Abstract
Gallium is an iron mimetic which has recently been repurposed as an antibacterial agent due to its capability to disrupt bacterial iron metabolism. In this study, the antibacterial activity of gallium nitrate [Ga(NO3)3] was investigated in complement-free human serum (HS) on 55 Pseudomonas aeruginosa clinical isolates from cystic fibrosis and non-cystic fibrosis patients. The susceptibility of P. aeruginosa to Ga(NO3)3 in HS was dependent on the bacterial ability to acquire iron from serum binding proteins (i.e., transferrin). The extent of serum protein degradation correlated well with P. aeruginosa growth in HS, while pyoverdine production did not. However, pyoverdine-deficient P. aeruginosa strains were unable to grow in HS and overcome iron restriction, albeit capable of releasing proteases. Predigestion of HS with proteinase K promoted the growth of all strains, irrespective of their ability to produce proteases and/or pyoverdine. The MICs of Ga(NO3)3 were higher in HS than in an iron-poor Casamino Acids medium, where proteolysis does not affect iron availability. Coherently, strains displaying high proteolytic activity were less susceptible to Ga(NO3)3 in HS. Our data support a model in which both pyoverdine and proteases affect the response of P. aeruginosa to Ga(NO3)3 in HS. The relatively high Ga(NO3)3 concentration required to inhibit the growth of highly proteolytic P. aeruginosa isolates in HS poses a limitation to the potential of Ga(NO3)3 in the treatment of P. aeruginosa bloodstream infections.
INTRODUCTION
Pseudomonas aeruginosa is a major nosocomial pathogen and the leading cause of chronic lung infection in cystic fibrosis (CF) patients. As for other pathogens, P. aeruginosa is faced with severe iron limitation in the mammalian host, since iron is withheld by the host's iron-binding proteins, such as transferrin (Tf) in serum and lactoferrin in mucosal secretions (1). Iron acquisition is crucial for P. aeruginosa pathogenicity, as inferred by the presence of multiple iron uptake systems, including the production of siderophores (i.e., pyoverdine [PVD] and pyochelin) (2, 3) and several proteases that cleave the host's iron binding proteins, thereby increasing iron availability in vivo (4).
The loss of efficacy of conventional antibiotic therapies for P. aeruginosa infection calls for the development of novel therapeutic options aimed at inhibiting both acute and chronic infections. In recent years, attention has been directed to antimicrobial approaches targeting bacterial iron metabolism, since iron is essential for bacteria to cause infection (5, 6). Due to its chemical similarity with iron, the metal gallium (Ga3+) perturbs several iron-dependent biological processes (7), thereby inhibiting the growth of many bacterial species, including P. aeruginosa (8–12). Therefore, the repurposing of gallium-based drugs for antibacterial therapy has attracted recent interest, as testified by the initiation of a clinical trial aimed at monitoring the effect of Ganite, the FDA-approved Ga(NO3)3 formulation, administered to CF patients chronically infected by P. aeruginosa (13, 14). Since sepsis represents a frequent complication of P. aeruginosa primary infection, with life-threatening consequences and a heavy impact on health care (15), drugs that counteract the bloodstream dissemination of P. aeruginosa are needed.
The aim of the present study was to test the efficacy of Ga(NO3)3 in inhibiting P. aeruginosa growth in complement-free human serum (HS), which provides a vehicle for systemic dissemination of infection. We demonstrate that the growth of P. aeruginosa in HS is dependent on the ability of individual strains to release proteases that cleave serum proteins, including Tf, as well as on the production of the PVD siderophore, ultimately causing iron release from serum proteins and stimulation of bacterial growth. This phenomenon should be taken into account when assessing the antibacterial efficacy of gallium, since the ability of bacteria to retrieve iron from the host would counteract growth inhibition based on iron mimetism (8–11).
MATERIALS AND METHODS
Bacterial strains, media, and chemicals.
The 55 P. aeruginosa clinical isolates used in this study are listed in Table S1 in the supplemental material. The collection included 27 isolates from respiratory secretions of CF patients and 28 from other pathological samples from non-CF patients, also including the reference strains PAO1 (ATCC 15692) and PA14 (16). P. aeruginosa was grown in iron-free Casamino Acids medium (DCAA) (17), Mueller-Hinton broth (MH; Difco), or HS supplemented or not with the appropriate FeCl3 concentration. Control experiments were conducted in HS that was predigested for 18 h at 37°C with 100 μg/ml proteinase K (Promega) and then inactivated for 30 min at 65°C. Complete HS hydrolysis has been qualitatively verified by SDS-PAGE (see Fig. S1 in the supplemental material).
HS was obtained from 125 healthy donors, following illustration, approval, and subscription of informed consent. Complement was inactivated by incubation at 56°C for 30 min, and the bulk of HS was sterilized by filtration as previously described (18) and then stored at +4°C until used. Bulk HS chemistry was as follows: total serum proteins, 80 mg/ml; total iron, 0.70 μg/ml; ferritin, 0.243 μg/ml; Tf, 2.63 mg/ml (64 μM total iron-binding sites); total iron binding capacity, 4.27 mg/ml (20% Tf saturation, equivalent to 51.5 μM unsaturated iron binding sites). Ga(NO3)3 was purchased from Sigma-Aldrich.
Bacterial growth and PVD determinations.
Bacterial growth (optical density at 600 nm [OD600]) was monitored in 96-well microtiter plates using a Wallac 1420 Victor3V multilabel plate reader (PerkinElmer). PVD levels in culture supernatants were measured as the OD405 upon dilution in 100 mM Tris-HCl (pH 8) (19).
PVD purification.
For PVD purification, the methods of Meyer et al. (20) were used with minor modifications. P. aeruginosa ΔpchD was grown in DCAA for 18 h. The culture supernatant was purified by filtration through a Sep-Pak C18 Vac-Cartridge 3cc (Waters). After the polymer packing was solvated with 10 hold-up volumes of 50% (vol/vol) methanol, the cartridge was flushed with 10 hold-up volumes of double-distilled water. The filtered culture supernatant containing PVD was loaded, and the unwanted components were eluted with double-distilled water. PVD was then eluted with a small quantity of 50% (vol/vol) methanol, evaporated to dryness in a desiccator, and dissolved in a small volume of double-distilled water. The PVD concentration was determined by spectrophotometric measurement of the apo form at OD405 (ε = 1.4 × 104 M−1 cm−1) (21).
Ga(NO3)3 susceptibility tests.
Ga(NO3)3 activity was tested in 96-well microtiter plates. Briefly, P. aeruginosa clinical isolates were grown overnight at 37°C in DCAA supplemented with 40 μM FeCl3 and then diluted to an OD600 of 0.01 in 200 μl (final volume) of HS or DCAA containing increasing Ga(NO3)3 concentrations (0 to 128 μM). Microtiter plates were incubated for up to 48 h at 37°C with shaking (120 rpm). Susceptibility assays in MH were performed using a Ga(NO3)3 concentration range of 0 to 512 μM and a standard broth microdilution experimental procedure (22). MICs were defined as the lowest Ga(NO3)3 concentrations that completely inhibited P. aeruginosa growth, as detected by visual inspection of the plates. Each strain was tested in at least two independent experiments.
Quantification of hydrolyzed HS proteins.
Supernatants from P. aeruginosa cultures in HS were analyzed both quantitatively and qualitatively to determine HS protein hydrolysis. For quantitative measurements, supernatants were supplemented with 13% trichloroacetic acid (TCA) in order to precipitate nonhydrolyzed proteins (23). Subsequently, the hydrolyzed (i.e., TCA-nonprecipitable) protein fraction was quantified by the Lowry assay (24). For qualitative measurements, supernatants were diluted 1:16 in gel loading buffer (0.25 M Tris-HCl, 2% SDS, 10% 2-mercaptoethanol, 20% glycerol), heated at 100°C for 10 min, and subjected to electrophoresis in 0.1% SDS–12% polyacrylamide gels (SDS-PAGE). Gels were stained with Coomassie brilliant blue.
Proteolytic activity.
For qualitative determination of proteolytic activity, P. aeruginosa strains were grown in LB, diluted to an OD600 of ∼1 in saline, and spotted (5 μl) onto Luria-Bertani agar (LA) plates containing 2% skim milk (Difco). Proteolytic activity was detected as a clear halo around the bacterial patch after 18 h of growth at 37°C.
Western blot detection of Tf in HS and the PVD biosynthetic enzyme PvdA in P. aeruginosa cells.
HS proteins and P. aeruginosa whole-cell lysates were separated by SDS-PAGE and electrotransferred onto nitrocellulose filters (Hybond C extra; Amersham) using a semidry transfer unit (Hoefer Scientific Instruments) for 1 h at 150 mA. Filters were blocked with 2× TBST (100 mM Tris-HCl [pH 8.0], 1.0 M NaCl, 0.1% Tween 20) containing 2% bovine serum albumin, washed with 2× TBST, incubated with a monoclonal anti-PvdA antibody (25) or an anti-human Tf rabbit polyclonal antibody (Epitomics) diluted 1:100 or 1:3,000 in 2× TBST, respectively. Proteins were detected with a secondary anti-mouse (Promega) or anti-rabbit (Calbiochem) alkaline phosphatase-conjugated antibody. Blots were developed with the 5-bromo-4-chloro-3-indoylphosphate (BCIP) and nitroblue tetrazolium chloride (NBT) reagents for colorimetric detection (Promega).
Detection of quorum sensing (QS) signal molecules.
To determine the production of N-3-oxododecanoyl homoserine lactone (3OC12-HSL) and N-butanoyl-l-homoserine lactone (C4-HSL), 100-μl volumes of overnight cultures of each clinical isolate diluted in LB at OD600 0.01 were cocultured with 100-μl volumes of overnight cultures of reporter strains PA14-R3 for 3OC12-HSL and PAO-JP2 (pKD-rhlA) for C4-HSL (26, 27) diluted in LB at an OD600 of 0.045. Microtiter plates (final volume, 200 μl) were incubated at 37°C with shaking, and cell density (OD600) and bioluminescence (light counts per second) were simultaneously measured after 6 h in a Wallac 1420 Victor3V multilabel plate reader (PerkinElmer). Luminescence values were normalized by the cell density (OD600).
Statistical analyses.
Spearman correlation coefficients (rs) were used as a measure of the relative validity between P. aeruginosa growth in HS and protein hydrolysis or PVD production. Student's t test was used to determine significant differences in Ga(NO3)3 MICs, using the GraphPad Prism software.
RESULTS
P. aeruginosa susceptibility to gallium is lower in HS than in an iron-poor laboratory medium.
The anti-P. aeruginosa activity of Ga(NO3)3 was preliminarily tested in complement-free HS on 55 P. aeruginosa clinical isolates collected from respiratory secretions of CF patients (27 isolates) and from a variety of pathological samples from non-CF patients (28 isolates) (see Table S1 in the supplemental material). All but four isolates, namely, SP18, SP20, SP21, and FM1, grew in HS. P. aeruginosa growth in HS was invariably characterized by a long lag phase of 10 to 18 h and attained stationary phase after 36 to 54 h (data not shown). The MIC of Ga(NO3)3 was determined both in HS and in the iron-free Casamino Acids medium DCAA (17), for comparison. The great majority of isolates (72%) displayed a ≥4-fold increase in Ga(NO3)3 MIC in HS compared with that in DCAA (Fig. 1; see also Table S1 in the supplemental material), indicating a reduced susceptibility of P. aeruginosa to Ga(NO3)3 in HS.
FIG 1.
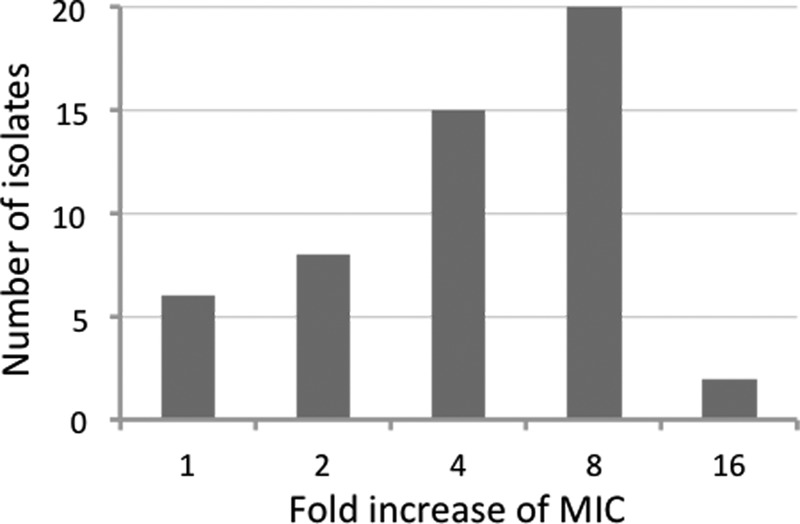
Fold increase of the Ga(NO3)3 MICs in HS, relative to that in DCAA, for 51 P. aeruginosa clinical isolates.
Both proteases and PVD contribute to P. aeruginosa growth in HS.
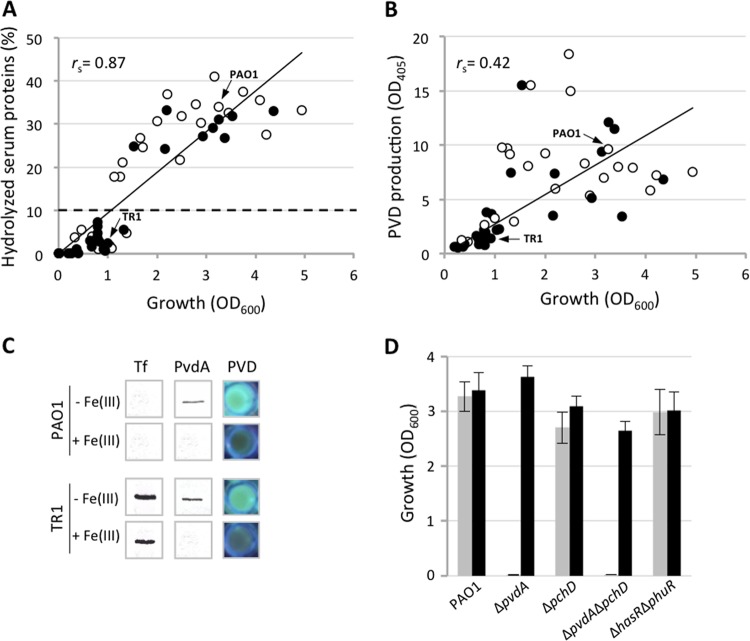
The antibacterial activity of gallium depends on iron availability, being evident only under iron-limiting conditions (8, 11). It was therefore surprising to observe a poor antibacterial activity for Ga(NO3)3 in HS, where the free iron concentration is known to be limiting for bacterial growth (1). This effect could be due to the ability of P. aeruginosa to acquire iron from Tf via siderophores, or proteases, or both (4, 28). Increased iron availability would thus promote growth and ultimately counteract the antibacterial activity of an iron-mimetic inhibitor, like Ga(NO3)3 (8–11). Therefore, the ability of clinical isolates to hydrolyze serum proteins and/or produce PVD was investigated. The amount of hydrolyzed proteins was measured in HS after bacterial growth, as TCA-nonprecipitable material (Fig. 2A), and qualitatively verified by SDS-PAGE analysis of serum proteins relative to the uninoculated control (see Fig. S1 in the supplemental material). The growth of P. aeruginosa clinical isolates in HS correlated well with their ability to hydrolyze serum proteins (rs = 0.87) (Fig. 2A), while poor correlation was observed between PVD production and growth in HS (rs = 0.42) (Fig. 2B). These data suggest that increased availability of nutrients (e.g., free iron and amino acids) and the consequent P. aeruginosa growth promotion in HS primarily depend on protein degradation rather than on PVD production, at least during late growth, when protease activity and PVD production were measured. Accordingly, Tf was completely degraded in spent HS obtained from HS-hydrolyzing strains such as the type strain PAO1, as opposed to weak HS hydrolyzers, such as the CF isolate TR1, regardless of the presence or absence of exogenously added FeCl3 (Fig. 2C and see Fig. S1 in the supplemental material). Interestingly, both PAO1 and TR1 produced PVD, as documented by their fluorescent phenotype in HS and expression of the iron-repressible PvdA enzyme, implicated in an early step of PVD biogenesis (Fig. 2C) (29). This observation confirms that HS is perceived by P. aeruginosa as an iron-poor environment, inductive of PVD production.
FIG 2.
(A) Correlation analysis between hydrolysis of HS proteins and P. aeruginosa growth in HS. Hydrolyzed HS proteins were quantified as TCA-nonprecipitable, Lowry-reactive material and are expressed as percentages relative to total HS proteins. An arbitrary cutoff value of 10% of hydrolyzed serum proteins was set to discriminate between weak and strong HS-hydrolyzing isolates (dotted line). (B) Correlation analysis between pyoverdine (PVD) production (OD405) and P. aeruginosa growth in HS. Black and white circles represent CF and non-CF isolates, respectively. Arrows indicate strains PAO1 and TR1, representative of HS hydrolyzing and nonhydrolyzing isolates, respectively. The Spearman rank correlation coefficients (rs) are indicated. (C) Western blot analysis of Tf and PVD biosynthetic enzyme (PvdA) levels in HS culture supernatants and bacterial cell lysates, respectively. P. aeruginosa strains PAO1 and TR1 were grown in HS supplemented or not with 100 μM FeCl3 [+Fe(III) and −Fe(III), respectively]. PVD production was qualitatively assayed by fluorescence emission under UV light. (D) Growth of P. aeruginosa PAO1 mutants impaired in different iron uptake systems in HS (gray bars) and in HS supplemented with 100 μM FeCl3 (black bars). In all experiments, the mean value of results for at least two independent microtiter plate assays was considered.
Of note, significantly lower HS proteolysis was observed for CF than for non-CF isolates (P < 0.05, chi-square test; data not shown), consistent with the notion that P. aeruginosa isolates from chronic CF infections generally display low protease activity (30).
To gain insight into the contribution of individual iron acquisition systems to P. aeruginosa growth in HS, the reference strain PAO1 and isogenic deletion mutants impaired in heme uptake (ΔhasR ΔphuR [31]) and siderophore-dependent iron uptake (ΔpvdA, impaired in PVD biosynthesis [32]; ΔpchD, impaired in pyochelin biosynthesis [33], or ΔpvdA ΔpchD, defective in production of both siderophores [34]) were cultured in HS supplemented or not with an excess of iron (Fig. 2D). Only the PVD-defective mutants (ΔpvdA and ΔpvdA ΔpchD) showed impaired growth in HS compared with the wild type, whereas addition of FeCl3 (100 μM) rescued the growth of the mutants to wild-type levels (Fig. 2D). Conversely, mutations in heme uptake or pyochelin biosynthesis genes did not affect bacterial growth in HS (Fig. 2D).
PVD is essential for P. aeruginosa growth initiation in HS.
The above-described results prompted us to investigate whether the lack of growth in HS of the clinical isolates SP18, SP20, SP21, and FM1 was due to a defect in PVD production. These strains displayed a PVD-defective phenotype when grown in the iron-poor minimal medium DCAA (Fig. 3) and were able to grow in predigested HS (Fig. 3), where iron is released from Tf and available to bacteria (Fig. 3). Moreover, all isolates except FM1 grew in HS supplemented with 100 μM FeCl3. Notably, FM1 was also the only strain that was impaired in protease production on milk agar plates (Fig. 3). Exogenously added PVD stimulated the growth of the PAO1 ΔpvdA mutant in HS in a dose-dependent manner, even at concentrations far below the maximum PVD levels achievable by P. aeruginosa PAO1 in iron-depleted laboratory cultures (>200 μM) (19). These results support the essential role of PVD for P. aeruginosa growth in HS.
FIG 3.
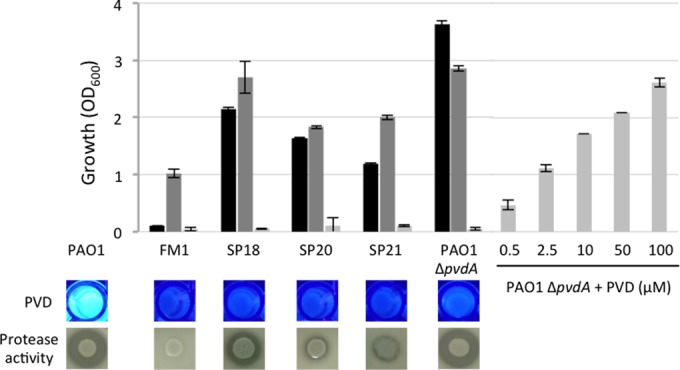
Growth (OD600) of PVD-defective P. aeruginosa strains in HS supplemented with 100 μM FeCl3 (black bars), predigested HS (dark gray bars), and HS (light gray bars). Growth of the PAO1 ΔpvdA mutant in HS was rescued by addition of increasing concentrations of PVD. Growth values (OD600) for the wild-type strain PAO1 were 3.39 ± 0.32, 4.56 ± 0.01, and 3.25 ± 0.27 in HS supplemented with 100 μM FeCl3, predigested HS, and HS, respectively. Qualitative data on PVD production in DCAA and proteolytic activity on skim milk agar plates are shown for each strain.
QS-controlled proteases increase P. aeruginosa growth in HS.
The inability of protease-proficient P. aeruginosa isolates SP18, SP20, and SP21 to grow in HS is in apparent contrast with the good correlation observed between protease production and growth in HS (Fig. 2A). Since the expression of protease in P. aeruginosa is a growth phase-dependent phenomenon regulated by quorum sensing (QS) (35), we hypothesized that bacteria need to reach a certain cell density to allow the protease-dependent growth increment. In this view, PVD might play a prominent role at early growth stages, when protease production is scarce. Then, in response to increased cell density and QS, protease-mediated iron mobilization from Tf would further support growth. To address this point, we determined the growth in HS of a P. aeruginosa PAO1 QS-defective mutant (lasR lasI) (36) and a protease-defective mutant (lasA lasB aprA) (37), both proficient in PVD production (data not shown). Both mutants produced barely detectable levels of proteases and showed a ca. 3-fold reduction in growth yields in HS, relative to that of the wild-type PAO1 (Fig. 4). Notably, both predigestion of HS and supplementation with 100 μM FeCl3 strongly stimulated the growth of QS- and protease-defective mutants (Fig. 4). These findings strongly suggest that QS-controlled protease production provides P. aeruginosa with incremental growth capabilities in HS, likely through proteolytic mobilization of Tf-bound iron. Moreover, a transposon mutant deficient in the iron- and PVD-regulated protease PrpL (38, 39) showed significant growth reduction in HS and decreased hydrolysis of HS proteins (20% and 35%, respectively, relative to the parental strain PAO1; data not shown), corroborating the importance of protease production for P. aeruginosa growth in HS.
FIG 4.
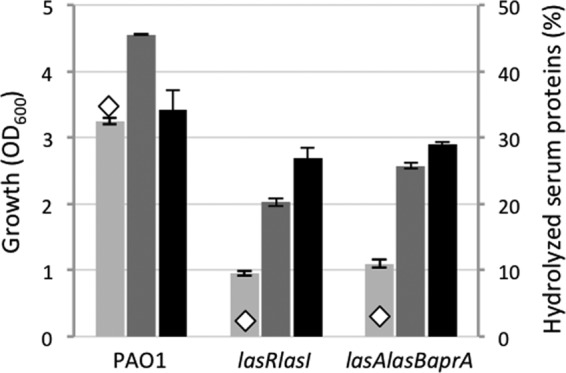
Growth (OD600) and hydrolysis of HS proteins (relative to total HS proteins; diamonds) by P. aeruginosa PAO1 and lasR lasI and lasA lasB aprA mutants, grown for 48 h at 37°C in HS (light gray bars), predigested HS (dark gray bars), and HS supplemented with 100 μM FeCl3 (black bars). Values represent the mean (±standard deviation) of results for three independent assays. Standard deviations are not visible when the bars representing them are smaller than the symbol of the corresponding data point.
HS proteolysis impairs gallium activity.
As anticipated, the growth of different P. aeruginosa clinical isolates in HS correlates well with their ability to produce proteases. Consequently, we hypothesized that proteolytic release of iron from HS proteins could be responsible for the loss of the antibacterial activity of gallium (8–11). After an arbitrary cutoff value of 10% hydrolyzed HS proteins was set (Fig. 2A, dotted line), isolates were split into two groups, defined as strong HS hydrolyzers (27 isolates composed of 9 CF and 18 non-CF isolates) and weak HS hydrolyzers (24 isolates composed of 17 CF and 7 non-CF isolates). Notably, the median Ga(NO3)3 MIC for weak HS hydrolyzers was 16 μM, while strong HS hydrolyzers displayed a median MIC of 64 μM (Fig. 5A). In line with this observation, the Ga(NO3)3 MICs of the protease-defective PAO1 lasR lasI and lasA lasB aprA mutants in HS were reproducibly 2-fold lower than that of their parental strain (32 μM versus 64 μM). The lower inhibitory effect of Ga(NO3)3 on strong HS hydrolyzers than on weak HS hydrolyzers (Fig. 5A) confirms that proteolytic activity, most plausibly Tf degradation, has a major impact on the anti-P. aeruginosa activity of Ga(NO3)3 in HS. This was corroborated by the finding that in predigested HS, the MIC of Ga(NO3)3 was >256 μM for all strains tested (data not shown), and stimulation of bacterial growth was much greater for weak HS hydrolyzers than strong HS hydrolyzers (see Fig. S2 in the supplemental material). Notably, weak and strong HS hydrolyzers displayed similar growth yields and Ga(NO3)3 susceptibilities when grown in the Casamino Acids-based iron-poor medium DCAA, where amino acids are readily available and proteolytic activity is dispensable for bacterial growth (no complexes with iron-binding proteins are present in this medium [17]) (Fig. 5B; see Fig. S3A in the supplemental material). As expected, the effect of Ga(NO3)3 in HS was completely abrogated by the addition of an excess of FeCl3 [Fe(III):Ga(III) molar ratio, 5:1]) (see Fig. S3B in the supplemental material), and MICs of >512 μM were determined for all strains in MH, where the iron concentration is high (14 μM [40]).
FIG 5.
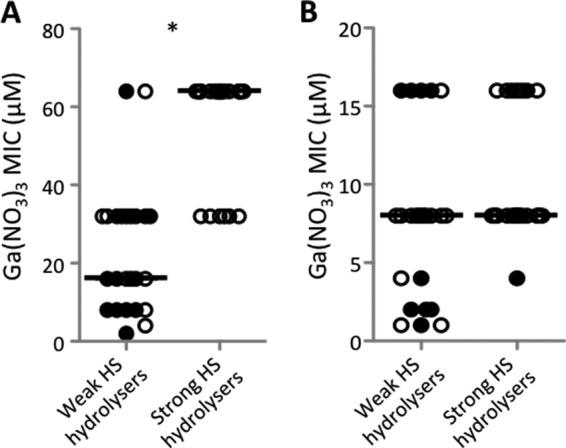
MICs of Ga(NO3)3 for 51 P. aeruginosa clinical isolates grown for 48 h in HS (A) and for 24 h in DCAA (B). PVD-defective isolates were not included. For each isolate, the mean value of results for two independent assays was considered. Isolates were grouped according to their ability to hydrolyze serum proteins (see Fig. 1A). The horizontal line is the median for each group. Black and white circles represent CF and non-CF isolates, respectively. An asterisk indicates a statistically significant difference (P < 0.001; Student's t test) between the two groups.
DISCUSSION
Interfering with P. aeruginosa iron metabolism represents a promising strategy to develop novel therapeutic options, and the repurposing of gallium-based drugs has recently been discussed as an alternative strategy for antibacterial chemotherapy (14).
In this work, we investigated the ability of Ga(NO3)3 to inhibit the growth of P. aeruginosa in HS. We report that P. aeruginosa is less susceptible to gallium-mediated growth inhibition in HS than in an iron-poor laboratory medium (Fig. 1), as opposed to what was recently reported for Acinetobacter baumannii, which is a scarcely proteolytic species (18, 41, 42). This different behavior depends on the ability of P. aeruginosa to acquire iron from Tf, by means of the PVD siderophore and hydrolysis of HS proteins, which ultimately provides sufficient iron to counteract gallium inhibition. Upon testing a representative collection of diverse P. aeruginosa clinical isolates, we report a very good correlation between HS proteolysis and the ability to grow in HS (Fig. 2A), indicating that iron availability in HS no longer limits bacterial growth when proteases are produced.
Interestingly, we found that clinical isolates and laboratory strains defective in PVD production (namely SP18, SP20, SP21, FM1, PAO1 ΔpvdA ΔpchD, and PAO1 ΔpvdA) cannot grow in HS (Fig. 2 and 3), in line with previous findings (28). Since protease production by P. aeruginosa is regulated by QS (35), we also observed that QS- and protease-defective mutants were inhibited by HS, although growth yields were restored to the wild-type levels by predigestion of HS proteins or supplementation of HS with an excess of FeCl3 (Fig. 4). Altogether, these data depict a scenario in which PVD production is essential to ensure initial growth in HS, while protease production, which likely starts during late growth being regulated by QS, allows P. aeruginosa to reach higher cell densities by making essential nutrients (e.g., iron and amino acids) readily available. Therefore, it appears that production of proteases is necessary but per se insufficient to allow P. aeruginosa growth in HS, since protease-proficient strains that are unable to produce PVD do not grow in HS (Fig. 3). In contrast, protease-deficient strains able to produce PVD grow in HS, albeit with reduced yields (Fig. 4). Accordingly, we found that QS-deficient clinical isolates impaired in protease production (about 15% in our strain collection) could grow in HS when they were proficient in PVD production (see Table S1 in the supplemental material).
Given the importance of protease production and iron release from Tf for P. aeruginosa growth in HS, we observed that weak HS hydrolyzers were more susceptible to gallium-mediated growth inhibition in HS than strong HS hydrolyzers (Fig. 5A) and that this difference was abolished when Ga(NO3)3 MICs were measured in DCAA (Fig. 5B) or in the presence of an excess of iron, e.g., in MH and predigested HS.
Overall, our findings highlight the need for appropriate in vitro conditions to assess growth inhibition by an iron mimetic. Compared with conventional laboratory media (e.g., DCAA and MH), HS provides a more realistic environment to assess the antibacterial activity of gallium (11). From a practical viewpoint, the Ga(NO3)3 concentrations required to inhibit the growth of the majority of P. aeruginosa isolates in HS are very close to or even higher than those achievable in plasma for cancer therapy. In fact, the recommended dosing regimens for the treatment of cancer-related hypercalcemia attain a peak serum concentration of Ga(NO3)3 of ca. 28 μM (7, 43). This gallium concentration is insufficient to suppress the growth in HS of highly proteolytic P. aeruginosa strains (Fig. 5A), which predominate among non-CF isolates, thus raising concern about the potential of Ga(NO3)3 in the treatment of systemic P. aeruginosa infection. It should also be considered that because of the high hemolytic activity of P. aeruginosa (44), some iron sources such as heme could be more readily available in whole blood. Thus, it cannot be excluded that iron acquisition strategies other than pyoverdine (e.g., heme uptake) could counteract the iron-mimetic activity of gallium in vivo. On the other hand, the antibacterial activity of the complement and the host's immune system are likely to synergize with gallium in vivo. Indeed, Ga(NO3)3 provided effective protection from P. aeruginosa lung infection in mice (8), and preliminary results point to its efficacy in reducing bacterial load during CF lung infection (13). However, the strain-dependent ability of P. aeruginosa to mobilize iron from the host iron-containing proteins and grow in serum (or other body fluids) should be taken into account in the future development of gallium-based antibacterial therapies.
Supplementary Material
ACKNOWLEDGMENTS
We thank Cornelia Reimmann and Dieter Haas, University of Lausanne, for supplying the lasR lasI strain, Suzanne M. J. Fleiszig, University of California, Berkeley, for supplying the lasA lasB aprA strain, and personnel from Policlinico Umberto I, Sapienza University of Rome, for help in collecting blood samples from healthy donors.
This work was supported by grants from the Italian Cystic Fibrosis Research Foundation (grant FFC#14/2010) and the Italian Ministry of University and Research-PRIN 2012 (prot. 2012WJSX8K) to P.V.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01097-15.
REFERENCES
- 1.Weinberg ED. 2009. Iron availability and infection. Biochim Biophys Acta 1790:600–605. doi: 10.1016/j.bbagen.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Faraldo-Gómez JD, Sansom MS. 2003. Acquisition of siderophores in gram-negative bacteria. Nat Rev Mol Cell Biol 4:105–116. doi: 10.1038/nrm1015. [DOI] [PubMed] [Google Scholar]
- 3.Wandersman C, Delepelaire P. 2004. Bacterial iron sources: from siderophores to hemophores. Annu Rev Microbiol 58:611–647. doi: 10.1146/annurev.micro.58.030603.123811. [DOI] [PubMed] [Google Scholar]
- 4.Döring G, Pfestorf M, Botzenhart K, Abdallah MA. 1988. Impact of proteases on iron uptake of Pseudomonas aeruginosa pyoverdin from transferrin and lactoferrin. Infect Immun 56:291–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foley TL, Simeonov A. 2012. Targeting iron assimilation to develop new antibacterials. Expert Opin Drug Discov 7:831–847. doi: 10.1517/17460441.2012.708335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imperi F, Massai F, Facchini M, Frangipani E, Visaggio D, Leoni L, Bragonzi A, Visca P. 2013. Repurposing the antimycotic drug flucytosine for suppression of Pseudomonas aeruginosa pathogenicity. Proc Natl Acad Sci U S A 110:7458–7463. doi: 10.1073/pnas.1222706110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernstein LR. 1998. Mechanisms of therapeutic activity for gallium. Pharmacol Rev 50:665–682. [PubMed] [Google Scholar]
- 8.Kaneko Y, Thoendel M, Olakanmi O, Britigan BE, Singh PK. 2007. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J Clin Invest 117:877–888. doi: 10.1172/JCI30783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banin E, Lozinski A, Brady KM, Berenshtein E, Butterfield PW, Moshe M, Chevion M, Greenberg EP, Banin E. 2008. The potential of desferrioxamine-gallium as an anti-Pseudomonas therapeutic agent. Proc Natl Acad Sci U S A 105:16761–16766. doi: 10.1073/pnas.0808608105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeLeon K, Balldin F, Watters C, Hamood A, Griswold J, Sreedharan S, Rumbaugh KP. 2009. Gallium maltolate treatment eradicates Pseudomonas aeruginosa infection in thermally injured mice. Antimicrob Agents Chemother 53:1331–1337. doi: 10.1128/AAC.01330-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minandri F, Bonchi C, Frangipani E, Imperi F, Visca P. 2014. Promises and failures of gallium as an antibacterial agent. Future Microbiol 9:379–397. doi: 10.2217/fmb.14.3. [DOI] [PubMed] [Google Scholar]
- 12.Rangel-Vega A, Bernstein LR, Mandujano-Tinoco EA, García-Contreras SJ, García-Contreras R. 2015. Drug repurposing as an alternative for the treatment of recalcitrant bacterial infections. Front Microbiol 6:282. doi: 10.3389/fmicb.2015.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goss CH, Hornick DB, Aitken ML, Caldwell E, Wilhelm E, Wolfstone A, Teresi M, Singh PK. 2012. Phase 1 pharmacokinetic and safety study of intravenous GaniteTM (gallium nitrate) in CF. Pediatric Pulmunol 47(Suppl 35):303. [Google Scholar]
- 14.Bonchi C, Imperi F, Minandri F, Visca P, Frangipani E. 2014. Repurposing of gallium-based drugs for antibacterial therapy. Biofactors 40:303–312. doi: 10.1002/biof.1159. [DOI] [PubMed] [Google Scholar]
- 15.Tam VH, Rogers CA, Chang KT, Weston JS, Caeiro JP, Garey KW. 2010. Impact of multidrug-resistant Pseudomonas aeruginosa bacteremia on patient outcomes. Antimicrob Agents Chemother 54:3717–3722. doi: 10.1128/AAC.00207-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rahme LG, Stevens EJ, Wolfort SF, Shao J, Tompkins RG, Ausubel FM. 1995. Common virulence factors for bacterial pathogenicity in plants and animals. Science 30:1899–1902. [DOI] [PubMed] [Google Scholar]
- 17.Visca P, Ciervo A, Sanfilippo V, Orsi N. 1993. Iron-regulated salicylate synthesis by Pseudomonas spp. J Gen Microbiol 139:1995–2001. doi: 10.1099/00221287-139-9-1995. [DOI] [PubMed] [Google Scholar]
- 18.Antunes LC, Imperi F, Minandri F, Visca P. 2012. In vitro and in vivo antimicrobial activities of gallium nitrate against multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 56:5961–5970. doi: 10.1128/AAC.01519-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imperi F, Tiburzi F, Visca P. 2009. Molecular basis of pyoverdine siderophore recycling in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 106:20440–20445. doi: 10.1073/pnas.0908760106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer JM, Stintzi A, De Vos D, Cornelis P, Tappe R, Taraz K, Budzikiewicz H. 1997. Use of siderophores to type pseudomonads: the three Pseudomonas aeruginosa pyoverdine systems. Microbiology 143:35–43. doi: 10.1099/00221287-143-1-35. [DOI] [PubMed] [Google Scholar]
- 21.James HE, Beare PA, Martin LW, Lamont IL. 2005. Mutational analysis of a bifunctional ferrisiderophore receptor and signal-transducing protein from Pseudomonas aeruginosa. J Bacteriol 187:4514–4520. doi: 10.1128/JB.187.13.4514-4520.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. 2010. Performance standards for antimicrobial susceptibility testing; 20th informational supplement, M100-S20. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 23.Haag M, Meyer HE, Schollmeyer P, Hörl WH. 1987. Characterization of non-TCA-precipitable ‘Lowry protein’ in plasma of patients with acute renal failure. Clin Chim Acta 170:181–186. doi: 10.1016/0009-8981(87)90126-4. [DOI] [PubMed] [Google Scholar]
- 24.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275. [PubMed] [Google Scholar]
- 25.Putignani L, Ambrosi C, Ascenzi P, Visca P. 2004. Expression of l-ornithine Ndelta-oxygenase (PvdA) in fluorescent Pseudomonas species: an immunochemical and in silico study. Biochem Biophys Res Commun 313:245–257. doi: 10.1016/j.bbrc.2003.11.116. [DOI] [PubMed] [Google Scholar]
- 26.Duan K, Surette MG. 2007. Environmental regulation of Pseudomonas aeruginosa PAO1 Las and Rhl quorum-sensing systems. J Bacteriol 189:4827–4836. doi: 10.1128/JB.00043-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massai F, Imperi F, Quattrucci S, Zennaro E, Visca P, Leoni L. 2011. A multitask biosensor for micro-volumetric detection of N-3-oxo-dodecanoyl-homoserine lactone quorum sensing signal. Biosens Bioelectron 26:3444–3449. doi: 10.1016/j.bios.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Ankenbauer R, Sriyosachati S, Cox CD. 1985. Effects of siderophores on the growth of Pseudomonas aeruginosa in human serum and transferrin. Infect Immun 49:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Visca P, Ciervo A, Orsi N. 1994. Cloning and nucleotide sequence of the pvdA gene encoding the pyoverdin biosynthetic enzyme l-ornithine N5-oxygenase in Pseudomonas aeruginosa. J Bacteriol 176:1128–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woods DE, Schaffer MS, Rabin HR, Campbell GD, Sokol PA. 1986. Phenotypic comparison of Pseudomonas aeruginosa strains isolated from a variety of clinical sites. J Clin Microbiol 24:260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minandri F, Frangipani E, Facchini M, Imperi F, Bonchi C, Bragonzi A, Visca P. 2013. Abstr 14th Int Conf Pseudomonas, abstr 167. [Google Scholar]
- 32.Imperi F, Putignani L, Tiburzi F, Ambrosi C, Cipollone R, Ascenzi P, Visca P. 2008. Membrane-association determinants of the omega-amino acid monooxygenase PvdA, a pyoverdine biosynthetic enzyme from Pseudomonas aeruginosa. Microbiology 154:2804–2813. doi: 10.1099/mic.0.2008/018804-0. [DOI] [PubMed] [Google Scholar]
- 33.Frangipani E, Bonchi C, Minandri F, Imperi F, Visca P. 2014. Pyochelin potentiates the inhibitory activity of gallium on Pseudomonas aeruginosa. Antimicrob Agents Chemother 58:5572–5575. doi: 10.1128/AAC.03154-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Visca P, Bonchi C, Minandri F, Frangipani E, Imperi F. 2013. The dual personality of iron chelators: growth inhibitors or promoters? Antimicrob Agents Chemother 57:2432–2433. doi: 10.1128/AAC.02529-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jimenez PN, Koch G, Thompson JA, Xavier KB, Cool RH, Quax WJ. 2012. The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol Mol Biol Rev 76:46–65. doi: 10.1128/MMBR.05007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pessi G, Haas D. 2000. Transcriptional control of the hydrogen cyanide biosynthetic genes hcnABC by the anaerobic regulator ANR and the quorum-sensing regulators LasR and RhlR in Pseudomonas aeruginosa. J Bacteriol 182:6940–6949. doi: 10.1128/JB.182.24.6940-6949.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cowell BA, Twining SS, Hobden JA, Kwong MS, Fleiszig SM. 2003. Mutation of lasA and lasB reduces Pseudomonas aeruginosa invasion of epithelial cells. Microbiology 143:2291–2299. [DOI] [PubMed] [Google Scholar]
- 38.Jacobs MA, Alwood A, Thaipisuttikul I, Spencer D, Haugen E, Ernst S, Will O, Kaul R, Raymond C, Levy R, Chun-Rong L, Guenthner D, Bovee D, Olson MV, Manoil C. 2003. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 100:14339–14344. doi: 10.1073/pnas.2036282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilderman PJ, Vasil AI, Johnson Z, Wilson MJ, Cunliffe HE, Lamont IL, Vasil ML. 2001. Characterization of an endoprotease (PrpL) encoded by a PvdS-regulated gene in Pseudomonas aeruginosa. Infect Immun 69:5385–5394. doi: 10.1128/IAI.69.9.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Girardello R, Bispo PJ, Yamanaka TM, Gales AC. 2012. Cation concentration variability of four distinct Mueller-Hinton agar brands influences polymyxin B susceptibility results. J Clin Microbiol 50:2414–2418. doi: 10.1128/JCM.06686-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antunes LC, Imperi F, Carattoli A, Visca P. 2011. Deciphering the multifactorial nature of Acinetobacter baumannii pathogenicity. PLoS One 6:e22674. doi: 10.1371/journal.pone.0022674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Léséleuc L, Harris G, KuoLee R, Chen W. 2012. In vitro and in vivo biological activities of iron chelators and gallium nitrate against Acinetobacter baumannii. Antimicrob Agents Chemother 56:5397–5400. doi: 10.1128/AAC.00778-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collery P, Keppler B, Madoulet C, Desoize B. 2002. Gallium in cancer treatment. Crit Rev Oncol Hematol 42:283–296. doi: 10.1016/S1040-8428(01)00225-6. [DOI] [PubMed] [Google Scholar]
- 44.Kurioka S, Liu PV. 1967. Effect of the hemolysin of Pseudomonas aeruginosa on phosphatides and on phospholipase c activity. J Bacteriol 93:670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.