Abstract
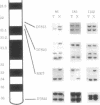
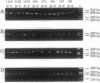
Loss of heterozygosity in human chromosome 7q was studied to determine the location of a putative tumor suppressor gene. Twenty-six of 31 cases studied presented loss of heterozygosity at one or more loci on chromosome 7q. Eighty-three percent loss of heterozygosity (in 11 informative cases) was detected by using the (C-A)n microsatellite repeat marker D7S522 at 7q31.1-7q31.2. These results suggest that a tumor suppressor gene relevant to the development of breast cancer is present in the 7q31.1-7q31.2 region, confirming our previous evidence for a tumor suppressor gene in this chromosome and frequent deletions of the long arm in human primary breast cancers.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkin N. B., Baker M. C. Chromosome 7q deletions: observations on 13 malignant tumors. Cancer Genet Cytogenet. 1993 Jun;67(2):123–125. doi: 10.1016/0165-4608(93)90164-h. [DOI] [PubMed] [Google Scholar]
- Atkin N. B., Baker M. C. Numerical chromosome changes in 165 malignant tumors. Evidence for a nonrandom distribution of normal chromosomes. Cancer Genet Cytogenet. 1991 Mar;52(1):113–121. doi: 10.1016/0165-4608(91)90061-x. [DOI] [PubMed] [Google Scholar]
- Barbry P., Simon-Bouy B., Mattéi M. G., Le Guern E., Jaume-Roig B., Chassande O., Ullrich A., Lazdunski M. Localization of the gene for amiloride binding protein on chromosome 7 and RFLP analysis in cystic fibrosis families. Hum Genet. 1990 Oct;85(6):587–589. doi: 10.1007/BF00193579. [DOI] [PubMed] [Google Scholar]
- Barrett J. C. Mechanisms of multistep carcinogenesis and carcinogen risk assessment. Environ Health Perspect. 1993 Apr;100:9–20. doi: 10.1289/ehp.931009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi A. B., Navone N. M., Aldaz C. M., Conti C. J. Overlapping loss of heterozygosity by mitotic recombination on mouse chromosome 7F1-ter in skin carcinogenesis. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7590–7594. doi: 10.1073/pnas.88.17.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bièche I., Champème M. H., Matifas F., Hacène K., Callahan R., Lidereau R. Loss of heterozygosity on chromosome 7q and aggressive primary breast cancer. Lancet. 1992 Jan 18;339(8786):139–143. doi: 10.1016/0140-6736(92)90208-k. [DOI] [PubMed] [Google Scholar]
- Callahan R., Cropp C. S., Merlo G. R., Liscia D. S., Cappa A. P., Lidereau R. Somatic mutations and human breast cancer. A status report. Cancer. 1992 Mar 15;69(6 Suppl):1582–1588. doi: 10.1002/1097-0142(19920315)69:6+<1582::aid-cncr2820691313>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Chen L. C., Dollbaum C., Smith H. S. Loss of heterozygosity on chromosome 1q in human breast cancer. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7204–7207. doi: 10.1073/pnas.86.18.7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. C., Kurisu W., Ljung B. M., Goldman E. S., Moore D., 2nd, Smith H. S. Heterogeneity for allelic loss in human breast cancer. J Natl Cancer Inst. 1992 Apr 1;84(7):506–510. doi: 10.1093/jnci/84.7.506. [DOI] [PubMed] [Google Scholar]
- Cooke A., Kim Y. T., Harvey T. I., Connor J. M., Nagy J., George W. D. Loss of heterozygosity on chromosome 7q31 in breast cancer. Lancet. 1993 May 15;341(8855):1289–1289. doi: 10.1016/0140-6736(93)91200-6. [DOI] [PubMed] [Google Scholar]
- Devilee P., van Vliet M., van Sloun P., Kuipers Dijkshoorn N., Hermans J., Pearson P. L., Cornelisse C. J. Allelotype of human breast carcinoma: a second major site for loss of heterozygosity is on chromosome 6q. Oncogene. 1991 Sep;6(9):1705–1711. [PubMed] [Google Scholar]
- Dutrillaux B., Gerbault-Seureau M., Zafrani B. Characterization of chromosomal anomalies in human breast cancer. A comparison of 30 paradiploid cases with few chromosome changes. Cancer Genet Cytogenet. 1990 Oct 15;49(2):203–217. doi: 10.1016/0165-4608(90)90143-x. [DOI] [PubMed] [Google Scholar]
- Estivill X., Farrall M., Scambler P. J., Bell G. M., Hawley K. M., Lench N. J., Bates G. P., Kruyer H. C., Frederick P. A., Stanier P. A candidate for the cystic fibrosis locus isolated by selection for methylation-free islands. 1987 Apr 30-May 6Nature. 326(6116):840–845. doi: 10.1038/326840a0. [DOI] [PubMed] [Google Scholar]
- Farrall M., Watson E., Bates G., Bell G., Bell J., Davies K. A., Estivill X., Kruyer H., Law H. Y., Lench N. Further data supporting linkage between cystic fibrosis and the met oncogene and haplotype analysis with met and pJ3.11. Am J Hum Genet. 1986 Dec;39(6):713–719. [PMC free article] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Fults D., Pedone C. Deletion mapping of the long arm of chromosome 10 in glioblastoma multiforme. Genes Chromosomes Cancer. 1993 Jul;7(3):173–177. doi: 10.1002/gcc.2870070311. [DOI] [PubMed] [Google Scholar]
- Iurlo A., Mecucci C., Van Orshoven A., Michaux J. L., Boogaerts M., Noens L., Bosly A., Louwagie A., Van Den Berghe H. Cytogenetic and clinical investigations in 76 cases with therapy-related leukemia and myelodysplastic syndrome. Cancer Genet Cytogenet. 1989 Dec;43(2):227–241. doi: 10.1016/0165-4608(89)90034-4. [DOI] [PubMed] [Google Scholar]
- James C. D., Carlbom E., Nordenskjold M., Collins V. P., Cavenee W. K. Mitotic recombination of chromosome 17 in astrocytomas. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2858–2862. doi: 10.1073/pnas.86.8.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Kere J., Donis-Keller H., Ruutu T., de la Chapelle A. Chromosome 7 long-arm deletions in myeloid disorders: terminal DNA sequences are commonly conserved and breakpoints vary. Cytogenet Cell Genet. 1989;50(4):226–229. doi: 10.1159/000132765. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson A. G., Jr The ninth Gordon Hamilton-Fairley memorial lecture. Hereditary cancers: clues to mechanisms of carcinogenesis. Br J Cancer. 1989 May;59(5):661–666. doi: 10.1038/bjc.1989.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson C., Byström C., Skoog L., Rotstein S., Nordenskjöld M. Genomic alterations in human breast carcinomas. Genes Chromosomes Cancer. 1990 Sep;2(3):191–197. doi: 10.1002/gcc.2870020305. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., O'Connell P., Leppert M., Nakamura Y., Farrall M., Tsui L. C., Lalouel J. M., White R. Twenty-five loci form a continuous linkage map of markers for human chromosome 7. Genomics. 1989 Nov;5(4):866–873. doi: 10.1016/0888-7543(89)90128-6. [DOI] [PubMed] [Google Scholar]
- Mamuris Z., Dumont J., Dutrillaux B., Aurias A. Chromosomal differences between acute nonlymphocytic leukemia in patients with prior solid tumors and prior hematologic malignancies. A study of 14 cases with prior breast cancer. Cancer Genet Cytogenet. 1989 Oct 1;42(1):43–50. doi: 10.1016/0165-4608(89)90006-x. [DOI] [PubMed] [Google Scholar]
- Neuman W. L., Rubin C. M., Rios R. B., Larson R. A., Le Beau M. M., Rowley J. D., Vardiman J. W., Schwartz J. L., Farber R. A. Chromosomal loss and deletion are the most common mechanisms for loss of heterozygosity from chromosomes 5 and 7 in malignant myeloid disorders. Blood. 1992 Mar 15;79(6):1501–1510. [PubMed] [Google Scholar]
- Ogata T., Ayusawa D., Namba M., Takahashi E., Oshimura M., Oishi M. Chromosome 7 suppresses indefinite division of nontumorigenic immortalized human fibroblast cell lines KMST-6 and SUSM-1. Mol Cell Biol. 1993 Oct;13(10):6036–6043. doi: 10.1128/mcb.13.10.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osella P., Carlson A., Wyandt H., Milunsky A. Cytogenetic studies of eight squamous cell carcinomas of the head and neck. Deletion of 7q, a possible primary chromosomal event. Cancer Genet Cytogenet. 1992 Mar;59(1):73–78. doi: 10.1016/0165-4608(92)90162-2. [DOI] [PubMed] [Google Scholar]
- Pandis N., Heim S., Bardi G., Idvall I., Mandahl N., Mitelman F. Chromosome analysis of 20 breast carcinomas: cytogenetic multiclonality and karyotypic-pathologic correlations. Genes Chromosomes Cancer. 1993 Jan;6(1):51–57. doi: 10.1002/gcc.2870060110. [DOI] [PubMed] [Google Scholar]
- Parkin D. M., Pisani P., Ferlay J. Estimates of the worldwide incidence of eighteen major cancers in 1985. Int J Cancer. 1993 Jun 19;54(4):594–606. doi: 10.1002/ijc.2910540413. [DOI] [PubMed] [Google Scholar]
- Stalsberg H., Thomas D. B. Age distribution of histologic types of breast carcinoma. Int J Cancer. 1993 Apr 22;54(1):1–7. doi: 10.1002/ijc.2910540102. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. The multistep nature of cancer. Trends Genet. 1993 Apr;9(4):138–141. doi: 10.1016/0168-9525(93)90209-z. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Tumor suppressor genes. Science. 1991 Nov 22;254(5035):1138–1146. doi: 10.1126/science.1659741. [DOI] [PubMed] [Google Scholar]
- Weissenbach J., Gyapay G., Dib C., Vignal A., Morissette J., Millasseau P., Vaysseix G., Lathrop M. A second-generation linkage map of the human genome. Nature. 1992 Oct 29;359(6398):794–801. doi: 10.1038/359794a0. [DOI] [PubMed] [Google Scholar]
- White R., Woodward S., Leppert M., O'Connell P., Hoff M., Herbst J., Lalouel J. M., Dean M., Vande Woude G. A closely linked genetic marker for cystic fibrosis. 1985 Nov 28-Dec 4Nature. 318(6044):382–384. doi: 10.1038/318382a0. [DOI] [PubMed] [Google Scholar]
- Zengerling S., Tsui L. C., Grzeschik K. H., Olek K., Riordan J. R., Buchwald M. Mapping of DNA markers linked to the cystic fibrosis locus on the long arm of chromosome 7. Am J Hum Genet. 1987 Mar;40(3):228–236. [PMC free article] [PubMed] [Google Scholar]
- Zenklusen J. C., Oshimura M., Barrett J. C., Conti C. J. Inhibition of tumorigenicity of a murine squamous cell carcinoma (SCC) cell line by a putative tumor suppressor gene on human chromosome 7. Oncogene. 1994 Oct;9(10):2817–2825. [PubMed] [Google Scholar]