Significance
We show that in-school music training changes the course of adolescent brain development. Relative to an active control group that shows the expected wane in subcortical response consistency, adolescents undertaking in-school music training maintained heightened neural consistency throughout high school. The music training group also exhibited earlier emergence of the adult cortical response, suggesting that in-school music accelerates neurodevelopment. These changes seem to benefit literacy skills: both groups improved in phonological awareness relative to the general population, but the music training group improved more compared with the active controls. Our results support the notion that the adolescent brain remains receptive to training, underscoring the importance of enrichment during teenage years.
Keywords: music, training, auditory
Abstract
Fundamental changes in brain structure and function during adolescence are well-characterized, but the extent to which experience modulates adolescent neurodevelopment is not. Musical experience provides an ideal case for examining this question because the influence of music training begun early in life is well-known. We investigated the effects of in-school music training, previously shown to enhance auditory skills, versus another in-school training program that did not focus on development of auditory skills (active control). We tested adolescents on neural responses to sound and language skills before they entered high school (pretraining) and again 3 y later. Here, we show that in-school music training begun in high school prolongs the stability of subcortical sound processing and accelerates maturation of cortical auditory responses. Although phonological processing improved in both the music training and active control groups, the enhancement was greater in adolescents who underwent music training. Thus, music training initiated as late as adolescence can enhance neural processing of sound and confer benefits for language skills. These results establish the potential for experience-driven brain plasticity during adolescence and demonstrate that in-school programs can engender these changes.
By age six, the brain has reached 90% of its adult size (1). However, the years between childhood and young adulthood are marked by a host of subtler neural developments. Myelination and synaptic pruning (2–5) lead to a decrease in gray matter and an increase in white matter (6–13). Resting-state oscillations decline (14–16), and passive evoked responses to sound change in complex ways. Cortically, the P1, which is a positive deflection at around 50 ms generated within lateral Heschl’s gyrus (17), declines whereas the N1, a negative deflection at around 100 ms generated within primary and secondary auditory cortices (18–20), increases (21–23). Subcortically, the trial-by-trial consistency of the response declines (24, 25). An open question is how experience interacts with this developmental plasticity during adolescence. Is the transition from the plasticity of childhood to the stability of adulthood malleable by experience? And if so, what types of enrichment have the greatest impact on the development of the neural mechanisms contributing to auditory and language skills?
Music training is an enrichment program commonly available to high school students, and its neural and behavioral consequences are well-understood (for a review, see ref. 26). Studies comparing nonmusicians with musicians who began training early in life have revealed a “signature” set of enhancements associated with musical experience (27, 28). Relative to nonmusician peers, musicians tend to show enhanced speech-in-noise perception (29–34), verbal memory (30–33, 35–38), phonological skills (39–45), and reading (46–50), although not without exception (51, 52). Music training has also been linked to enhancements in the encoding of sound throughout the auditory system. For example, musicians show an enhanced N1 (53–56). These enhancements extend to the subcortical auditory system, with musicians showing responses to sound that are faster (55, 57–61), are degraded less by background noise (32, 61), represent speech formant structure more robustly (32, 62–64). differentiate speech sounds to a greater extent (65–67), track stimulus pitch more accurately (68, 69), and are more consistent across trials (59, 70). In adolescence, music training leads to faster responses to speech in noise (71), but the extent to which adolescent music training can confer other aspects of the musician signature remains unknown.
Motivated by a conceptual framework in which auditory enrichment interacts with the auditory processes that remain under development during adolescence, we undertook a school-based longitudinal study of adolescent auditory enrichment. We focused on objective biological measures of sound processing that (i) have shown developmental plasticity during adolescence in the absence of intervention and (ii) contribute to the “neural signature” of musicianship: the consistency of the subcortical response to speech and the magnitude of the cortical onset response to speech. Subcortical response consistency peaks in childhood, waning into young adulthood (24), coinciding with a period when learning a second language becomes more difficult than earlier in life (72). Response consistency tracks with language skills (73) and is enhanced in musicians (59, 70). Accordingly, we predicted that music training in adolescence prolongs this period of heightened auditory stability. Moreover, given that the cortical N1 onset response emerges during adolescence while the P1 response declines (17, 18, 21–23), and that N1 is enhanced in younger and older musicians (53–56), we predicted that music training during adolescence would accelerate the development of the cortical onset response.
To test these hypotheses, we followed two groups of high school students longitudinally, testing them just before they entered high school (mean age 14.7) and again 4 y later during their last year of school. One group (n = 19) engaged in music training in which they performed music from written notation in a group setting whereas the active control group (n = 21) engaged in Junior Reserve Officers Training Corps (JROTC) training. Both types of training required investment of time and effort and emphasized the development of self-discipline, dedication, and determination; however, only the music training targeted auditory function. Both activities were part of the high school curriculum, which was otherwise identical for both groups. We also tested students’ language skills (phonological memory, phonological awareness, and rapid naming ability) to determine whether in-school music engendered benefits for literacy skills, a prediction consistent with cross-sectional studies (39–45). The two groups were matched demographically and on all outcome measures at the start of the study (see Table S1 for demographic information for the two groups).
Table S1.
Demographic information at pretest for the music and JROTC training groups, listed as mean (standard deviation)
| Demographic information | Music training | JROTC training |
| No. female | 8 | 8 |
| Age at pretest | 14.66 (0.42) | 14.72 (0.38) |
| Nonverbal IQ scores at pretest | 51.74 (9.88) | 51.14 (4.75) |
| Avg degree of maternal education* | 2.53 (0.84) | 2.4 (0.75) |
1, less than high school; 2, high school; 3, college; 4, graduate training.
Results
Neural.
Subcortical response consistency.
The JROTC group exhibited the waning of response consistency characteristically observed between adolescence and young adulthood (24, 25). The music group, however, maintained high response consistency throughout high school. There was a year-by-training group interaction: [F(1,36) = 7.36, P = 0.01, partial eta squared = 0.17] (Fig. 1). Response consistency decreased between year 1 and year 4 for the JROTC group [t(20) = 3.83, P = 0.0011, partial eta squared = 0.42], but did not for the music group (P > 0.1). (See Table S2 for means and SDs of all measures across years and groups.) Although the two groups did not differ at year 1 (P > 0.2), in year 4, the music training group had higher response consistency than the JROTC group [t(36) = 2.62, P = 0.013, partial eta squared = 0.16].
Fig. 1.
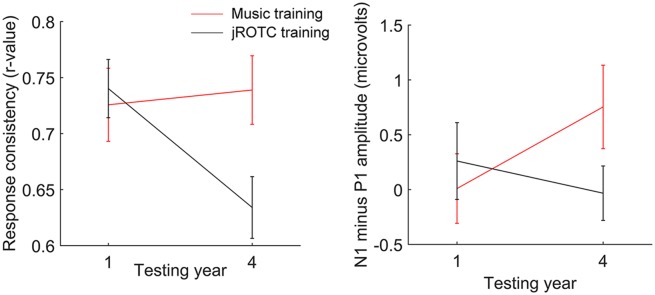
(Left) Response consistency declined with age in the JROTC training group but not the music training group [group by time-point interaction: F(1,36) = 7.36, P = 0.01]. (Right) The difference between N1 and P1 amplitude (a marker of cortical maturation) increased in the music training group but did not change in the JROTC training group [group by time-point interaction: F(1,34) = 6.41, P = 0.016]. Error bars, 1 SEM.
Table S2.
Mean (standard deviation) of all measures in years 1 and 4 for the music and JROTC training groups
| Measure | Year 1 | Year 4 |
| Music training | ||
| Response consistency (z-score) | 1.154 (0.402) | 1.152 (0.394) |
| N1 minus P1 (microvolts) | 0.010 (1.307) | 0.754 (1.568) |
| Phonological awareness | 98.0 (8.6) | 105.2 (10.2) |
| Phonological memory | 95.4 (11.9) | 96.2 (11.9) |
| Rapid naming | 97.2 (11.0) | 95.5 (10.3) |
| JROTC training | ||
| Response consistency (z-score) | 1.260 (0.449) | 0.984 (0.331) |
| N1 minus P1 (microvolts) | 0.261 (1.526) | −0.033 (1.081) |
| Phonological awareness | 99.9 (9.5) | 102.6 (9.0) |
| Phonological memory | 96.9 (11.0) | 94.6 (11.2) |
| Rapid naming | 95.6 (11.2) | 92.8 (10.9) |
Cortical onset response.
Consistent with the known developmental trajectory of the cortical onset response, there was an increase in the difference between N1 and P1 from year 1 to year 4 for the music group [t(16) = 2.22, P = 0.041, partial eta squared = 0.24]. The relationship between N1 and P1, however, did not change for the JROTC group [P > 0.1, year-by-training group interaction, F(1,34) = 6.41, P = 0.016, partial eta squared = 0.159] (Fig. 1). Fig. 2 illustrates group mean cortical responses across fronto-central channels at year 1 and year 4 for the two groups. The groups did not differ in the relationship between N1 and P1 at year 1 (P > 0.1), indicating that the different cortical maturation trajectories between the groups were not driven by preexisting differences. In year 4, cortical differences between music training and the JROTC groups were emerging: there was a trend suggesting a greater difference in amplitude between N1 and P1 (i.e., a more mature cortical onset response) in the music group relative to the JROTC group [t(34) = 1.77, P = 0.086, partial eta squared = 0.084]. Across all subjects, cortical maturation from year 1 to year 4 did not correlate with change in response consistency from year 1 to year 4 (r = 0.21, P > 0.1).
Fig. 2.
Average cortical waveforms across fronto-central electrodes in year 1 and year 4 in music (Left) and JROTC (Right) training groups. Shaded regions, 1 SEM.
Behavioral.
Phonological awareness.
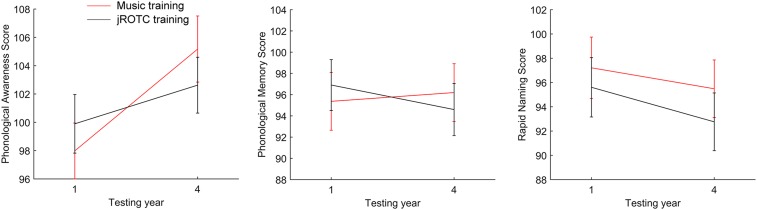
Both groups showed gains on phonological awareness [main effect of year, F(1,36) = 26.6, P < 0.001, partial eta squared = 0.41], but the music group showed larger gains: there was an interaction between year and training group [F(1,38) = 5.38, P = 0.026, partial eta squared = 0.12] (Fig. 3). Post hoc paired t tests revealed that phonological awareness score increased between year 1 and year 4 for both the music [t(18) = 4.53, P < 0.001, partial eta squared = 0.53] and JROTC [t(20) = 2.41, P = 0.026, partial eta squared = 0.23] groups. The groups did not differ on phonological awareness at year 1 (P > 0.2).
Fig. 3.
(Left) Phonological awareness ability increased in both training groups but did so to a greater extent in the music training group [group by time-point: F(1,38) = 5.38, P = 0.026]. (Center) Training had no significant effects on phonological memory ability [no group by time-point interaction: F(1,38) = 1.56, P = 0.22]. (Right) Training had no significant effects on rapid naming ability [no group by time-point interaction: F(1,38) = 0.15, P = 0.70]. Error bars, 1 SEM.
Phonological memory.
The two training groups did not differ longitudinally on phonological memory. There was no interaction between year and training group (P > 0.2) (Fig. 3) and no main effects (P > 0.2). The two training groups did not differ on phonological memory at year 1 (P > 0.2).
Rapid naming.
The two training groups did not differ longitudinally on rapid naming. There was no interaction between year and training group (P > 0.2) (Fig. 3) and no main effects (P > 0.2). The two training groups did not differ on rapid naming score at year 1 (P > 0.2).
Discussion
Studies of child music lessons have established a signature set of neurophysiological and behavioral benefits, but is it too late to see these gains in children who initiate music training during high school? We investigated the effects of music training versus JROTC training on adolescent auditory development by testing auditory neural encoding and language skills in adolescents before, and 3 years after, they entered high school. Although adolescents undergoing JROTC training exhibited the typical waning of the consistency of the subcortical response to speech (24, 25), music training maintained high response consistency throughout high school. An increase in the N1/P1 amplitude ratio from year 1 to year 4, known to emerge in adolescence (21–23), was observed in the music group but had not yet emerged in the JROTC group. Phonological awareness improved in both training groups from year 1 to year 4, but these gains were larger in the adolescents who underwent in-school music training. Two other language tests, phonological memory and rapid naming, showed no group differences. Taken together, these results establish that high school music classes engender gains in brain function and behavior that, although small, demonstrate the potential of enrichment to jump-start adolescent neurodevelopment.
The consistency of neural responses to sound tracks with language skills, suggesting that stable perceptual encoding is vital for the acquisition and maintenance of phonological categories (73). Response consistency peaks in childhood (∼8–11 y of age), declining steadily until young adulthood (24, 25); we show that this adolescent decline is mitigated by in-school music lessons. What mechanisms underlie this developmental trend and, perhaps, training effect? Synaptic density follows a similar developmental trajectory, increasing in early childhood and subsequently declining during adolescence (2–5). Moreover, gray matter volume has been linked to the power of resting oscillations in the brain (74), suggesting that an abundance of synapses might lead to more phase-locked neural populations and less variable responses. Consistent with previous cross-sectional studies showing enhanced response consistency in musicians (59, 70) and in participants using assistive listening devices (75), the music training group maintained a higher level of response consistency between years 1 and 4. Thus, music training may maintain heightened synaptic density within the auditory system to enable the learning and performance of challenging auditory tasks, much as songbirds show seasonal increases in synaptogenesis that coincide with the onset of the preferential period for learning new songs (76). The maintenance of response consistency in the music training group may prolong sensitivity to auditory learning. Future work could test this hypothesis by measuring auditory learning in adolescents with or without prior musical experience. Learning to produce and understand a foreign language becomes more difficult with age as auditory sensitivity declines (72); music training might extend the time window during which auditory sensitivity is enhanced. Supporting this idea, adults with more musical experience show enhanced auditory plasticity (77) and more proficient second language learning (78).
During adolescence, N1 amplitude increases whereas P1 amplitude declines (17, 18, 21–23). This process is not complete until young adulthood, by which time N1 has become the largest component in the cortical response to sound (17, 18, 21–23). In adults, music training amplifies the N1 response (53–56). Here, we find an increase in N1 amplitude relative to P1 amplitude only in the music group. Thus, music training may have accelerated cortical development. The change in response consistency from year 1 to year 4 did not correlate with cortical maturation across all participants, suggesting that different mechanisms underlie the development of subcortical response consistency and the maturation of the cortical onset response across adolescence. Although synaptic pruning is a likely candidate for driving response consistency, recruitment of a larger pool of neurons involved in the generation of the cortical onset response may underlie the emergence of N1 in adolescence.
Music training leads to greater gains in auditory and motor function when begun in young childhood; by adolescence, the plasticity that characterizes childhood has begun to decline (79). Nevertheless, our results establish that music training impacts the auditory system even when it is begun in adolescence, suggesting that a modest amount of training begun later in life can affect neural function. Plasticity within the auditory system is enhanced when attention is directed to sound, as well as when auditory perceptual learning is tied to reward (79–82). Music training, therefore, may be a particularly effective strategy for inducing neural change because it requires attention to sound (83) and recruits cognitive, sensory, and reward circuits (84) as sound-to-meaning connections are learned. Although JROTC training requires discipline and time investment, it does not mandate fine auditory perceptual judgments, which may explain why we do not find auditory system enhancements in the JROTC group. However, JROTC training likely leads to a separate set of benefits outside the auditory domain. One possibility, for example, is that the mental discipline acquired and practiced over the course of JROTC training strengthens attentional control.
Both music and JROTC training groups experienced enhancements on a test of phonological awareness, normed to the general population, with the greatest gains observed in the music group. Thus, these seemingly different types of training may share a common characteristic capable of bolstering certain phonological skills. A feature common to both music and JROTC training is synchronization to perceptual cues. The music training that our participants underwent was in-school group training, which required them to synchronize playing both with their fellow students and with the visual signals presented by the teacher. A chief component of JROTC training was synchronized marching, during which students used perceptual cues to synchronize with the other students. Perceptual–motor synchronization ability has been linked to phonological skills (85–87), suggesting that synchronization and the knowledge of speech sounds rely on shared neural resources. One possibility is that both phonological awareness and auditory–motor synchronization draw on the ability to precisely track sound event timing (88). Given that both music training and JROTC training enhance phonological awareness and involve synchronization with perceptual cues, future work comparing music training to a passive control group could reveal a divergence not reported here. On the other hand, we found no gains in rapid naming or phonological memory, despite the fact that both reading (46–50) and verbal memory (30–38) have been associated with music training in other studies, suggesting either that the training studied here was not optimally designed to enhance these skills or that enhancing these skills requires a greater amount of training or training begun earlier in life. A third possibility is that the link between phonological processes and beat synchronization is restricted to phonological awareness. Perhaps rapid automatized naming, which is dissociable from phonological awareness and makes an independent contribution to reading skill (89), relies on precise perception of auditory timing to a lesser extent than does phonological awareness.
An unavoidable limitation of this study was that, due to working with in-school programs, we were not able to randomly assign participants to one or the other training group. Thus, our groups were differentiated not only by the training that they received over the 3 y but also by their motivation to begin that training in the first place. Nonetheless, given that the two training groups were matched on measures of auditory function before training began, we attribute study outcomes to the training itself. Moreover, the fact that students were required to select a form of training as a requirement for graduation means that our subject population was not limited to those who were motivated to seek out training.
We found effects of music versus control training despite the large amount of between-subjects variation on neural and behavioral measures. For example, training group accounts for 16% of the variance in the year-to-year change in N1/P1 ratio, suggesting that there are other factors at play. Socioeconomic status, sex, and maturational progress could account for some of this variance because all three of these variables have been shown to affect auditory processing (25, 90, 91).
These results inform the debate about music’s place in the high school curriculum. Faced with dwindling funds and increasing costs, administrators must often make difficult decisions about which fields of study will remain a part of the curriculum. Because the ability to play music seems irrelevant to most career paths, music training has often been sacrificed: the percentage of children receiving music instruction before age 18 dropped from 53% in 1982 to 36% in 2008 (92). Increasingly, however, longitudinal studies of music training present converging evidence that music training confers gains in skills vital for everyday life. Therefore, although learning to play music does not train skills directly relevant to most careers, music may engender “learning to learn,” the development of skills that will enhance the ability to acquire knowledge and talents in the future (60, 61, 93).
Methods
Participants.
Participants were recruited from three Chicago-area public high schools and enrolled in the study during the summer before their freshman year of high school [average age at first test = 14.7 (standard deviation 0.39) y]. Year 1 data were collected on 68 participants. Twenty-eight participants were excluded from analysis due to hearing loss (n = 3), failed IQ screening (n = 1), external diagnosis of a reading (n = 2) or learning (n = 3) disorder, failure to return for testing after training (n = 4), and switching from one training regimen to the other (n = 15), leaving 40 total participants. Participants were recruited by visiting the classrooms and speaking to students directly. Participants were not required to participate by their teachers; they volunteered, and, as such, our subject population was limited to only a subset of the students in each class. As a requirement of these schools’ curricula, participants enrolled in either music classes (n = 19, 8 females) or Junior Reserve Officers Training Corps (JROTC) (n = 21, 8 females). Students were told about the study after they made their choice of training program, and thus the existence of the study did not influence their choice of training. Participants were tested before training to provide a baseline measure of neural processing and language abilities. They were tested again during the summer preceding their senior year of high school to evaluate changes in auditory neurophysiology and language skills. At both test points, parental/guardian informed consent and adolescent informed assent (or consent if the participant was 18 y old) were obtained. All procedures were approved by the Institutional Review Board of Northwestern University. Participants were compensated $10 an hour, with an extra $100 given at posttest.
At both test points, participants were screened to ensure they met the inclusionary criteria: no diagnosis of a learning or neurological disorder, normal IQ (standard score of >85 on the Wechsler Abbreviated Scale of Intelligence) (94), normal hearing thresholds (<20-dB normal hearing level for octaves between 125 and 8,000 Hz) and an 80-dB sound pressure level (SPL) click-evoked wave V latency within laboratory-internal normal limits (5.24–5.99 ms). Groups did not differ at pretraining with respect to IQ, sex, age, and amount of maternal education (a proxy for socioeconomic status). (See Table S1 for demographic information for both groups.) Participating schools were in low-income neighborhoods (with 90% qualifying for subsidized lunch). Unpaired t tests were used to evaluate year 1 group differences in IQ, age, and maternal education, with results as follows: IQ t = 0.25, P = 0.81; age t = 0.48, P = 0.63; maternal education t = 0.49, P = 0.62. A binomial test found that sex ratio did not differ between the two groups with P = 0.445. JROTC participants had no prior music training whereas two musician students had a small amount of formal music training (1 and 6 y). However, because the groups did not differ on neural and linguistic performance at pretest (all P > 0.2), we attribute any prospective group differences at the end of the study to the in-school training programs.
Training Regimens.
In-school music curriculum.
Band class provides students with between 2 h 20 min and 3 h of in-school instrumental music instruction per week. The goal of this curriculum is to provide students with a level of musical knowledge that will ready them for college-level music performance classes by the end of their senior year. Classes combine active music making with intellectual and pragmatic aspects of musicianship, including playing technique, sight reading, performing in an ensemble, practice caring for musical instruments, and regular assessments of student progress. These assessments include written examinations related to music theory, playing examinations that address continuous growth as well as concert readiness, and content-based writing assignments. Students participated in at least two public performances each year in which the students performed high-school level orchestral material. (By their junior year all participants mastered their instruments sufficiently to be placed in “advanced band.”) Classes comprised 25–30 students, and thus the musical training primarily consisted of learning to play in a large ensemble. The students included in this study were learning to play the following instruments: percussion (2 students), tuba (1), baritone saxophone (1), trumpet (3), clarinet (6), bass (1), alto sax (3), euphonium (1), hammered dulcimer (1), and trombone (1). Practice outside of class was left at the discretion of the student to prepare for concerts and weekly quizzes.
JROTC curriculum.
Band and JROTC classes were held at the same time, so the JROTC group had the same amount of class time as the band group. For both the JROTC and music training curricula, all class time was spent on instructed learning via direct contact with instructors. The goal of the JROTC curriculum is to hone leadership skills, strengthen character, and promote self-discipline through classroom-based instruction and fitness-based training. As part of the program, students engage in regular group-based synchronized marching and fitness routines that occur in response to spoken commands. Students are graded and promoted based on demonstrating knowledge and mastery of the concepts covered in the classroom as well as attainment of muscular and cardiovascular fitness milestones. Students participated in public performances, such as parades, as well as marching drill competitions with neighboring high schools. Classes comprised 25–30 students. As for the music curriculum, practice outside of class was left at the discretion of the student to prepare for competitions and parades. In-class assessments were also given on knowledge of military rules, regimens, and regulations.
Neurophysiological Testing.
Stimuli.
The stimulus for the brainstem recording was a 40-ms synthesized “da,” which is a five-formant Klatt-synthesized syllable (20-kHz sampling rate). The stimulus for the cortical recording was a 170-ms speech sound “da,” which is a six-formant Klatt-synthesized syllable (20-kHz sampling rate). See Supporting Information for a detailed description of these stimuli.
Recording parameters.
Participants sat in a comfortable reclining chair in a soundproof, electromagnetically shielded booth and watched a self-selected movie with the soundtrack presented in free field at <40 dB SPL. The left ear remained unoccluded so that the participant could hear the movie’s soundtrack.
Subcortical responses were collected with the Bio-logic Navigator Pro System (Natus Medical Incorporated) at a sampling rate of 12,000 Hz using Ag-AgCl electrodes applied to the participant in an ipsilateral vertical montage, with the active electrode at Cz, reference at the right earlobe, and ground on the forehead. Individual electrode impedance was kept below 5 kΩ. The stimulus was presented to the participant’s right ear in alternating polarity at 80 ± 1 dB SPL at a rate of 10.9 Hz. Responses were online filtered from 100 to 2,000 Hz, a frequency range that captures the phase-locking limits of the inferior colliculus, the putative generator of the brainstem response (95, 96). Responses were segmented into epochs (−15 to 58 ms relative to stimulus onset) and then baseline corrected to the average prestimulus amplitude. Epochs in which the amplitude exceeded ± 23.8 µV were considered artifact and rejected. Artifacts were monitored online during data collection, and two artifact-free 3,000-epoch averaged responses were collected.
Cortical responses were collected at a sampling rate of 500 Hz using a cloth cap in which 31 tin electrodes were embedded (Compumedics), with the earlobes as reference. Electrodes were placed above the left pupil and outer canthus of the left eye to track eye movements. Individual electrode impedance was kept below 10 kΩ. The stimulus was presented to the participant’s right ear in alternating polarity at 80 ± 1 dB SPL and a rate of 0.99 Hz. Cortical data were processed in Matlab (The Mathworks, Inc.) using EEGLAB (97) and ERPLAB (98). The data were filtered offline from 1 to 35 Hz using a second order IIR Butterworth filter (12 dB per octave rolloff) and epoched from −100 to 500 ms relative to stimulus onset. Epochs were baseline corrected to the average amplitude of the prestimulus period. Epochs containing eyeblinks, eye movements, or large amplitude spikes (±100 µV) were automatically detected and excluded from further analysis. Artifact rejection was monitored online, and 400 artifact-free epochs were collected. Responses were then averaged separately for each channel and participant.
Data processing.
Consistency of the subcortical response for each subject was calculated by constructing a pair of 3,000-sweep averages from the first and second halves of the recording. A Pearson product-moment correlation (r-value) was calculated for this pair to estimate response consistency (24). A consistency score of 0 would indicate a completely inconsistent response whereas a consistency score of 1 would indicate a perfectly consistent response across trials. This procedure was run for the entire response (0–58 ms). R-values were converted to z-scores via the Fisher transform before statistical analysis. The Bio-logic Navigator Pro System is incapable of storing individual trials during data collection, necessitating the use of subaverages for analysis of response consistency. This procedure has been validated (74): response consistency calculated by comparing waveforms collected in the first and last half of a recording session correlates with response consistency calculated by averaging “even” and “odd” epochs at r = 0.8, confirming that this procedure reflects trial-by-trial response consistency rather than neural fatigue.
Cortical analyses were conducted on a fronto-central montage consisting of FP1, FPZ, FP2, F3, FZ, F4, C3, CZ, C4, CP3, CPZ, and CP4 because P1 and N1 were most prominent at these sites. P1 latency was automatically detected as the largest positive maximum found in the latency range of 40–100 ms. N1 latency was automatically detected as the largest negative maximum found in the latency range of 70–170 ms. These latencies were then verified by an expert who simultaneously viewed global field power and average waveforms for every channel. Those subjects with a P1 or N1 that was not prominent enough to be clearly picked were assigned the mean latency of all subjects with a clear P1/N1. Average waveforms across the entire fronto-central montage were then computed, and P1 and N1 amplitude for each subject was taken as the average amplitude in a 50-ms time window centered around the peak latency for that subject. Cortical onset response maturation was calculated as the difference in amplitude between N1 and P1: specifically, because N1 is a negative potential whereas P1 is a positive potential, P1 amplitude was subtracted from inverse N1 amplitude.
Behavioral.
Phonological awareness, phonological memory, and rapid naming abilities were measured with the Comprehensive Test of Phonological Processing (99). See Supporting Information for a detailed description of these tests.
Statistical Analyses.
Analyses were carried out with MATLAB version R2012B (The MathWorks, Inc.) and R (R Core Team), using EEGLAB (97), ERPLAB (98), and custom scripts written by the authors. Year-to-year changes were determined through repeated measures analysis of variance [two group × two test point repeated measures ANOVA (RMANOVA)], using Hyunh–Feldt-corrected P values when Mauchley’s test revealed that the assumption of sphericity was violated (P < 0.05). Then, t tests between years 1 and 4 were conducted for all measures that showed a main effect of test point in the RMANOVA. To ensure that results were not driven by outliers, before analysis, outliers for any variable were corrected to two SDs from the mean. Three data points were corrected for cortical maturation, 5 for subcortical response consistency, 5 for phonological awareness, 4 for rapid naming, and 5 for phonological memory. Our results were largely unaffected by this manipulation; not correcting for outliers strengthened the year-by-training group interaction for both N1/P1 ratio (F = 7.011, P = 0.012) and subcortical response consistency (F = 7.88, P = 0.008). However, not correcting for outliers reduced the significance of the year-by-training group interaction for phonological awareness (F = 3.87, P = 0.057).
Subcortical Recording Stimulus
The stimulus for the brainstem recording was a 40-ms synthesized “da,” which is a five-formant Klatt-synthesized syllable (20-kHz sampling rate). The first 10 ms correspond to the sound’s onset and are composed of a short burst of broadband energy. The consonant-vowel transition lasts from 10 to 40 ms; although there is no steady-state period of the stimulus, it nonetheless is heard by listeners as “da.” During the formant transition, the fundamental frequency (F0) ramps from 103 to 125 Hz, the first formant (F1) increases from 220 to 720 Hz, the second formant (F2) declines from 1,700 to 1,240 Hz, and the third formant (F3) declines from 2,580 to 2,500 Hz. The fourth and fifth formants are constant at 3,500 and 4500 Hz, respectively.
Cortical Recording Stimulus
The stimulus for the cortical recording was a 170-ms speech sound “da,” which is a six-formant Klatt-synthesized syllable (20-kHz sampling rate). The first 5 ms correspond to the sound’s onset and are composed of a short burst of broadband energy. The consonant-vowel formant transition lasts from 5 to 50 ms. During the formant transition, the fundamental frequency (F0) remains at 100 Hz, the first formant (F1) increases from 400 to 720 Hz, the second formant (F2) declines from 1,700 to 1,240 Hz, and the third formant (F3) declines from 2,580 Hz to 2,500 Hz. The steady-state vowel occurs from 50 to 170 ms and F1–F3 are steady at 720, 1,240, and 2,500 Hz over this region. From 5 to 170 ms, the fourth, fifth, and sixth formants are constant at 3,330, 3,750, and 4,900 Hz, respectively.
Behavioral Tests
Phonological awareness, phonological memory, and rapid naming abilities were measured with the Comprehensive Test of Phonological Processing (CTOPP) (99). Phonological Awareness is a composite score made up of the elision subtest, in which participants are asked to create a new word by dropping a syllable or phoneme from a spoken word, and the blending words subtest, in which participants blend spoken syllables to create a new word. The phonological memory composite score consists of the digit repetition and nonword repetition subtests, in which the participants repeat back a list of digits or increasingly longer English-based nonwords presented via speaker as accurately as they can. The rapid naming composite score is composed of rapid letter naming and rapid number naming, two subtests in which the participants read aloud a list of letters or digits as quickly and accurately as they can. These three composite measures are age-normed standard scores.
Acknowledgments
We thank teachers Kate Johnston, Steve Sanders, Kelsey Tortorice, Katie Foster, Pat Hansen-Schmidt, Brian Pavloff, Ben Das, and Mike Tooker and the participants and their families. We thank the members of the Auditory Neuroscience Laboratory, past and present, for their help with data collection and processing, especially Erika Skoe, Ahren B. Fitzroy, Margaret Touny, Samantha O’Connell, Rafael Escobedo Quiroz, Yurie Kim, Emily Spitzer, Evan Davies, Manto Agouridou, and Hillary Sigale. We also thank Trent Nicol, Jess Slater, and Travis White-Schwoch for helpful comments on an earlier version of the manuscript. This research is funded by National Science Foundation Grant SMA1015614, NIH Grant DC009399, the Mathers Foundation, the National Association of Music Merchants, and the Knowles Hearing Center, Northwestern University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. P.T. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1505114112/-/DCSupplemental.
References
- 1.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 2.Huttenlocher PR. Synaptic density in human frontal cortex: Developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 3.Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28(6):517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- 4.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387(2):167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Paus T, et al. Structural maturation of neural pathways in children and adolescents: In vivo study. Science. 1999;283(5409):1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- 6.Pfefferbaum A, et al. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51(9):874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- 7.Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children: A volumetric imaging study. Brain. 1996;119(Pt 5):1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- 8.Sowell ER, et al. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999;9(6 Pt 1):587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- 9.Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21(22):8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sowell ER, et al. Mapping cortical change across the human life span. Nat Neurosci. 2003;6(3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 11.Giedd JN, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 12.Courchesne E, et al. Normal brain development and aging: Quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216(3):672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- 13.Gogtay N, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benninger C, Matthis P, Scheffner D. EEG development of healthy boys and girls: Results of a longitudinal study. Electroencephalogr Clin Neurophysiol. 1984;57(1):1–12. doi: 10.1016/0013-4694(84)90002-6. [DOI] [PubMed] [Google Scholar]
- 15.Clarke AR, Barry RJ, McCarthy R, Selikowitz M. Age and sex effects in the EEG: Development of the normal child. Clin Neurophysiol. 2001;112(5):806–814. doi: 10.1016/s1388-2457(01)00488-6. [DOI] [PubMed] [Google Scholar]
- 16.Campbell IG, Feinberg I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proc Natl Acad Sci USA. 2009;106(13):5177–5180. doi: 10.1073/pnas.0812947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liégeois-Chauvel C, Musolino A, Badier JM, Marquis P, Chauvel P. Evoked potentials recorded from the auditory cortex in man: Evaluation and topography of the middle latency components. Electroencephalogr Clin Neurophysiol. 1994;92(3):204–214. doi: 10.1016/0168-5597(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 18.Näätänen R, Picton T. The N1 wave of the human electric and magnetic response to sound: A review and an analysis of the component structure. Psychophysiology. 1987;24(4):375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 19.Woods DL. The component structure of the N1 wave of the human auditory evoked potential. Electroencephalogr Clin Neurophysiol. 1995;44(Suppl):102–109. [PubMed] [Google Scholar]
- 20.Godey B, Schwartz D, de Graaf JB, Chauvel P, Liégeois-Chauvel C. Neuromagnetic source localization of auditory evoked fields and intracerebral evoked potentials: A comparison of data in the same patients. Clin Neurophysiol. 2001;112(10):1850–1859. doi: 10.1016/s1388-2457(01)00636-8. [DOI] [PubMed] [Google Scholar]
- 21.Mahajan Y, McArthur G. Maturation of auditory event-related potentials across adolescence. Hear Res. 2012;294(1-2):82–94. doi: 10.1016/j.heares.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Sharma A, Kraus N, McGee TJ, Nicol TG. Developmental changes in P1 and N1 central auditory responses elicited by consonant-vowel syllables. Electroencephalogr Clin Neurophysiol. 1997;104(6):540–545. doi: 10.1016/s0168-5597(97)00050-6. [DOI] [PubMed] [Google Scholar]
- 23.Eggermont JJ, Ponton CW. Auditory-evoked potential studies of cortical maturation in normal hearing and implanted children: Correlations with changes in structure and speech perception. Acta Otolaryngol. 2003;123(2):249–252. doi: 10.1080/0036554021000028098. [DOI] [PubMed] [Google Scholar]
- 24.Skoe E, Krizman J, Anderson S, Kraus N. Stability and plasticity of auditory brainstem function across the lifespan. Cereb Cortex. 2015;25(6):1415–1426. doi: 10.1093/cercor/bht311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krizman J, et al. 2015. Continued maturation of auditory brainstem function during adolescence: A longitudinal approach. Clin Neurophysiol 2015:S1388-2457(15)00083-8.
- 26.Strait DL, Kraus N. Biological impact of auditory expertise across the life span: musicians as a model of auditory learning. Hear Res. 2014;308:109–121. doi: 10.1016/j.heares.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kraus N, Anderson S. Identifying neural signatures of auditory function. Hear J. 2015;68:38–40. [Google Scholar]
- 28.Kraus N, Strait DL. Emergence of biological markers of musicianship with school-based music instruction. Ann N Y Acad Sci. 2015;1337:163–169. doi: 10.1111/nyas.12631. [DOI] [PubMed] [Google Scholar]
- 29.Slater J, et al. Music training improves speech-in-noise perception: Longitudinal evidence from a community-based music program. Behav Brain Res. 2015;291:244–252. doi: 10.1016/j.bbr.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 30.Parbery-Clark A, Skoe E, Lam C, Kraus N. Musician enhancement for speech-in-noise. Ear Hear. 2009;30(6):653–661. doi: 10.1097/AUD.0b013e3181b412e9. [DOI] [PubMed] [Google Scholar]
- 31.Parbery-Clark A, Strait DL, Anderson S, Hittner E, Kraus N. Musical experience and the aging auditory system: Implications for cognitive abilities and hearing speech in noise. PLoS One. 2011;6(5):e18082. doi: 10.1371/journal.pone.0018082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strait DL, Parbery-Clark A, Hittner E, Kraus N. Musical training during early childhood enhances the neural encoding of speech in noise. Brain Lang. 2012;123(3):191–201. doi: 10.1016/j.bandl.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zendel BR, Alain C. Musicians experience less age-related decline in central auditory processing. Psychol Aging. 2012;27(2):410–417. doi: 10.1037/a0024816. [DOI] [PubMed] [Google Scholar]
- 34.Swaminathan J, Mason C, Streeter T, Kidd G, Patel A. Musical training, individual differences and the cocktail party problem. Scientific Reports. 2015;5:11628. doi: 10.1038/srep11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan AS, Ho YC, Cheung MC. Music training improves verbal memory. Nature. 1998;396(6707):128. doi: 10.1038/24075. [DOI] [PubMed] [Google Scholar]
- 36.Ho YC, Cheung MC, Chan AS. Music training improves verbal but not visual memory: Cross-sectional and longitudinal explorations in children. Neuropsychology. 2003;17(3):439–450. doi: 10.1037/0894-4105.17.3.439. [DOI] [PubMed] [Google Scholar]
- 37.Jakobson L, Lewycky S, Kilgour A, Stoesz B. Memory for verbal and visual material in highly trained musicians. Music Percept. 2008;26:21–55. [Google Scholar]
- 38.Tierney AT, Bergeson-Dana TR, Pisoni DB. Effects of early musical experience on auditory sequence memory. Empir Musicol Rev. 2008;3(4):178–186. doi: 10.18061/1811/35989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolinsky R, Cuvelier H, Goetry V, Peretz I, Morais J. Music training facilitates lexical stress processing. Music Percept. 2009;26:235–246. [Google Scholar]
- 40.Franҫois C, Chobert J, Besson M, Schön D. Music training for the development of speech segmentation. Cerebral Cortex. 2013;23(9):2038–2043. doi: 10.1093/cercor/bhs180. [DOI] [PubMed] [Google Scholar]
- 41.Zuk J, et al. Enhanced syllable discrimination thresholds in musicians. PLoS One. 2013;8(12):e80546. doi: 10.1371/journal.pone.0080546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Overy K. Dyslexia, temporal processing and music: The potential of music as an early learning aid for dyslexic children. Psychol Music. 2000;28:218–229. [Google Scholar]
- 43.Degé F, Schwarzer G. The effect of a music program on phonological awareness in preschoolers. Front Psychol. 2011;2:124. doi: 10.3389/fpsyg.2011.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herrera L, Lorenzo O, Defior S, Fernandez-Smith G, Costa-Giomi E. Effects of phonological and music training on the reading readiness of native- and foreign-Spanish-speaking children. Psychol Music. 2011;39:68–81. [Google Scholar]
- 45.Moritz C, Yampolsky S, Papadelis G, Thomson J, Wolf M. Links between early rhythm skills, music training, and phonological awareness. Read Writ. 2013;26:739–769. [Google Scholar]
- 46.Moreno S, et al. Musical training influences linguistic abilities in 8-year-old children: More evidence for brain plasticity. Cereb Cortex. 2009;19(3):712–723. doi: 10.1093/cercor/bhn120. [DOI] [PubMed] [Google Scholar]
- 47.Taub GE, Lazarus PJ. The effects of training in timing and rhythm on reading achievement. Contemp Issues Educ Res. 2012;5:343–350. [Google Scholar]
- 48.Bhide A, Power A, Goswami U. A rhythmic musical intervention for poor readers: A comparison of efficacy with a letter-based intervenction. Mind Brain Educ. 2013;7:113–123. [Google Scholar]
- 49.Cogo-Moreira H, Brandão de Ávila CR, Ploubidis GB, Mari JdeJ. Effectiveness of music education for the improvement of reading skills and academic achievement in young poor readers: A pragmatic cluster-randomized, controlled clinical trial. PLoS One. 2013;8(3):e59984. doi: 10.1371/journal.pone.0059984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slater J, et al. Longitudinal effects of group music instruction on literacy skills in low-income children. PLoS One. 2014;9(11):e113383. doi: 10.1371/journal.pone.0113383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Costa-Giomi E. Effects of three years of piano instruction on children’s academic achievement, school performance and self-esteem. Psychol Music. 2004;32:139–152. [Google Scholar]
- 52.Ruggles DR, Freyman RL, Oxenham AJ. Influence of musical training on understanding voiced and whispered speech in noise. PLoS One. 2014;9(1):e86980. doi: 10.1371/journal.pone.0086980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shahin A, Bosnyak DJ, Trainor LJ, Roberts LE. Enhancement of neuroplastic P2 and N1c auditory evoked potentials in musicians. J Neurosci. 2003;23(13):5545–5552. doi: 10.1523/JNEUROSCI.23-13-05545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shahin A, Roberts LE, Trainor LJ. Enhancement of auditory cortical development by musical experience in children. Neuroreport. 2004;15(12):1917–1921. doi: 10.1097/00001756-200408260-00017. [DOI] [PubMed] [Google Scholar]
- 55.Musacchia G, Strait D, Kraus N. Relationships between behavior, brainstem and cortical encoding of seen and heard speech in musicians and non-musicians. Hear Res. 2008;241(1-2):34–42. doi: 10.1016/j.heares.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Franҫois C, Schön C. Musical expertise boosts implicit learning of both musical and linguistic structures. Cereb Cortex. 2011;21(10):2357–2365. doi: 10.1093/cercor/bhr022. [DOI] [PubMed] [Google Scholar]
- 57.Musacchia G, Sams M, Skoe E, Kraus N. Musicians have enhanced subcortical auditory and audiovisual processing of speech and music. Proc Natl Acad Sci USA. 2007;104(40):15894–15898. doi: 10.1073/pnas.0701498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parbery-Clark A, Anderson S, Hittner E, Kraus N. Musical experience offsets age-related delays in neural timing. Neurobiol Aging. 2012;33(7):1483.e1–1483.e4. doi: 10.1016/j.neurobiolaging.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 59.Parbery-Clark A, Anderson S, Hittner E, Kraus N. Musical experience strengthens the neural representation of sounds important for communication in middle-aged adults. Front Aging Neurosci. 2012;4:30. doi: 10.3389/fnagi.2012.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.White-Schwoch T, Woodruff Carr K, Anderson S, Strait DL, Kraus N. Older adults benefit from music training early in life: Biological evidence for long-term training-driven plasticity. J Neurosci. 2013;33(45):17667–17674. doi: 10.1523/JNEUROSCI.2560-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kraus N, et al. Auditory learning through active engagement with sound: Biological impact of community music lessons in at-risk children. Front Neurosci. 2014;8:351. doi: 10.3389/fnins.2014.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parbery-Clark A, Skoe E, Kraus N. Musical experience limits the degradative effects of background noise on the neural processing of sound. J Neurosci. 2009;29(45):14100–14107. doi: 10.1523/JNEUROSCI.3256-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Strait DL, Chan K, Ashley R, Kraus N. Specialization among the specialized: Auditory brainstem function is tuned in to timbre. Cortex. 2012;48(3):360–362. doi: 10.1016/j.cortex.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 64.Strait DL, Parbery-Clark A, O’Connell S, Kraus N. Biological impact of preschool music classes on processing speech in noise. Dev Cogn Neurosci. 2013;6:51–60. doi: 10.1016/j.dcn.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parbery-Clark A, Tierney A, Strait DL, Kraus N. Musicians have fine-tuned neural distinction of speech syllables. Neuroscience. 2012;219:111–119. doi: 10.1016/j.neuroscience.2012.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Strait D, O’Connell S, Parbery-Clark A, Kraus N. Musicians’ enhanced neural differentiation of speech sounds arises early in life: Developmental evidence from ages 3 to 30. Cereb Cortex. 2014;24(9):2512–2521. doi: 10.1093/cercor/bht103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kraus N, et al. Music enrichment programs improve the neural encoding of speech in at-risk children. J Neurosci. 2014;34(36):11913–11918. doi: 10.1523/JNEUROSCI.1881-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong PC, Skoe E, Russo NM, Dees T, Kraus N. Musical experience shapes human brainstem encoding of linguistic pitch patterns. Nat Neurosci. 2007;10(4):420–422. doi: 10.1038/nn1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bidelman GM, Gandour JT, Krishnan A. Cross-domain effects of music and language experience on the representation of pitch in the human auditory brainstem. J Cogn Neurosci. 2011;23(2):425–434. doi: 10.1162/jocn.2009.21362. [DOI] [PubMed] [Google Scholar]
- 70.Skoe E, Kraus N. Musical training heightens auditory brainstem function during sensitive periods in development. Front Psychol. 2013;4:622. doi: 10.3389/fpsyg.2013.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tierney A, Krizman J, Skoe E, Johnston K, Kraus N. High school music classes enhance the neural processing of speech. Front Psychol. 2013;4:855. doi: 10.3389/fpsyg.2013.00855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hakuta K, Bialystok E, Wiley E. Critical evidence: A test of the critical-period hypothesis for second-language acquisition. Psychol Sci. 2003;14(1):31–38. doi: 10.1111/1467-9280.01415. [DOI] [PubMed] [Google Scholar]
- 73.Hornickel J, Kraus N. Unstable representation of sound: A biological marker of dyslexia. J Neurosci. 2013;33(8):3500–3504. doi: 10.1523/JNEUROSCI.4205-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Whitford TJ, et al. Brain maturation in adolescence: Concurrent changes in neuroanatomy and neurophysiology. Hum Brain Mapp. 2007;28(3):228–237. doi: 10.1002/hbm.20273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hornickel J, Zecker SG, Bradlow AR, Kraus N. Assistive listening devices drive neuroplasticity in children with dyslexia. Proc Natl Acad Sci USA. 2012;109(41):16731–16736. doi: 10.1073/pnas.1206628109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tramontin AD, Brenowitz EA. Seasonal plasticity in the adult brain. Trends Neurosci. 2000;23(6):251–258. doi: 10.1016/s0166-2236(00)01558-7. [DOI] [PubMed] [Google Scholar]
- 77.Seppänen M, Hämäläinen J, Pesonen AK, Tervaniemi M. Music training enhances rapid neural plasticity of n1 and p2 source activation for unattended sounds. Front Hum Neurosci. 2012;6:43. doi: 10.3389/fnhum.2012.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Slevc LR, Miyake A. Individual differences in second-language proficiency: Does musical ability matter? Psychol Sci. 2006;17(8):675–681. doi: 10.1111/j.1467-9280.2006.01765.x. [DOI] [PubMed] [Google Scholar]
- 79.Penhune VB. Sensitive periods in human development: Evidence from musical training. Cortex. 2011;47(9):1126–1137. doi: 10.1016/j.cortex.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 80.Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13(1):87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blake DT, Heiser MA, Caywood M, Merzenich MM. Experience-dependent adult cortical plasticity requires cognitive association between sensation and reward. Neuron. 2006;52(2):371–381. doi: 10.1016/j.neuron.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xiong Y, Zhang Y, Yan J. The neurobiology of sound-specific auditory plasticity: A core neural circuit. Neurosci Biobehav Rev. 2009;33(8):1178–1184. doi: 10.1016/j.neubiorev.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 83.Patel AD. Why would music training benefit the neural encoding of speech? The OPERA hypothesis. Front Psychol. 2011;2:142. doi: 10.3389/fpsyg.2011.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Salimpoor VN, et al. Interactions between the nucleus accumbens and auditory cortices predict music reward value. Science. 2013;340(6129):216–219. doi: 10.1126/science.1231059. [DOI] [PubMed] [Google Scholar]
- 85.Thomson J, Fryer B, Maltby J, Goswami U. Auditory and motor rhythm awareness in adults with dyslexia. J Res Read. 2006;29:334–348. [Google Scholar]
- 86.Thomson JM, Goswami U. Rhythmic processing in children with developmental dyslexia: Auditory and motor rhythms link to reading and spelling. J Physiol Paris. 2008;102(1-3):120–129. doi: 10.1016/j.jphysparis.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 87.Corriveau KH, Goswami U. Rhythmic motor entrainment in children with speech and language impairments: Tapping to the beat. Cortex. 2009;45(1):119–130. doi: 10.1016/j.cortex.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 88.Tierney A, Kraus N. Auditory-motor entrainment and phonological skills: Precise auditory timing hypothesis (PATH) Front Hum Neurosci. 2014;8:949. doi: 10.3389/fnhum.2014.00949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Norton ES, Wolf M. Rapid automatized naming (RAN) and reading fluency: Implications for understanding and treatment of reading disabilities. Annu Rev Psychol. 2012;63:427–452. doi: 10.1146/annurev-psych-120710-100431. [DOI] [PubMed] [Google Scholar]
- 90.Krizman J, Skoe E, Kraus N. Sex differences in auditory subcortical function. Clin Neurophysiol. 2012;123(3):590–597. doi: 10.1016/j.clinph.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Skoe E, Krizman J, Kraus N. The impoverished brain: Disparities in maternal education affect the neural response to sound. J Neurosci. 2013;33(44):17221–17231. doi: 10.1523/JNEUROSCI.2102-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rabkin N, Hedberg EC. 2011. Arts Education in America: What the Declines Mean for Arts Participation (National Endowment for the Arts, Washington, DC), Research Report 52 (Based on the 2008 Survey of Public Participation in the Arts)
- 93.Green CS, Bavelier D. Learning, attentional control, and action video games. Curr Biol. 2012;22(6):R197–R206. doi: 10.1016/j.cub.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- 95.Liu LF, Palmer AR, Wallace MN. Phase-locked responses to pure tones in the inferior colliculus. J Neurophysiol. 2006;95(3):1926–1935. doi: 10.1152/jn.00497.2005. [DOI] [PubMed] [Google Scholar]
- 96.Chandrasekaran B, Kraus N. The scalp-recorded brainstem response to speech: Neural origins and plasticity. Psychophysiology. 2010;47(2):236–246. doi: 10.1111/j.1469-8986.2009.00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 98.Lopez-Calderon J, Luck SJ. ERPLAB: An open-source toolbox for the analysis of event-related potentials. Front Hum Neurosci. 2014;8:213. doi: 10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wagner R, Torgesen J, Rashotte C. CTOPP: Comprehensive Test of Phonological Processing. Pro-ed; Austin, TX: 1999. [Google Scholar]