Abstract
Azoles are commonly used as antifungal drugs or pesticides to control fungal infections in medicine and agriculture. Fungi adapt to azole stress by rapidly activating the transcription of a number of genes, and transcriptional increases in some azole-responsive genes can elevate azole resistance. The regulatory mechanisms that control transcriptional responses to azole stress in filamentous fungi are not well understood. This study identified a bZIP transcription factor, ADS-4 (antifungal drug sensitive-4), as a new regulator of adaptive responses and resistance to antifungal azoles. Transcription of ads-4 in Neurospora crassa cells increased when they were subjected to ketoconazole treatment, whereas the deletion of ads-4 resulted in hypersensitivity to ketoconazole and fluconazole. In contrast, the overexpression of ads-4 increased resistance to fluconazole and ketoconazole in N. crassa. Transcriptome sequencing (RNA-seq) analysis, followed by quantitative reverse transcription (qRT)-PCR confirmation, showed that ADS-4 positively regulated the transcriptional responses of at least six genes to ketoconazole stress in N. crassa. The gene products of four ADS-4-regulated genes are known contributors to azole resistance, including the major efflux pump CDR4 (Pdr5p ortholog), an ABC multidrug transporter (NcAbcB), sterol C-22 desaturase (ERG5), and a lipid transporter (NcRTA2) that is involved in calcineurin-mediated azole resistance. Deletion of the ads-4-homologous gene Afads-4 in Aspergillus fumigatus caused hypersensitivity to itraconazole and ketoconazole, which suggested that ADS-4 is a functionally conserved regulator of adaptive responses to azoles. This study provides important information on a new azole resistance factor that could be targeted by a new range of antifungal pesticides and drugs.
INTRODUCTION
Filamentous fungi cause over 70% of plant diseases, and some can cause deadly infections in humans (1–5). Azoles (e.g., itraconazole [ITA], fluconazole [FLC], and ketoconazole [KTC]) are the most commonly used antifungal drugs in medicine, and some azoles, such as triadimenol and propiconazole, are also used to control fungal diseases in plants (6). Antifungal azoles inhibit 14α-methyl sterol demethylase (encoded by ERG11), a key enzyme involved in fungal ergosterol biosynthesis. This leads to changes in membrane consistency (7, 8). In addition to blocking ergosterol production, the inhibition of ERG11 by azoles results in the accumulation of toxic 14α-methylated sterol intermediates (9, 10).
Fungi can adapt to azole stress by rapidly increasing the expression of a number of genes. Increased expression of some azole-responsive genes, such as genes encoding azole efflux pumps and genes involved in ergosterol biosynthesis, can increase resistance to azoles (11, 12). Previous studies in Saccharomyces cerevisiae and Candida albicans identified a number of regulatory genes that mediate azole responses. The transcription factors Pdr1p and Pdr3p in S. cerevisiae and their homologs in C. albicans regulate azole responses by controlling multidrug efflux pump genes (13–15). However, filamentous fungi do not have such homologs. The transcription factor Upc2p in C. albicans and its ortholog Ecm22p in S. cerevisiae regulate azole responses by upregulating ergosterol synthesis genes and multidrug efflux pump genes (16–19). Although Upc2p homologs are present in filamentous fungi, a deletion mutant (FGSC 11076) of the Neurospora crassa Upc2p homolog gene (NCU03686) was not hypersensitive to ketoconazole (20). To date, only one transcription factor, AP-1, is known to play a role in the azole responses of both yeasts and filamentous fungi (21–23). It is possible that filamentous fungi have azole response regulation mechanisms that are different from those found in yeasts.
Neurospora crassa, which has knockout mutants for over 6,000 genes, was recently used as a model to study how filamentous fungi respond to azole stress. This led to the discovery of a number of new azole-responsive genes that play important roles in azole resistance (20, 24, 25). One of the newly identified genes encodes a transcription factor (CCG-8) in N. crassa that regulates the transcriptional responses to ketoconazole of 78 genes, including the azole-target-coding gene erg11 and the Pdr5p-like ABC-transporter-coding gene cdr4. Its homolog in another filamentous fungus, Fusarium verticillioides, has a similar role (20).
Using N. crassa as a model, this study identified another new transcription factor, ADS-4, which is essential for normal azole resistance in both N. crassa and Aspergillus fumigatus. ADS-4 regulated the transcriptional responses to ketoconazole of at least six genes.
MATERIALS AND METHODS
Strain cultivation.
The Neurospora crassa wild-type (WT) strain and knockout mutants used in this study were purchased from the Fungal Genetics Stock Center (FGSC) (Kansas City, MO) and are listed in Table S1 in the supplemental material. Vogel's medium (26), supplemented with 2% (wt/vol) sucrose for slants or 2% glucose for liquid and plate media, were used to culture N. crassa. All of the N. crassa strains were cultured at 28°C.
Aspergillus fumigatus wild-type strain YJ407 and the CEA17 strain (ΔpyrG89) were grown in complete medium (CM). All Aspergillus fumigatus cultures were grown at 37°C.
Drug sensitivity tests.
Ketoconazole, itraconazole, and fluconazole were dissolved in dimethyl sulfoxide (DMSO) and then aseptically added to autoclaved medium before it was poured into agar plates. The final DMSO concentration was below 0.25% (vol/vol). The plates (diameter, 9 cm) were inoculated with 2 μl of conidial suspension, with or without antifungal drugs, and incubated in the dark.
Complementation of ads-4 deletion mutant.
To complement the ads-4-knockout mutant, the ads-4-knockout mutant (FGSC 11386) was crossed with mutant FGSC 6103 (his-3; type A), which cannot synthesize histidine, to generate the ads-4-knockout strain named NCW 1, which has a his-3 background. To create the complementary plasmid, the whole length of the ads-4 coding sequence (1,260 bp), with a 1,948-bp upstream region and a 1,976-bp downstream region, was amplified using primers ads4-F and ads4-R (Table 1), to create a 5,184-bp complementation fragment. The PCR product was inserted into the pBM61 vector (27) at the SmaI site to form the complementary plasmid pBM61-ads4. The PBM61-ads4 construct was transformed into the ads-4 deletion mutant with a his-3 background (NCW 1) by using a previously reported method (28). Transformants were screened on Vogel's medium without histidine and were verified by PCR using primers ads4v-F and ads4v-R (Table 1).
TABLE 1.
Gene-specific primer pairs
| Process and gene | Primer name | Nucleotide sequence (5′ to 3′) |
|---|---|---|
| Complementation | ||
| ads-4 | ads4-F | TGGTTACGTGTTCTGCGTCAGTATC |
| ads4-R | TCAGTCCCTATAACGAACACCTCAC | |
| N. crassa transformant and double-mutant screening | ||
| ads-4 | ads4v-F | CTTTCCAACCCAACCATC |
| ads4v-R | GTCCGCTATACTGCTGTCC | |
| Construction of ads-4 overexpression strain | ||
| cfp | cfp-F | CGACCTCAAACCTCAACAAAC |
| cfp-R | ATATCAGATCCGATGCTCTCTCTTTAGGGTGAG | |
| ads-4 | hismycads4-F | GAGAGAGCATCGGATCTGATATCATCGATTTAAAGC |
| hismycads4-R | TTTGCCCTCGCGAGCACTAACGTGGAAAATC | |
| trpc | trpc-F | TTAGTGCTCGCGAGGGCAAAGGAATAGAGTAG |
| trpc-R | AAGCAGCCCAGTAGTAGGTTGA | |
| Construction of Afu1g16460-knockout strain | ||
| Afu1g16460 upstream | Afu1g16460U-XhoI-F | CCGCTCGAGGCGGCCGCGATGTCCGAAAAAAGGCAGAGG |
| Afu1g16460U-ClaI-SmaI-R | CCATCGATCCCGGGGATGTGCGTATGCACGAGGTTC | |
| Afu1g16460 downstream | Afu1g16460D-ClaI-F | CCATCGATCCCCCCTCTTTCCTTTGTTCATG |
| Afu1g16460D-BamHI-R | CGGGATCCCGTAGGTTTTCCCTCGTCTGAA | |
| A. fumigatus transformant screening | ||
| Afu1g16460 | Afu1g16460V-F | GGCTTCATTGGTCCGTGC |
| Afu1g16460V-R | AAGCCATTCTCGCAAGCC | |
| qRT-PCR analysis | ||
| ads-4 | Qncu08744-F | TCGAACTCTTGGGACTGCCAGAAA |
| Qncu08744-R | AAGGCATTCCGATTGAGTCCGCTA | |
| Ncmmt2 | Qncu07879-F | ACACCGTTTCTGCCCTCT |
| Qncu07879-R | CCGCCAGCTCTATATCCA | |
| erg5 | Qncu05278-F | TTTCACCTTCCTCTTCGCTTCCCA |
| Qncu05278-R | TCATCGACTCAAGCTGCTCCATGT | |
| cdr4 | Qncu05591-F | ACGCTTTGGAAATGGATGGTGACG |
| Qncu05591-R | ATGAACAAGGCGACGGAAATGCAG | |
| Ncrta2 | Qncu05209-F | TGAGCAAGATCATTGTCCTAAT |
| Qncu05209-R | AAATACCACAGCCATCTCAC | |
| NcabcB | Qncu03776-F | CGGTGATGCAGGAAGTTATC |
| Qncu03776-R | CTTCAACACCGCCACTAAA | |
| Ncmnn4 | Qncu03213-F | GGTGGTGGAACAAGCAGAT |
| Qncu03213-R | GGTCTCCGTTGGAGAAGTTAG |
Overexpression of ads-4.
The cfp promoter was used to overexpress ads-4 (29). The cfp promoter (888 bp) was amplified from the wild-type N. crassa genome by PCR using primers cfp-F and cfp-R (Table 1). The ads-4 coding region, tagged with 5×cMyc-6×His, was amplified from the Qa5myc6his-ads4 vector (constructed by inserting the ads-4 coding sequence into the Qa5myc6his plasmid) by PCR using the primers hismycads4-F and hismycads4-R (Table 1). The trpc terminator (997 bp) was amplified from the pCSN43 vector using primers trpc-F and trpc-R (Table 1). The three fragments were purified and fused together by fusion PCR. The fused fragment (3,423 bp) was ligated to the pCSN43 vector, which produced the ads-4 overexpression vector pCSN43-ads4OE. The pCSN43-ads4OE vector was transformed into the N. crassa wild-type strain (FGSC 4200) by protoplast transformation, as reported previously (20). The transformants were screened on Vogel's medium with hygromycin and were verified by PCR using primers cfp-F and trpc-R (Table 1).
RNA extraction and transcriptional analysis by qRT-PCR.
RNA extraction and cDNA synthesis were performed according to previously described methods (24). Quantitative reverse transcription (qRT)-PCR was carried out using the iQ5 multicolor real-time PCR detection system (Bio-Rad, Hercules, CA) with SYBR green detection (SYBR PrimeScript RT-PCR kit; TaKaRa Biotechnology Co., Ltd.), according to the manufacturer's instructions. Each cDNA sample was analyzed in triplicate, and the average threshold cycle was calculated. Relative expression levels were calculated using the 2−ΔΔCt method (30). The results were normalized to the β-tubulin expression level. Gene-specific primers are shown in Table 1.
Transcript profile analysis.
Briefly, conidia from the wild-type strain and the ads-4 deletion mutant were added to 20 ml Vogel's liquid medium in a plate (diameter, 9 cm) and incubated at 28°C in the dark for 24 h until a mycelial mat formed on the surface of the liquid medium. The mycelial mat was then cut into small pieces (diameter, 10 mm) and transferred to Vogel's liquid medium (two pieces in 100 ml) in 150-ml flasks. The cultures were incubated at 28°C for 12 h with shaking. KTC, at a final concentration of 2.5 μg/ml, was then added to the medium. After 24 h of incubation, total RNA was extracted and subjected to transcriptome sequencing (RNA-seq) analysis. Genes with transcriptional ratios of more than 2.0 or less than 0.5 in two samples were considered to be differentially expressed.
Knockout of ads-4-homologous gene in Aspergillus fumigatus.
A deletion cassette containing the pyrG gene as the selectable marker was constructed to knock out Afu1g16460 in Aspergillus fumigatus. PCR primers Afu1g16460U-XhoI-F and Afu1g16460U-ClaI-SmaI-R (Table 1) were designed to amplify the upstream sequence (1,462 bp) of Afu1g16460 before the ATG start codon, and Afu1g16460D-ClaI-F and Afu1g16460D-BamHI-R (Table 1) were used to amplify the downstream flanking sequence (821 bp) of Afu1g16460 after the stop codon. The upstream and downstream noncoding fragments were digested with XhoI/ClaI and ClaI/BamHI, respectively, and then cloned into the pBlueScript (pSK) vector to form the pSK-UD vector. The pyrG gene selectable marker, which was released from pCDA14 (31) by HpaI digestion, was inserted into pSK-UD at the SmaI site between the upstream and downstream Afu1g16460 sequences. The deletion vectors were linearized by digestion with NotI. They were then transformed into A. fumigatus CEA17 protoplasts and plated under uridine and uracil autotrophy selection. The deletion mutants were confirmed using PCR analysis with primers Afu1g16460V-F and Afu1g16460V-R (Table 1), to amplify the coding sequence for Afu1g16460 (32).
RESULTS
ADS-4 responds transcriptionally to ketoconazole in Neurospora crassa.
NCU08744 is a transcription factor composed of 430 amino acids with a bZIP DNA-binding domain. NCU08744 was named ADS-4 (antifungal drug sensitive-4) according to the azole-hypersensitive phenotype of its deletion mutant. RNA-seq analysis showed that KTC (2.5 μg/ml) treatment (24 h) resulted in a 6.7-fold increase in ads-4 transcript levels (see Data Set S1 in the supplemental material). The ads-4 transcriptional increase after treatment with KTC was confirmed by a time course experiment in which ads-4 transcript levels were measured after 2 h, 6 h, 12 h, 18 h, 24 h, and 30 h of KTC treatment. Figure 1A shows that the ads-4 transcript levels increased 0.68-fold after 2 h of KTC treatment and continued to increase with time. Thus, ADS-4 is a transcriptional factor that responds to KTC stress.
FIG 1.
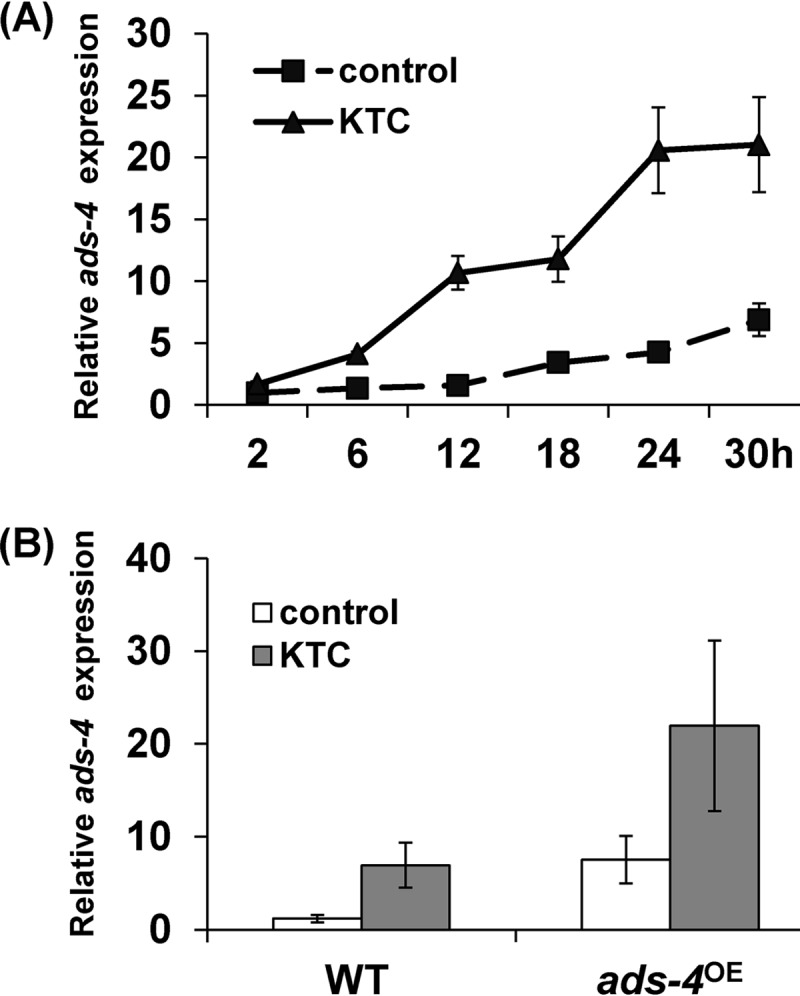
Effects of ketoconazole treatment and ads-4 overexpression on the transcription of ads-4. (A) ads-4 transcriptional responses to ketoconazole (KTC) in N. crassa WT cells. The WT cells were inoculated and cultured for 12 h at 28°C with shaking (200 rpm), and then KTC was added to reach a final concentration of 2.5 μg/ml. The control medium did not contain KTC. Mycelia were harvested at 2 h, 6 h, 12 h, 18 h, 24 h, and 30 h, and the transcription of ads-4 was detected by qRT-PCR. The ads-4 transcript levels are shown in terms of relative quantity (the ads-4 transcript level at 2 h in WT cells without KTC treatment was defined as 1). The ads-4 transcript levels in WT cells with or without KTC are shown. (B) Detection of ads-4 transcripts in the strain with ads-4 overexpression (ads-4OE) and the WT strain. Expression of ads-4 was detected by qRT-PCR 24 h after treatment with 2.5 μg/ml KTC. The control medium did not contain KTC.
Deletion of ads-4 causes hypersensitivity to azoles in N. crassa.
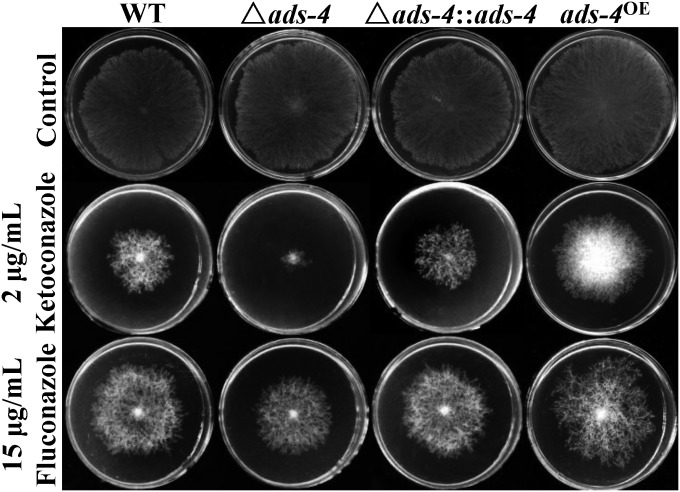
To test whether ADS-4 makes a potential contribution to azole resistance, the sensitivities of an ads-4 deletion mutant (FGSC 11386) and the WT strain (FGSC 4200) to two azole drugs were analyzed comparatively. When grown on medium without drugs, the ads-4 mutant had a growth rate similar to that of the WT strain. When grown on medium containing KTC or fluconazole (FLC), however, the ads-4 mutant grew significantly more slowly than the WT strain (Fig. 2). The inhibition rates for the WT strain treated with KTC and FLC were 72.29% and 61.25%, respectively, while the inhibition rates for the mutant strain were 90.33% and 69.96%, respectively. Statistical analysis indicated that ads-4 deletion significantly increased the sensitivity to KTC and FLC (Table 2).
FIG 2.
Role of ADS-4 under azole stress. Two-microliter aliquots of conidial suspensions (1 × 106 conidia/ml) of the N. crassa WT strain, the ads-4-knockout mutant (Δads-4), the ads-4-complemented strain (Δads-4::ads-4), and the ads-4 overexpression strain (ads-4OE) were spotted onto the center of Vogel's plates (diameter, 9 cm) with or without antifungal drugs. The final concentrations of ketoconazole and fluconazole were 2 μg/ml and 15 μg/ml, respectively. Plates were incubated at 28°C in the dark. Photographs were captured at 24 h for the control plates and at 48 h for the ketoconazole- and fluconazole-treated plates.
TABLE 2.
Inhibition rates with azoles for Neurospora crassa WT, Δads-4, Δads-4::ads-4, and ads-4OE strains
| Drug | Inhibition rate (%)a |
|||
|---|---|---|---|---|
| WT | Δads-4 | Δads-4::ads-4 | ads-4OE | |
| KTC | 72.29 ± 0.02 | 90.33 ± 0.02b | 72.92 ± 0.02 | 67.93 ± 0.02c |
| FLC | 61.25 ± 0.01 | 69.96 ± 0.01b | 62.63 ± 0.01 | 58.51 ± 0.01c |
Tested strains are the wild-type (WT) strain, the ads-4-knockout strain (Δads-4), the ads-4-complemented strain (Δads-4::ads-4), and the ads-4 overexpression strain (ads-4OE). Radii of colonies were measured after 24 h of inoculation for nontreated plates and 48 h for drug-treated plates, and growth rates of strains were calculated by the equation colony radius (mm)/incubation time (h). The means of the relative inhibition rates for each fungicide were calculated with the following equation: (growth rate on plates without fungicide − growth rate on plates with fungicide)/growth rate on plates without fungicide. Differences between the mutants and the WT strain were statistically analyzed with the Waller-Duncan test.
Significantly different from the WT strain, P < 0.01.
Significantly different from the WT strain, P < 0.05.
To confirm the role of ads-4 in azole resistance, the NCW 2 strain (Δads-4::ads-4), with complementation of the ads-4 deletion, was created. As shown in Fig. 2 and Table 2, the complemented strain exhibited wild-type sensitivities to KTC and FLC, which indicated that ADS-4 is required if normal resistance to azoles is to be maintained.
Overexpression of ads-4 increases azole resistance in N. crassa.
To identify the role of ads-4 during azole resistance, an ads-4 overexpression strain, ads-4OE, was generated, in which ADS-4 was tagged with 5×cMyc-6×His at its N terminus and was driven by a cfp promoter (29). The ads-4 transcriptional levels in the ads-4OE strain were 5.25-fold higher than those in the WT strain in liquid medium without azoles (Fig. 1B). When cells were treated with KTC (2.5 μg/ml) for 24 h, the ads-4 transcriptional levels in the ads-4OE strain were 17.26-fold higher than those in the WT strain (Fig. 1B). On plates without azoles, the ads-4OE strain grew at a rate similar to that of the WT strain. On plates with KTC or FLC, the ads-4OE strain grew faster than the WT strain (Fig. 2). The inhibition rates for the WT strain treated with KTC and FLC were 72.29% and 61.25%, respectively, while the inhibition rates for the ads-4oe strain were 67.93%, and 58.51%, respectively. The Waller-Duncan t test showed that the ads-4oe inhibition rates with these azoles were significantly lower than the WT rates (Table 2). These results indicate that the ads-4 transcriptional increase during azole stress improves azole resistance.
Deletion of ADS-4 affects genome-wide transcriptional responses to ketoconazole in N. crassa.
The ads-4 deletion strain and the WT genome-wide transcriptional responses to KTC were comparatively analyzed by RNA-seq in order to ascertain whether ADS-4 mediates transcriptional responses to azoles. The RNA-seq data showed that, with KTC treatment, 488 genes were upregulated in the WT strain, while only 398 genes were upregulated in the ads-4 deletion mutant. KTC treatment also caused downregulation of 427 genes in the WT strain, whereas only 342 genes were downregulated by KTC treatment in the ads-4 deletion mutant (see Data Set S1 in the supplemental material).
ADS-4 regulates transcriptional responses by the sterol C-22 desaturase ERG5 to ketoconazole.
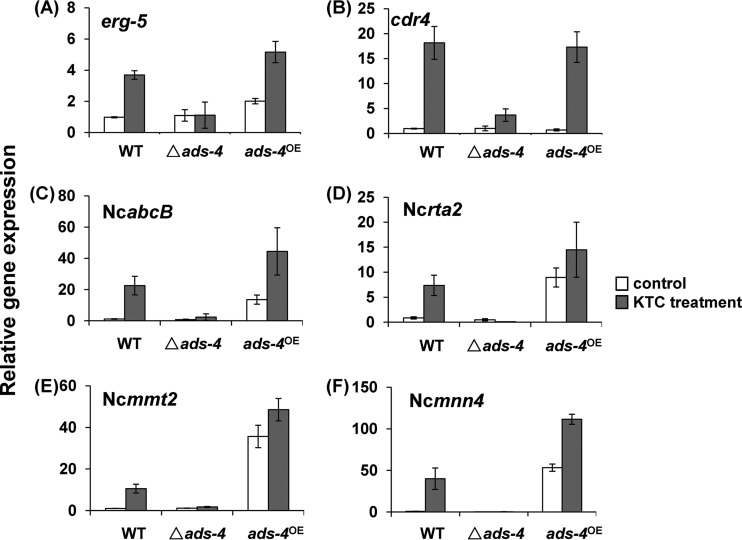
ERG5 is a sterol C-22 desaturase that plays a role in ergosterol biosynthesis. Deletion of erg5 in N. crassa and Fusarium verticillioides caused hypersensitivity to azoles (25). The RNA-seq data showed that the erg5 transcript level in KTC-treated WT cells was 2.1-fold higher than that in non-KTC-treated WT cells. In the ads-4-knockout mutant, KTC treatment did not significantly change the erg5 transcript level (see Data Set S1 in the supplemental material). Consistent with the RNA-seq data, qRT-PCR analysis showed that the expression of erg5 in KTC-treated WT cells was 3.76-fold greater than that in non-KTC-treated WT cells. In contrast, no significant erg5 transcriptional increase was detected in the ads-4-knockout mutant after KTC treatment (Fig. 3A).
FIG 3.
Differential expression of genes in the WT strain, the ads-4 deletion mutant (Δads-4), and the ads-4 overexpression strain (ads-4OE). Strains were grown in Vogel's liquid medium at 28°C, with shaking at 180 rpm, for 12 h before treatment. Ketoconazole (KTC) was then added to the medium to reach a final concentration of 2.5 μg/ml. After incubation for 24 h, transcripts of erg5, cdr4, NcabcB, Ncrta2, Ncmmt2, and Ncmnn4 were analyzed by qRT-PCR. Values shown are the means of three independent replicates. Standard deviations are indicated by error bars.
In the strain with ads-4 overexpression, ads-4OE, the erg5 transcript level was 2.06-fold higher than that the WT strain in liquid medium without KTC. With KTC treatment, the erg5 transcript level in the ads-4OE strain was also significantly higher than that in the WT strain (Fig. 3A).
These results show that ADS-4 is a transcription factor that regulates the expression of erg5 and is essential for the erg5 transcriptional response to KTC stress. The ads-4 transcriptional increase during KTC stress should promote the expression of erg5. Promotion of erg5 expression is probably a mechanism by which ADS-4 regulates the adaptation to azole stress.
ADS-4 activates transcriptional responses by the azole efflux pump CDR4 to ketoconazole.
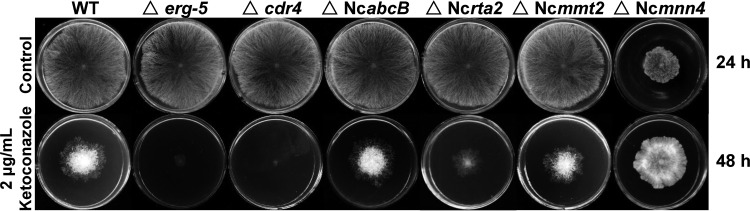
CDR4, the ortholog of Cdr1p in C. albicans, is the only Pdr5p-like ABC transporter that has a detectable role in azole resistance in N. crassa (24). Deletion of cdr4 significantly increases the sensitivity to azoles (24) (Fig. 4). The RNA-seq data showed that the cdr4 transcript level increased 68.5-fold in the WT strain after 24 h of KTC treatment, whereas the increase was only 16.1-fold in the ads-4-knockout mutant (see Data Set S1 in the supplemental material). The qRT-PCR analysis showed that deletion of ads-4 did not significantly affect the expression of cdr4 in liquid medium without KTC. KTC treatment resulted in an 18.0-fold cdr4 transcriptional increase. KTC treatment also increased the cdr4 transcriptional level in the ads-4-knockout mutant but only by 2.7-fold (Fig. 3B).
FIG 4.
Ketoconazole susceptibility analyses of the N. crassa knockout mutant for genes regulated by ADS-4. Two-microliter aliquots of conidial suspension (1 × 106 conidia/ml) were inoculated onto the center of Vogel's plates (diameter, 9 cm) with or without ketoconazole (2 μg/ml). The plates were incubated at 28°C in the dark. Photographs were taken at 24 h for the control plates and at 48 h for the ketoconazole-treated plates (at 24 h, no colony for any tested strain was formed on the plates with ketoconazole).
The expression of cdr4 was not significantly higher in the strain with ads-4 overexpression, ads-4OE, compared to the WT strain, under both KTC-treated and non-KTC-treated conditions. Thus, ADS-4 is important in the transcriptional responses of cdr4 to azoles, but upregulation of ads-4 may not promote the expression of cdr4.
ADS-4 regulates transcriptional responses by the ABC multidrug transporter NcAbcB to ketoconazole.
The RNA-seq data showed that 24 h of KTC treatment resulted in a 18.1-fold increase in NCU03776 transcription. However, only a 4.3-fold increase in NCU03776 transcription was seen in the ads-4-knockout mutant (see Data Set S1 in the supplemental material). Phylogenic analysis of NCU03776 with its homologs in S. cerevisiae and Aspergillus fumigates showed that NCU03776 is the ortholog of Ycf1p in S. cerevisiae and AbcB (Afu1g10390) in A. fumigates (see Fig. S2 in the supplemental material). S. cerevisiae Ycf1p is an ABC transporter that transports a broad range of toxins into vacuoles (33, 34). Transcription of A. fumigates abcB can be induced by voriconazole, and the abcB-knockout mutant is hypersensitive to azoles (35, 36). NCU03776 was named NcabcB in this study. The qRT-PCR analysis showed that deletion of ads-4 did not significantly affect the transcription of NcabcB in medium without KTC. KTC treatment caused an 18.85-fold increase in the NcabcB transcript level in the WT strain. However, the NcabcB transcript level increased only 2.28-fold in the ads-4-knockout mutant after KTC treatment (Fig. 3C).
NcabcB expression increased 10.96-fold in the ads-4 overexpression strain ads-4OE, compared with the WT strain, with no KTC treatment. With KTC treatment, the transcriptional level of NcabcB in the ads-4OE strain was significantly higher than that in the WT strain (Fig. 3C). Thus, the ads-4 transcriptional increase with exposure to KTC probably promotes NcabcB expression. Although the NcabcB homolog abcB is known to contribute to azole resistance in A. fumigates (35, 36), the deletion of NcabcB does not affect the sensitivity to KTC in N. crassa (Fig. 4), which indicates that NcabcB is not a major contributor to azole resistance in N. crassa.
ADS-4 regulates transcriptional responses by NcRTA2 to azole stress.
C. albicans Rta2p was thought to be a lipid transporter and has predicted transmembrane domains. Rta2p is also involved in calcineurin-mediated azole resistance (37, 38). Our RNA-seq data showed that transcriptional responses by the RTA2 homolog NCU05209 (named Ncrta2 in this study) were affected by ads-4 deletion. The Ncrta2 transcription level increased 5.17-fold in the WT strain during KTC treatment, but there was not a significant Ncrta2 transcriptional increase in the ads-4-knockout strain (see Data Set S1 in the supplemental material). This result was confirmed by qRT-PCR, which showed that there was a 7.48-fold increase in Ncrta2 transcription in the WT strain after KTC treatment but no significant Ncrta2 transcriptional increase in the ads-4-knockout strain (Fig. 3D). This indicated that ADS-4 is required for the Ncrta2 transcriptional response to KTC.
Without KTC treatment, the Ncrta2 transcript level in the strain with ads-4 overexpression, ads-4OE, was 10.29-fold higher than that in the WT strain; with KTC treatment, the Ncrta2 transcript level was 16.63-fold higher than that in the non-KTC-treated WT cells, which was significantly higher than that in the KTC-treated WT cells. These results indicate that the Ncrta2 transcriptional response is regulated by ADS-4.
The Ncrta2-knockout mutant was hypersensitive to KTC (Fig. 4), which was consistent with observations for the C. albicans RTA2-knockout mutant (37, 38). Thus, the Ncrta2 transcriptional increase should improve resistance to azoles.
ADS-4 regulates transcriptional responses by the mitochondrial metal transporter NcMMT2 to ketoconazole.
From the remainder of the differentially expressed genes, whose roles in azole resistance were not previously known, we chose two genes for which transcriptional responses to KTC were completely abolished by ads-4 deletion for further analysis by qRT-PCR. The first gene chosen was NCU07879. The homolog of NCU07879 in S. cerevisiae is MMT2 (mitochondrial metal transporter), a gene responsible for iron homeostasis (39, 40). NCU07879 was named Ncmmt2 in this study. The RNA-seq data showed that Ncmmt2 transcription rose 24.5-fold in the WT strain after KTC treatment, whereas no significant transcriptional increase was seen in the ads-4-knockout mutant (see Data Set S1 in the supplemental material). The qRT-PCR analysis showed that Ncmmt2 transcription was 10.3-fold higher in KTC-treated WT cells than in non-KTC-treated WT cells, whereas Ncmmt2 transcription was only 1.48-fold higher in KTC-treated ads-4-knockout mutant cells than in non-KTC-treated WT cells (Fig. 3E). Overexpression of ads-4 resulted in a 33.68-fold increase in Ncmmt2 transcription (Fig. 3E), which indicated that Ncmmt2 expression was controlled by ADS-4. The Ncmmt2-knockout mutant had no obvious growth defects and showed almost wild-type sensitivity to KTC (Fig. 4).
ADS-4 controls transcriptional responses by the mannosylphosphorylation protein NcMNN4 to azole stress.
The other gene analyzed by qRT-PCR was NCU03213. NCU03213 shares significant homology with S. cerevisiae Mnn4p. Mnn4p is mannosyl phosphate transferase, which is essential for the transfer of mannosyl phosphate to N- and O-oligosaccharides in S. cerevisiae cell walls (41, 42). NCU03213 was named Ncmnn4 in this study. RNA-seq data showed that Ncmnn4 had a strong response to azole treatment; Ncmnn4 transcription increased 23.78-fold after KTC treatment in the WT strain, whereas there was no transcriptional increase in the ads-4-knockout strain (see Data Set S1 in the supplemental material). This trend was verified by qRT-PCR, which showed that Ncmnn4 transcription increased 53.18-fold after KTC treatment in the WT strain. KTC treatment did not cause significant Ncmnn4 transcriptional changes in the ads-4-knockout strain (Fig. 3F).
Without KTC treatment, the Ncmnn4 transcript level in the ads-4OE strain was 71.11-fold higher than that in non-KTC-treated WT cells. After 24 h of KTC treatment, the Ncmnn4 transcript level in the ads-4OE strain was 150.01-fold higher than that in non-KTC-treated WT cells, which was also significantly higher than that in KTC-treated WT cells (Fig. 3F).
The Ncmnn4-knockout mutant grew more slowly than the WT strain. On KTC-treated medium, however, the mutant was less sensitive to KTC than was the WT strain (Fig. 4).
ADS-4 is critical for azole resistance in the pathogenic fungus A. fumigatus.
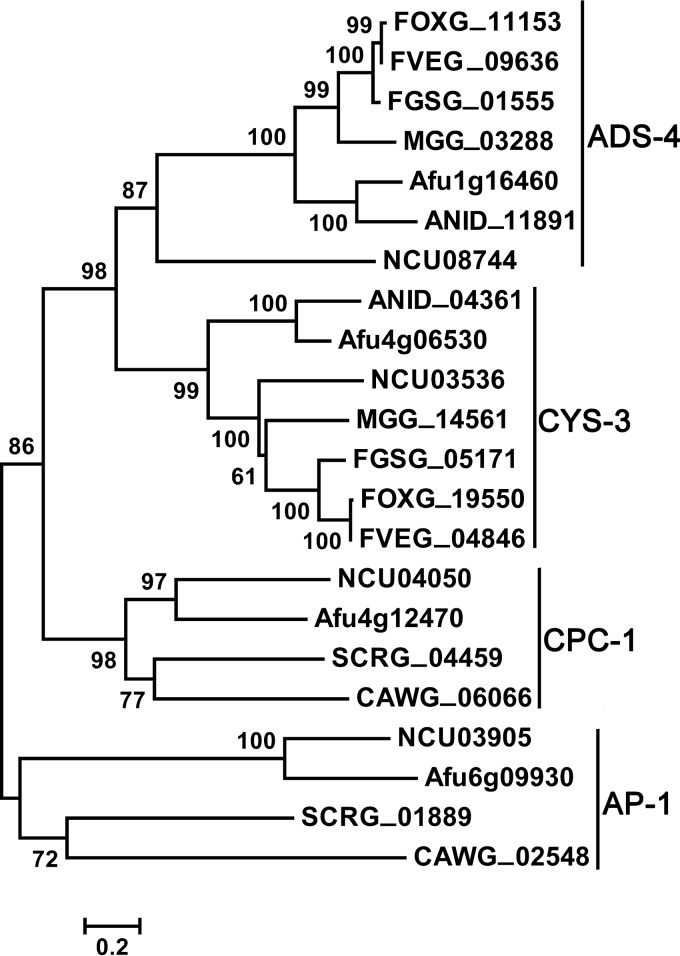
A BLASTp search revealed that a large number of ADS-4 homologs are present in filamentous ascomycetes but not in yeasts. Alignment analysis showed that ADS-4 homologs are highly conserved (see Fig. S1 in the supplemental material). Among fungal bZIP transcription factors, the functions of CYS-3, CPC-1, and AP-1 are relatively clear. Phylogenic analysis of fungal bZIP transcription factors showed that ADS-4, CYS-3, CPC-1, and AP-1 were distributed in four distinct clades (Fig. 5).
FIG 5.
Phylogenic analysis of N. crassa bZIP transcription factors and their homologs. Protein sequences were aligned and the neighbor-joining tree was constructed using MEGA version 6 (61), with cutoff bootstrap values of 50% obtained from 1,000 replicates. NCU, Neurospora crassa; FOXG, Fusarium oxysporum; FVEG, Fusarium verticillioides; FGSG, Fusarium graminearum; MGG, Magnaporthe oryzae; Afu, Aspergillus fumigatus; ANID, Aspergillus nidulans; SCRG, Saccharomyces cerevisiae; CAWG, Candida albicans.
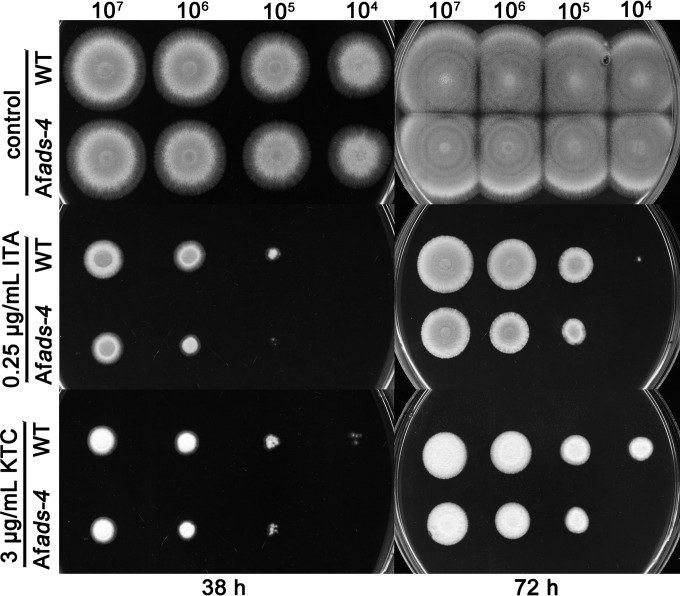
The protein sharing the greatest similarity with ADS-4 in A. fumigatus is Afu1g16460 (33% sequence identical [45/135] and 49% sequence positive [67/135]), which was named AfADS-4 in this study. Afu1g16460 is the ortholog of ADS-4 (Fig. 5). Afads-4 was deleted in A. fumigatus in order to test the functional conservation of ADS-4 among fungi. Figure 6 shows that the Afads-4-knockout strain was hypersensitive to itraconazole and KTC, compared to the WT strain, which indicated that ADS-4 orthologs are functionally conserved with regard to adaptation and resistance to antifungal azoles.
FIG 6.
Hypersensitivity of Aspergillus fumigatus ADS-4 homolog Afu1g16460 (Afads-4)-knockout mutant to azoles. A series of conidial suspensions (1 × 107, 1 × 106, 1 × 105, and 1 × 104 conidia/ml) of the A. fumigatus WT strain was prepared, and 2 μl of each conidial suspension was inoculated onto CM agar plates (diameter, 15 cm) with or without 3 μg/ml ketoconazole or 0.25 μg/ml itraconazole (ITA). Images of the plates were captured after 38 h and 72 h of cultivation at 37°C. The experiment was independently repeated twice.
DISCUSSION
To survive under antifungal azole stress, fungi adjust the transcriptional levels of a number of genes (20, 36, 43–45). Many of these azole-responsive genes are known to contribute to azole adaptation and resistance (17, 20, 23, 46–49). In filamentous fungi, only two transcription factors, AP-1 and CCG-8, are known to be involved in the regulation of azole responses and adaptation (20, 23). This study identified the third transcription factor controlling azole adaptation. The only reported homolog of ADS-4 is ZipA (ANID_11891) in Aspergillus nidulans (Fig. 5). ZipA negatively regulates resistance to oxidative stress (50). However, its role in antifungal drug resistance has not been studied. The functional conservation of ADS-4 in N. crassa and A. fumigatus suggests that it is possible to use ADS-4 as a new target for antifungal drug design. Our study also identified some new azole-responsive genes, such as Ncmmt2 and Ncmnn4.
Since overexpression of ads-4 increased azole resistance, spontaneous mutations that cause overexpression of ads-4 might increase azole resistance in pathogenic fungi. Gain-of-function mutations that cause overexpression of the transcription factor Mrr1p in C. albicans increase resistance to azoles (51). Similarly, gain-of-function mutations that improve Pdr1p transcriptional activity in S. cerevisiae increase resistance to azoles (52). Therefore, azole resistance caused by ads-4 overexpression might exist in nature.
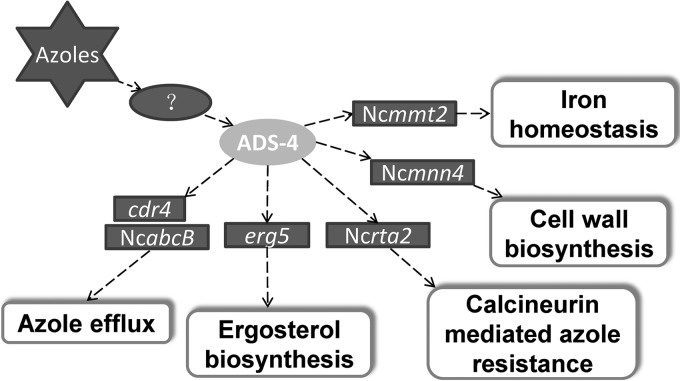
ADS-4 mediates transcriptional responses to ketoconazole for a number of genes, among which at least six genes, including cdr4, erg5, NcabcB, Ncmmt2, NcRTA2, and Ncmnn4, might be related to azole adaptation and resistance. On the basis of the functions of these genes, we propose that ADS-4 regulates azole adaptation and resistance by the following possible mechanisms (Fig. 7).
FIG 7.
Proposed mechanisms of ADS-4 in azole responses and resistance. Azole stress activates ads-4 transcription by an unknown signaling pathway, and then ADS-4 activates transcription of downstream genes (indicted in rectangles) involved in biological processes important to azole adaptation and resistance.
First, ADS-4 should positively contribute to azole efflux. CDR4 is the ortholog of the azole efflux pump Cdr1p in C. albicans (24). The roles of cdr4 and its orthologs in azole resistance in many fungi have been demonstrated previously (24, 25, 53). The ortholog of NcabcB in A. fumigates is the ABC multidrug transporter AbcB, which positively contributes to azole resistance (35, 36). Increases in cdr4 and NcabcB transcript levels should reduce the accumulation of azoles in cells. Thus, upregulation of cdr4 and NcabcB by ADS-4 should contribute positively to azole efflux.
Second, ADS-4 is linked to calcineurin-mediated azole resistance. Azole stress could activate the calcineurin signaling cascade, which leads to dephosphorylation of the transcription factor Crz1p in C. albicans (54). Dephosphorylated Crz1p activates the expression of Rta2p, which transports sphingolipid long-chain bases from the inner leaflet to the outer leaflet of the plasma membrane. The disruption of RTA2 affects raft formation, making C. albicans more susceptible to azoles (37, 38, 55). The Rta2p homolog Rsb1p in S. cerevisiae has the same function (56). Thus, ADS-4 might be linked to calcineurin-mediated azole resistance by activating Ncrta2 transcription under azole stress.
Third, ADS-4 might mediate ergosterol biosynthesis under azole stress. Azole treatment affects the composition of sterols in fungal membranes (9, 25). The sterol C-22 desaturase ERG5 is an essential enzyme in ergosterol biosynthesis. Deletion of erg5 in N. crassa completely blocked ergosterol biosynthesis and increased azole susceptibilities (25). Thus, the promotion of erg5 expression by ADS-4 might be beneficial for maintaining the proper sterol composition in membranes under azole stress.
Fourth, ADS-4 might mediate mitochondrial iron transport during azole stress. The mitochondrial iron transporter NcMMT-2 had a strong response to KTC, and its response was completely controlled by ADS-4. Although its role in azole resistance has not been reported previously, a linkage between iron and azole susceptibility has been reported. In S. cerevisiae, mitochondria serve as an iron reservoir, and mitochondrial iron transporters redistribute iron from intracellular compartments into the mitochondria (39). Iron deprivation can enhance membrane fluidity and increase the passive diffusion of drugs, which leads to increased drug susceptibility in C. albicans (57). In addition, ERG-25, an enzyme involved in ergosterol biosynthesis, needs iron to fulfill its function (58). Thus, iron plays an important role in azole resistance. The increase in Ncmmt-2 expression produced by ADS-4 might enhance iron transport into mitochondria and counteract membrane iron leakage.
Fifth, ADS-4 might mediate the adaptive alterations in cell wall composition during azole stress. Mnn4p transfers mannosyl phosphate to N- and O-oligosaccharides in S. cerevisiae cell walls (41, 42, 59, 60). The Ncmnn4 transcriptional increase during ketoconazole treatment suggests that the composition of cell walls might have adaptive alterations under azole stress. In Candida glabrata, structural changes in cell walls could lead to the strain hypersensitive to itraconazole (60). Thus, ADS-4 might mediate cell wall biosynthesis through upregulation of Ncmnn4 under azole stress.
In addition, this study partially revealed the relationship between ADS-4 and CCG-8, two transcription factors that positively regulate responses to azoles. The RNA-seq data showed that ADS-4 and CCG-8 did not regulate each other. A comparison of the genes regulated by ADS-4 and the genes regulated by CCG-8 showed that only cdr4 was regulated by both ADS-4 and CCG-8. Deletion of either ads-4 or ccg-8 did not completely block the cdr4 transcriptional response to ketoconazole (20). Therefore, the cdr4 transcriptional responses to azole stresses are probably regulated by a number of transcription factors. In addition to ads-4 and ccg-8, genes encoding other transcription factors were also found in the ketoconazole-responsive gene group. Thus, it should be possible to find new transcription factors that regulate the mechanisms controlling azole responses and resistance.
In summary, this study identified a new regulator of azole responses and partially revealed its mechanism. However, its detailed regulatory mechanisms, such as the consensus DNA motifs that ADS-4 might recognize and bind to and the signaling pathway that activates ads-4 transcription during azole stress, remain to be further clarified.
Supplementary Material
ACKNOWLEDGMENTS
This project was supported by grants from the National Natural Science Foundation of China (grants 31461143002 and 31371986) and the China Ocean Mineral Resources R & D Association (grant DY125-15-T-07).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00542-15.
REFERENCES
- 1.Lucas JA, Hawkins NJ, Fraaije BA. 2015. The evolution of fungicide resistance. Adv Appl Microbiol 90:29–92. [DOI] [PubMed] [Google Scholar]
- 2.Hobbelen PH, Paveley ND, van den Bosch F. 2014. The emergence of resistance to fungicides. PLoS One 9:e91910. doi: 10.1371/journal.pone.0091910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornet M, Fleury L, Maslo C, Bernard JF, Brucker G. 2002. Epidemiology of invasive aspergillosis in France: a six-year multicentric survey in the greater Paris area. J Hosp Infect 51:288–296. doi: 10.1053/jhin.2002.1258. [DOI] [PubMed] [Google Scholar]
- 4.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik JA, Wingard JR, Patterson TF. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 5.Leslie JF, Pearson CAS, Nelson PE, Toussoun TA. 1990. Fusarium spp. from corn, sorghum, and soybean fields in the central and eastern United States. Phytopathology 80:343–350. doi: 10.1094/Phyto-80-343. [DOI] [Google Scholar]
- 6.Cools HJ, Hawkins NJ, Fraaije BA. 2013. Constraints on the evolution of azole resistance in plant pathogenic fungi. Plant Pathol 62:36–42. doi: 10.1111/ppa.12128. [DOI] [Google Scholar]
- 7.Forastiero A, Mesa-Arango AC, Alastruey-Izquierdo A, Alcazar-Fuoli L, Bernal-Martinez L, Pelaez T, Lopez JF, Grimalt JO, Gomez-Lopez A, Cuesta I, Zaragoza O, Mellado E. 2013. Candida tropicalis antifungal cross-resistance is related to different azole target (Erg11p) modifications. Antimicrob Agents Chemother 57:4769–4781. doi: 10.1128/AAC.00477-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White TC, Marr KA, Bowden RA. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev 11:382–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapiro RS, Robbins N, Cowen LE. 2011. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol Mol Biol Rev 75:213–267. doi: 10.1128/MMBR.00045-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chau AS, Gurnani M, Hawkinson R, Laverdiere M, Cacciapuoti A, McNicholas PM. 2005. Inactivation of sterol Δ5,6-desaturase attenuates virulence in Candida albicans. Antimicrob Agents Chemother 49:3646–3651. doi: 10.1128/AAC.49.9.3646-3651.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snelders E, Melchers WJ, Verweij PE. 2011. Azole resistance in Aspergillus fumigatus: a new challenge in the management of invasive aspergillosis? Future Microbiol 6:335–347. doi: 10.2217/fmb.11.4. [DOI] [PubMed] [Google Scholar]
- 12.Vandeputte P, Larcher G, Berges T, Renier G, Chabasse D, Bouchara JP. 2005. Mechanisms of azole resistance in a clinical isolate of Candida tropicalis. Antimicrob Agents Chemother 49:4608–4615. doi: 10.1128/AAC.49.11.4608-4615.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mamnun YM, Pandjaitan R, Mahe Y, Delahodde A, Kuchler K. 2002. The yeast zinc finger regulators Pdr1p and Pdr3p control pleiotropic drug resistance (PDR) as homo- and heterodimers in vivo. Mol Microbiol 46:1429–1440. doi: 10.1046/j.1365-2958.2002.03262.x. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Talibi D, Weber S, Poisson G, Raymond M. 2001. Functional isolation of the Candida albicans FCR3 gene encoding a bZip transcription factor homologous to Saccharomyces cerevisiae Yap3p. Yeast 18:1217–1225. doi: 10.1002/yea.770. [DOI] [PubMed] [Google Scholar]
- 15.Shen H, An MM, Wang DJ, Xu Z, Zhang JD, Gao PH, Cao YY, Cao YB, Jiang YY. 2007. Fcr1p inhibits development of fluconazole resistance in Candida albicans by abolishing CDR1 induction. Biol Pharm Bull 30:68–73. doi: 10.1248/bpb.30.68. [DOI] [PubMed] [Google Scholar]
- 16.Oliver BG, Song JL, Choiniere JH, White TC. 2007. cis-Acting elements within the Candida albicans ERG11 promoter mediate the azole response through transcription factor Upc2p. Eukaryot Cell 6:2231–2239. doi: 10.1128/EC.00331-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunkel N, Liu TT, Barker KS, Homayouni R, Morschhauser J, Rogers PD. 2008. A gain-of-function mutation in the transcription factor Upc2p causes upregulation of ergosterol biosynthesis genes and increased fluconazole resistance in a clinical Candida albicans isolate. Eukaryot Cell 7:1180–1190. doi: 10.1128/EC.00103-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Znaidi S, Weber S, Al-Abdin OZ, Bomme P, Saidane S, Drouin S, Lemieux S, De Deken X, Robert F, Raymond M. 2008. Genomewide location analysis of Candida albicans Upc2p, a regulator of sterol metabolism and azole drug resistance. Eukaryot Cell 7:836–847. doi: 10.1128/EC.00070-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marie C, Leyde S, White TC. 2008. Cytoplasmic localization of sterol transcription factors Upc2p and Ecm22p in S. cerevisiae. Fungal Genet Biol 45:1430–1438. doi: 10.1016/j.fgb.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun X, Wang K, Yu X, Liu J, Zhang H, Zhou F, Xie B, Li S. 2014. Transcription factor CCG-8 as a new regulator in the adaptation to antifungal azole stress. Antimicrob Agents Chemother 58:1434–1442. doi: 10.1128/AAC.02244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alarco AM, Balan I, Talibi D, Mainville N, Raymond M. 1997. AP1-mediated multidrug resistance in Saccharomyces cerevisiae requires FLR1 encoding a transporter of the major facilitator superfamily. J Biol Chem 272:19304–19313. doi: 10.1074/jbc.272.31.19304. [DOI] [PubMed] [Google Scholar]
- 22.Chen KH, Miyazaki T, Tsai HF, Bennett JE. 2007. The bZip transcription factor CgAp1p is involved in multidrug resistance and required for activation of multidrug transporter gene CgFLR1 in Candida glabrata. Gene 386:63–72. doi: 10.1016/j.gene.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Qiao J, Liu W, Li R. 2010. Truncated Afyap1 attenuates antifungal susceptibility of Aspergillus fumigatus to voriconazole and confers adaptation of the fungus to oxidative stress. Mycopathologia 170:155–160. doi: 10.1007/s11046-010-9309-2. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Zhang Z, Zhang X, Zhang H, Sun X, Hu C, Li S. 2012. CDR4 is the major contributor to azole resistance among four Pdr5p-like ABC transporters in Neurospora crassa. Fungal Biol 116:848–854. doi: 10.1016/j.funbio.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Sun X, Wang W, Wang K, Yu X, Liu J, Zhou F, Xie B, Li S. 2013. Sterol C-22 desaturase ERG5 mediates the sensitivity to antifungal azoles in Neurospora crassa and Fusarium verticillioides. Front Microbiol 4:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogel HJ. 1956. A convenient growth medium for Neurospora (medium N). Microb Genet Bull 13:43. [Google Scholar]
- 27.Margolin BS, Freitag M, Selker EU. 1997. Improved plasmids for gene targeting at the his-3 locus of Neurospora crassa by electroporation. Fungal Genet Newsl 44:34–36. [Google Scholar]
- 28.Royer JC, Yamashiro CT. 1992. Generation of transformable spheroplasts from mycelia, macroconidia, microconidia and germinating ascospores of Neurospora crassa. Fungal Genet Newsl 39:76–79. [Google Scholar]
- 29.Temporini ED, Alvarez ME, Mautino MR, Folco HD, Rosa AL. 2004. The Neurospora crassa cfp promoter drives a carbon source-dependent expression of transgenes in filamentous fungi. J Appl Microbiol 96:1256–1264. doi: 10.1111/j.1365-2672.2004.02249.x. [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.d'Enfert C. 1996. Selection of multiple disruption events in Aspergillus fumigatus using the orotidine-5′-decarboxylase gene, pyrG, as a unique transformation marker. Curr Genet 30:76–82. doi: 10.1007/s002940050103. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Zhang L, Wang D, Zhou H, Ouyang H, Ming J, Jin C. 2008. Deletion of the msdS/AfmsdC gene induces abnormal polarity and septation in Aspergillus fumigatus. Microbiology 154:1960–1972. doi: 10.1099/mic.0.2008/017525-0. [DOI] [PubMed] [Google Scholar]
- 33.Sasser TL, Lawrence G, Karunakaran S, Brown C, Fratti RA. 2013. The yeast ATP-binding cassette (ABC) transporter Ycf1p enhances the recruitment of the soluble SNARE Vam7p to vacuoles for efficient membrane fusion. J Biol Chem 288:18300–18310. doi: 10.1074/jbc.M112.441089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jungwirth H, Wendler F, Platzer B, Bergler H, Hogenauer G. 2000. Diazaborine resistance in yeast involves the efflux pumps Ycf1p and Flr1p and is enhanced by a gain-of-function allele of gene YAP1. Eur J Biochem 267:4809–4816. doi: 10.1046/j.1432-1327.2000.01537.x. [DOI] [PubMed] [Google Scholar]
- 35.Paul S, Diekema D, Moye-Rowley WS. 2013. Contributions of Aspergillus fumigatus ATP-binding cassette transporter proteins to drug resistance and virulence. Eukaryot Cell 12:1619–1628. doi: 10.1128/EC.00171-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.da Silva Ferreira ME, Malavazi I, Savoldi M, Brakhage AA, Goldman MH, Kim HS, Nierman WC, Goldman GH. 2006. Transcriptome analysis of Aspergillus fumigatus exposed to voriconazole. Curr Genet 50:32–44. doi: 10.1007/s00294-006-0073-2. [DOI] [PubMed] [Google Scholar]
- 37.Jia X-M, Ma Z-P, Jia Y, Gao P-H, Zhang J-D, Wang Y, Xu Y-G, Wang L, Cao Y-Y, Cao Y-B, Zhang L-X, Jiang Y-Y. 2008. RTA2, a novel gene involved in azole resistance in Candida albicans. Biochem Biophys Res Commun 373:631–636. doi: 10.1016/j.bbrc.2008.06.093. [DOI] [PubMed] [Google Scholar]
- 38.Jia XM, Wang Y, Jia Y, Gao PH, Xu YG, Wang L, Cao YY, Cao YB, Zhang LX, Jiang YY. 2009. RTA2 is involved in calcineurin-mediated azole resistance and sphingoid long-chain base release in Candida albicans. Cell Mol Life Sci 66:122–134. doi: 10.1007/s00018-008-8409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L, Kaplan J. 1997. Characterization of two homologous yeast genes that encode mitochondrial iron transporters. J Biol Chem 272:28485–28493. doi: 10.1074/jbc.272.45.28485. [DOI] [PubMed] [Google Scholar]
- 40.Li LT, Miao R, Jia X, Ward DM, Kaplan J. 2014. Expression of the yeast cation diffusion facilitators Mmt1 and Mmt2 affects mitochondrial and cellular iron homeostasis: evidence for mitochondrial iron export. J Biol Chem 289:17132–17141. doi: 10.1074/jbc.M114.574723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Odani T, Shimma Y, Wang X-H, Jigami Y. 1997. Mannosylphosphate transfer to cell wall mannan is regulated by the transcriptional level of the MNN4 gene in Saccharomyces cerevisiae. FEBS Lett 420:186–190. doi: 10.1016/S0014-5793(97)01513-5. [DOI] [PubMed] [Google Scholar]
- 42.Odani T, Shimma Y, Tanaka A, Jigami Y. 1996. Cloning and analysis of the MNN4 gene required for phosphorylation of N-linked oligosaccharides in Saccharomyces cerevisiae. Glycobiology 6:805–810. doi: 10.1093/glycob/6.8.805. [DOI] [PubMed] [Google Scholar]
- 43.Liu X, Jiang J, Shao J, Yin Y, Ma Z. 2010. Gene transcription profiling of Fusarium graminearum treated with an azole fungicide tebuconazole. Appl Microbiol Biotechnol 85:1105–1114. doi: 10.1007/s00253-009-2273-4. [DOI] [PubMed] [Google Scholar]
- 44.Agarwal AK, Rogers PD, Baerson SR, Jacob MR, Barker KS, Cleary JD, Walker LA, Nagle DG, Clark AM. 2003. Genome-wide expression profiling of the response to polyene, pyrimidine, azole, and echinocandin antifungal agents in Saccharomyces cerevisiae. J Biol Chem 278:34998–35015. doi: 10.1074/jbc.M306291200. [DOI] [PubMed] [Google Scholar]
- 45.Yu L, Zhang W, Wang L, Yang J, Liu T, Peng J, Leng W, Chen L, Li R, Jin Q. 2007. Transcriptional profiles of the response to ketoconazole and amphotericin B in Trichophyton rubrum. Antimicrob Agents Chemother 51:144–153. doi: 10.1128/AAC.00755-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vik A, Rine J. 2001. Upc2p and Ecm22p, dual regulators of sterol biosynthesis in Saccharomyces cerevisiae. Mol Cell Biol 21:6395–6405. doi: 10.1128/MCB.21.19.6395-6405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silver PM, Oliver BG, White TC. 2004. Role of Candida albicans transcription factor Upc2p in drug resistance and sterol metabolism. Eukaryot Cell 3:1391–1397. doi: 10.1128/EC.3.6.1391-1397.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacPherson S, Akache B, Weber S, De Deken X, Raymond M, Turcotte B. 2005. Candida albicans zinc cluster protein Upc2p confers resistance to antifungal drugs and is an activator of ergosterol biosynthetic genes. Antimicrob Agents Chemother 49:1745–1752. doi: 10.1128/AAC.49.5.1745-1752.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Talibi D, Raymond M. 1999. Isolation of a putative Candida albicans transcriptional regulator involved in pleiotropic drug resistance by functional complementation of a pdr1 pdr3 mutation in Saccharomyces cerevisiae. J Bacteriol 181:231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin WB, Reinke AW, Szilagyi M, Emri T, Chiang YM, Keating AE, Pocsi I, Wang CC, Keller NP. 2013. bZIP transcription factors affecting secondary metabolism, sexual development and stress responses in Aspergillus nidulans. Microbiology 159:77–88. doi: 10.1099/mic.0.063370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morschhauser J, Barker KS, Liu TT, Bla BWJ, Homayouni R, Rogers PD. 2007. The transcription factor Mrr1p controls expression of the MDR1 efflux pump and mediates multidrug resistance in Candida albicans. PLoS Pathog 3:e164. doi: 10.1371/journal.ppat.0030164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kolaczkowska A, Kolaczkowski M, Delahodde A, Goffeau A. 2002. Functional dissection of Pdr1p, a regulator of multidrug resistance in Saccharomyces cerevisiae. Mol Genet Genomics 267:96–106. doi: 10.1007/s00438-002-0642-0. [DOI] [PubMed] [Google Scholar]
- 53.Paul S, Moye-Rowley WS. 2014. Multidrug resistance in fungi: regulation of transporter-encoding gene expression. Front Physiol 5:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cyert MS. 2003. Calcineurin signaling in Saccharomyces cerevisiae: how yeast go crazy in response to stress. Biochem Biophys Res Commun 311:1143–1150. doi: 10.1016/S0006-291X(03)01552-3. [DOI] [PubMed] [Google Scholar]
- 55.Thewes S. 2014. Calcineurin-Crz1 signaling in lower eukaryotes. Eukaryot Cell 13:694–705. doi: 10.1128/EC.00038-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kihara A, Igarashi Y. 2002. Identification and characterization of a Saccharomyces cerevisiae gene, RSB1, involved in sphingoid long-chain base release. J Biol Chem 277:30048–30054. doi: 10.1074/jbc.M203385200. [DOI] [PubMed] [Google Scholar]
- 57.Prasad T, Chandra A, Mukhopadhyay CK, Prasad R. 2006. Unexpected link between iron and drug resistance of Candida spp.: iron depletion enhances membrane fluidity and drug diffusion, leading to drug-susceptible cells. Antimicrob Agents Chemother 50:3597–3606. doi: 10.1128/AAC.00653-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hameed S, Dhamgaye S, Singh A, Goswami SK, Prasad R. 2011. Calcineurin signaling and membrane lipid homeostasis regulates iron mediated multidrug resistance mechanisms in Candida albicans. PLoS One 6:e18684. doi: 10.1371/journal.pone.0018684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hazen KC, Singleton DR, Masuoka J. 2007. Influence of outer region mannosylphosphorylation on N-glycan formation by Candida albicans: normal acid-stable N-glycan formation requires acid-labile mannosylphosphate addition. Glycobiology 17:1052–1060. doi: 10.1093/glycob/cwm080. [DOI] [PubMed] [Google Scholar]
- 60.Takahashi S, Kudoh A, Okawa Y, Shibata N. 2012. Significant differences in the cell-wall mannans from three Candida glabrata strains correlate with antifungal drug sensitivity. FEBS J 279:1844–1856. doi: 10.1111/j.1742-4658.2012.08564.x. [DOI] [PubMed] [Google Scholar]
- 61.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.