Abstract
Background: The potential association between IGF-1 polymorphisms and high myopia has been investigated in previous studies, but the actual relationship remains controversial. Accordingly, we conducted a meta-analysisincludingcase-control and cohort studies to assess the existing relationship between high myopia and IGF-1 polymorphisms. We searched MEDLINE, EMBASE, and OVID. Odds ratios (OR) with 95% confidence intervals (CI) were derived for single-nucleotide polymorphisms (SNPs) involved in the studies obtained from the retrospective database search. Analyses of heterogeneity, sensitivity, and publication bias were also conducted. The findings from this meta-analysis were based on approximately 2,187 high myopia cases and 1,183 controls, and were used to assess the association between three IGF-1 genetic polymorphisms (rs6214, rs12423791, and rs5742632) and high myopia risks. We investigated the association of the IGF-1 gene SNP rs6214, but no statistical association was observed in the resulting odds ratios (OR) in the allelic (OR = 1.06, 95% CI = 0.89-1.25), dominant (OR = 1.07, 95% CI = 0.90-1.27), or recessive models (OR = 1.06, 95% CI = 0.89-1.26), or in the homozygote (OR = 1.12, 95% CI = 0.91-1.38) and heterozygote comparisons (OR = 1.06, 95% CI = 0.88-1.27). Simultaneously, two other selected SNPs, rs12423791 and rs5742632, were also studied, but similarly, no statistical association existed between these polymorphisms and the risk of high myopia. In conclusions, no statistical association between IGF-1 polymorphisms (rs6214, rs12423791, and rs5742632) and the risk of high myopia was observed following the reported meta-analysis.
Keywords: IGF-1, polymorphisms, high myopia, meta-analysis
Introduction
Myopia is a prevalent ocular disorder that can adverselyimpactsocial, educational, and economic circumstances, as well asaffect the quality of life [1]. The global prevalence of myopia varies widely, affecting approximately 500 million people worldwide. High myopia as an extreme form of myopia, which can be also termed pathologic myopia, is usually defined as a refractive error of at least -6.00 diopter (D). Individuals with high myopia are predisposed to the development of ocular abnormalities including cataracts, retinal detachment, glaucoma, or chorioretinal degeneration resulting from pathological changes in ocular construction [2,3], which may also lead to irreversible vision impairment, sometimes even blindness.Combinations of genetic and environmental factors have been associated with modulationsinthe pathogenesis of myopia [4,5]. Accordingly, the intervention of hereditary factorsrelatedto high myopia has been studied intensively. Epidemiological, experimental, and clinical studies have demonstrated a significant genetic contribution to high myopia. Additionally, a number of affirmative genetic loci are known to be correlated to high myopia, including Xq28 (MYP1), 12q21-q23 (MYP3), 7q36 (MYP4), and so on [2,6]. As well, single-nucleotide polymorphisms (SNPs) of Insulin-like growth factor-1 (IGF-1) are currently considered additional candidate genes associated with high myopia [7].
IGF-1 is a member of a protein family involved in mediating growth and development, and the IGF-1 gene is located at a well-replicated myopia susceptibility locus, known as MYP3 [8-10]. Recent studies have provided evidence that IGF-1 plays a role inocular growth, even axial myopia [11]. Additionally, a number of previously published studies have focused on the relationship between IGF-1 SNPs and high myopia, though the accuracy of theassociation remains controversial.
To date, no meta-analysis has been conducted to evaluate the potential relationship between IGF-1 SNPs and high myopia. In the current study, we performed a meta-analysis, using strictly controlledcriteria for the inclusion and exclusion of previously published studies, with the objective of assessingthe association between IGF-1 and high myopia.
Methods
Search strategy
The current study was performed according to the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines and thePreferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [12,13] for the meta-analysis of observational studies. A systematic literature searchof MEDLINE (1966 to June 1, 2014), EMBASE (1980 to June 1, 2014), and OVID (1950 to June 1, 2014) was conducted, using medical subject headings (MeSH), or free text words. Search terms including “insulin growth factor”, “insulin like growth factor”, “IGF-1”, “IGF”, “polymorphism(s)”, “variant(s)”, “mutation(s)”, and outcomes (“myopia”, “refraction”, “refraction error”, “refractive error”) were combined. The reference sections of all identified relevant publications were also searched.
Selection criteria
For inclusion in the meta-analysis, studies had to meet the following two criteria: (1) the study had to be an original case-control or cohort study that evaluated the relationship between IGF-1 polymorphisms and high myopia, and (2) the study had to provide sufficient data on each genotype and/or alleles in both case and control groups. Reviewers independently evaluated the published quantitative estimates of the association between IGF-1 and high myopia for inclusion in the meta-analysis. Studies that did not meet the above inclusion criteria were excluded during the initial review. If the suitability of the study was not agreed upon unanimously, the study in question was discussed by the reviewers until a consensus was reached.
Data extraction
The data extracted from each study included the last nameof the first author, publication year, country in which the data were collected, ethnicity of participants, gender, age, and study size distributions of case and controls in study populations. Specific SNPs of IGF-1 available in the study, genotyping method, source of controls, extent of refractive degree and axial length for case and control groups, and number of eligible and genotyped cases and controls were also extracted. The reviewers independently extracted all of the data from the previously published studies using a standardized data collection form. Discrepancies were resolved through reviewer discussion, and by referring to the original articles.
Statistical analyses
The Hardy-Weinberg equilibrium (HWE) of genetic frequency distributions for the controls was evaluated using achi-square test, and a resulting P-value < 0.05 was considered a statistically significant inequality of genetic distributions. Genetic comparisons, including the allelic model (a vs. A), dominant model (aa+Aa vs. AA), recessive model (aa vs. AA+Aa), homozygote (aa vs. AA) and heterozygote models (Aa vs. AA) were conducted, where “A” denoted a major allele, and “a” denoted a minor allele. The odds ratios (OR) and 95% confidence intervals (95% CI) were used as the common measure across studies, in both fixed- and random-effects models [14]. The Cochran Q statistic was calculated to assess heterogeneity across studies. If the result of the Q test was P > 0.1, ORs were pooled according to the fixed effects model (Mantel-Haenszel); otherwise, the random-effects model (DerSimonian and Larid) was used. The Cochran (I2) statistic, whichquantifies the proportion of total variation attributable to between-study heterogeneity, was also calculated [15]. As suggested by Higgins et al., I2 values of 25%, 50%, and 75% were considered to be low, moderate, and high heterogeneity, respectively [16]. A funnel plot of the overall OR was generated, and a standard error (SE) was calculated. As well, potential publication biaswas measured by Egger’s and Begg’s regression tests. Ananalysis of sensitivitywas likewise conducted by omitting each study to assess potential outliers.
Stratified analyses were conducted for further investigation of the associations between IGF-1 and high myopia, according to ethnicity, source of controls, and study size. All analyses were conducted using Stata 12 (Stata Corp, College Station, TX). Statistical significance was defined as a p-value < 0.05.
Results
There were five publications in total that met the inclusion criteria [7,17-20], and one was written in Chinese. The Chinese article was excluded because the same patient population was included in a separate publication, and the more complete study had already been includedin the current meta-analysis. Consequently, the remaining four studies containing 2,187 cases and 1,183 controls were eligible for inclusion in the meta-analysis (Figure 1).
Figure 1.
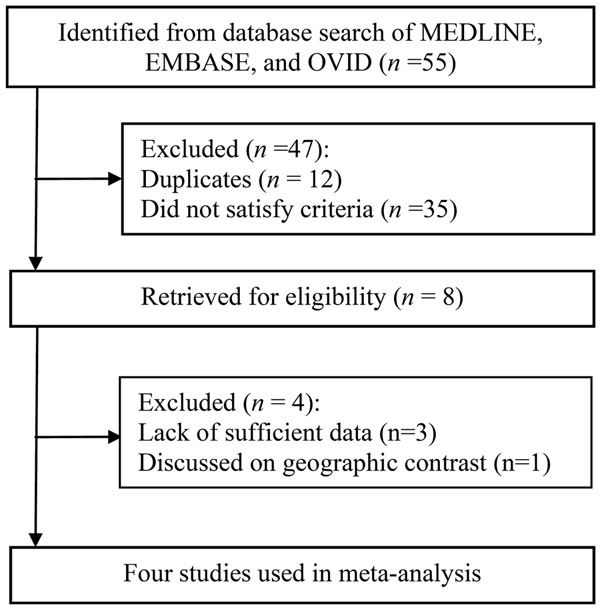
Flowchart of the study selection process.
Characteristics and quality of the studies
The main characteristics of the studies included in the meta-analysisare listed in Table 1. More than 19 SNPs were included in the articles, and the genetic variants studied most frequently were rs6214, rs12423791, and rs5742632. Thus, our meta-analysis focused on these three SNPs. Of the included articles, all four studies focused on the association between rs6214and high myopia, with three studies including rs12423791, and two studies involving rs5742632. None of the controls in the included studies had a statistically significant deviation from HWE at P < 0.01.
Table 1.
Characteristics of the included studies regarding polymorphisms in IGF-1 gene and high myopia risk
| Study (year) | Country | Ethnicity | Source of controls | Genotyping method | SNP ID | Gender (M/F) | Age (mean±SD, a) | Sample size | Refractive degree (diopter) | Axial length (mm) | HWE | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | |||||||
| Miyake et al. (2013) | Japan | Asian | HB | TaqMan | rs6214 | 442/897 | 132/202 | 57.2±14.9 | 74.8±8.12 | 1,339 | 334 | Right eyes: -12.83±4.48 | Right eyes: -0.459±3.24 | ≥ 26 | < 25 | rs6214: 0.512 |
| rs5742632 | Left eyes: -12.55±4.60 | Left eyes: -0.294±2.76 | rs12423791: 0.162 | |||||||||||||
| rs12423791 | rs5742632: 0.381 | |||||||||||||||
| Zhuang et al. (2012) | China | Asian | PB | MALDI-TOF | rs6214 | 165/256 | 172/229 | 38.29±16.57 | 68.77±10.65 | 421 | 401 | < -8.00 | -0.50~+2.00 | > 26 | < 24 | rs6214: 0.960 |
| rs12423791 | ||||||||||||||||
| rs12423791: 0.414 | ||||||||||||||||
| Mak et al. (2012) | China | Asian | HB | Restriction fragment length polymorphism analysis | rs12423791 | NA | NA | 27.6 | 24.6 | 300 | 300 | ≤ -8.00 | -1.00~+1.00 | 27.76±1.13 | 23.85±0.83 | rs6214: 0.136 |
| rs12423791: 0.657 | ||||||||||||||||
| rs5742632 | ||||||||||||||||
| rs5742632: 0.591 | ||||||||||||||||
| rs6214 | ||||||||||||||||
| Rydzanicz et al. (2011) | Poland | Caucasian | PB | PCR-RFLP | rs6214 | 50/77 | 69/79 | 40.2±20.43 | 38.6±18.54 | 127 | 148 | ≤ -6.00 | -4.0~-0.5 | 27.27±2.03 | 23.39±0.82 | rs6214: 0.118 |
HB, hospital based; PB, population based; PCR, Polymerase chain reaction; RFLP, Restriction fragment length polymorphism; NA, Not applicable; HWE, Hardy–Weinberg equilibrium.
Main analysis
The primary results of our meta-analysis for IGF-1 polymorphisms are presented in Table 2. For rs6214, no significant association was observed when all eligible studies were pooled into our analysis, either in the allelic, dominant, or recessive models, or in the homozygote and heterozygote models (OR = 1.06, 95% CI = 0.89-1.25; OR = 1.07, 95% CI = 0.90-1.27; OR = 1.06, 95% CI = 0.89-1.26; OR = 1.12, 95% = CI 0.91-1.38; OR = 1.06, 95% CI = 0.88-1.27, respectively, using fixed-effects). No significant statistical heterogeneity was discovered among the studies (PHeterogeneity = 0.608, I2 = 0% for the allelic model; PHeterogeneity = 0.563, I2 = 0% forthe dominant model; PHeterogeneity = 0.495, I2 = 0% for recessive model; PHeterogeneity = 0.552, I2 = 0% for homozygote model; PHeterogeneity = 0.591, I2 = 0% for heterozygote model). Similar results were also observed in terms of rs12423791 and rs5742632. The pooled OR was 1.12 (95% CI = 0.93-1.36) and 1.06 (95% CI = 0.83-1.34) for the dominant model, 0.91 (95% CI 0.74-1.12) and 0.89 (95% CI 0.72-1.11) for the recessive model, 1.01 (95% CI 0.75-1.37) and 1.00 (95% CI 0.75-1.32) for the homozygote model, and 1.14 (95% CI 0.93-1.40) and 1.10 (95% CI 0.86-1.42) for the heterozygote model, respectively.
Table 2.
Meta-analysis of studies for rs6214, rs12423791, rs5742632 polymorphisms of IGF-1 gene and high myopia risk
| SNPs | Models tested | Number of studies | Pooled OR (95% CL) | p | Heterogeneity | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| FEM | REM | FEM | REM | Q | PQ | I 2, % | |||
| rs6214 | Allelic model | 2 | 1.06 (0.89, 1.25) | 1.06 (0.89, 1.25) | 0.517 | 0.517 | 0.26 | 0.608 | 0 |
| Dominant model | 4 | 1.07 (0.90, 1.27) | 1.07 (0.91, 1.28) | 0.423 | 0.411 | 2.05 | 0.563 | 0 | |
| Recessive model | 4 | 1.06 (0.89, 1.26) | 1.06 (0.89, 1.26) | 0.513 | 0.515 | 2.39 | 0.495 | 0 | |
| Homozygote | 4 | 1.12 (0.91, 1.38) | 1.12 (0.91, 1.38) | 0.300 | 0.298 | 2.10 | 0.552 | 0 | |
| Heterozygote | 4 | 1.06 (0.88, 1.27) | 1.06 (0.89, 1.27) | 0.529 | 0.522 | 1.91 | 0.591 | 0 | |
| rs12423791 | Dominant model | 3 | 1.12 (0.93, 1.36) | 1.09 (0.81, 1.46) | 0.231 | 0.587 | 4.38 | 0.112 | 54.3 |
| Recessive model | 3 | 0.91 (0.74, 1.12) | 0.91 (0.73, 1.14) | 0.359 | 0.422 | 2.10 | 0.349 | 5.0 | |
| Homozygote | 3 | 1.01 (0.75, 1.37) | 1.02 (0.73, 1.44) | 0.928 | 0.901 | 2.48 | 0.289 | 19.3 | |
| Heterozygote | 3 | 1.14 (0.93, 1.40) | 1.12 (0.87, 1.45) | 0.196 | 0.383 | 3.07 | 0.215 | 34.9 | |
| rs5742632 | Dominant model | 2 | 1.06 (0.83, 1.34) | 1.06 (0.84, 1.34) | 0.647 | 0.645 | 0.09 | 0.765 | 0 |
| Recessive model | 2 | 0.89 (0.72, 1.11) | 0.91 (0.70, 1.17) | 0.303 | 0.444 | 1.31 | 0.253 | 23.5 | |
| Homozygote | 2 | 1.00 (0.75, 1.32) | 1.00 (0.75, 1.32) | 0.973 | 0.974 | 0.19 | 0.66 | 0 | |
| Heterozygote | 2 | 1.10 (0.86, 1.42) | 1.10 (0.86, 1.42) | 0.449 | 0.442 | 0.59 | 0.443 | 0 | |
FEM, fixed-effects model; REM, random-effects model; “A”, major allele; “a”, minor allele; allelic model, a vs. A; dominant model, aa+Aa vs. AA; recessive model, aa vs. AA+Aa; homozygote comparison, aa vs. AA; heterozygote, Aa vs. AA.
Stratified analysis
The results of the stratified analyses for rs6214 are provided in Table 3. The results of the analysis indicated no influence on the relationship between the rs6214 polymorphism and high myopia with ORs of 1.11 (95% CI = 0.92-1.33), 1.05 (95% = 0.87-1.26), 1.12 (95% CI = 0.89-1.40), and 1.11 (95% CI = 0.91-1.34) for the dominant, recessive, homozygote, and heterozygote models in the Asian population. When considering different sources of controls, the enrolled population-based studies returnedORs of 1.01 (95% CI = 0.77-1.32), 1.16 (95% CI = 0.87-1.55), 1.16 (95% CI = 0.82-1.64), and 0.96 (95% CI = 0.72-1.27), respectively. Regarding thehospital-based studies, the resulting ORs were 1.12, 0.99, 1.07, and 1.14, respectively. We also further investigatedthe varying populations included in each of the studies. No statistical association was observed in studies that included ≤600 participants (OR = 0.93, 95% CI = 0.69-1.24; OR = 0.91, 95% CI = 0.64-1.30; OR = 0.91, 95% CI = 0.62-1.33); and OR = 0.94, 95% CI = 0.69-1.29, respectively) as well as those that included > 600 participants (OR = 1.16, 95% CI =0.94-1.44; OR = 1.13, 95% CI = 0.92-1.39; OR = 1.23, 95% CI = 0.95-1.58; and OR = 1.13, 95% CI = 0.90-1.41, respectively).
Table 3.
Result of meta-analysis for IGF-1 rs6214 polymorphism and high myopia risk
| Number of studies | Dominant model | Recessive model | Homozygote | Heterozygote | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| OR (95% CI) | P | P for heterogeneity | OR (95% CI) | P | P for heterogeneity | OR (95% CI) | P | P for heterogeneity | OR (95% CI) | P | P for heterogeneity | ||
| Total | 4 | 1.07 (0.91, 1.28) | 0.411 | 0.563 | 1.06 (0.89, 1.26) | 0.515 | 0.495 | 1.12 (0.91, 1.38) | 0.298 | 0.552 | 1.06 (0.89, 1.27) | 0.522 | 0.591 |
| Asian | 3 | 1.11 (0.92, 1.33) | 0.266 | 0.578 | 1.05 (0.87, 1.26) | 0.632 | 0.345 | 1.12 (0.89, 1.40) | 0.338 | 0.350 | 1.11 (0.91, 1.34) | 0.312 | 0.750 |
| Source of controls | |||||||||||||
| Population based | 2 | 1.01 (0.77, 1.32) | 0.955 | 0.434 | 1.16 (0.87, 1.55) | 0.301 | 0.787 | 1.16 (0.82, 1.64) | 0.396 | 0.930 | 0.96 (0.72, 1.27) | 0.761 | 0.412 |
| Hospital based | 2 | 1.12 (0.89, 1.41) | 0.328 | 0.303 | 0.99 (0.74, 1.32) | 0.927 | 0.196 | 1.07 (0.72, 1.58) | 0.751 | 0.155 | 1.14 (0.90, 1.45) | 0.274 | 0.548 |
| Study size | |||||||||||||
| ≤ 600 | 2 | 0.93 (0.69, 1.24) | 0.602 | 0.697 | 0.91 (0.64, 1.30) | 0.604 | 0.299 | 0.91 (0.62, 1.33) | 0.624 | 0.539 | 0.94 (0.69, 1.29) | 0.716 | 0.427 |
| > 600 | 2 | 1.16 (0.94, 1.44) | 0.163 | 0.551 | 1.13 (0.92, 1.39) | 0.247 | 0.914 | 1.23 (0.95, 1.58) | 0.116 | 0.754 | 1.13 (0.90, 1.41) | 0.291 | 0.505 |
Sensitivity analysis
For most of the studiesfocusedon thers6214 polymorphism, we evaluated the influence of eachindividual study on the overall OR. Figure 2A and 2B show the influence of individual studies on the summary OR in the dominant and recessive models. No individual study influenced the overall OR in both the dominant and recessive models, since the omission of any of the included studiesdid not result in statistical significance.
Figure 2.
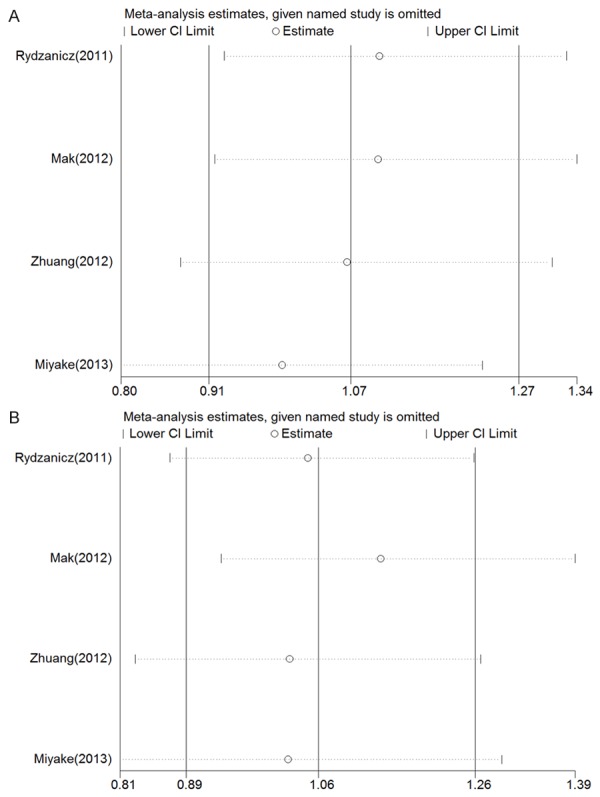
Sensitivity analysis for rs6214. A and B show the influence of individual studies on the summary ORin the dominant and recessive models, respectively. The vertical axis indicates the overall OR, and the two vertical axes show the 95% CI. Every hollow circle indicates the pooled OR when the study was omitted from the meta-analysis. The two ends of every broken line represent the respective 95% CI.
Publication bias
Figure 3A and 3B containfunnel plots for the dominant and recessive models of studies enrolled in themeta-analysis. Asymmetry was not apparent on the funnel plot. Moreover, the Begg’s and Egger’s test were also conducted to identify publication bias for the polymorphism rs6214. For the four studies included, the Begg’s (P = 0.308) and Egger’s (P = 0.258) tests suggested no evidence of publication bias in the dominant model, and similar resultswereobserved in the recessive model (P = 0.734 and 0.607, respectively).
Figure 3.
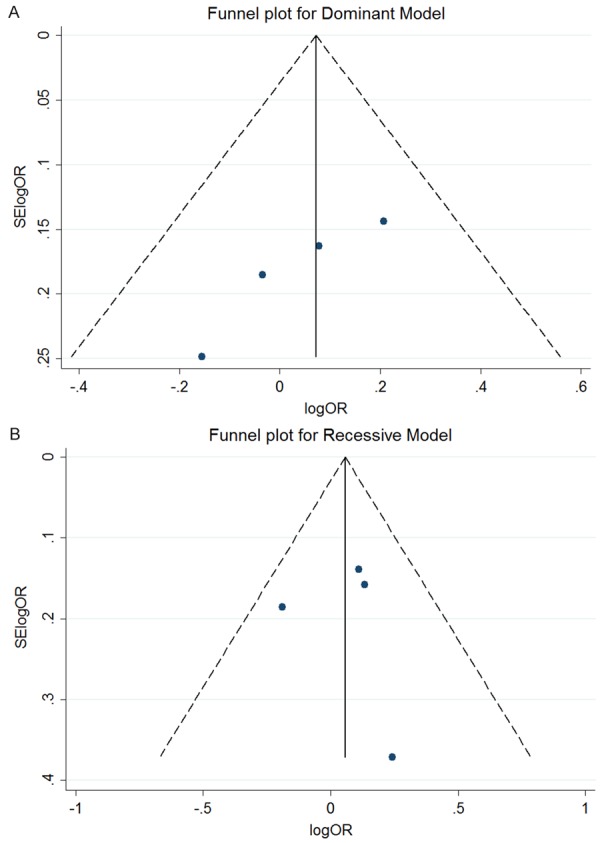
Publication bias analysis for rs6214. A and B show the funnel plot of included studies in the dominant and recessive models, respectively. The horizontal axis indicates the logOR, and the vertical axis indicates the standard error of logOR (SElogOR). The vertical and sloping lines in the funnel plot demonstrate the fixed-effects summary OR, and the expected 95% CI for a given standard error, respectively.
Discussion
The currentmeta-analysiswas based on approximately 2,187 high myopia cases and 1,183 controls, andevaluated the association between three IGF-1 gene polymorphisms (rs6214, rs12423791, and rs5742632) and high myopia risk. We investigated the association between the rs6214 IGF-1 gene SNP and high myopia risks, butno statistically significant association was observed in either the allelic, dominant, or the recessive models, orin the homozygote and heterozygote comparisons. Concurrently, two other selected SNPs, rs12423791 and rs5742632, were studied, butno statistically significant association between these polymorphisms and risk of high myopia was detected. For the stratified analysis of the rs6214 polymorphism, no significant difference was found when stratified for ethnicity, source of controls, orstudy size. In addedsensitivity analysis, no individual study was found to have an influence on the overall OR in the dominant and recessive models. As well, the results of the Begg’s and Egger’s tests indicated no evidence of publication bias.
The IGF-1 gene has been suggested as a candidate gene for several genetic diseases including diabetes and osteoarthritis [21,22], but is now likewise implicated in ocular genetic diseases, such as proliferative diabetic retinopathy, retinopathy prematurity, and age-related macular degeneration [23-27].
In recent studies, possible associations between IGF-1 polymorphisms and myopia have been explored. Animal experiments have been conducted to investigate the possible role of IGF-1 in the development and progression of myopia, and studies involving poultry models have demonstrated an impact on eye growth, as well as elongation [11,28]. However, the results of studies involving mammalian models did not correspond with the outcomes of the avian studies [29,30]. In regards to human experimental studies, Cordian et al. observed enhanced scleral growth which may have resulted from increased levels of insulin and insulin-like growth hormones [31]. Conversely, other studie sreportedno difference in axial length between IGF-1 treated Laron syndrome patients, and healthy controls [32].
The rs6214 SNP is located in the 3’-untranslated region (UTR) of the IGF-1 gene. The minor allelic frequencies in different ethnicities differ. According to HapMap data (www.hapmap.org), as in Eur-opean, Han Chinese, Yoruba, and Japanese populations, the frequencies are 0.421, 0.467, 0.533, and 0.568 respectively. The 3’UTR is known as a noncoding sequence, which contains regulatory motifs crucial for gene expression, mRNA stability, and cellular location of mRNA of the binding of microRNA. Accordingly, 3’UTR variants may play an important role in genetic disease by down-regulating gene expression through mRNA cleavage as well as translational repression [33-36]. Further, other high myopia candidate genes, including PAX6, are also located in this critical site [37].
The rs12423791 SNP, which is located in the intron region of IGF-1, has minor allelicfrequencies of 0.009, 0.25, and 0.209 among Europeans, Han Chinese, and Japanese respect to HapMap data, respectively. In our meta-analysis, this SNP was only consideredin the Asian population, and relatedstudies reported confusing results. Miyake et al. found no association between rs12423791 and high myopia, or even extreme myopia. However, for Zhuang and colleagues, a positive association was discovered [18], while Mak et al. detectedhigh-risk rs12423791 haplotypes CAC in their study [7]. Regarding rs5742632, only two experimental studies selected this SNP for genotyping [7,20], which further indicates that these potentially functional SNPs are worthy of further investigation.
Various studies have been conducted to investigate the association between the IGF-1 gene and high myopia; however, no statistically significant association was established followingour meta-analysis. Nonetheless, it is common in the study of complex and multigenic diseasesfor significant association sdeterminedin one investigation to appear to be negative in subsequent analyses, owing primarily to the involvement ofseveral overlapping signaling pathways [18]. Attention should be paid tothe failure to demonstrate asignificant association between any of the three IGF-1 gene polymorphisms and high myopia risk, which does not eliminate the possibility that other polymorphisms, or combinations of IGF-1 gene alleles, may prove to be extremely relevant to the risk of high myopia. Aside from rs6214, rs12423791, and rs5742632, other IGF-1 polymorphisms were investigated in other experimental studies. For instance, a Japanese case-control study conducted by Yoshida et al. demonstrated that the association between the A allele of rs5742629 and a moderately increased risk of high myopia approached statistical significance [38]. Therefore, attention should be paid to studies that involvea comprehensive haplotype-based approach, which could yieldbetter evidence on the genetic contribution of the IGF-1 gene to the risk of high myopia.
In the current meta-analysis, anaccepted, stringent strategy was adopted regarding inclusion of studiesonthe IGF-1 gene and high myopia, and the tests for potential biases revealed adequate comprehensiveness. However, several limitations existed in the understanding of IGF-1 gene in genetics related tohigh myopia. First, the differences in ethnic background should be considered, as the studies involved were mainly based onmembers of the Asian population. Considering the minor allelic frequencies of SNPs differ among various ethnicities, inclusion of a predominantly Asian study population may have restricted our conclusion, and indicated the need for related studies of other ethnic populations. Second, this meta-analysis was based on a limited number of studies, which potentially affected the stringency of the statistical analyses. Therefore, additional studies should be conducted to validate our conclusions. Third, the definition of controls and high-grade myopia varied across the studies included in the analyses. One study characterizedhigh-grade myopia as lower than -6.00 D, while others regarded high myopia as -8.00 D. As well, the refractive degree of controls differed, and ranged from -4.00 to +2.00 D, which may have likewise decreased the power of the statistical analyses. Increased stringency in the criteria applied to case and control subjects could aid in obtaining more sufficient proof regardingthe associationof IGF-1 polymorphisms and high myopia, and minimize potential bias.
Conclusions
In conclusion, the results of the currentmeta-analysis did not reveal anyevidence to support the existence of a genetic association betweenthe IGF-1 gene polymorphisms rs6214, rs12423791, and rs5742632, and high myopia. The association between IGF-1 polymorphisms and extreme myopia should thus be investigated further usinglarge cohort studies.
Disclosure of conflict of interest
None.
References
- 1.Saw SM, Katz J, Schein OD, Chew SJ, Chan TK. Epidemiology of myopia. Epidemiol Rev. 1996;18:175–87. doi: 10.1093/oxfordjournals.epirev.a017924. [DOI] [PubMed] [Google Scholar]
- 2.Hornbeak DM, Young TL. Myopia genetics: a review of current research and emerging trends. Curr Opin Ophthalmol. 2009;20:356–62. doi: 10.1097/ICU.0b013e32832f8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005;25:381–91. doi: 10.1111/j.1475-1313.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 4.Liang CL, Yen E, Su JY, Liu C, Chang TY, Park N, Wu MJ, Lee S, Flynn JT, Juo SH. Impact of family history of high myopia on level and onset of myopia. Invest Ophthalmol Vis Sci. 2004;45:3446–52. doi: 10.1167/iovs.03-1058. [DOI] [PubMed] [Google Scholar]
- 5.Sherwin JC, Reacher MH, Keogh RH, Khawaja AP, Mackey DA, Foster PJ. The association between time spent outdoors and myopia in children and adolescents: a systematic review and meta-analysis. Ophthalmology. 2012;119:2141–51. doi: 10.1016/j.ophtha.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Wojciechowski R. Nature and nurture: the complex genetics of myopia and refractive error. Clin Genet. 2011;79:301–20. doi: 10.1111/j.1399-0004.2010.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mak JY, Yap MK, Fung WY, Ng PW, Yip SP. Association of IGF1 gene haplotypes with high myopia in Chinese adults. Arch Ophthalmol. 2012;130:209–16. doi: 10.1001/archophthalmol.2011.365. [DOI] [PubMed] [Google Scholar]
- 8.Li YJ, Guggenheim JA, Bulusu A, Metlapally R, Abbott D, Malecaze F, Calvas P, Rosenberg T, Paget S, Creer RC, Kirov G, Owen MJ, Zhao B, White T, Mackey DA, Young TL. An international collaborative family-based whole-genome linkage scan for high-grade myopia. Invest Ophthalmol Vis Sci. 2009;50:3116–27. doi: 10.1167/iovs.08-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nurnberg G, Jacobi FK, Broghammer M, Becker C, Blin N, Nurnberg P, Stephani U, Pusch CM. Refinement of the MYP3 locus on human chromosome 12 in a German family with Mendelian autosomal dominant high-grade myopia by SNP array mapping. Int J Mol Med. 2008;21:429–38. [PubMed] [Google Scholar]
- 10.Farbrother JE, Kirov G, Owen MJ, Pong-Wong R, Haley CS, Guggenheim JA. Linkage analysis of the genetic loci for high myopia on 18p, 12q, and 17q in 51 U. K. families. Invest Ophthalmol Vis Sci. 2004;45:2879–85. doi: 10.1167/iovs.03-1156. [DOI] [PubMed] [Google Scholar]
- 11.Feldkaemper MP, Neacsu I, Schaeffel F. Insulin acts as a powerful stimulator of axial myopia in chicks. Invest Ophthalmol Vis Sci. 2009;50:13–23. doi: 10.1167/iovs.08-1702. [DOI] [PubMed] [Google Scholar]
- 12.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins J, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 17.Zhao JJ, Yang XQ, Li SS, Li ZL, Zhuang WJ. [Association study of insulin-like growth factor-1 polymorphisms with extreme high myopia] . Zhonghua Yan Ke Za Zhi. 2013;49:334–9. [PubMed] [Google Scholar]
- 18.Zhuang W, Yang P, Li Z, Sheng X, Zhao J, Li S, Yang X, Xiang W, Rong W, Liu Y, Zhang F. Association of insulin-like growth factor-1 polymorphisms with high myopia in the Chinese population. Mol Vis. 2012;18:634–44. [PMC free article] [PubMed] [Google Scholar]
- 19.Rydzanicz M, Nowak DM, Karolak JA, Frajdenberg A, Podfigurna-Musielak M, Mrugacz M, Gajecka M. IGF-1 gene polymorphisms in Polish families with high-grade myopia. Mol Vis. 2011;17:2428–39. [PMC free article] [PubMed] [Google Scholar]
- 20.Miyake M, Yamashiro K, Nakanishi H, Nakata I, Akagi-Kurashige Y, Tsujikawa A, Moriyama M, Ohno-Matsui K, Mochizuki M, Yamada R, Matsuda F, Yoshimura N. Insulin-like growth factor 1 is not associated with high myopia in a large Japanese cohort. Mol Vis. 2013;19:1074–81. [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang F, Tang YT, Guo L, Jiao XY. The role of insulin-like growth factor I and hypoxia inducible factor 1alpha in vascular endothelial growth factor expression in type 2 diabetes. Ann Clin Lab Sci. 2013;43:37–44. [PubMed] [Google Scholar]
- 22.Wang M, Liu C, Zhang Y, Hao Y, Zhang X, Zhang YM. Protein interaction and microRNA network analysis in osteoarthritis meniscal cells. Genet Mol Res. 2013;12:738–46. doi: 10.4238/2013.March.13.2. [DOI] [PubMed] [Google Scholar]
- 23.Ruberte J, Ayuso E, Navarro M, Carretero A, Nacher V, Haurigot V, George M, Llombart C, Casellas A, Costa C, Bosch A, Bosch F. Increased ocular levels of IGF-1 in transgenic mice lead to diabetes-like eye disease. J Clin Invest. 2004;113:1149–57. doi: 10.1172/JCI19478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simo R, Lecube A, Segura RM, Garcia Arumi J, Hernandez C. Free insulin growth factor-I and vascular endothelial growth factor in the vitreous fluid of patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2002;134:376–82. doi: 10.1016/s0002-9394(02)01538-6. [DOI] [PubMed] [Google Scholar]
- 25.Rietveld I, Ikram MK, Vingerling JR, Hofman A, Pols HA, Lamberts SW, de Jong PT, van Duijn CM, Janssen JA. An igf-I gene polymorphism modifies the risk of diabetic retinopathy. Diabetes. 2006;55:2387–91. doi: 10.2337/db06-0021. [DOI] [PubMed] [Google Scholar]
- 26.Hellstrom A, Perruzzi C, Ju M, Engstrom E, Hard AL, Liu JL, Albertsson-Wikland K, Carlsson B, Niklasson A, Sjodell L, LeRoith D, Senger DR, Smith LE. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc Natl Acad Sci U S A. 2001;98:5804–8. doi: 10.1073/pnas.101113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambooij AC, van Wely KH, Lindenbergh-Kortleve DJ, Kuijpers RW, Kliffen M, Mooy CM. Insulin-like growth factor-I and its receptor in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2003;44:2192–8. doi: 10.1167/iovs.02-0410. [DOI] [PubMed] [Google Scholar]
- 28.Zhu X, Wallman J. Opposite effects of glucagon and insulin on compensation for spectacle lenses in chicks. Invest Ophthalmol Vis Sci. 2009;50:24–36. doi: 10.1167/iovs.08-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie L, Chen H, Overbeek PA, Reneker LW. Elevated insulin signaling disrupts the growth and differentiation pattern of the mouse lens. Mol Vis. 2007;13:397–407. [PMC free article] [PubMed] [Google Scholar]
- 30.Shirke S, Faber SC, Hallem E, Makarenkova HP, Robinson ML, Overbeek PA, Lang RA. Misexpression of IGF-I in the mouse lens expands the transitional zone and perturbs lens polarization. Mech Dev. 2001;101:167–74. doi: 10.1016/s0925-4773(00)00584-0. [DOI] [PubMed] [Google Scholar]
- 31.Cordain L, Eaton SB, Brand Miller J, Lindeberg S, Jensen C. An evolutionary analysis of the aetiology and pathogenesis of juvenile-onset myopia. Acta ophthalmol Scand. 2002;80:125–35. doi: 10.1034/j.1600-0420.2002.800203.x. [DOI] [PubMed] [Google Scholar]
- 32.Bourla DH, Laron Z, Snir M, Lilos P, Weinberger D, Axer-Siegel R. Insulinlike growth factor I affects ocular development: a study of untreated and treated patients with Laron syndrome. Ophthalmology. 2006;113:1197, e1–5. doi: 10.1016/j.ophtha.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 33.Chen JM, Ferec C, Cooper DN. A systematic analysis of disease-associated variants in the 3’ regulatory regions of human protein-coding genes II: the importance of mRNA secondary structure in assessing the functionality of 3’ UTR variants. Hum Genet. 2006;120:301–33. doi: 10.1007/s00439-006-0218-x. [DOI] [PubMed] [Google Scholar]
- 34.Conne B, Stutz A, Vassalli JD. The 3’ untranslated region of messenger RNA: A molecular’ hotspot’ for pathology? Nat Med. 2000;6:637–41. doi: 10.1038/76211. [DOI] [PubMed] [Google Scholar]
- 35.Lee EK, Gorospe M. Minireview: posttranscriptional regulation of the insulin and insulin-like growth factor systems. Endocrinology. 2010;151:1403–8. doi: 10.1210/en.2009-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazumder B, Seshadri V, Fox PL. Translational control by the 3’-UTR: the ends specify the means. Trends Biochem Sci. 2003;28:91–8. doi: 10.1016/S0968-0004(03)00002-1. [DOI] [PubMed] [Google Scholar]
- 37.Tang SM, Rong SS, Young AL, Tam PO, Pang CP, Chen LJ. PAX6 Gene Associated with High Myopia: A Meta-analysis. Optom Vis Sci. 2014;91:419–29. doi: 10.1097/OPX.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida M, Meguro A, Yoshino A, Nomura N, Okada E, Mizuki N. Association study of IGF1 polymorphisms with susceptibility to high myopia in a Japanese population. Clin Ophthalmol. 2013;7:2057–62. doi: 10.2147/OPTH.S52726. [DOI] [PMC free article] [PubMed] [Google Scholar]


