Significance
Targeting cancer metabolism requires personalized diagnostics for clinical success. Pancreatic ductal adenocarcinoma (PDAC) is characterized by metabolism addiction. To identify metabolic dependencies within PDAC, we conducted broad metabolite profiling and identified three subtypes that showed distinct metabolite profiles associated with glycolysis, lipogenesis, and redox pathways. Importantly, these profiles significantly correlated with enriched sensitivity to a variety of metabolic inhibitors including inhibitors targeting glycolysis, glutaminolysis, lipogenesis, and redox balance. In primary PDAC tumor samples, the lipid subtype was strongly associated with an epithelial phenotype, whereas the glycolytic subtype was strongly associated with a mesenchymal phenotype, suggesting functional relevance in disease progression. Our findings will provide valuable predictive utility for a number of metabolic inhibitors currently undergoing phase I testing.
Keywords: metabolite profiling, metabolic subtypes in PDAC, glycolysis, lipid synthesis, biomarkers for metabolic inhibitors
Abstract
Although targeting cancer metabolism is a promising therapeutic strategy, clinical success will depend on an accurate diagnostic identification of tumor subtypes with specific metabolic requirements. Through broad metabolite profiling, we successfully identified three highly distinct metabolic subtypes in pancreatic ductal adenocarcinoma (PDAC). One subtype was defined by reduced proliferative capacity, whereas the other two subtypes (glycolytic and lipogenic) showed distinct metabolite levels associated with glycolysis, lipogenesis, and redox pathways, confirmed at the transcriptional level. The glycolytic and lipogenic subtypes showed striking differences in glucose and glutamine utilization, as well as mitochondrial function, and corresponded to differences in cell sensitivity to inhibitors of glycolysis, glutamine metabolism, lipid synthesis, and redox balance. In PDAC clinical samples, the lipogenic subtype associated with the epithelial (classical) subtype, whereas the glycolytic subtype strongly associated with the mesenchymal (QM-PDA) subtype, suggesting functional relevance in disease progression. Pharmacogenomic screening of an additional ∼200 non-PDAC cell lines validated the association between mesenchymal status and metabolic drug response in other tumor indications. Our findings highlight the utility of broad metabolite profiling to predict sensitivity of tumors to a variety of metabolic inhibitors.
Metabolic reprogramming during tumorigenesis is an essential process in nearly all cancer cells. Tumors share a common phenotype of uncontrolled cell proliferation and must efficiently generate the energy and macromolecules required for cellular growth. The first example of metabolic reprogramming was discovered more than 80 y ago by Otto Warburg: tumor cells can shift from oxidative to fermentative metabolism in the course of oncogenesis (1). More recently, there has been a resurgence of interest in targeting cancer metabolism (2–4) because it may not only be effective in inhibiting tumor growth, but may also provide a therapeutic window (5, 6). For example, inactivation of lactate dehydrogenase-A (LDHA), an enzyme that catalyzes the final step of aerobic glycolysis, thereby reducing pyruvate to lactate, decreases tumorigenesis and induces regression of established tumors in mouse models of lung cancer driven by oncogenic KRAS or epidermal growth factor receptor (EGFR) while minimally affecting normal cell function (7). The finding that cancers have altered metabolism has prompted substantial investigation, both preclinically and in clinical trials, of several metabolically targeted agents, including those that elevate reactive oxygen species (ROS) or block glycolysis, lipid synthesis, mitochondrial function, and glutamine synthesis pathways (8).
The identification of distinct metabolic reprogramming events or metabolic subtypes in cancer may inform patient selection for investigational metabolic inhibitors and in the selection of new therapeutic targets (9, 10). Just as tumors vary greatly in genomic alterations that impact signaling and regulatory pathways, metabolic transformation is also heterogeneous and dependent on tissue type, proliferation rate, and isoenzyme use (9, 11). In addition, the observed differences in the dependence on and utilization of the major nutrients—glutamine and glucose—are linked to oncogenic signaling and the genomic features of a cancer cell (12).
Large-scale pharmacogenomic screening is a powerful method for identifying biomarkers of drug response and can accelerate the search for new cancer therapies (13, 14). In this study, we used broad baseline metabolite profiling in cell line models of pancreatic ductal adenocarcinoma (PDAC), a disease context previously associated with altered metabolism (15–18), to identify metabolic subtypes within PDAC and predict their sensitivity to various metabolic inhibitors.
Results
Baseline Metabolite Profiling Identifies Three Metabolic Subtypes in PDAC.
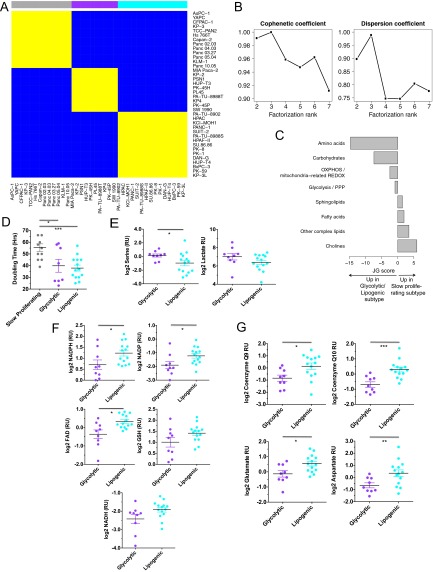
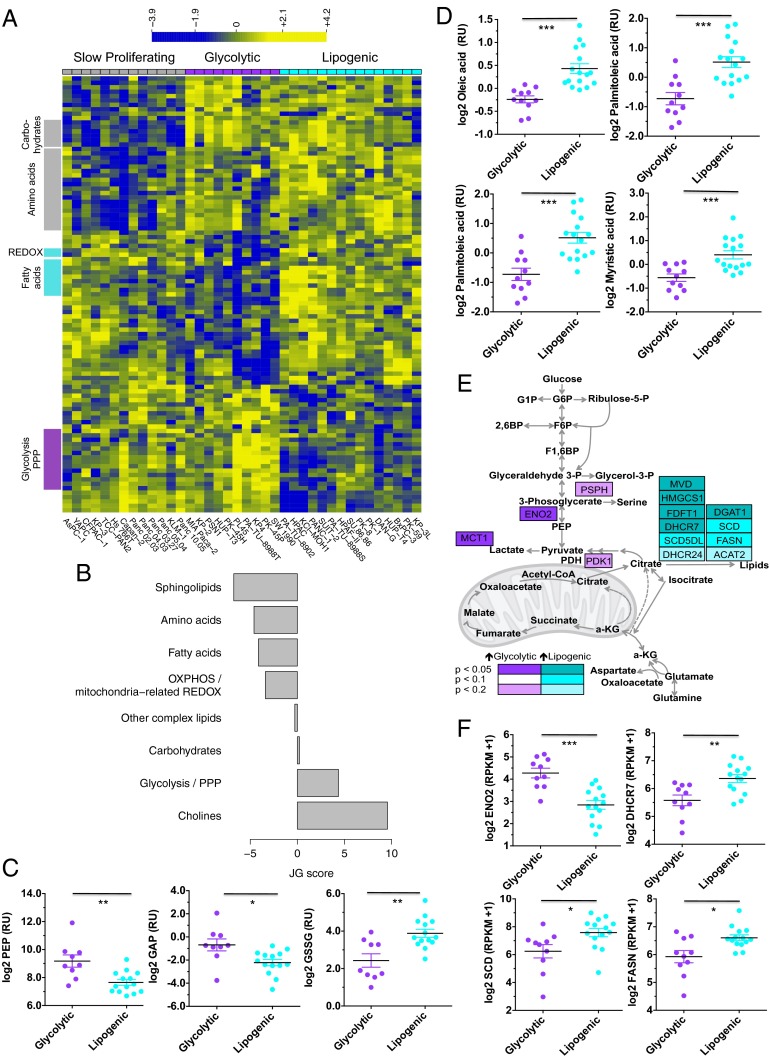
We examined cell lines derived from naturally occurring tumors because they recapitulate many aspects of the tissue type and genomic context of cancer (13, 14, 19, 20). Levels of 256 metabolites were quantified in 38 pancreatic cancer cell lines (five biological replicates per cell line) in logarithmic growth phase using media with physiological glucose and glutamine concentrations (Datasets S1–S3). We applied nonnegative matrix factorization (NMF) (21), a recently established approach for consensus clustering (22–24), to 153 metabolites with reproducible variation, allowing the capture of the strongest signal of metabolic dependency (SI Materials and Methods). This analysis revealed three stable and reproducible subtypes with adequate data coherence (Fig. S1 A and B). The metabolite profiles of the cell lines ordered by subtype are shown in Fig. 1A for metabolites with distinct intensities in at least one subtype compared with the other two subtypes (F test, P < 0.05). These three subtypes provided a useful and interpretable basis for further analysis.
Fig. S1.
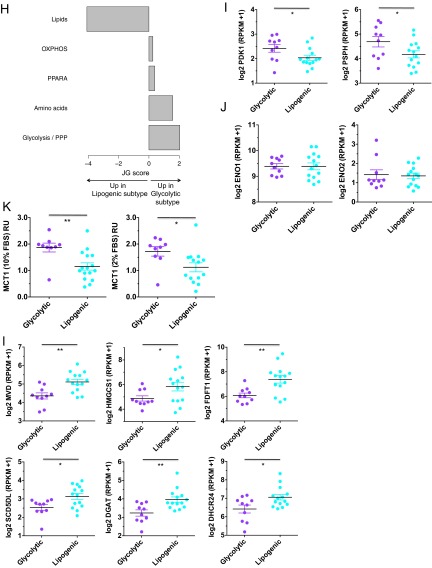
Related to Fig. 1. (A) Cell line-by-cell line consensus heatmap shows the clustering consensus obtained with nonnegative matrix factorization (NMF) based on 200 runs (21); yellow color indicates similar metabolic profiles and blue indicates dissimilar. The three identified subtypes are colored on top: slow proliferating subtype in gray, glycolytic subtype in purple, lipogenic subtype in cyan. (B) Cophenetic coefficient (measure of subtype stability) and dispersion coefficient (measure of subtype robustness) in function of the number of subtypes ranging from 2 to 7. The cophenetic coefficient equals 1 for a perfect consensus matrix with entries 0 and 1 and decreases when entries become scattered between 0 and 1. (C) Relative enrichment of the eight metabolic ontology classes in the slow proliferating subtype vs. the glycolytic/lipogenic subtypes, represented by JG score (47). Positive scores represent ontologies enriched for metabolites with high intensities in the slow proliferating subtype. Negative scores represent ontologies characteristic of the glycolytic/lipogenic subtypes. See Dataset S1 for a list of metabolites per ontology and Dataset S4 for the list of differentially expressed metabolites. (D) Doubling time for all cell lines grouped by subtype, with a lower proliferation rate for cell lines in the slow proliferating subtype. Proliferation was measured using CyQUANT Cell Proliferation Assays. Data from Dataset S7. (E) Normalized metabolite intensity level for lactate involved in glycolysis. RU stands for relative unit, similar to Fig. 1C. (F) Normalized metabolite intensity levels for metabolites involved in redox pathways that were differentially expressed between glycolytic and lipogenic lines. RU stands for relative unit, similar to Fig. 1C. (G) Normalized metabolite intensity levels for metabolites involved in the electron transport chain and aspartate/malate shuttle that were differentially expressed between glycolytic and lipogenic subtype lines. RU stands for relative unit, similar to Fig. 1C. (H) Relative enrichment of the five curated metabolism gene sets in the glycolytic and lipogenic subtypes, represented by JG score. Positive scores represent gene sets enriched in the glycolytic subtype. Negative scores represent gene sets characteristic of the lipogenic subtype. The transcriptomic profile of the glycolytic subtype is enriched with genes involved in glycolysis and pentose phosphate. Cell lines from the lipogenic subtype show higher expression of lipid synthesis genes. Dataset S5 lists genes per gene set, and Dataset S6 lists differentially expressed genes. (I) Expression of several of the glycolysis genes that were differentially expressed between glycolytic and lipogenic lines (Dataset S5 and Fig. 1E). (J) Enolase homologs ENO1 and ENO3 show no differential expression between glycolytic and lipogenic lines. The expression profile for ENO2 is shown in Fig. 1F. (K) Western blots and quantification of Mct1 protein in glycolytic and lipogenic lines (quantification normalized to HSP90). (L) Expression of several of the fatty acid synthesis genes (cholesterol and lipids) that were differentially expressed between glycolytic and lipogenic lines (Dataset S5 and Fig. 1F). Asterisks denote a statistically significant difference by t test (*P < 0.05, **P < 0.01, ***P < 0.001).
Fig. 1.
Identification of distinct metabolic subtypes in PDAC through baseline metabolite profiling. (A) Hierarchical clustering of identifiable metabolites with significant intensity differences between any of the three subtypes (F test, P < 0.05; 99 metabolites). Cell lines were grouped by subtype, with the order per subtype defined by unsupervised clustering. Log2 intensity ratio data per metabolite are scaled across all cell lines to mean = 0 and SD = 1. Blue indicates low scaled intensity, and yellow indicates high for each metabolite. Highlighted in gray are functionally related metabolites. Slow proliferating lines are labeled in gray, glycolytic lines in purple, and lipogenic in cyan. (B) Relative enrichment of the eight metabolic ontology classes in the glycolytic and lipogenic subtypes, represented by JG score (47). Positive scores represent ontologies enriched for metabolites with high intensities in the glycolytic subtype. See Dataset S1 for a description and list of metabolites per ontology and Dataset S4 for the list of differentially expressed metabolites. (C) Normalized metabolite intensity levels for metabolites involved in glycolysis/pentose phosphate and redox pathways that were differentially expressed between glycolytic and lipogenic lines. RU stands for relative unit, with intensity levels normalized to a reference pool of samples for metabolites from the Broad Profiling platform (Dataset S2) and to a universal 13C-labeled internal standard for metabolites from the Energy platform (Dataset S3). (D) Normalized metabolite intensity levels for metabolites involved in lipid synthesis that were differentially expressed between glycolytic and lipogenic lines. (E) Detailed metabolite map with genes differentially expressed between cell lines from the glycolytic vs. lipogenic subtype indicated with various shades of color depending on P value corrected for multiple testing. For MCT1, P value is based on protein expression level. We refer to Dataset S6 for a list of differentially expressed genes. (F) Expression levels of ENO2, DHCR7, SCD, and FASN involved in glycolysis and lipid synthesis that were differentially expressed between glycolytic and lipogenic lines (Dataset S6). Asterisks denote a statistically significant difference by unpaired t test with Welch’s correction (*P < 0.05, **P < 0.01, ***P < 0.001).
Metabolic Characterization Reveals a Slow Proliferating, Glycolytic, and Lipogenic Subtype.
The metabolite intensities within each subtype were then mapped to known, previously established metabolic ontologies (Dataset S1 and SI Materials and Methods) (25). One subtype (34% of all lines) was especially low in amino acids and carbohydrates (Fig. 1A, left subtype, and Fig. S1C). Cell lines in this subtype had an average doubling time that was significantly higher (Fig. S1D) and were named the slow proliferating subtype. Doubling times for cell lines from the other two subtypes were more similar (Fig. S1D); however, these two subtypes displayed strikingly distinct metabolic profiles, independent of proliferation rate (SI Materials and Methods). Thus, these metabolic subtypes have unique metabolic profiles that are independent of growth rate.
We further explored the metabolic differences between the two subtypes with similar proliferation rates. One subtype (27% of all lines; Fig. 1A) exhibited, on average, elevated levels of various components of the glycolytic and serine pathways, mainly phosphoenolpyruvate (PEP), glyceraldehyde-3-phosphate, lactate, and serine (Fig. 1 B and C and Fig. S1E), and was named the glycolytic subtype. This subtype was also distinguished by lower levels of metabolites important for redox potential such as nicotinamide adenine dinucleotide (NAD) reduced (NADH), NAD phosphate (NADP), NAD phosphate reduced (NADPH), glutathione disulfide (GSSG), glutathione (GSH), and flavine adenine dinucleotide (Fig. 1 B and C, Fig. S1F, and Dataset S4). In contrast, the other subtype (39% of all lines; Fig. 1A) was enriched for various lipid metabolites such as palmitic acid (C16:0), oleic acid (C18:cis[9]1), palmitoleic acid (C16:cis[9]1), and myristic acid (C14:0) (Fig. 1 B and D and Dataset S4), as well as mitochondrial [oxidative phosphorylation (OXPHOS)] metabolites important for the electron transport chain such as coenzyme Q10 and coenzyme Q9 and components of the aspartate-malate shuttle such as aspartate and glutamate (Fig. S1G and Dataset S4), and was named the lipogenic subtype.
Differences Between Glycolytic and Lipogenic Subtypes Are Confirmed Transcriptionally.
We next determined whether differences in metabolite levels observed between the glycolytic and lipogenic subtypes could be explained by differences in the expression of genes known to be associated with the metabolic ontologies (Dataset S5 and SI Materials and Methods). Consistent with the differences in metabolite levels, expression of genes associated with glycolysis and the pentose phosphate pathway were found to be relatively elevated in cell lines from the glycolytic subtype (Fig. 1 E and F, Fig. S1 H and I, and Dataset S6). For example, most glycolytic lines demonstrated higher expression of neuron-specific enolase [ENO2; adjusted P = 0.0016; Fig. 1 E and F], along with higher levels of its product PEP, whereas other enolase homologs were not differentially expressed (Fig. S1J). We also noted that protein (and not mRNA) abundance of the lactate transporter, monocarboxylate transporter 1 (MCT1) was elevated in the glycolytic lines compared with the lipogenic lines (P < 0.05; Fig. 1E and Fig. S1K). In contrast, cell lines within the lipogenic subtype were enriched for expression of lipogenesis genes involved in cholesterol and de novo lipid synthesis including 7-dehydrocholesterol reductase (DHCR7), stearoyl-CoA desaturase (SCD), and fatty acid synthase (FASN) (adjusted P < 0.1; Fig. 1 E and F, Fig. S1 H and L, and Dataset S6). Thus, PDAC-derived cell lines can be clustered by their metabolite profiles and these differences appear to be determined in part by differences in gene expression.
Glycolytic and Lipogenic Subtypes Use Glucose and Glutamine in a Different Manner.
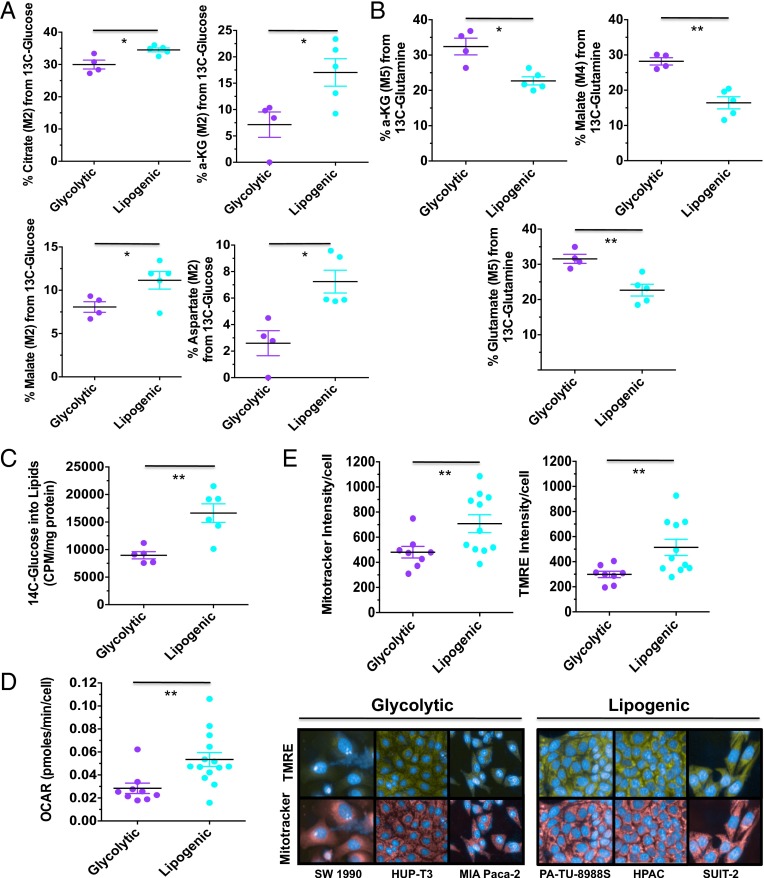
The metabolic and transcriptional profiles suggested that these two subtypes may differ in their use of glucose and glutamine, the most abundant carbon sources available to cancer cells. We predicted that the lipogenic subtype would preferentially use glucose for the tricarboxylic acid (TCA) cycle and lipid synthesis, whereas the glycolytic subtype would use glucose more for aerobic glycolysis, and consequently, use more glutamine for TCA anaplerosis. 13C metabolic mass isotopomer distribution analysis (MIDA) using either [U-13C5]glutamine or [U-13C6]glucose revealed a significant increase in the contribution of [U-13C6]glucose to TCA metabolites in representative cell lines from the lipogenic subtype relative to the glycolytic subtype (Fig. 2A; P < 0.05). In contrast, representative glycolytic lines incorporated [U-13C5]glutamine into TCA metabolites at significantly higher levels than lines from the lipogenic subtype (Fig. 2B; P < 0.05). Moreover, lipogenic cell lines incorporated 14C-glucose into lipid metabolites at a significantly higher level than cell lines from the glycolytic subtype (Fig. 2C; P < 0.01). Consistent with these observations, lipogenic lines showed on average higher O2 consumption (Fig. 2D; P < 0.01) and a greater mitochondrial content [Mitotracker and tetramethylrhodamine ethyl ester (TMRE) intensity] compared with glycolytic subtype lines (Fig. 2E; P < 0.01; Dataset S7). Thus, cell lines from the glycolytic and lipogenic subtypes appear to use glucose and glutamine in a different manner.
Fig. 2.
Functional characterization of glycolytic and lipogenic subtypes. (A) Comparison of relative contribution of glucose oxidation to the TCA metabolites, determined by M2 labeling from [U-13C6]glucose for citrate, αKG, malate, and aspartate between glycolytic and lipogenic cell lines. (B) Comparison of relative contribution of reductive glutamine metabolism to TCA metabolites, determined by M5 labeling from [U-13C5]glutamine for αKG and glutamate, and M4 labeling for malate between glycolytic and lipogenic cell lines. (C) Comparison of relative contribution of glucose metabolism to de novo lipid synthesis between glycolytic and lipogenic cell lines. Cells were labeled with 1 μCi/mL d[U-14C] glucose for 6 h, and lipids were extracted. The incorporation of 14C into lipids was determined by scintillation counting. (D) Comparison of oxygen consumption rates (OCRs) between glycolytic and lipogenic cell lines. (E) Comparison of relative mitochondria number (Mitotracker intensity per cell) and potential/fitness (TMRE per cell) between glycolytic and lipogenic cell lines. For A–E, the mean and SD between cell lines belonging to the glycolytic subtype vs. lipogenic subtype is plotted where each cell line is shown as one dot, representing the mean of three replicates. Data are normalized to sample protein content (A–C) or cell number (D and E). Asterisks denote a statistically significant difference by unpaired t test with Welch’s correction (*P < 0.05, **P < 0.01, ***P < 0.001).
Glycolytic and Lipogenic Cell Lines Show Distinct Sensitivity to Various Metabolic Inhibitors in Vitro.
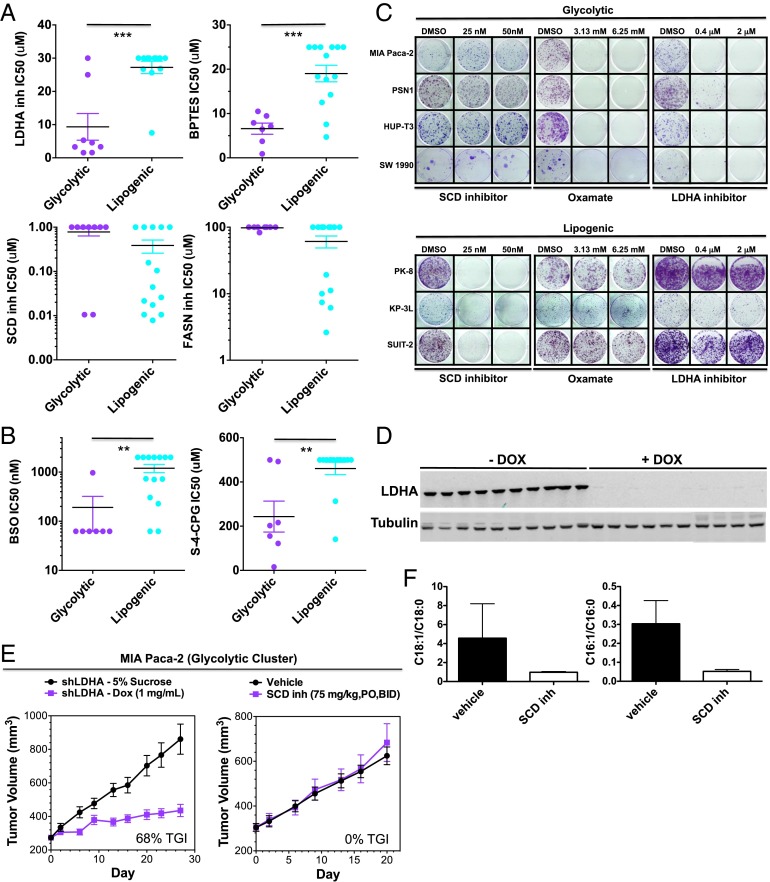
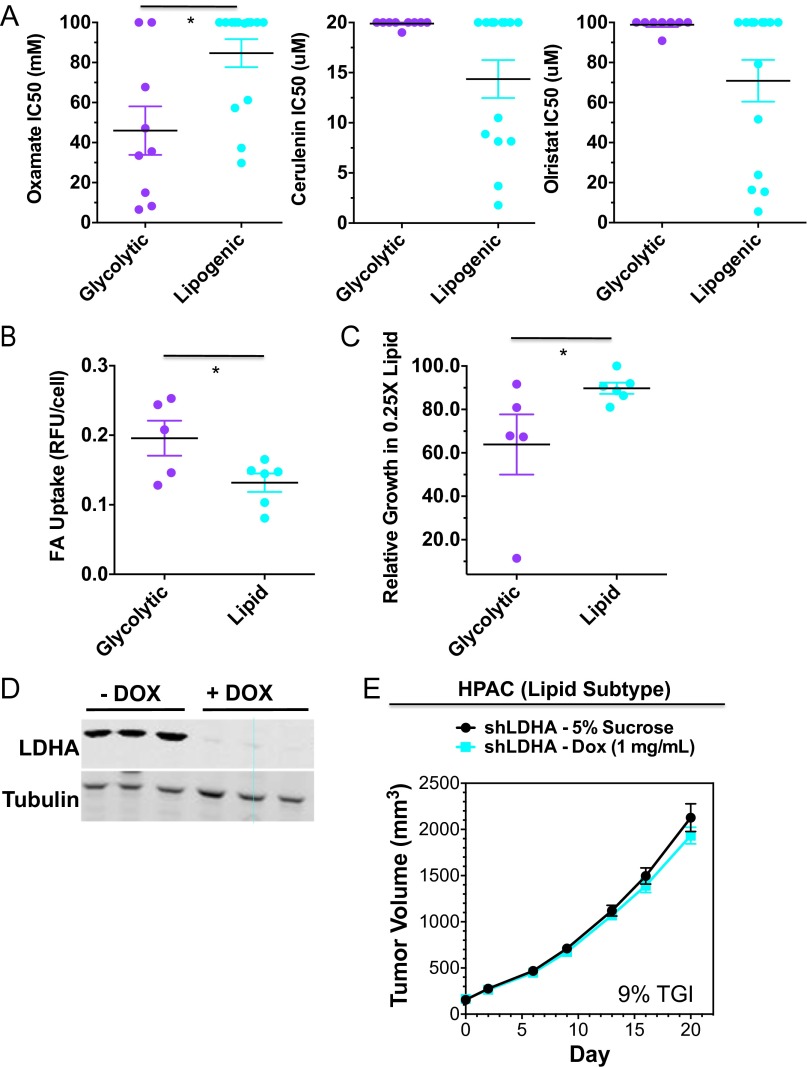
Based on their distinct metabolic wiring, we predicted that glycolytic and lipogenic cell lines would show differential sensitivity to inhibitors targeting aerobic glycolysis (oxamate and the LDHA inhibitor GNE-140) (26), glutaminolysis [bis-2-(5-phenylacetimido-1,2,4,thiadiazol-2-yl)ethyl sulfide (BPTES)], and de novo lipid synthesis [FASN inhibitor GSK1195010 (27), SCD inhibitor (28), cerulenin, and orlistat]. Indeed, as predicted, the glycolytic subtype was enriched for lines that were sensitive to the LDHA inhibitor, oxamate, and BPTES, whereas the lipogenic subtype was enriched for lines that were sensitive to inhibitors targeting lipid synthesis (Fig. 3A and Fig. S2A; P < 0.05; Dataset S7 and SI Materials and Methods). Moreover, glycolytic cell lines showed higher rates of fatty acid (FA) uptake (Fig. S2B) and increased sensitivity to media with reduced lipid content (Fig. S2C), suggesting these lines may be more reliant on FA pathways for generating lipids.
Fig. 3.
Glycolytic and lipogenic cell lines show distinct sensitivity to various metabolic inhibitors both in vitro and in vivo. (A) Comparison of IC50 values to various metabolic inhibitors between representative glycolytic vs. lipogenic cell lines in short-term (3 d) viability assays. Saturated IC50 values correspond to cell lines where an IC50 was not reached at the maximum drug concentration. The mean and SD between cell lines belonging to the glycolytic vs. lipogenic subtype is plotted where each cell line is shown as one dot, representing the mean of three replicates. Asterisks denote a statistically significant difference by Mann–Whitney test (*P < 0.05, **P < 0.01, ***P < 0.001). (B) Comparison of IC50 values to various ROS-inducing agents between representative glycolytic vs. lipogenic cell lines in short-term (3 d) viability assays, similar to A. (C) Comparison of sensitivity to oxamate, LDHA, or SCD inhibitors between representative glycolytic vs. lipogenic cell lines in longer-term (12 d), low seeding density growth assays. (D) Western blots showing 98% in vivo knockdown of LDHA levels in MIA Paca-2 xenografts administered with doxycycline (1 mg/mL) for 8 d vs. 5% sucrose. (E) In vivo knockdown of LDHA (n = 10 for each group) results in 68% TGI, 95% confidence interval [48, 83] in the MIA Paca-2 shLDHA model of a glycolytic subtype tumor, whereas treatment with an SCD inhibitor (75 mg/kg, orally, BID) resulted in no significant change in tumor volume. (F) Confirmed pharmacodynamic inhibition of lipid metabolism by SCD inhibitor. The SCD inhibitor reduces desaturation of palmitate and stearate in MIA Paca-2 shLDHA xenograft tumor tissues and in mouse liver and plasma (n = 5 per group). Data are presented as mean ± SD.
Fig. S2.
Related to Fig. 3. (A) Comparison of IC50 values of lipid synthesis inhibitors cerulenin and orlistat between representative glycolytic and lipogenic cell lines in short-term (3 d) viability assays. The mean and SD between cell lines belonging to the glycolytic vs. lipogenic subtype is plotted where each cell line is shown as one dot, representing the mean of three replicates. Asterisks denote a statistically significant difference by Mann–Whitney test (*P < 0.05, **P < 0.01, ***P < 0.001). Data from Dataset S7. (B) Comparison in baseline fatty acid (FA) uptake between representative glycolytic and lipogenic cell lines. Asterisks denote a statistically significant difference by t test (*P < 0.05, **P < 0.01, ***P < 0.001). (C) Comparison in percent growth in 3.75% delipidated serum:1.25% FBS (relative to 5% FBS) between representative glycolytic and lipogenic cell lines. Asterisks denote a statistically significant difference by Mann–Whitney test (*P < 0.05, **P < 0.01, ***P < 0.001). (D) Western blots showing 98% in vivo knockdown of LDHA levels in HPAC xenografts administered with doxycycline (1 mg/mL) for 8 d vs. 5% sucrose. (E) In vivo knockdown of LDHA results in 9% TGI in the HPAC shLDHA model of a lipogenic subtype tumor.
Maintaining redox balance is another key requirement for cancer cells (29). The differences in redox-related metabolites between glycolytic and lipogenic cell lines suggested that they may also show differential sensitivity to ROS-inducing agents or inhibitors of enzymes or transporters important for maintaining glutathione synthesis and NADP/NADPH balance in cells. Indeed, we found that cell lines within the glycolytic subtype showed enhanced sensitivity to a variety of such agents including inhibitors of gamma-glutamylcysteine synthetase [buthionine sulphoximine (BSO)], and the cystine transporter xCT {(S)-4-carboxyphenylglycine [(S)-4-CPG]} (Fig. 3B and Dataset S7).
In addition to short-term (3 d) culture assays, we tested the efficacy profile of LDHA inhibitor, oxamate, and the SCD inhibitor in long-term (12 d) culture assays and observed similar results (Fig. 3 A and C).
Functional Confirmation of the Glycolytic and Lipogenic Subtype in Vivo.
To translate these findings in vivo and generate proof-of-concept findings for our two metabolic subtypes, we evaluated xenografts of MIA Paca-2, a glycolytic cell line, and HPAC, a lipogenic cell line, for their sensitivity to glycolysis vs. lipid synthesis inhibition. Because oxamate and LDHA inhibitors have poor pharmacokinetics in mice (26), we inhibited glycolysis by engineering MIA Paca-2 and HPAC cells to express a doxycline (DOX)-inducible shRNA against LDHA. MIA Paca-2 xenograft tumors treated with DOX showed undetectable levels of LDHA (Fig. 3D) and 68% tumor growth inhibition (TGI) compared with tumors expressing LDHA (Fig. 3E). In contrast, administration of an SCD inhibitor showed no efficacy (Fig. 3E), although pharmacodynamic inhibition of SCD was seen (Fig. 3F). In contrast, HPAC xenograft tumors showed minimal sensitivity to LDHA knockdown (9% TGI; Fig. S2 D and E) but showed significant tumor growth inhibition to SCD inhibitor treatment (52% TGI) (30). Thus, glycolytic and lipogenic subtypes are functionally distinct and show differential sensitivity to glycolytic and lipid biosynthesis inhibition.
Glycolytic and Lipogenic Subtypes Are Associated with Known Subtypes of PDAC, Driven by Mesenchymal Status.
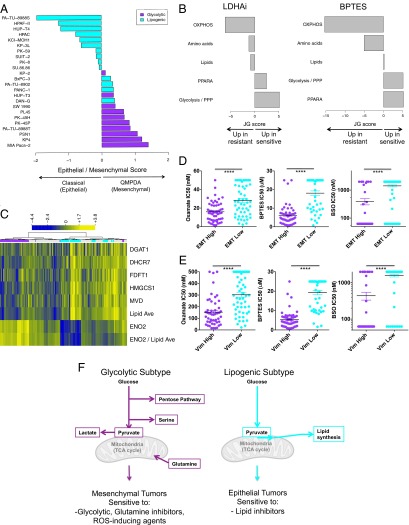
We next set out to determine how our defined metabolic subtypes associated with primary PDAC tumor samples from patients. Three clinical subtypes of PDAC were recently identified through molecular profiling of PDAC tumors: classical (characterized by high expression of adhesion-associated and epithelial genes), quasi-mesenchymal (QM-PDA, characterized by mesenchyme-associated genes), and exocrine-like (22). Because exocrine-like cell lines have not been reported, we simplified the three-subtype PDAC signature to a 42-gene expression signature that distinguishes classical from QM-PDA (22), and applied it to our cell line panel. We found that all cell lines within the glycolytic subtype associated with the quasimesenchymal subtype, whereas most lipogenic lines were associated with the classical subtype (Fig. 4A; P = 0.0006; Dataset S7). Thus, our metabolite subtypes derived from pancreatic cell lines strongly correlate with known subtypes of PDAC tumors, with the glycolytic subtype strongly associating with mesenchymal features and the lipogenic subtype associating with epithelial features.
Fig. 4.
Metabolic and mesenchymal markers predict response to glycolytic and glutaminolytic inhibitors in PDAC and other tumor types. (A) Epithelial/mesenchymal score for the glycolytic and lipogenic cell lines based on a 42-gene set characteristic of the classical and QM-PDA subtypes (22). The score is defined as the difference in average expression of QM-PDA vs. classical genes, with a positive score indicative of QM-PDA and a negative score of classical. Cell lines are colored by metabolic subtype, with glycolytic lines in purple and lipogenic lines in cyan. All glycolytic cell lines are of the QM-PDA subtype, whereas lipogenic cell lines are associated with the classical subtype (Fisher’s exact test, P = 0.0006). (B) Relative enrichment of the five curated metabolism gene sets in cell lines that are sensitive (positive JG score) or resistant (negative JG score) to LDHA inhibitor or BPTES in a pan-cancer panel of 204 and 167 nonpancreatic cell lines, respectively, after exclusion of cell lines with intermediate response. See Dataset S5 for a list of genes per gene set. (C) Metabolic dependency preference in the panel of ∼200 nonpancreatic cell lines is based on the ratio of ENO2 expression to the average expression of five lipid genes, and labeled on top of the heatmap as glycolytic in purple (ratio > third quartile), lipogenic in cyan (ratio < lower quartile), and undefined type in gray (ratio between lower and third quartile). Shown are expression (log2 RPKM + 1) of glycolytic gene ENO2, five lipid genes DGAT1, DHCR7, FDFT1, HMGCS1, and MVD, average expression of the five lipid genes (Lipid Ave), and the ratio of ENO2 to average lipid expression (ENO2/Lipid Ave). Data from Dataset S8. (D) High expression of a pan-cancer EMT signature (EMT) associates with sensitivity to oxamate, BPTES, and BSO across a variety of tumor types. EMT low is defined by RPKM values < lower quartile, EMT high = RPKM values > third quartile. Asterisks denote a statistically significant difference by Mann–Whitney test (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). (E) High expression of mesenchymal marker vimentin (Vim) associates with sensitivity to oxamate, BPTES, and BSO across a variety of tumor types. Vim low is defined by RPKM values < lower quartile, Vim high = RPKM values > third quartile. Asterisks as per D. (F) Model of preferential glucose and glutamine utilization in the glycolytic vs. lipogenic subtype.
Metabolic and Mesenchymal Markers Predict Response to Glycolytic and Glutaminolytic Inhibitors in PDAC and Other Tumor Types.
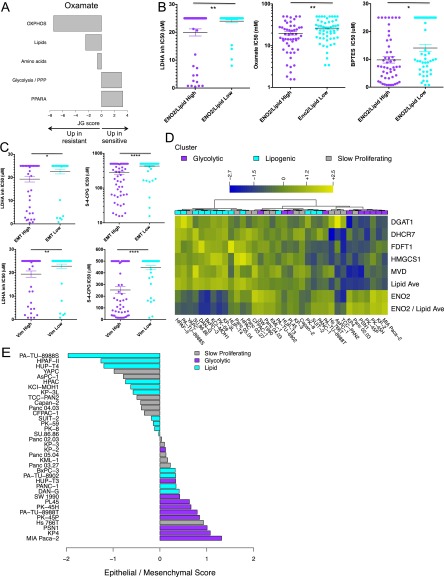
Carcinomas with mesenchymal features (including PDAC) tend to be more aggressive and typically have an overall poorer prognosis (22, 31, 32). Given the strong association between quasi-mesenchymal status and glycolytic dependency in the PDAC lines, we asked whether this association might also exist in other tumor types. We screened ∼200 nonpancreatic cancer cell lines, representing various tumor types, for sensitivity to inhibitors of aerobic glycolysis and glutaminolysis, as well as to ROS-inducing agents (Dataset S8). As in PDAC (Fig. 1E and Fig. S1H), we found that cell lines most sensitive to the LDHA inhibitor, oxamate, and BPTES were associated with a glycolytic signature, whereas cell lines that were most resistant to these inhibitors were associated with an OXPHOS signature (Fig. 4B and Fig. S3A). We next assigned each cell line to a metabolic subtype (glycolytic vs. lipogenic) using the glycolytic and lipid genes that were most differentially expressed in the PDAC metabolic subtypes (adjusted P < 0.05). Using the ratio of expression of glycolytic gene ENO2 to the average expression of lipid genes diacylglycerol O-acyltransferase 1 (DGAT1), DHCR7, farnesyl-diphosphate farnesyltransferase 1 (FDFT1), 3-hydroxy-3-methylglutaryl-CoA synthase 1 (HMGCS1), and mevalonate (diphospho) decarboxylase (MVD) clearly distinguished nonpancreatic cell lines by metabolic dependency preference (Fig. 4C). A glycolytic preference in nonpancreatic lines associated with sensitivity to LDHA inhibitor, oxamate, and BPTES (Fig. S3B; P < 0.05; Dataset S8). In addition, consistent with our findings in the PDAC tumors, mesenchymal tumors [according to a pan-cancer epithelial-mesenchymal transition (EMT) signature (33) or vimentin] were more sensitive to the LDHA inhibitor, oxamate, BPTES, and ROS-inducing agents [BSO and (S)-4-CPG] across a variety of tumor types (Fig. 4 D and E and Fig. S3C; P < 0.001; Dataset S8). A similar discrepant dependency was observed in the slow proliferating PDAC cell lines, with six lines more glycolytic and/or mesenchymal and six lines more lipogenic and/or epithelial, despite their slower proliferation (Fig. S3 D and E). Thus, mesenchymal tumors, regardless of indication, appear to share common metabolic vulnerabilities, and agents that block glycolysis, glutamine metabolism, or redox balance may be particularly effective. These results support a model in which metabolic plasticity with regard to bioenergetic pathways is limited, and, consequently, unique metabolic dependencies exist in tumors that can be exploited for cancer therapy based on tumor subtype.
Fig. S3.
Related to Fig. 4. (A) Relative enrichment of the five curated metabolism gene sets in cell lines that are sensitive (positive JG score) or resistant (negative JG score) to oxamate in a pan-cancer panel of 133 nonpancreatic cell lines after exclusion of cell lines with intermediate response. See Dataset S5 for a list of genes per gene set. (B) Ratio of ENO2 expression to average lipid gene expression associates with sensitivity to LDHA inhibitor, oxamate, and BPTES across a variety of tumor types. Saturated values correspond to cell lines where an IC50 was not reached at the maximum drug concentration. Low is defined by RPKM values < lower quartile; high = RPKM values > third quartile. (C) High expression of a pan-cancer EMT signature (EMT) and mesenchymal marker vimentin (Vim) associates with sensitivity to LDHA inhibitor and (S)-4-CPG across a variety of tumor types. EMT and Vim low are defined by RPKM values < lower quartile, EMT and Vim high = RPKM values > third quartile. Asterisks denote a statistically significant difference by Mann–Whitney test (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). (D) Metabolic dependency preference in the panel of 36 PDAC cell lines is based on the ratio of ENO2 expression to the average expression of five lipid genes. Shown are expression (log2 RPKM + 1) of glycolytic gene ENO2, five lipid genes DGAT1, DHCR7, FDFT1, HMGCS1, and MVD, average expression of the five lipid genes (Lipid Ave), and the ratio of ENO2 to average lipid expression (ENO2/Lipid Ave), as per Fig. 4C. Slow proliferating lines are labeled in gray, glycolytic lines in purple, and lipogenic in cyan. Six Slow proliferating lines favor the glycolytic phenotype, and six favor the lipogenic phenotype. (E) Epithelial/mesenchymal score for all PDAC lines based on a 42-gene set characteristic of the classical and QM-PDA subtypes (22). The score is defined as the difference in average expression of QM-PDA vs. classical genes, with a positive score indicative of QM-PDA and a negative score of classical. Cell lines are colored by metabolic subtype, with slow proliferating lines in gray, glycolytic lines in purple, and lipogenic lines in cyan. Six slow proliferating lines are strongly epithelial (of which four are more lipogenic based on expression profiling in D), and six are more mesenchymal (of which four are more glycolytic based on expression profiling).
Discussion
Using broad metabolite profiling, we successfully stratified PDAC-derived cell lines into discrete metabolic subtypes. Previous metabolic profiling studies have been conducted in tumors and in cell lines of the NCI-60 panel with different end points (9). However, this study is the first, to our knowledge, to successfully identify metabolic subtypes through profiling of a large number of samples within one tissue type and to demonstrate that each subtype is enriched for drug sensitivity to unique classes of metabolic inhibitors.
Although metabolic clustering accounted for a substantial fraction of the drug response variation observed across cancer cell lines, some heterogeneity in drug response within the lipogenic subtype remained (see SI Text and Figs. S4 and S5 for a discussion on heterogeneity). Some cell lines were clearly “hard-wired” for lipogenesis and showed sensitivity to all lipid inhibitors tested, whereas the more refractory lines appeared to be capable of switching to alternative pathways, perhaps those involving fatty acid uptake. Further understanding of the nature and plasticity of metabolic networks in these cancer cells will be required to more accurately predict their sensitivity to specific classes of metabolic inhibitors. In addition, although we successfully translated our in vitro findings in vivo, additional factors within the tumor microenvironment (tumor-stroma signaling, angiogenesis, and hypoxia) will influence sensitivity and adaptation to metabolic inhibition in vivo.
Fig. S4.
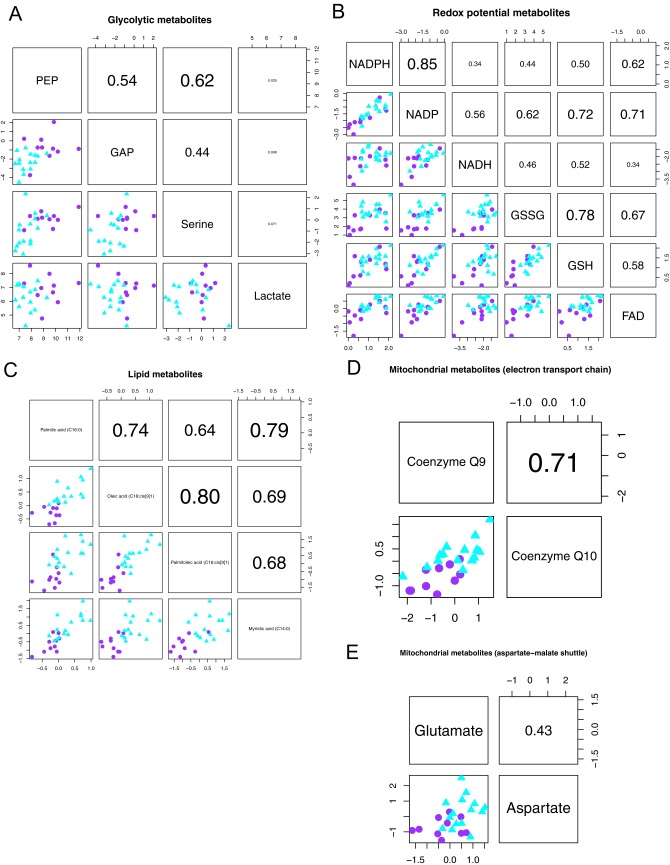
Related to SI Text. Overlap in metabolite intensities between the glycolytic and lipogenic subtypes is not indicative of a phenotype that partially reflects the glycolytic and lipogenic subtypes. Association plots are shown per set of metabolites: A, glycolytic metabolites; B, redox potential metabolites; C, lipid metabolites; D, mitochondrial metabolites important for the electron transport chain; E, mitochondrial metabolites from the aspartate-malate shuttle. Shown below the diagonal are scatter plots for each pairwise comparison of metabolites, with relative intensity levels on the x and y axes. Each dot represents a cell line, with glycolytic lines in purple (circle) and lipogenic lines in cyan (triangle). Above the diagonal are the respective Spearman correlation coefficients.
Fig. S5.
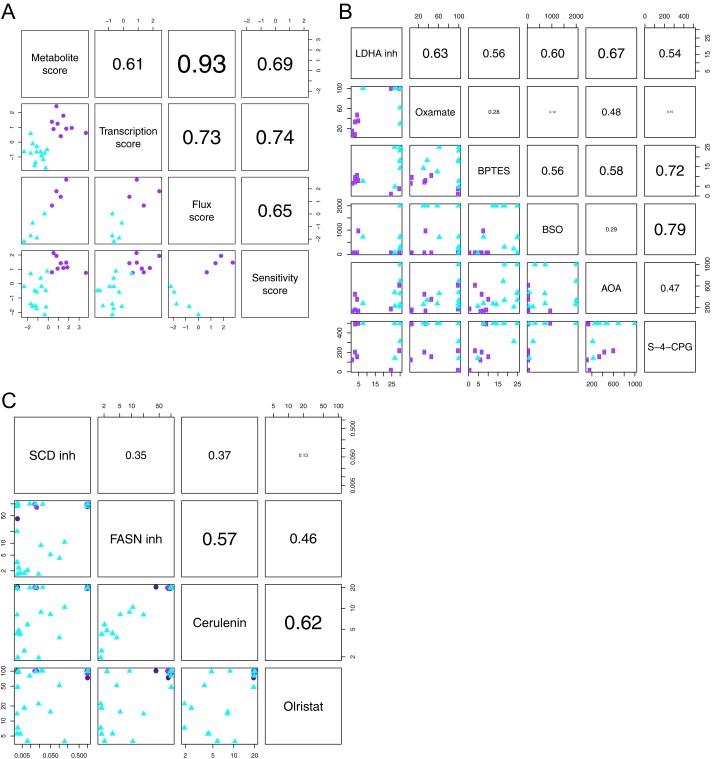
Related to SI Text. (A) The metabolomics data, transcription profiles, flux experiments, and drug sensitivity confirm robust differences between glycolytic and lipogenic subtype cell lines. PDAC-derived lines were ranked by metabolic dependency for each data type separately, defined as the difference in profile between the glycolytic and lipogenic subtype cell lines, and labeled as metabolite score, transcription score, flux score, and sensitivity score. Shown below the diagonal are scatter plots for each pairwise comparison of scores. (B) Concordance in drug sensitivity to inhibitors of aerobic glycolysis, glutaminolysis, and ROS. Shown below the diagonal are scatter plots for each pairwise comparison of compounds, with IC50 values on the x and y axes. Data are from Dataset S7. (C) Concordance in sensitivity to lipid synthesis inhibitors. Shown below the diagonal are scatter plots for each pairwise comparison of compounds, with IC50 values on log10 scale on the x and y axes. Data are from Dataset S7. For A–C, each dot represents a cell line, with glycolytic lines in purple (circle) and lipogenic lines in cyan (triangle). Above the diagonal are the respective Spearman correlation coefficients.
Our study also identified PEP as one of the most differentially expressed metabolites between glycolytic and lipogenic cell lines. ENO2, which converts 2-phosphoglycerate (2-PG) to PEP, was also one of the most differentially expressed genes between these two subtypes, suggesting that inhibitors of ENO2 may be particularly effective against glycolytic tumors. Enolases act downstream of phosphoglycerate mutase (PGAM1) and regulate pyruvate kinase (PK) M2 isoform (PKM2), genes that are particularly active in glycolytic tumors and have recently attracted attention for their role in serine biosynthesis through regulation of 3-phosphoglycerate dehydrogenase (PHGDH) (34). ENO2 has also been proposed as a target in ENO1-deleted glioblastomas (35). Our findings further substantiate the biological rationale for targeting ENO2 in a subset of cancers.
Finally, we demonstrated that the observed metabolic subtypes correlate with epithelial vs. (quasi)-mesenchymal cell states both in PDAC and other cancer types. We propose a model (Fig. 4F) in which mesenchymal tumors are metabolically wired to preferentially use glucose for glycolysis and lactate production and use glutamine for generating TCA metabolites, whereas epithelial tumors preferentially use glucose for the TCA cycle and de novo lipogenesis. Moreover, our analysis suggests that mesenchymal tumors may be more vulnerable to ROS-inducing agents, potentially through differences in NADPH balance and antioxidant responses (36).
Such differences in metabolic vulnerabilities between epithelial and mesenchymal states could arise from the activation of signaling pathways associated with these states. For example, epithelial subtypes have previously been shown to be enriched for activating mutations in receptor tyrosine kinases (RTK) such as EGFR (37) and PI3K/AKT signaling pathways (23), leading to activation of the mechanistic target of rapamycin (mTOR). mTOR increases both protein synthesis and lipogenesis through mechanisms including enzyme phosphorylation and transcriptional activation of EIF1A (38) and SREBP1 (39–41). In contrast, mesenchymal states are associated with increased c-Myc expression and HIF1A, which have been shown to drive a glycolytic profile (42, 43). Regardless of the nature or mechanism of action for the metabolic variation we observed, our data provide valuable predictive utility and thereby inform clinical evaluation of a variety of metabolic inhibitors such as MCT and glutaminase inhibitors currently undergoing phase I testing across a variety of tumor indications.
Materials and Methods
Detailed materials and methods can be found in SI Materials and Methods. All cell lines listed in Dataset S9 were grown in RPMI (without glucose, without glutamine) media (US Biological #R9011) supplemented with 6 mM glucose, 2 mM glutamine, 5% FBS, 100 μg/mL penicillin, and 100 U/mL streptomycin. Metabolite profiling was performed as previously described (44). For flux analysis, cells were cultured for ∼18 h in RPMI with 10% (vol/vol) dialyzed FBS supplemented with either 3 mM d[U-13C]glucose or 1 mM l[U-13C]glutamine. Data analysis was carried out with the MultiQuant software. For short-term viability assays, cells were plated using optimal seeding densities in 384-well plates. The following day, cells were treated with LDHA inhibitor GNE-140 (26), oxamate (Sigma cat# O2751), SCD inhibitor (28), FASN inhibitor GSK1195010 (27), cerulenin, orlistat, BSO, S-4-CPG, aminooxyacetic acid (AOA), and BPTES (45), using a 6-pt dose titration scheme. After 72 h, cell viability was assessed using the CellTiter-Glo Luminescence Cell Viability assay. Absolute inhibitory concentration (IC) values were calculated using four-parameter logistic curve fitting and are averages from a minimum of two independent experiments. For long-term growth assays, glycolytic cell lines (MIA Paca-2, SW 1990, PSN1, and HUP-T3) and lipogenic cell lines (PA-TU-8902, PK-8, KP-3L, and SUIT-2) were seeded in a 6-well dish at 3,000 cells per well overnight (RPMI, 5% serum, 2 mM glutamine) and then treated in media with indicated concentrations of oxamate, SCD inhibitor, or DMSO for 12 d at 37 °C and 5% CO2. Fatty uptake assays were performed using the Free Fatty Acid Uptake Assay Kit (ab176768) according to the manufacturer’s protocol. Reduced serum experiments were carried out using 3% delipidated serum (SeraCare 502099) and 1% FBS (SeraCare CC5010-500). Seahorse Bioscience assays were used for oxygen consumption. All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Genentech and carried out in an AAALAC (Association for the Assessment and Accreditation of Laboratory Animal Care) accredited facility. All statistical analyses were performed in R 3.0.0 (46). The optimal number of metabolic subtypes was obtained with nonnegative matrix factorization, using the NMF package. The DESeq2 package was used for differential expression analysis. Metabolic ontology and gene set enrichment analyses were based on GSEAlm.
SI Text
Supplementary Note on Metabolic, Transcriptional, and Drug Response Heterogeneity Within the Glycolytic and Lipogenic Subtypes.
Although we successfully identified three metabolic subtypes for PDAC through metabolite profiling, we observed heterogeneity within each subtype. We investigated whether this heterogeneity was due to the existence of additional subtypes (or additional subtype complexity) that we did not capture with our three metabolic subtypes. NMF clustering resulted in the identification of three subtypes (Fig. S1A). The metabolite data did not justify four subtypes, with stability and robustness substantially less in case of four subtypes compared with three (Fig. S1B). This robustness argues that—at least at the metabolite level —there is no well-defined set of lines with a phenotype partially shared between multiple subtypes.
Analysis by ranking of the glycolytic and lipogenic cell lines from low to high intensity per metabolite further suggested that additional intermediate groups do not likely exist (Fig. S4). For glycolytic metabolites (Fig. 1C and Fig. S1E), cell lines with intermediate intensity levels (either low expressing glycolytic or high expressing lipogenic lines) are not shared between PEP, glyceraldehyde 3-phosphate (GAP), serine, and lactate. For redox potential metabolites (Fig. 1C and Fig. S1F), there is consistency in intensity levels for interconvertible metabolites GSSG and GSH and NADPH and NADP, but less so for other pairs of redox metabolites. Although palmitic acid, oleic acid, and palmitoleic acid show consistent levels for lipogenic cell lines, there is no correlation among these fatty acids within the set of glycolytic cell lines (Fig. 1D). Mitochondrial metabolites coenzyme Q9 and Q10 represent similar readouts of the electron transport chain, whereas there is minimal consistency for aspartate and glutamate (Fig. S1G). Although no individual metabolite can perfectly separate one subtype from the other subtypes, a panel of multiple metabolite measurements robustly separates glycolytic from lipogenic cell lines (Fig. S5A).
We also investigated the degree to which transcription profiles, MIDA experiments, and sensitivity to metabolic inhibitors align with the metabolic dependency derived from metabolomics data. Although no perfect separation of glycolytic from lipogenic lines could be achieved based on any one individual measurement, the analyses and experiments showed good concordance when taking all measurements per analysis/experiment into account and support the existence of the stable and reproducible glycolytic and lipogenic subtypes (Fig. S5A). We also ranked the glycolytic and lipogenic cell lines by metabolic dependency based on each data type separately and compared these rankings. The flux experiments on the use of glucose and glutamine are in highest concordance with the glycolytic and lipogenic metabolic subtypes. Within the lipogenic subtype, limited heterogeneity was present at transcriptional level and in drug sensitivity. At the transcriptional level, the lipogenic cell line PANC-1 showed a glycolytic transcription profile. In terms of sensitivity profiles, lipogenic cell lines PANC-1, SU.86.86, and PK-59 showed a glycolytic sensitivity profile. Correlation between any of the four rankings shown in Fig. S5A ranges from 0.61 (metabolite vs. transcription score) to 0.93 (metabolite vs. flux score). Overall, the metabolomics data, transcription profiles, flux experiments, and drug sensitivity confirm robust differences between glycolytic and lipogenic cell lines.
Our metabolite profiling classification succeeded in enriching for drug sensitivity. The class of glycolytic cell lines responds consistently to inhibitors of aerobic glycolysis, glutaminolysis, and ROS (Fig. S5B). All glycolytic cell lines responded to BPTES, all but two cell lines responded to the LDHA inhibitor and oxamate, and all but two cell lines responded to S-4-CPG. Although the lipogenic cell lines showed more drug response heterogeneity, there was good concordance in drug sensitivity between all of the lipid inhibitors (SCD inhibitor, FASN inhibitor, cerulenin, and olristat) (Fig. S5C). In addition, the five lipogenic lines that were resistant to all lipid synthesis inhibitors were also resistant to LDHA inhibitor, BPTES, and oxamate treatment.
SI Materials and Methods
Metabolite Profiling.
All cell lines were grown in RPMI (without glucose, without glutamine) media (US Biological #R9011) supplemented with 6 mM glucose (Teknova # G9005), 2 mM glutamine (Gibco #25030–081), 5% (vol/vol) FBS (Sigma F4135), 100 μg/mL penicillin, and 100 U/mL streptomycin (Gibco 15140–122).
Briefly, cells were plated on LUMOX gas permeable membranes (Sarstedt no. 94.6077.331) in six-well plates at a concentration range of 5–7.5 × 105 per well. After 48 h, LUMOX membranes containing the cells were removed from the well with a scalpel. Samples subjected to MxP broad profiling (44) were washed two times in 0.9% NaCl. Samples subjected to MxP energy profiling were washed two times in 0.9% NaCl and 4.5 g/L glucose. Membranes were then immediately transferred to an Eppendorf tube containing quenching solution (45% dichloromethane, 55% ethanol) on dry ice and transferred to liquid nitrogen.
Sample preparation and metabolite profiling of intracellular metabolites (MxP broad profiling) (44).
Samples were prepared and subjected to LC-MS/MS and GC-MS analysis as described below. Several groups of metabolites were analyzed semiquantitatively or quantitatively including amino acids, carbohydrates, fatty acids, mono, di-, and triglycerides, other lipids, organic acids, coenzymes, vitamins, and secondary metabolites.
Adherent cells were cultured on LUMOX plates (Sarstedt no. 94.6077.331). Once the incubation time was completed, the membranes were cut out of the LUMOX plates and were washed in 5 mL isotonic NaCl (0.9%) at 37 °C. This washing step was repeated a second time in a fresh solution of the same composition. Cells were then quenched by placing the membranes in a polypropylene tube and the addition of 600 mL of a dichloromethane/ethanol 9:11 (vol/vol) solution at −80 °C. After addition of water, the samples were extracted using a ball mill (Retsch), filtered through a centrifuge filter (Millipore; mesh size, 0.2 mm), and fractionated into an aqueous, polar phase and an organic, lipophilic phase.
For the transmethanolysis of the lipid extracts (lipophilic phase), a mixture of 140 µL chloroform, 37 µL hydrochloric acid (37% by weight HCl in water), 320 µL methanol, and 20 µL toluene was added to the evaporated extract. The vessel was sealed tightly and heated for 2 h at 100 °C, with shaking. The solution was subsequently evaporated to dryness. The residue was dried completely.
The methoximation of the carbonyl groups was carried out by reaction with methoxyamine hydrochloride (20 mg/mL in pyridine, 100 µL for 1.5 h at 60 °C) in a tightly sealed vessel. Twenty microliters of a solution of odd-numbered, straight-chain fatty acids [solution of each 0.3 mg/mL of fatty acids from 7 to 25 carbon atoms and each 0.6 mg/mL of fatty acids with 27, 29, and 31 carbon atoms in 3/7 (vol/vol) pyridine/toluene] was added as time standards. Finally, the derivatization with 100 µL of N-methyl-N-(trimethylsilyl)-2,2,2-trifluoroacetamide (MSTFA) was carried out for 30 min at 60 °C, again in the tightly sealed vessel. The final volume before injection into the GC was 200 µL.
For the dried polar phase, the derivatization was performed in the following way. The methoximation of the carbonyl groups was carried out by reaction with methoxyamine hydrochloride (20 mg/mL in pyridine, 50 µL for 1.5 h at 60 °C) in a tightly sealed vessel. Ten microliters of a solution of odd-numbered, straight-chain fatty acids [solution of each 0.3 mg/mL of fatty acids from 7 to 25 carbon atoms and each 0.6 mg/mL of fatty acids with 27, 29 and 31 carbon atoms in 3/7 (vol/vol) pyridine/toluene] was added as time standards. Finally, the derivatization with 50 µL MSTFA was carried out for 30 min at 60 °C, again in the tightly sealed vessel. The final volume before injection into the GC was 100 µL.
The GC-MS systems consist of an Agilent 6890 GC coupled to an Agilent 5973 MSD (Agilent), and autosamplers were CompiPal or GCPal from CTC.
In LC-MS analysis, both fractions were reconstituted in appropriate solvent mixtures. HPLC was performed by gradient elution on reversed phase separation columns. MS detection, which allows target and high-sensitivity multiple reaction monitoring (MRM) profiling in parallel to a full screen analysis, was performed as previously outlined in Patent WO2003073464 on mass spectrometry methods for analyzing mixtures of substances. The HPLC instruments were Agilent 1100 (Agilent), and the MS instruments were API4000 from SCIEX (AB SCIEX).
The data were normalized to the protein content, determined as the median of protein contents of three parallel cultures per treatment group.
Sample preparation and measurement of intracellular energy metabolites (MxP energy profiling).
Phosphorylated or carboxylated metabolites like ATP, NADH, intermediates of glycolysis, mevalonate pathway, purine and pyrimidine metabolism, pentose phosphate cycle, and Krebs cycle were extracted and analyzed semiquantitatively with a special ultra performance liquid chromatography (UPLC)-MS/MS method. Adherent cells were cultured on LUMOX plates (Sarstedt no. 94.6077.331). Once the incubation time was completed, the membranes were cut out of the lumox plates and were washed with 5 mL isotonic NaCl (0.9%) solution containing 4.5 g/L glucose, preconditioned at 37 °C. This washing step was repeated a second time using a fresh solution of the same composition. Cells were then quenched by placing the membranes in a polypropylene tube containing metal beads (Peqlab tubes) and 900 mL of a dichloromethane/ethanol 2:1 (vol/vol) solution at −80 °C.
Ammonium acetate 1.5 M (100 mL) and a uniformly 13C labeled internal standard (50 mL) were added to the membranes for extraction. The internal standard consisted of an aqueous cell extract from yeast grown on U13C glucose substrate. The samples were homogenized using a high-speed benchtop homogenizer for 30 s at 6.5 m/s (FastPrep-24; MP Biomedicals). The samples were centrifuged for 2 min at 4 °C (20,000 × g), and 100-mL portions from the aqueous phases were transferred to a centrifugal filter (Millipore; mesh size, 0.2 mm). A second portion of ammonium acetate 1.5 M (150 mL) was added in the polypropylene tubes for a second extraction. The samples were again homogenized using the high-speed homogenizer and centrifuged as above. A 200-mL fraction from the aqueous phase was transferred to the centrifugal filter and combined with the first aqueous phase fraction that was collected. The samples were centrifuged for 5 min (20,000 × g) at 4 °C. The filters were rinsed with water portions (200 mL), which were combined with the samples and transferred to high recovery glass vials. The samples were freeze dried for 20 h.
Chromatographic separation of polar metabolites was achieved with an ultra-high pressure ion pairing liquid chromatography (IP-UPLC) system (Acquity; Waters). A chromatographic gradient between a solvent A (deionized water) and a solvent B [50% acetonitrile, 50% water (vol/vol)] was used, with a flow rate of 0.4 mL/min and a column oven temperature of 45 °C. Tributylamine was added as ion pairing agent to both eluents. The lyophilized samples were dissolved in 100 µL water. Injection volume was 5 µL.
Negative mode electrospray tandem MS (−ESI-MS/MS) was used to assess the polar metabolites separated by UPLC. The tandem MS/MS (API 5500; AB SCIEX) was operated in MRM mode. Isotopically labeled and nonlabeled forms of individual metabolites were distinguished by different mass traces.
All metabolite signals were normalized to their isotopically labeled counterpart. Subsequently, the data were normalized to the protein content, determined as the median of protein contents of three parallel cultures per treatment group.
Metabolite Data Processing.
With five technical replicates per cell line, outliers were defined as replicates with a correlation <0.7 with at least three of the four other replicates for a particular cell line. Ten samples from the broad profiling platform fulfilled this criterion and were excluded (one replicate for eight cell lines and two replicates for one cell line). Data were subsequently median normalized per sample. Metabolites with missing values for all replicates in at least one cell line were also excluded. Other missing values were imputed by the average intensity level of a particular metabolite with missing value(s) in a particular cell line. These processing steps excluded 15 metabolites from the broad profiling platform and 2 from the energy platform. Finally, metabolite intensities were averaged across replicates for each cell line. Data from both platforms were analyzed together. Both known and unknown metabolites were included in analyses, unless stated otherwise.
Subtypes were identified using metabolites with reproducible variation (153 of 256). Metabolites with reproducible variation were defined as those with a SD across all samples exceeding two times the pooled SD among replicates of each cell line. The percentage of metabolites with reproducible variation greatly differed between platforms, with 71% (n = 137) of metabolites from the broad profiling platform showing larger overall variation compared with within-replicate variation and 26% (n = 16) of metabolites measured on the energy platform.
Metabolic Subtype Identification.
Consensus NMF clustering was applied for the identification of an optimal number of subtypes. The Brunet algorithm was used from the NMF package in R version 3.0.0 (21, 46, 48, 49). We applied NMF to log10 intensity ratio data for the subset of the 153 most reproducible metabolites in 38 pancreatic cell lines, with intensity levels averaged across replicates per cell line. Metabolites measured on the broad profiling and energy platforms were combined. For each number of subtypes varying from 2 to 7, a consensus matrix was obtained from 50 runs. This matrix represents the average cell line-by-cell line connectivity matrix across all runs. Entries range from 0 to 1 and represent the fraction of runs in which cell lines are assigned to the same subtype. From the consensus matrices, two summary measures were obtained: the cophenetic coefficient as measure of subtype stability and the dispersion coefficient as measure of subtype reproducibility (21). After determining the optimal number of subtypes corresponding to high cophenetic and dispersion coefficients, the final subtype assignment was regenerated for this number of subtypes, using 200 runs.
For the heatmap with log2 intensity ratio data in Fig. 1A, metabolites in rows were clustered using Ward’s method for hierarchical clustering with Euclidean distance. Cell lines in columns were grouped by metabolic subtype, using prior unsupervised clustering to obtain an optimal order of cell lines per subtype. The heatmap includes data for the 99 metabolites that are significantly different in at least one subtype compared with the other two subtypes (F test, P < 0.05), and for which ontology information is available. Sixty-four of the 99 metabolites had reproducible variation. Metabolites are scaled across all cell lines, centered to mean = 0 and scaled to SD = 1.
RNAseq Expression Data Processing.
For 36 of 38 pancreatic cell lines (not available for lines PK-1 and Panc 10.05) and for the pan-cancer panel of cell lines, RNA libraries were made with the TruSeq RNA Sample Preparation kit (Illumina) according to the manufacturer’s protocol. The libraries were sequenced on an Illumina HiSEq. 2000, using one to four lanes per cell line. We generated a median of 61 million reads per sample, of which we were able to map a median of 49 million reads uniquely to the human genome and concordant with established gene models. Reads were trimmed to 75 bp and filtered for quality and rRNA contamination. Genomic alignment was performed using GSNAP (50, 51). RPKM (reads per kilobase per million mapped reads) values on log2 scale were used in all analyses. RNA sequencing data are available at the European Genome-phenome Archive (www.ebi.ac.uk/ega/), under accession no. EGAS00001000610 (51).
To find metabolism-associated genes differentially expressed between the glycolytic and lipogenic cell lines shown in Fig. 1E and Dataset S6, we started from a comprehensive list of 2,581 metabolism genes (52). The DESeq2 R package for R version 3.0.0 was used for differential expression analysis.
Before differential expression and gene set enrichment analysis, RNAseq data for 2,581 metabolism genes were investigated in 40 pancreatic cell lines and 194 nonpancreatic cell lines with response to oxamate and/or BPTES. We excluded 588 poorly expressed metabolism genes, with average RPKM and 90th percentile of RPKM across all 234 cell lines below 1. The remaining 1,993 metabolism genes were included in subsequent analyses.
Metabolic Ontology Enrichment Analysis.
For metabolite-based enrichment analysis, we started from established biological categories from ref. 25, listed in Dataset S1 as super- and subpathways, and derived ontology groups for enrichment analysis. We merged small, related superpathways and divided large superpathways into informative ontology groups. We combined superpathways amino acids and amino acids related into the ontology group amino acids. We distinguished fatty acids, cholines, sphingolipids, and other complex lipids in the broader superpathway complex lipids, fatty acids and related, after a preliminary observation that complex lipids and fatty acids of distinct type show different associations with the metabolic subtypes. Similarly, vitamins, cofactors and related was divided into REDOX (mitochondria-related) and other vitamins and cofactors. We left other medium-sized superpathways intact. Each of these ontology groups in Dataset S1 was tested for enrichment of metabolites with high or low intensity in one particular subtype compared with other subtypes. All 256 metabolites were included, regardless of variation reproducibility. Ranked lists of metabolites based on the T-statistic were obtained from all representative lines of each subtype using the limma package in R version 3.0.0 (46). Permutation-based P values for ontology enrichment were derived from 10,000 permutations of the cell line subtype labels, using the GSEAlm package in R. For each ontology, a JG statistic was calculated proportional to the sum of T-statistics across that ontology’s metabolite set and rescaled to the square root of the ontology set size (47). Ontology information for all 256 metabolites is available in Dataset S1.
RNAseq data were used for enrichment analysis of specific gene sets (Dataset S5) that cover the established metabolic ontologies for which we have metabolite intensity data. These gene sets were chosen before data analysis. We used publicly available metabolism-associated gene sets where appropriate: branched chain amino acid catabolism (amino acids) from Reactome, regulation via peroxisome proliferator-activated receptor alpha (PPARA) from BioCarta, genes involved in oxidative phosphorylation from ref. 53 (oxidative phosphorylation), and a glycolysis signature from ref. 54. The metabolic ontology energy includes both glycolytic and pentose phosphate metabolites (Dataset S1). We therefore extended the glycolysis signature with an in-house set of 16 pentose phosphate genes (glycolysis/PPP). To cover lipogenesis, we used an in-house set of 22 lipid-associated genes (lipids). All gene sets except for the glycolysis set and two in-house sets were downloaded from the Molecular Signature Database (MSigDB), v4.0 (55). After exclusion of consistently low-expressed metabolism genes, ranked lists of genes differential expressed between glycolytic subtype vs. lipogenic subtype cell lines or between cell lines sensitive vs. resistant to the glycolytic inhibitors oxamate and BPTES were based on the Wald statistic and obtained with the DESeq2 R package using R version 3.0.2. The GSEAlm R package was used to calculate JG statistics and P values based on 1,000 permutations for each gene set. As reference, the list of metabolism genes obtained from ref. 52 was included as gene set.
Metabolic Subtype Characterization and Dependence on Proliferation Rate.
We investigated the degree to which transcription profiles, flux experiments, and sensitivity to metabolic inhibitors align with the metabolic dependency derived from metabolomics data. We ranked the glycolytic and lipogenic cell lines based on each data type separately and compared these rankings. To rank cell lines, we defined a metabolic dependency score for each data type as the difference in profile between the glycolytic and lipogenic subtype cell lines. Fifty-three metabolites in the metabolomics data were significantly different between glycolytic and lipogenic cell lines (t test, adjusted P < 0.05; Dataset S4). The metabolite score was defined as the difference in average z-score intensity of 22 metabolites high in glycolytic lines and 31 metabolites high in lipogenic lines. Based on RNAseq data, 129 metabolism genes fulfilled the differential expression criteria (t test, adjusted P < 0.05; Dataset S6). The transcription score was defined as the difference in average z-score expression of 42 genes high in glycolytic lines and 87 genes high in lipogenic lines. The flux score was defined as the difference in average z-score flux of four metabolites from the [U-13C6]glucose labeled experiment and four metabolites from the [U-13C5]glutamine-labeled experiment (Dataset S7). Finally, the sensitivity score was defined as the difference in average z-score sensitivity to five glycolytic inhibitors (oxamate, BPTES, AOA, BSO, and S-4-CPG) and four lipid synthesis inhibitors (SCD inhibitor, FASN inhibitor, cerulenin, and olristat).
A multivariate logistic regression model for the prediction of subtype entity (glycolytic vs. lipogenic subtype) was fitted to doubling time and metabolite intensity, for 89 metabolites that were significantly different between the glycolytic and lipogenic subtype according to a t test with adjusted P < 0.05. The glm function from the stats package in R was used for logistic regression modeling. Fifty-seven metabolites were predictive of subtype entity when taking into account doubling time, with a multivariate P < 0.05. An additional 22 metabolites tended to be predictive when keeping doubling time fixed, with a multivariate P < 0.1, totaling 79 of 89 metabolites.
PDAC Signature Validation.
Of three clinical subtypes of PDAC identified by molecular profiling (classical, QM-PDA, and exocrine-like), two subtypes are represented in cell lines (22). The original PDAssigner signature for PDAC subtyping was therefore reduced from 62 genes to 42 genes, retaining genes representative of the QM-PDA and classical subtypes and excluding genes for the exocrine-like subtype (22). Class prediction was calculated as the mean expression value of the Z-scores of genes characteristic of the QM-PDA subtype minus the mean expression value of the Z-scores of genes characteristic of the classical subtype. A positive score indicates the QM-PDA subtype and a negative score indicates the classical subtype. Z-scores were derived from log2 RPKM data of 36 cell lines.
Pan-Cancer EMT Signature.
We associated sensitivity to the LDHA inhibitor, oxamate, BPTES, and ROS-inducing agents [BSO and (S)-4-CPG] with mesenchymal status, represented by a pan-cancer EMT signature (33) (Fig. 4E) and vimentin (Fig. S3A). The pan-cancer EMT signature we used contains ten genes that are mostly associated with EMT and that were independently obtained in six datasets from three cancer types (ovary, colon, and breast) (33, 56). Those 10 genes are COL5A2, VCAN, SPARC, THBS2, FBN1, COL1A2, COL5A1, FAP, AEBP1, and CTSK. The EMT score in non-PDAC cell lines was calculated as the average expression value (log2 RPKM) of those 10 genes.
In Vitro [U-13C5]Glutamine and [U-13C6]Glucose Experiments.
Cells were cultured for ∼18 h in RPMI with 10% dialyzed FBS supplemented with either 3 mM d[U-13C]glucose or 1 mM l[U-13C]glutamine. The final concentrations of d-glucose and l-glutamine were adjusted to 6 and 2 mM, respectively, using unlabeled d-glucose and l-glutamine. Metabolic activity was quenched with a solution containing 4.5:4.5:1 ratio of methanol, acetonitrile, and water (−20 °C). The cells were incubated with constant shaking for 15 min at 4 °C and then collected in tubes by scraping the wells. The extracts were sonicated for 30 s at 4 °C and centrifuged at 16,000 × g for 10 min at 4 °C. The supernatant was collected and subjected to LC-MS analysis. Metabolic fluxes and the contribution of tracers to TCA metabolites were calculated with the MultiQuant software.
Analysis of Total Fatty Acid Synthesis.
Cells were grown in RPMI medium 1640 supplemented with 10% FBS, 100 U/mL penicillin, 0.1 mg/mL streptomycin, and 2 mM glutamine. For lipid synthesis experiments, cells were seeded overnight in 12-well plates in RPMI medium 1640 containing 2% FBS, 2 mM glutamine, and 2.5 mM glucose. d[U-14C] glucose (Perkin-Elmer) was added to the media at a final concentration of 1 μCi/mL. After 6 h of incubation, the cells were washed twice with ice cold PBS, and then lipids were extracted from plates two times with 500 μL hexane:isoproponal (3:2). Phase separation was achieved by addition of 300 μL PBS, and the nonpolar phase was collected. An additional 300 μL PBS was added to the hexane fraction to wash off the remaining polar radioactivity. The hexane fraction was dried under nitrogen gas, and radioactivity was quantified by liquid scintillation counting.
Fatty Acid Uptake Assays.
Lipid uptake was measured using a fluorescent free fatty acid uptake assay (Abcam; ab176768), following the manufacturer’s instructions. Briefly, cells were seeded at a density of 50,000 cells per well in 96-well plate, and the following day, cells were deprived of serum for 1 h. Cells were then incubated with labeled C12 fatty acid at room temperature, and fluorescence per well (excitation: 485 nm, emission: 515 nm) was measured in 26-s intervals using a SpectraMax M5 plate reader (Molecular Devices). Cells were then fixed in 4% paraformaldehyde and counterstained with Hoescht 33342 (Life Technologies; H3570) to normalize lipid uptake to cell number; cells were imaged using an IN Cell Analyzer 2000 (GE Healthcare) under a 10× objective (four fields per well), and the number of cells per field was calculated using IN Cell Analyzer Workstation v3.7.1 software (GE Healthcare).
In Vitro Drug Treatment Experiments.
For short-term viability assays, cells were plated using optimal seeding densities in 384-well plates using RPMI (without glucose, without glutamine) media (US Biological #R9011) supplemented with 6 mM Glucose (Teknova #G9005), 2 mM glutamine (Gibco #25030-081), 5% FBS (Sigma F4135), 100 μg/mL penicillin, and 100 U/mL streptomycin (Gibco 15140–122). Optimal seeding densities were established for each cell line to reach 75–80% confluence at the end of the assay. The following day, cells were treated with various small molecule inhibitors using a 6-pt dose titration scheme. After 72 h, cell viability was assessed using the CellTiter-Glo Luminescence Cell Viability assay. Absolute inhibitory concentration (IC) values were calculated using four-parameter logistic curve fitting and are averages from a minimum of two independent experiments. In cases where an IC50 could not be reached with the maximum concentration of drug, the IC50 equivalent to the maximum drug concentration was used for statistical analysis.
For long-term growth assays, glycolytic subtype cell lines (MIA Paca-2, SW 1990, PSN1, and HUP-T3) and lipid subtype cell lines (PA-TU-8902, PK-8, KP-3L, and SUIT-2) were seeded in a six-well dish at 3,000 cells per well overnight (RPMI, 5% serum, 2 mM glutamine) and then treated in media with indicated concentrations of oxamate, SCD inhibitor, or DMSO for 12 d at 37 °C and 5% CO2. Media (±drug) were changed every 3 d. After 12 d, cells were washed with PBS and stained with 0.5% crystal violet for 30 min, followed by washes with water until background was removed, and then imaged.
Oxygen Consumption (Seahorse) Assays.
Cells were plated at 20,000 cells per well in XF 96-well cell culture microplates (Seahorse Bioscience) pretreated with poly-d-lysine and incubated for 24 h at 37 °C in a 5% CO2 incubator. To assay OCR, the growth media were replaced with bicarbonate-free, serum-free prewarmed medium and the plate was loaded into the XF96 Extracellular Flux Analyzer (Seahorse Bioscience). Measurements are plotted as pmoles of O2 per minute per cell for OCR. Cell numbers used for normalization were determined by fixing the plate after analysis with 4% paraformaldehyde, staining with Hoechst, imaging four quadrants per well on a Molecular Devices ImageXpress HCS, and counting the average nuclei number per quadrant.
Mitotracker and TMRE Experiments.
Cells were plated at 10,000 cells per well in 384-well cell culture microplates pretreated with poly-d-lysine and incubated for 24 h at 37 °C in a 5% CO2 incubator overnight. Cells were then stained with 25 µg/mL Hoechst 33342 (Life Technologies), 200 nM TMRE (Life Technologies), and 200 nM MitoTracker Deep Red (Life Technologies) for 30 min (37 °C, 5% CO2). Images were acquired with a Perkin-Elmer Opera confocal imaging system, using a 20× water immersion objective (0.6 NA). Mean pixel intensity of the 565- (TMRE) and 690-nm (MitoTracker) emission channels within cytoplasmic regions was determined on a per-cell basis using Acapella image analysis software (Perkin-Elmer).
In Vivo Experiments.
All mice were housed and treated in accordance with protocols approved by the Institutional Animal Care and Use Committee at Genentech. MIA Paca-2 and HPAC shLDHA human pancreatic cell lines were generated by stably transducing with inducible shRNA constructs targeting LDHA using the lentivirus pHush–shRNA system (57) and shLDHA sequence GGCAAAGACTATAATGTAA.
To determine in vivo knockdown efficiency of LDHA, tumors were allowed to establish to between 125 and 250 mm3 and then treated with 5% sucrose (control) or doxycycline (1 mg/mL in 5% sucrose) to induce knockdown of LDHA. After 8 d, tumors were collected and subjected to immunoblot analysis using human-specific anti-LDHA (Cell Signaling; CS3582), and tubulin (Sigma; T6074).
The MIA Paca-2 shLDHA model was used to evaluate the effects of LDHA knockdown vs. therapeutic response to SCD inhibition. Briefly, 5 × 106 MIA Paca-2 shLDHA cells were implanted s.c. in Matrigel in the right flank of NCR nude (nu/nu) mice, and tumors were allowed to establish to between 125 and 250 mm3. Animals were grouped out to ensure even distribution of tumor sizes, and treatment was initiated. For shRNA experiments, control (5% sucrose) or doxycycline (1 mg/mL in 5% sucrose) was administered in the animals’ drinking water ad libidum. For SCD inhibition, animals were treated with vehicle (6.5% DMSO, 43.5% PEG-400) or SCD inhibitor G01523403 at 75 mg/kg by oral gavage, twice a day (BID) for 21 d. Tumor volumes were measured in two dimensions (length and width) using Ultra Cal-IV calipers (model 54-10-111; Fred V. Fowler Co.) and analyzed using Excel, version 14.2.5 (Microsoft Corporation). The tumor volume was calculated with the following formula:
Reductions in tumor size were tracked as partial responses (PRs) (>50% decrease from the initial tumor volume) or complete responses (CRs) (100% decrease in tumor volume).
Animal body weights were measured using an Adventura Pro AV812 scale (Ohaus Corporation). Percent weight change was calculated using the following formula:
To appropriately analyze the repeated measurement of tumor volumes from the same animals over time, a mixed modeling approach was used (58). This approach addresses both repeated measurements and modest dropouts due to any non–treatment-related death of animals before end of study.
Cubic regression splines were used to fit a nonlinear profile to the time courses of log2 tumor volume at each dose level. These nonlinear profiles were then related to dose within the mixed model. Tumor growth inhibition as a percentage of vehicle (%TGI) was calculated as the percentage of the area under the fitted curve (AUC) for the respective dose group per day in relation to the vehicle, using the following formula:
To calculate uncertainty intervals (UIs) for %TGI, the fitted curve and the fitted covariance matrix were used to generate a random sample as an approximation to the distribution of %TGI. The random sample is composed of 1,000 simulated realizations of the fitted-mixed model, where the %TGI has been recalculated for each realization. Our reported UI is the values for which 95% of the time, the recalculated values of %TGI will fall in this region given the fitted model. The 2.5 and 97.5 percentiles of the simulated distribution were used as the upper and lower UIs.
Plotting was performed and generated using R version 2.8.1 and Excel, version 14.2.5 (Microsoft). Data were analyzed using R version 2.8.1 (46), and the mixed models were fit within R using the nlme package, version 3.1−89 (58). Efficacy at 50% maximal efficacy observed (ED50) and 90% maximal efficacy observed (ED90) were calculated using PRISM based on nonlinear regression.
Fatty Acid Desaturation Studies of Xenograft Tissues.
To analyze fatty acid desaturation in MIA Paca-2 shLDHA tumor tissues and mouse plasma and liver, samples were collected at the end of the efficacy study (2 h after the last dose) and snap frozen. Fatty acid profiling was performed by Microbial ID using a standard sample preparation method for saponification and methylation. The fatty acid methyl esters were extracted and analyzed by gas chromatography. Desaturation index was expressed as the ratio of oleic on stearic methyl ester acids or palmitoleic on palmitic methyl ester acids.
Cell Line Authentication/Quality Control.
Short tandem repeat profiling.
Short tandem repeat (STR) profiles were determined for each line using the Promega PowerPlex 16 System. STR profiling was performed once and compared with external STR profiles of cell lines (when available) to determine cell line ancestry. The loci analyzed were as follows: detection of 16 loci (15 STR loci and Amelogenin for sex identification), including D3S1358, TH01, D21S11, D18S51, Penta E, D5S818, D13S317, D7S820, D16S539, CSF1PO, Penta D, AMEL, vWA, D8S1179, and TPOX (Dataset S9).
SNP fingerprinting.
SNP profiles were performed each time new stocks were expanded for cryopreservation. Cell line identity was verified by high-throughput SNP profiling using Fluidigm multiplexed assays. SNPs were selected based on minor allele frequency and presence on commercial genotyping platforms. SNP profiles were compared with SNP calls from available internal and external data (when available) to determine or confirm ancestry. In cases where data were unavailable or cell line ancestry was questionable, DNA or cell lines were repurchased to perform profiling to confirm cell line ancestry. The SNPs analyzed were as follows: rs11746396, rs16928965, rs2172614, rs10050093, rs10828176, rs16888998, rs16999576, rs1912640, rs2355988, rs3125842, rs10018359, rs10410468, rs10834627, rs11083145, rs11100847, rs11638893, rs12537, rs1956898, rs2069492, rs10740186, rs12486048, rs13032222, rs1635191, rs17174920, rs2590442, rs2714679, rs2928432, rs2999156, rs10461909, rs11180435, rs1784232, rs3783412, rs10885378, rs1726254, rs2391691, rs3739422, rs10108245, rs1425916, rs1325922, rs1709795, rs1934395, rs2280916, rs2563263, rs10755578, rs1529192, rs2927899, rs2848745, and rs10977980.
Mycoplasma testing.
All stocks were tested for mycoplasma before and after cells were cryopreserved. Two methods were used to avoid false-positive/negative results: Lonza Mycoalert and Stratagene Mycosensor. Cell growth rates and morphology were also monitored for any batch-to-batch changes.
Supplementary Material
Acknowledgments
We thank Richard Bourgon, Eva Lin, Billy Lam, Yihong Yu, and Arjan Gower for help with cell-based drug screens and data analysis, Mandy Kwong for advice on 13C metabolic mass isotopomer distribution analysis (MIDA), Allison Bruce for assistance with the metabolic diagram, and Metanomics Health (Lisette Leonhardt, Ulrike Rennefarhrt, Oliver Schmitz, and Hajo Schiewe) for technical support on metabolite profiling.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1501605112/-/DCSupplemental.
References
- 1.Warburg O, Posener K, Negelein E. On the metabolism of carcinoma cells. Biochem Z. 1924;152:309–344. [Google Scholar]
- 2.Dang L, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462(7274):739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: Metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18(1):54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134(5):703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Anastasiou D, et al. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat Chem Biol. 2012;8(10):839–847. doi: 10.1038/nchembio.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le A, et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012;15(1):110–121. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie H, et al. Targeting lactate dehydrogenase–a inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor-initiating cells. Cell Metab. 2014;19(5):795–809. doi: 10.1016/j.cmet.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Available at www.clinicaltrials.gov. Accessed December, 2014.
- 9.Jain M, et al. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012;336(6084):1040–1044. doi: 10.1126/science.1218595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sreekumar A, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457(7231):910–914. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 11.Hu J, et al. Heterogeneity of tumor-induced gene expression changes in the human metabolic network. Nat Biotechnol. 2013;31(6):522–529. doi: 10.1038/nbt.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dang CV. Glutaminolysis: Supplying carbon or nitrogen or both for cancer cells? Cell Cycle. 2010;9(19):3884–3886. doi: 10.4161/cc.9.19.13302. [DOI] [PubMed] [Google Scholar]
- 13.Barretina J, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483(7391):603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garnett MJ, et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature. 2012;483(7391):570–575. doi: 10.1038/nature11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ying H, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149(3):656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Son J, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496(7443):101–105. doi: 10.1038/nature12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang G, et al. Integration of metabolomics and transcriptomics revealed a fatty acid network exerting growth inhibitory effects in human pancreatic cancer. Clin Cancer Res. 2013;19(18):4983–4993. doi: 10.1158/1078-0432.CCR-13-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Commisso C, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497(7451):633–637. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDermott U, et al. Identification of genotype-correlated sensitivity to selective kinase inhibitors by using high-throughput tumor cell line profiling. Proc Natl Acad Sci USA. 2007;104(50):19936–19941. doi: 10.1073/pnas.0707498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heiser LM, et al. Subtype and pathway specific responses to anticancer compounds in breast cancer. Proc Natl Acad Sci USA. 2012;109(8):2724–2729. doi: 10.1073/pnas.1018854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunet JP, Tamayo P, Golub TR, Mesirov JP. Metagenes and molecular pattern discovery using matrix factorization. Proc Natl Acad Sci USA. 2004;101(12):4164–4169. doi: 10.1073/pnas.0308531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collisson EA, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17(4):500–503. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mutch DM, et al. Metabolite profiling identifies candidate markers reflecting the clinical adaptations associated with Roux-en-Y gastric bypass surgery. PLoS One. 2009;4(11):e7905. doi: 10.1371/journal.pone.0007905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Brien T, et al. Abstract 964: Inhibiting glycolysis with an LDHA inhibitor: A new solution to an old problem. Cancer Res. 2014;74(19) Suppl:964. [Google Scholar]
- 27.Chaudhari AM, Hallman J, Laudeman CP, Musso DL, Parrish CA. 2011. Azabenzimidazoles as fatty acid synthase inhibitors and their preparation and use for the treatment of cancer. US Patent WO 2011066211 A1 (June 3, 2011)
- 28.Du X, et al. FGFR3 stimulates stearoyl CoA desaturase 1 activity to promote bladder tumor growth. Cancer Res. 2012;72(22):5843–5855. doi: 10.1158/0008-5472.CAN-12-1329. [DOI] [PubMed] [Google Scholar]
- 29.Acharya A, Das I, Chandhok D, Saha T. Redox regulation in cancer: A double-edged sword with therapeutic potential. Oxid Med Cell Longev. 2010;3(1):23–34. doi: 10.4161/oxim.3.1.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashkenazi A, Du X, Qing J. 2013. Methods of using scd1 antagonists. US Patent WO2013056148 A2 (April 18, 2013)
- 31.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7(6):415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 33.Cheng WY, Ou Yang TH, Anastassiou D. Development of a prognostic model for breast cancer survival in an open challenge environment. Sci Transl Med. 2013;5(181):181ra50. doi: 10.1126/scitranslmed.3005974. [DOI] [PubMed] [Google Scholar]
- 34.Hitosugi T, et al. Phosphoglycerate mutase 1 coordinates glycolysis and biosynthesis to promote tumor growth. Cancer Cell. 2012;22(5):585–600. doi: 10.1016/j.ccr.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muller FL, et al. Passenger deletions generate therapeutic vulnerabilities in cancer. Nature. 2012;488(7411):337–342. doi: 10.1038/nature11331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12(12):931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 37.Yauch RL, et al. Epithelial versus mesenchymal phenotype determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patients. Clin Cancer Res. 2005;11(24 Pt 1):8686–8698. doi: 10.1158/1078-0432.CCR-05-1492. [DOI] [PubMed] [Google Scholar]
- 38.Lomakin IB, Steitz TA. The initiation of mammalian protein synthesis and mRNA scanning mechanism. Nature. 2013;500(7462):307–311. doi: 10.1038/nature12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15(7):807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- 40.Luyimbazi D, et al. Rapamycin regulates stearoyl CoA desaturase 1 expression in breast cancer. Mol Cancer Ther. 2010;9(10):2770–2784. doi: 10.1158/1535-7163.MCT-09-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7(10):763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 42.Kim JW, Gao P, Liu YC, Semenza GL, Dang CV. Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol Cell Biol. 2007;27(21):7381–7393. doi: 10.1128/MCB.00440-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoo YG, Christensen J, Huang LE. HIF-1α confers aggressive malignant traits on human tumor cells independent of its canonical transcriptional function. Cancer Res. 2011;71(4):1244–1252. doi: 10.1158/0008-5472.CAN-10-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Ravenzwaay B, et al. The use of metabolomics for the discovery of new biomarkers of effect. Toxicol Lett. 2007;172(1-2):21–28. doi: 10.1016/j.toxlet.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 45.Shukla K, et al. Design, synthesis, and pharmacological evaluation of bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide 3 (BPTES) analogs as glutaminase inhibitors. J Med Chem. 2012;55(23):10551–10563. doi: 10.1021/jm301191p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.R Development Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna: 2009. [Google Scholar]
- 47.Jiang Z, Gentleman R. Extensions to gene set enrichment. Bioinformatics. 2007;23(3):306–313. doi: 10.1093/bioinformatics/btl599. [DOI] [PubMed] [Google Scholar]
- 48.Gaujoux R, Seoighe C. A flexible R package for nonnegative matrix factorization. BMC Bioinformatics. 2010;11:367. doi: 10.1186/1471-2105-11-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim PM, Tidor B. Subsystem identification through dimensionality reduction of large-scale gene expression data. Genome Res. 2003;13(7):1706–1718. doi: 10.1101/gr.903503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu TD, Nacu S. Fast and SNP-tolerant detection of complex variants and splicing in short reads. Bioinformatics. 2010;26(7):873–881. doi: 10.1093/bioinformatics/btq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klijn C, et al. A comprehensive transcriptional portrait of human cancer cell lines. Nat Biotechnol. 2015;33(3):306–312. doi: 10.1038/nbt.3080. [DOI] [PubMed] [Google Scholar]
- 52.Possemato R, et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476(7360):346–350. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mootha VK, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34(3):267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 54.McCleland ML, et al. Lactate dehydrogenase B is required for the growth of KRAS-dependent lung adenocarcinomas. Clin Cancer Res. 2013;19(4):773–784. doi: 10.1158/1078-0432.CCR-12-2638. [DOI] [PubMed] [Google Scholar]
- 55. Subramanian A, et al. (2005) Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102(43):15545–15550. [DOI] [PMC free article] [PubMed]
- 56.Cheng WY, Ou Yang TH, Anastassiou D. Biomolecular events in cancer revealed by attractor metagenes. PLOS Comput Biol. 2013;9(2):e1002920. doi: 10.1371/journal.pcbi.1002920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gray DC, et al. pHUSH: A single vector system for conditional gene expression. BMC Biotechnol. 2007;7:61. doi: 10.1186/1472-6750-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pinheiro J, Bates D, DebRoy S, Sarkar D, R Development Core Team (2009) nlme: Linear and nonlinear mixed effects models. R Package Version 3.1-96. Available at CRAN.R-project.org/package=nlme. Accessed July 8, 2015.
- 59.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57(1):289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.