 | ||
| Need-Related Factors | |||
| FIM Score at Admission | 59.2 | 15.4 | |
| Number of Comorbid Conditions | 8.3 | 2.2 | |
| Comorbidity Tier Level | |||
| Tier 1 | 1.6 | ||
| Tier 2 | 6.1 | ||
| Tier 3 | 16.3 | ||
| No Tier Comorbidities a | 75.8 | ||
| Health Behavior Factor | |||
| LOS | 13.3 | 4.9 | |
| Outcomes | |||
| Discharged Home | |||
| No | 34.5 | ||
| Yes | 65.5 | ||
| FIM Score at Discharge | 86.0 | 19.2 | |
FIM = Functional Independence Measure
Indicates reference group in regression models.
Pearson correlations between the outcome variable (FIM score at discharge) and independent variables (age, total comorbidities, FIM score at admission, LOS) were all statistically significant (untabled). Older patients had lower FIM scores at admission and discharge, more comorbid conditions, and longer lengths of stay. Patients with more comorbid conditions also had lower FIM scores at admission and discharge and longer lengths of stay. Patients with longer LOS had lower FIM scores when discharged to home.
Predictors: FIM Score at Discharge
Table 2 presents results of hierarchical regression models of predictors of FIM score at discharge. Model 1 (predisposing variables) explained 9% of the variance. FIM scores at discharge were lower in older and male patients and in patients of Black, Hispanic, and Other race/ethnicity (as compared to White); Asian patients had FIM ratings at discharge similar to Whites. In Model 2, added social support (enabling factor), FIM score at discharge was significantly lower in patients who lived with someone before hospital admission than in patients who lived alone. Model 3, which added FIM scores at admission and comorbidity-related variables (need-related factors), explained 51% of the variance. Higher FIM scores at admission predicted higher FIM scores at discharge, but having tier comorbidities at any level was significantly associated with lower FIM scores at discharge. Model 4, which added LOS (health behavior factor), explained 58% of the variance in FIM score at discharge. In this model, greater length of stay was associated with higher FIM scores at discharge; in addition, all variables that were significant predictors of FIM score at discharge in the first three models retained significance.
Table 2.
Hierarchical Regression Models Predicting FIM Score at Discharge: Results.
| Variables
|
Model 1
|
Model 2
|
Model 3
|
Model 4
|
||||
|---|---|---|---|---|---|---|---|---|
| b (SE) | p | b (SE) | p | b (SE) | p | b (SE) | p | |
| Age | −0.66 (.01) | <.001 | −0.70 (.01) | <.001 | −0.24 (.01) | <.001 | −0.28 (.01) | <.001 |
| Race/Ethnicity a | ||||||||
| Black | −8.81 (.54) | <.001 | −8.66 (.54) | <.001 | −3.73 (.40) | <.001 | −4.28 (.38) | <.001 |
| Asian | −0.92 (.97) | .344 | −0.13 (.97) | .897 | −0.28 (.71) | .694 | −0.38 (.67) | .573 |
| Hispanic | −8.00 (.47) | <.001 | −7.17 (.47) | <.001 | −2.01 (.35) | <.001 | −1.82 (.33) | <.001 |
| Other | −2.22 (.60) | <.001 | −2.02 (.60) | <.001 | −2.07 (.44) | <.001 | −2.05 (.42) | <.001 |
| Female b | 4.37 (.22) | <.001 | 3.54 (.22) | <.001 | 1.57 (.16) | <.001 | 1.57 (.15) | <.001 |
| Living with Someone c | −4.35 (.21) | <.001 | −1.60 (.15) | <.001 | −1.15 (.15) | <.001 | ||
| FIM Score at Admission | 0.87 (.01) | <.001 | 0.95 (.01) | <.001 | ||||
| # Comorbid Conditions | 0.07 (.03) | .053 | −0.13 (.03) | <.001 | ||||
| Comorbidity Tier Level d | ||||||||
| Tier 1 | −3.76 (.57) | <.001 | −5.06 (.54) | <.001 | ||||
| Tier 2 | −3.56 (.31) | <.001 | −4.44 (.29) | <.001 | ||||
| Tier 3 | −1.35 (.20) | <.001 | −1.95 (.19) | <.001 | ||||
| LOS e | 0.91 (.01) | <.001 | ||||||
| Properties of Model: | Model 1
|
Model 2
|
Model 3
|
Model 4
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | R2 | F | p | R2 | F | p | R2 | F | p | R2 | |
| F Value | 547.6 | <.001 | 0.086 | 538.2 | <.001 | 0.097 | 3086.8 | <.001 | 0.514 | 3645.2 | <.001 | 0.563 |
|
| ||||||||||||
| Change in R2 from Previous Model: | – | +0.011 | +0.417 | +0.049 | ||||||||
Notes. FIM=Functional Independence Measure. b=unstandardized regression coefficient.
Reference group=White;
Reference group=Male;
Reference group=Living alone;
Reference group=No tier comorbidities.
Analysis used square root of LOS.
Predictors: Discharged Home
Odds of discharged home associated with predisposing, enabling, need, and health behavior variables are presented in Table 3. Younger and female patients were more likely to be discharged home. Black, Asian, and Hispanic patients were respectively 28%, 35%, and 49% more likely to be discharged home than non-Hispanic Whites. Patients living with someone before hospital admission, higher FIM scores at admission, and fewer comorbidities were more likely to be discharged home. Patients with comorbidities in tiers 1, 2, and 3 were respectively 39%, 25%, and 19% less likely to be discharged home than those without tier comorbidities. Patients with longer LOS were more likely to be discharged home.
Table 3.
Hierarchical Logistic Regression Analyses Predicting Discharged Home: Results.
| Variables | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| OR (95% CI), p | OR (95% CI), p | OR (95% CI), p | OR (95% CI), p | |
| Age | 0.94 (0.94–0.94), <.001 | 0.94 (0.94–0.95), <.001 | 0.96 (0.96–0.97), <.001 | 0.96 (0.96–0.96), <.001 |
| Race/Ethnicity a | ||||
| Black | 0.99 (0.87–1.12), .86 | 0.97 (0.85–1.10), .64 | 1.33 (1.16–1.52), <.001 | 1.28 (1.12–1.47), <.001 |
| Asian | 1.45 (1.14–1.85), .002 | 1.33 (1.05–1.70), .02 | 1.35 (1.04–1.73), .02 | 1.35 (1.04–1.74), .02 |
| Hispanic | 1.14 (1.02–1.27), .02 | 1.04 (0.93–1.16), .53 | 1.45 (1.29–1.63), <.001 | 1.49 (1.32–1.68), <.001 |
| Other | 0.94 (0.82–1.08), .40 | 0.92 (0.80–1.06), .25 | 0.91 (0.78–1.05), .20 | 0.91 (0.78–1.10), .21 |
| Female b | 1.15 (1.10–1.21), <.001 | 1.27 (1.21–1.34), <.001 | 1.14 (1.08–1.20), <.001 | 1.14 (1.08–1.20), <.001 |
| Living with Someone c | 1.59 (1.51–1.66), <.001 | 2.02 (1.92–2.13), <.001 | 2.12 (2.01–2.23), <.001 | |
| FIM Score at Admission | 1.06 (1.05–1.06), <.001 | 1.06 (1.06–1.06), <.001 | ||
| # Comorbid Conditions | 0.99 (0.98–1.00), .21 | 0.98 (0.97–0.99), .001 | ||
| Comorbidity Tier Level d | ||||
| Tier 1 | 0.66 (0.55–0.80), <.001 | 0.61 (0.50–0.73), <.001 | ||
| Tier 2 | 0.81 (0.73–0.89), <.001 | 0.76 (0.68–0.83), <.001 | ||
| Tier 3 | 0.84 (0.79–0.90), <.001 | 0.81 (0.75–0.86), <.001 | ||
| LOS e | 1.61 (1.56–1.67), <.001 | |||
Notes. FIM=Functional Independence Measure.
Reference group=White;
Reference group=Male;
Reference group=Living alone;
Reference group=No tier comorbidities.
Analysis used square root of LOS.
Discussion
This study is the first to use the CMS IRF-PAI dataset to examine relationships between multiple patient characteristics and rehabilitation outcomes in a national sample of Medicare beneficiaries with hip fracture following inpatient rehabilitation. Significant effects of predisposing factors (age, race, gender), an enabling factor (social support), need-related factors (FIM score at admission, total number of comorbidities, tier comorbidities), and a health behavior factor (LOS) on two rehabilitation outcomes (FIM at discharge and discharged home) were identified. The magnitudes of the contributions of FIM score at admission and tier comorbidities to FIM score at discharge were striking. Several factors predicted significantly higher odds of being discharged home. Living with someone prior to hospital admission more than doubled the odds of discharged home, and longer LOS in the IRF increased the odds of discharged home by about 60%. Race/ethnicity, gender, tier comorbidities, and FIM at admission were also significant predictors.
Predisposing Factors
Race/ethnicity
In this study, FIM score at discharge was lower for patients of Black, Hispanic, and Other race/ethnicity than for non-Hispanic Whites. These results support earlier findings of racial/ethnic disparities in functional outcomes among hip fracture patients (Graham et al., 2008; Ottenbacher et al., 2003; Sterling, 2011). These differences may be associated with clinical and/or nonclinical correlates of race/ethnicity. Disparities in functional outcomes could be related to racial/ethnic differences in health status that originate prior to IRF admission. Prevalence of diabetes is higher in both African Americans (Kountz, 2012; Sentell, He, Gregg, & Schillinger, 2012) and Hispanic Americans (Peek, Cargill, & Huang, 2007) than in Whites; in addition, African Americans have higher prevalence of hypertension (Kramer et al., 2004), stroke (G. Howard et al., 2013; V. J. Howard et al., 2011), and certain types of cancer (Siegel, Naishadham, & Jemal, 2013). All of these comorbidities are known to hinder functional improvement during inpatient rehabilitation and post-discharge (Hunter & Baltisberger, 2013; Matthew, Hsu, & Young, 2013).
Nonclinical correlates of race/ethnicity might also explain disparities in functional outcomes. For example, an early study by Hoenig, Rubenstein, & Kahn (1996) reported significant racial disparities in the use of inpatient rehabilitation therapy among Medicare patients following hospitalization for hip fracture: intensity of rehabilitation therapy was significantly lower among African Americans than among Whites. By 2009, when this study was conducted, all IRF patients were receiving an intensive level of rehabilitation therapy (minimum 3 hours per day); therefore, disparities in duration of use of rehabilitation therapy would not be expected for this sample. Nonetheless, for post-acute stroke patients in IRFs, recent studies have reported significant variations between African American and White patients in therapy session duration and types of interventions received, although these differences were not correlated with functional outcomes (Horn, Deutscher, Smout, DeJong, & Putman, 2010; Deutscher, Horn, Smout, DeJong, & Putman, 2010). To our knowledge, such relationships have yet to be explored among post-acute hip fracture patients.
Furthermore, while equal access to post-acute rehabilitation services should be available to all Medicare beneficiaries regardless of race/ethnicity, a number of nonclinical factors correlated with race/ethnicity might impede access to these services for patients of minority race/ethnicity. These include financial barriers (i.e., primary and supplemental insurance coverage), attitudinal barriers (i.e., patient preferences, provider attitudes and practice habits), and structural barriers (i.e., geographic location of providers/facilities and poorly developed referral systems) (Ottenbacher & Graham, 2007). These factors warrant further study, as they have potential to improve our understanding of possible causes of racial/ethnic disparities in functional outcomes for post-acute hip fracture patients.
We observed higher rates of discharge to the home setting in patients of minority race/ethnicity. Higher odds of being discharged home for Black, Hispanic, and Asian hip fracture patients may be attributable to stronger or more extensive family/social networks (Graham et al., 2008; Ottenbacher et al., 2003; Sterling, 2011). However, Black and Hispanic patients in this sample also had significantly poorer function at discharge – a matter of concern, as these groups may have difficulty accessing the community-based services needed to restore pre-fracture levels of function. Social support factors that promote discharge of hip fracture patients to home and help these patients access services deserve further exploration.
Gender
This study also revealed gender disparities in both outcome variables: men had lower FIM scores at discharge and lower odds of being discharged home than women. Two previous studies (Arinzon, Shabat, Peisakh, Gepstein, & Berner, 2010; Lieberman & Lieberman 2004) reported no significant gender differences in total FIM scores of hip fracture patients on discharge following inpatient rehabilitation, although one of these (Arinzon et al., 2010) did report better transfer and locomotion scores in men. Results of our study are more consistent with Sterling’s (2011) observations of higher postoperative morbidity and mortality in male hip fracture patients, and with recent research reporting poorer functional outcomes after inpatient rehabilitation in male as opposed to female hip fracture patients (Di Monaco, Castiglioni, Vallero, Di Monaco, & Tappero, 2012).
Age
Our results are consistent with previous findings that age predicts higher risk of poor functional outcomes among adults receiving inpatient rehabilitation for hip fracture (Arinzon, Fidelman, Zuta, Peisakh, & Berner, 2005; Lieberman, Friger, & Lieberman, 2006).
Enabling Factor
Patients who had lived with someone prior to hospitalization had poorer function at discharge but were more likely to be discharged home, a combination not without risk. Readmission rates six months post-hospital discharge for hip fracture have varied from 4% to 32% (Ottenbacher et al., 2003; Riggs, Roberts, Aronow, & Younan, 2010). Follow-up studies post-discharge could assess transitional care and community-based factors which may inform strategies designed to prevent costly hospital readmissions.
Need-Related Factors
In this sample, poorer function at admission predicted increased risk of poor outcomes (lower FIM scores at discharge and lower odds of being discharged home), consistent with previous research (Arinzon et al., 2010; Graham et al., 2008; Lieberman et al., 2006; Ottenbacher et al., 2003). Severity (and, to a lesser extent, number) of comorbidities also increased the risk of poor outcomes. Two studies (Mathew, Hsu, & Young, 2013; Semel et al., 2010) have reported poorer outcomes in hip fracture patients with greater numbers of comorbidities, while a third (Lew, Lee, Date, and Zeiner, 2002) found no significant association between number of comorbidities and functional outcomes.
Health Behavior Factor: Use of IRF Services
Longer LOS had direct, significant, and positive effects on functional status at discharge and the likelihood of returning home, without significantly diminishing any of the direct effects of individual predisposing, enabling, and need-related factors. Although both shorter LOS and better functional outcomes characterize IRFs compared to SNFs (Herbold et al., 2011; Munin et al., 2005), longer LOS remains a strong predictor of better outcomes within the intensive environment of IRFs.
Limitations
The structure of IRF-PAI data limited the variables that could be examined in this analysis. Examples: 1) the IRF-PAI dataset includes only a limited number of race and ethnic categories, effects of cultural differences within a racial or ethnic minority group (e.g., Caribbean- versus U.S.-born Blacks) could not be assessed in this study. 2) The social support measure (living alone vs. with someone else) was not sufficiently specific to elucidate effects of relationship quantity and quality on outcomes. Current literature suggests that social support embodies both quantity and quality of an individual’s relationships with others (Chronister, Chou, Frain, & da Silva Cardoso, 2008). 3) Tier comorbidity variables were used to measure comorbidity severity in this analysis, but these variables could not address potential effects of specific medical conditions or complications on outcomes. 4) IRF service use was operationalized as a single measure (LOS); effects of variation among IRFs in type (e.g., the balance between physical and occupational therapy) or intensity of services delivered could not be assessed because such information is not available in the IRF-PAI dataset.
Another potential limitation of this study is that the outcome variable “discharged home” was operationalized as a dichotomous variable. Discharged home was coded as yes only for patients discharged from the IRF to their own private residence in the community, as indicated by a discharge to living setting code of 01 (=“home”) on the IRF-PAI (CMS, 2012). This distinction was made because “home” is the only community-based discharge setting where individuals are assumed to be responsible for their own (self) care; outside of informal resources such as family and friends, other formal supportive services may not be readily accessible at home (Holland, Mistiaen, & Bowles, 2011). Discharge home was coded as no for patients discharged from the IRF to any other setting, including other rehabilitation facilities, skilled nursing facilities, hospitals, and community discharge settings such as board and care, transitional living, and assisted living which provide personalized assistance and/or formal support such as transportation, laundry, and meals to their residents (CMS, 2012). Although these community discharge settings may be considered “home” by some of their residents, the patients in this study would not have regarded these settings as “home”, because this sample was restricted to patients who had lived in their own private residence before hip fracture.
A specific issue with the measure of functional independence (total FIM score) is that each item contributing to the total score is scored 0 (missing data) if the activity it measures is not observed during the initial assessment, which occurs within three calendar days of IRF admission. If a patient is not observed performing an activity assessed by a FIM item during the assessment period, a zero score (=missing data) is assigned for that item. Per CMS guidelines, this analysis converted the value of each zero item score at admission to “1”, a value indicating that the activity can be performed only with total assistance. This conversion can lead to an underestimate of functional status at admission if a patient who actually had the ability to perform the measured activity at some level received a zero item score because the activity was not observed at that time. These considerations do not pertain to FIM scores at discharge, because IRF staff continuously monitor patient functional status throughout the entire IRF stay and are therefore able to assign an actual score for each item at discharge. Given that total FIM scores are more likely to skew toward underestimates at admission than at discharge, it is possible for clinicians and researchers to misinterpret the magnitude of improvement in function between admission and discharge.
Clinical and Policy Implications
Robust conclusions about effectiveness of treatment of patients with a hip fracture and other conditions requiring post-acute rehabilitation await the implementation of a standardized patient assessment tool that allow comparisons of patients of similar conditions and severity across all settings. When development of such a tool was mandated under the Deficit Reduction Act of 2005, CMS contracted with RTI International (trade name of Research Triangle Institute) to develop and test the Continuity Assessment Record and Evaluation (CARE) tool, which collects patient and system level data at discharge from acute care hospitals and at admission and discharge from each PAC setting. A series of demonstration projects have been conducted with promising results; however, implementation of the CARE tool has yet to be finalized (Gage et al., 2012). Until that time, the current study has at least three important implications.
First, our finding of poorer outcomes for African American and Hispanic hip fracture patients receiving inpatient rehabilitation suggests that more effort is needed to understand racial/ethnic disparities in rehabilitation outcomes and develop interventions to reduce or eliminate their effects. As pay for performance emerges, reduction of such disparities may require system-level interventions to ensure that the performance metrics used to determine payment include reduction of disparities as one component of healthcare quality improvement (Weissman et al., 2012). Chien, Chin, Davis, and Casalino (2007) have pointed out that collecting racial and ethnic data and identifying subgroups that need specifically tailored interventions are essential first steps in development of performance-based health systems that will reduce disparities. The identification of African Americans and Hispanics as the racial/ethnic subgroups with the poorest inpatient rehabilitation outcomes in this study sets the stage for further research on interventions tailored to their specific needs. Such interventions should be developed and tested in partnership with members of the appropriate subgroups, as recommended by Gelman, Faul, & Yankeelov (2013).
Second, IRF admission policies regarding medical necessity and the 60% rule do not yet include standardized methods for assessing severity or number of comorbidities, which can affect recovery and functional independence (Granger et al., 2009). Findings from this study concerning effects of tier comorbidity category and number of comorbidities might help standardize IRF admission criteria and highlight the prognostic indicators of discharge function and odds of returning home that could guide clinical decision-making on admission of hip fracture patients to IRFs.
Third, Medicare policy should consider the role of longer LOS in achieving better functional outcomes. Reducing LOS for hospitalized hip fracture patients under the Prospective Payment System (PPS) has achieved cost savings, but extending post-acute LOS may reduce hospital readmissions and total Medicare expenditures while promoting health-related quality of life. A strategy that reinforces incentives inherent in a bundled payment system would encourage providers to identify and deliver combinations of setting and service to improve the probability of optimal outcomes (DeJong, 2010).
Conclusions
This national study identifies characteristics of Medicare hip fracture patients and subgroups therein that benefit from inpatient rehabilitation. Specifically, individuals who were younger, female, and living alone prior to admission, and those with higher functional status at admission, lesser need, and longer LOS had the best function at discharge. These characteristics were also associated with higher odds of being discharged home. However, Black and Hispanic individuals, who had poorer function at discharge in comparison to members of other racial/ethnic groups, were also more likely to be discharged home. Racial/ethnic and gender disparities were found for both functional status at discharge and discharged home.
This research may help health policymakers identify those who most need and are most likely to benefit from inpatient rehabilitation, and highlights subgroups in need of greater intervention to achieve better outcomes. Further examination of racial/ethnic and gender differences in rehabilitation outcomes are needed to better understand underlying causes of these disparities and develop interventions to reduce barriers to, and improve mediators of equitable health care for all.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institute of Nursing Research at the National Institutes of Health (Grant 1F31- NR012402-01A1) and by the University of Virginia School of Nursing Barbara Brodie Scholars Endowment Award. The primary author, Dr. Michael Cary, received this support while at the University of Virginia when completing the requirements for his PhD
Biographies
Michael P. Cary, Jr. is an assistant professor in the School of Nursing, senior fellow in the Center for the Study of Aging and Human Development, and core faculty member in the Center for Biobehavioral Health Disparities Research at Duke University. His research focuses on improving function among older adults and quality care delivery in post-acute care settings.
Elizabeth I. Merwin is the Ann Henshaw Gardiner Professor of nursing at the Duke University School of Nursing. She is a methodological expert in the use of secondary data. Her nursing and health care delivery research has focused on improving care for underserved and rural populations, particularly those in rural communities and minority populations.
M. Norman Oliver is the Walter M. Seward Professor and Chair of the Department of Family Medicine at the University of Virginia, and holds joint appointments in the University of Virginia (UVA) Departments of Public Health Sciences and Anthropology. His research focuses on investigating social determinants of health, particularly their effect on racial and ethnic health inequities.
Ishan C. Williams, PhD, is an assistant professor at the University of Virginia, School of Nursing. Her current research focuses on older adults with cognitive impairment and chronic illnesses, and the health and well-being of family caregivers. She focuses on understanding the health care needs of older adults and their family caregivers within social, cultural, and geographical contexts.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
Contributor Information
Michael P. Cary, Jr., Duke University, Durham, NC, USA
Elizabeth I. Merwin, Duke University, Durham, NC, USA
M. Norman Oliver, University of Virginia, Charlottesville, USA.
Ishan C. Williams, University of Virginia, Charlottesville, USA
References
- Abrahamsen B, van Staa T, Ariely R, Olson M, Cooper C. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporosis International. 2009;20(10):1633–1650. doi: 10.1007/s00198-009-0920-3. [DOI] [PubMed] [Google Scholar]
- Ahmed I, Graham JE, Karmarkar AM, Granger CV, Ottenbacher KJ. In-patient rehabilitation outcomes following lower extremity fracture in patients with pneumonia. Respiratory Care. 2013;58(4):601–606. doi: 10.4187/respcare.02022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen RM. National health surveys and the behavioral model of health services use. Medical Care. 2008;46(7):647–653. doi: 10.1097/MLR.0b013e31817a835d. [DOI] [PubMed] [Google Scholar]
- Arinzon Z, Fidelman Z, Zuta A, Peisakh A, Berner YN. Functional recovery after hip fracture in old-old elderly patients. Archives of Gerontology and Geriatrics. 2005;40(3):327–336. doi: 10.1016/j.archger.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Arinzon Z, Shabat S, Peisakh A, Gepstein R, Berner YN. Gender differences influence the outcome of geriatric rehabilitation following hip fracture. Archives of Gerontology and Geriatrics. 2010;50(1):86–91. doi: 10.1016/j.archger.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality and costs. Journal of the American Geriatrics Society. 2003;51(3):364–370. doi: 10.1046/j.1532-5415.2003.51110.x. [DOI] [PubMed] [Google Scholar]
- Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573–1579. doi: 10.1001/jama.2009.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter GM, Buntin MB, Hayden O, Paddock SM, Relles DA, Ridgeway G, Wynn BO. Analyses for the initial implementation of the Inpatient Rehabilitation Facility Prospective Payment System (MR-1500-CMS) Santa Monica, CA: RAND Corporation; 2002. [Google Scholar]
- Carter GM, Totten ME. Preliminary analyses for refinement of the tier comorbidities in the Inpatient Rehabilitation Facility Prospective Payment System. Santa Monica, CA: RAND Corporation; 2005. pp. 1–51. [Google Scholar]
- Centers for Medicare & Medicaid Services [CMS] The Inpatient Rehabilitation Facility –Patient Assessment Instrument (IRF-PAI) Training Manual: Effective 4/01/04. Baltimore, MD: CMS; 2004. Retrieved March 13, 2014 from https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientRehabFacPPS/downloads/irfpaimanual040104.pdf. [Google Scholar]
- Centers for Medicare & Medicaid Services [CMS] Medicare program: Inpatient Rehabilitation Facility Prospective Payment System for Federal Fiscal Year 2010. Final Rule. Washington, DC: Government Printing Office; 2009. Federal Register Doc. E9-18616 of August 7, 2009 (74 FR 39762) Retrieved December 12, 2013 from http://www.gpo.gov/fdsys/pkg/FR-2009-08-07/pdf/E9-18616.pdf. [PubMed] [Google Scholar]
- Centers for Medicare & Medicaid Services [CMS] Medicare Benefit Policy Manual, Chapter 1 - Inpatient hospital services covered under Part A, (Section 110. Inpatient Rehabilitation Facility (IRF) Services) Washington, DC: CMS; 2010. Retrieved May 17, 2013 from http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/bp102c01.pdf. [Google Scholar]
- Centers for Medicare & Medicaid Services [CMS] The Inpatient Rehabilitation Facility - Patient Assessment Instrument (IRF-PAI) Training Manual. Baltimore, MD: CMS; 2012. Retrieved June 26, 2013 from http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientRehabFacPPS/IRFPAI.html. [Google Scholar]
- Centers for Medicare & Medicaid Services [CMS] IRF Patient Assessment Instrument. 2013 Retrieved January 9, 2014, from http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientRehabFacPPS/IRFPAI.html.
- Chien AT, Chin MH, Davis AM, Casalino LP. Pay for performance, public reporting, and racial disparities in health care: how are programs being designed? Medical Care Research and Review. 2007;64(5 Suppl):283S–304S. doi: 10.1177/1077558707305426. [DOI] [PubMed] [Google Scholar]
- Chronister JC, Cou CC, Frain M, da Silva Cardoso E. The relationship between social support and rehabilitation related outcomes: a meta-analysis. Journal of Rehabilitation. 2008;74(2):16–32. [Google Scholar]
- DeJong G. Bundling acute and postacute payment: from a culture of compliance to a culture of innovation and best practice. Physical Therapy. 2010;90(5):658–662. doi: 10.2522/ptj.2010.90.5.658. [DOI] [PubMed] [Google Scholar]
- Deutsch A, Granger CV, Fiedler RC, DeJong G, Kane RL, Ottenbacher KJ, Trevisan M. Outcomes and reimbursement of inpatient rehabilitation facilities and subacute rehabilitation programs for Medicare beneficiaries with hip fracture. Medical Care. 2005;43(9):892–901. doi: 10.1097/01.mlr.0000173591.23310.d5. [DOI] [PubMed] [Google Scholar]
- Deutscher D, Horn SD, Smout RI, DeJong G, Putman K. Black-white disparities in motor function outcomes taking into account patient characteristics, nontherapy ancillaries, therapy activities, and therapy interventions. Archives of Physical Medicine and Rehabilitation. 2010;91(11):1722–1730. doi: 10.1016/j.apmr.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Di Monaco M, Castiglioni C, Vallero F, Di Monaco R, Tappero R. Men recover ability to function less than women do: an observational study of 1094 subjects after hip fracture. American Journal of Physical Medicine and Rehabilitation. 2012;91(4):309–315. doi: 10.1097/PHM.0b013e3182466162. [DOI] [PubMed] [Google Scholar]
- Freburger JK, Holmes GM, Ku LJ. Postacute rehabilitation care for hip fracture: who gets the most care? Journal of the American Geriatrics Society. 2012;60(10):1929–1935. doi: 10.1111/j.1532-5415.2012.04149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage B, Ingber MJ, Morley M, Smith L, Deutsch A, Kline T, Mallinson T. Post-acute care payment reform demonstration: Final report. Baltimore, MD: Centers for Medicare and Medicaid Services; 2012. Volume 4 of 4. [Google Scholar]
- Gelman C, Faul AC, Yankeelov PA. Intervention research with minority older adults: challenges encountered, solutions enacted, and implications for future work. Journal of Applied Gerontology. 2013;32(2):207–225. doi: 10.1177/0733464811416812. [DOI] [PubMed] [Google Scholar]
- Government Accountability Office [GAO] Medicare: More specific criteria needed to classify inpatient rehabilitation facilities: Report to the Senate Committee on Finance and the House Committee on Ways and Means (GAO-05-366), April 2005. 2005 (GAO-05-366) Retrieved May 17, 2013 from http://www.gao.gov/assets/250/246084.pdf.
- Graham JE, Chang PF, Bergés IM, Granger CV, Ottenbacher KJ. Race/ethnicity and outcomes following inpatient rehabilitation for hip fracture. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2008;63(8):860–866. doi: 10.1093/gerona/63.8.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger CV, Carlin M, Diaz P, Dorval J, Forer S, Kessler C, Roberts P. Medical necessity: is current documentation practice and payment denial limiting access to inpatient rehabilitation? American Journal of Physical Medicine and Rehabilitation. 2009;88(9):755–765. doi: 10.1097/PHM.0b013e3181aa71a8. [DOI] [PubMed] [Google Scholar]
- Herbold JA, Bonistall K, Walsh MB. Rehabilitation following total knee replacement, total hip replacement, and hip fracture: a case-controlled comparison. Journal of Geriatric Physical Therapy. 2011;34(4):155–160. doi: 10.1519/JPT.0b013e318216db81. [DOI] [PubMed] [Google Scholar]
- Hoenig H, Rubenstein L, Kahn K. Rehabilitation after hip fracture--equal opportunity for all? Archives of Physical Medicine and Rehabilitation. 1996;77(1):58–63. doi: 10.1016/s0003-9993(96)90221-x. [DOI] [PubMed] [Google Scholar]
- Horn SD, Deutscher D, Smout RJ, DeJong G, Putman K. Black-white differences in patient characteristics, treatments, and outcomes in inpatient stroke rehabilitation. Archives of Physical Medicine and Rehabilitation. 2010;91(11):1712–1721. doi: 10.1016/j.apmr.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Howard G, Lackland DT, Kleindorfer DO, Kissela BM, Moy CS, Judd SE, Howard VJ. Racial differences in the impact of elevated systolic blood pressure on stroke risk. JAMA Internal Medicine. 2013;173(1):46–51. doi: 10.1001/2013.jamainternmed.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, Howard G. Disparities in stroke incidence contributing to disparities in stroke mortality. Annals of Neurology. 2011;69(4):619–627. doi: 10.1002/ana.22385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter EG, Baltisberger J. Functional outcomes by age for inpatient cancer rehabilitation: a retrospective chart review. Journal of Applied Gerontology. 2013;32(4):443–456. doi: 10.1177/0733464811432632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer H, Han C, Post W, Goff D, Diez-Roux A, Cooper R, Shea S. Racial/ethnic differences in hypertension and hypertension treatment and control in the multi-ethnic study of atherosclerosis (MESA) American Journal of Hypertension. 2004;17(10):963–970. doi: 10.1016/j.amjhyper.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Kountz D. Special considerations of care and risk management for African American patients with type 2 diabetes mellitus. Journal of the National Medical Association. 2012;104(5–6):265–273. doi: 10.1016/s0027-9684(15)30158-9. [DOI] [PubMed] [Google Scholar]
- Lew HL, Lee E, Date ES, Zeiner H. Influence of medical comorbidities and complications on FIM change and length of stay during inpatient rehabilitation. American Journal of Physical Medicine and Rehabilitation. 2002;81(11):830–837. doi: 10.1097/01.phm.0000030723.10483.f7. [DOI] [PubMed] [Google Scholar]
- Lieberman D, Friger M, Lieberman D. Inpatient rehabilitation outcome after hip fracture surgery in elderly patients: a prospective cohort study of 946 patients. Archives of Physical Medicine and Rehabilitation. 2006;87(2):167–171. doi: 10.1016/j.apmr.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Lieberman D, Lieberman D. Rehabilitation following hip fracture surgery: a comparative study of females and males. Disability and Rehabilitation. 2004;26(2):85–90. doi: 10.1080/196538280310001629660. [DOI] [PubMed] [Google Scholar]
- Mathew RO, Hsu WH, Young Y. Effect of comorbidity on functional recovery after hip fracture in the elderly. American Journal of Physical Medicine and Rehabilitation. 2013;92(8):686–696. doi: 10.1097/PHM.0b013e318282bc67. [DOI] [PubMed] [Google Scholar]
- Medicare Payment Advisory Commission [MedPAC] Washington, DC: MedPAC; 2010. Post-acute care (Section 9) in A data book: Health care spending and the Medicare program; pp. 121–146. Retrieved December 20, 2013 from http://www.medpac.gov/chapters/Jun09DataBookSec9.pdf. [Google Scholar]
- Medicare Payment Advisory Commission [MedPAC] Report to the Congress: Medicare Payment Policy. Washington, DC: MedPAC; 2011. Retrieved June 19, 2013 from http://medpac.gov/documents/Mar11_EntireReport.pdf. [Google Scholar]
- Holland DE, Mistiaen P, Bowles KH. Problems and unmet needs of patients discharged “home to self-care”. Professional Case Management. 2011;16(5):240–250. doi: 10.1097/NCM.0b013e31822361d8. quiz 251–242. [DOI] [PubMed] [Google Scholar]
- Moran Company. Utilization trends in inpatient rehabilitation: Update through Q II 2006. 2006 Sep; Retrieved June 19, 2013, from http://www.aha.org/content/00-10/2006septmoranreport.pdf.
- Munin MC, Seligman K, Dew MA, Quear T, Skidmore ER, Gruen G, Lenze EJ. Effect of rehabilitation site on functional recovery after hip fracture. Archives of Physical Medicine and Rehabilitation. 2005;86(3):367–372. doi: 10.1016/j.apmr.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Nguyen-Oghalai TU, Ottenbacher KJ, Granger CV, Smith ST, Goodwin JS. Impact of osteoarthritis on rehabilitation for persons with hip fracture. Arthritis and Rheumatism. 2006;55(6):920–924. doi: 10.1002/art.22345. [DOI] [PubMed] [Google Scholar]
- Ottenbacher KJ, Graham JE. The state-of-the-science: Access to postacute care rehabilitation services. A review. Archives of Physical Medicine and Rehabilitation. 2007;88(11):1513–1521. doi: 10.1016/j.apmr.2007.06.761. [DOI] [PubMed] [Google Scholar]
- Ottenbacher KJ, Hsu Y, Granger CV, Fiedler RC. The reliability of the functional independence measure: a quantitative review. Archives of Physical Medicine and Rehabilitation. 1996;77(12):1226–1232. doi: 10.1016/s0003-9993(96)90184-7. [DOI] [PubMed] [Google Scholar]
- Ottenbacher KJ, Smith PM, Illig SB, Linn RT, Gonzales VA, Ostir GV, Granger CV. Disparity in health services and outcomes for persons with hip fracture and lower extremity joint replacement. Medical Care. 2003;41(2):232–241. doi: 10.1097/01.mlr.0000044902.01597.54. [DOI] [PubMed] [Google Scholar]
- Peek ME, Cargill A, Huang ES. Diabetes health disparities: a systematic review of health care interventions. Medical Care Research and Review. 2007;64(5 Suppl):101s–156s. doi: 10.1177/1077558707305409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reistetter TA, Graham JE, Deutsch A, Markello SJ, Granger CV, Ottenbacher KJ. Diabetes comorbidity and age influence rehabilitation outcomes after hip fracture. Diabetes Care. 2011;34(6):1375–1377. doi: 10.2337/dc10-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs RV, Roberts PS, Aronow H, Younan T. Joint replacement and hip fracture readmission rates: impact of discharge destination. PM & R: the Journal of Injury, Function, and Rehabilitation. 2010;2(9):806–810. doi: 10.1016/j.pmrj.2010.05.008. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS Version 9.2 Product Documentation. Cary, NC: SAS Institute Inc; 2013. [Google Scholar]
- Semel J, Gray JM, Ahn HJ, Nasr H, Chen JJ. Predictors of outcome following hip fracture rehabilitation. PM & R: The Journal of Injury, Function, and Rehabilitation. 2010;2(9):799–805. doi: 10.1016/j.pmrj.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Sentell TL, He G, Gregg EW, Schillinger D. Racial/ethnic variation in prevalence estimates for United States prediabetes under alternative 2010 American Diabetes Association criteria: 1988–2008. Ethnicity and Disease. 2012;22(4):451–458. [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: A Cancer Journal for Clinicians. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- Sterling RS. Gender and race/ethnicity differences in hip fracture incidence, morbidity, mortality, and function. Clinical Orthopaedics and Related Research. 2011;469(7):1913–1918. doi: 10.1007/s11999-010-1736-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stineman MG, Hamilton BB, Granger CV, Goin JE, Escarce JJ, Williams SV. Four methods for characterizing disability in the formation of function related groups. Archives of Physical Medicine and Rehabilitation. 1994;75(12):1277–1283. [PubMed] [Google Scholar]
- Weissman JS, Hasnain-Wynia R, Weinick RM, Kang R, Vogeli C, Iezzoni L, Landrum MB. Pay-for-performance programs to reduce racial/ethnic disparities: what might different designs achieve? Journal of Health Care for the Poor and Underserved. 2012;23(1):144–160. doi: 10.1353/hpu.2012.0030. [DOI] [PubMed] [Google Scholar]
- Wright NC, Saag KG, Curtis JR, Smith WK, Kilgore ML, Morrisey MA, Delzell ES. Recent trends in hip fracture rates by race/ethnicity among older US adults. Journal of Bone and Mineral Research. 2012;27(11):2325–2332. doi: 10.1002/jbmr.1684. [DOI] [PubMed] [Google Scholar]


