Abstract
Cytokine production is necessary to protect against pathogens and promote tissue repair, but excessive cytokine release can lead to systemic inflammation, organ failure and death. Inflammatory responses are finely regulated to effectively guard from noxious stimuli. The central nervous system interacts dynamically with the immune system to modulate inflammation through humoral and neural pathways. The effect of glucocorticoids and other humoral mediators on inflammatory responses has been studied extensively in the past decades. In contrast, neural control of inflammation has only been recently described. We summarize autonomic regulation of local and systemic inflammation through the ‘cholinergic anti-inflammatory pathway’, a mechanism consisting of the vagus nerve and its major neurotransmitter, acetylcholine, a process dependent on the nicotinic acetylcholine receptor α7 subunit. We recapitulate additional sources of acetylcholine and their contribution to the inflammatory response, as well as acetylcholine regulation by acetylcholinesterase as a means to attenuate inflammation. We discuss potential therapeutic applications to treat diseases characterized by acute or chronic inflammation, including autoimmune diseases, and propose future research directions.
Keywords: alpha7, cholinergic, inflammation, innate immunity
Introduction
Inflammation is the physiological response to pathogen invasion and tissue damage that manifests clinically by swelling, pain, redness and heat. During the inflammatory process, cells of the immune system release cytokines including TNF and other mediators that mediate bacterial clearance and promote tissue repair. Typically, local regulatory mechanisms adjust the magnitude of the response such that the injurious condition is resolved and homeostasis is maintained. Regulatory mechanisms of inflammation include mediators of humoral or neural origin that maintain the inflammatory process within physiological range. Humoral anti-inflammatory mediators like IL-10 and glucocorticoids inhibit the release or the effect of proinflammatory cytokines; others facilitate tissue healing, such as lipoxins and resolvins [1]. Humoral mediators reach target cells by local diffusion and through circulation, and act not only on target cells located within the tissue source but also on other organs. In contrast to humoral mediators, effectors released by nerve terminals like norepinephrine and acetylcholine reach discrete groups of cells in specific organs with minimum delay.
Sepsis, the systemic inflammatory response to infection, is characterized by dysregulated production of cytokines, a pathologic state that causes tissue injury, which leads to organ dysfunction and death. In its early stages, unrestrained production of pro-inflamma-tory cytokines, such as TNF and IL-1β triggers a systemic inflammatory cascade mediated by chemokines, vasoactive amines, the complement and coagulation systems, and reactive oxygen species, amongst others. The combined effect of these mediators may cause increased vascular permeability, hypotension and septic shock [2]. Late mediators of sepsis like MIF and HMGB1, released actively or passively because of cell damage, perpetuate the inflammatory response ultimately leading to multiple organ failure and death [3–5].
In the process of developing new strategies to modulate the inflammatory response in sepsis, we discovered that signals arising in the brain and conveyed by the vagus nerve attenuate inflammatory cytokine production and improve survival in experimental models of sepsis. Here, we summarize how the autonomic nervous system regulates inflammation through the ‘cholinergic anti-inflammatory pathway’, a mechanism consisting of the vagus nerve, its major neurotransmitter, acetylcholine, and dependent on the nicotinic acetylcholine receptor subunit alpha7 (α7). We summarize results obtained from experimental models of local and systemic inflammation in which electrical stimulation of the vagus nerve or cholinergic drugs acting through α7 effectively modulate inflammation. We comment on the possible anti-inflammatory role of acetylcholine derived from sources other than neurons and describe modulation of the inflammatory response through inhibition of acetylcholinesterase, the enzyme that hydrolyzes acetylcholine. Finally, we propose future research directions and potential therapeutic approaches to treat specific inflammatory diseases.
Immune-to-brain communication via the vagus nerve
The vagus nerve, the major nerve of the parasympathetic division of the autonomic nervous system, regulates organ function including heart rate, gut motility and bronchial constriction through efferent motor fibres. Approximately 80% of the vagus nerve's fibres are sensory fibres that gather information from the airways, heart, liver and gastric tract via receptors that respond to pressure and temperature [6, 7]. Recent experimental evidence suggests that the afferent component of the vagus nerve also conveys information to the brain regarding inflammatory processes occurring in the periphery. For example, intraperitoneal administration of the pro-inflammatory cytokine IL-1β induces the expression of the neural activation marker c-fos in afferent neurons of the vagus nerve [8], which express IL-1β receptors [9]. Vagotomy abrogates the illness behaviour originating in the central nervous system mounted in response to intraperitoneal injections of IL-1β or LPS [10]. The immune system thus gathers information generated in the periphery and serves as a sensory organ informing the brain of noxious stimuli [11, 12].
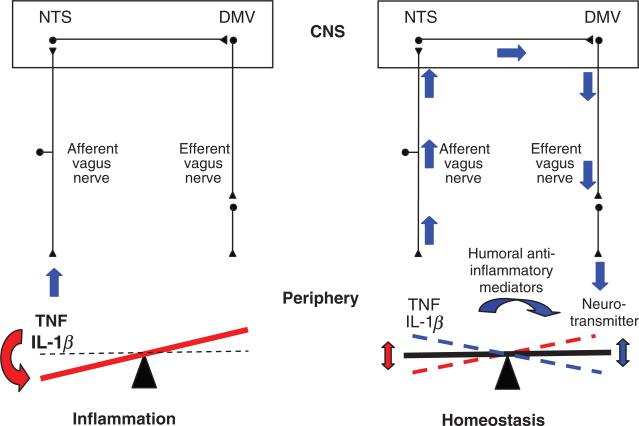
Afferent fibres of the vagus nerve reach the medulla oblongata and terminate in the nucleus tractus solitarius where release of glutamate is enhanced in response to peripheral administration of LPS or IL-1β [13]. Information reaching the nucleus tractus solitarius is delivered to the dorsal motor nucleus of the vagus, the origin of preganglionic neurons whose axons embody the efferent component of the vagus nerve. The connection between the nucleus tractus solitarius and the dorsal motor nucleus of the vagus coordinates vagal afferent signals and vagal efferent responses. The autonomic nervous system, through this anatomical layout, gathers information from peripheral inflammatory responses and responds in real-time through efferent fibres of the vagus nerve maintaining homeostasis, a mechanism known as the inflammatory reflex [14] (Fig. 1).
Fig. 1.
Inflammatory reflex. Pathogens and tissue damage induce release of cytokines, which serve to limit the extent of infection and promote tissue repair. Humoral and neural regulatory pathways regulate the magnitude of the inflammatory response. Cytokines released at the inflammatory site activate afferent fibres of the vagus nerve and reach the nucleus tractus solitarius in the brain stem, thus providing the autonomic nervous system information regarding peripheral inflammatory status. Compensatory signals are conveyed by the efferent vagus nerve and reach the site of inflammation where neurotransmitters act upon macrophages and other cells of the immune system to attenuate the inflammatory response. NTS, nucleus tractus solitarius; DMV, dorsal motor nucleus of the vagus; CNS, central nervous system.
Cholinergic anti-inflammatory pathway
The cholinergic anti-inflammatory pathway, the efferent arm of the inflammatory reflex, is composed of the efferent vagus nerve, the neurotransmitter acetylcholine and the α7 subunit of the nicotinic acetylcholine receptor. Initial experiments established that acetylcholine attenuates the production of TNF, IL-1β, IL-6 and IL-18 by human macrophages at the post-transcriptional stage [15]. Acetylcholine does not alter IL-10 release, which indicates a direct inhibitory effect of acetylcholine on pro-inflammatory cytokine production [15]. In a model of endotoxaemia, electrical stimulation of the cervical vagus nerve significantly reduced serum and liver TNF levels, prevented development of haemodynamic shock and improved survival without significantly altering IL-10 or corticosterone serum levels.
The molecular link between the brain and the immune system in the cholinergic anti-inflammatory pathway is the nicotinic acetylcholine receptor subunit α7. Nicotine, the prototypical nicotinic acetylcholine receptor agonist, attenuates TNF release in LPS-stimulated human macrophages. Transfection of specific anti-sense oligonucleotides against α7 significantly inhibits nicotine's TNF-suppressing effect in macrophages. Vagus nerve stimulation fails to reduce serum TNF levels in α7 knockout mice, indicating that α7 is required for the functional integrity of the cholinergic anti-inflammatory pathway in vivo. Endotoxaemic α7 knockout mice develop significantly increased TNF levels in serum, spleen and liver as compared with wild type mice, indicating that the cholinergic anti-inflammatory pathway exerts tonic inhibition of cytokine production and functions as an essential regulator of inflammation via α7 [16].
Vagus nerve stimulation specifically modulates immune function through a mechanism that is dissociable from cardiac control, because the voltage and frequency parameters of vagus nerve stimulation used to attenuate endotoxin-induced TNF in serum are below the threshold to reduce heart rate [17]. The vagus nerve controls bodily function through muscarinic receptors expressed on target organs. Intravenous administration of atropine methyl nitrate, a peripheral muscarinic receptor antagonist that does not cross the blood–brain barrier, does not abrogate the TNF suppressive effect of vagus nerve stimulation [18]. Moreover, intravenous injection of muscarine fails to inhibit serum TNF in endotoxaemic rats [18], which indicates that the cholinergic anti-inflammatory pathway does not utilize peripheral muscarinic signalling to modulate inflammation. These findings suggest that electrical stimulation of the vagus nerve attenuates inflammation without unwanted secondary effects on organ functions, such as respiratory and heart rate, and gut motility.
Whilst muscarinic signalling in peripheral organs is not required for vagus nerve control of inflammation, central muscarinic transmission is important in attenuating inflammatory responses. Intracerebroventricular administration of muscarine or the M1 muscarinic receptor agonist McN-A-343 attenuated serum TNF levels in a rat model of entodotoxemia [18]. Intracerebroventricular administration of methoctramine, a M2 receptor antagonist that enhances acetylcholine release in brain, attenuated systemic TNF in endotoxaemic rats and augmented vagus nerve activity [18]. Cholinergic signalling through brain muscarinic receptors modulates peripheral cytokine production by activation of the cholinergic anti-inflammatory pathway.
Recent work on the anatomical basis of the cholinergic anti-inflammatory pathway indicates that the spleen is required for vagus nerve control of inflammation [19]. The spleen, a highly innervated secondary lymphoid organ, is a major source of serum TNF during endotoxaemia [19, 20]. In splenectomized rats injected with endotoxin, serum TNF is reduced by 70% and vagus nerve stimulation fails to further suppress TNF. Vagus nerve stimulation attenuates TNF mRNA and protein levels in spleen, and selective surgical ablation of the common celiac branches of the vagus prevents TNF inhibition by electrical stimulation of the vagus nerve [19].
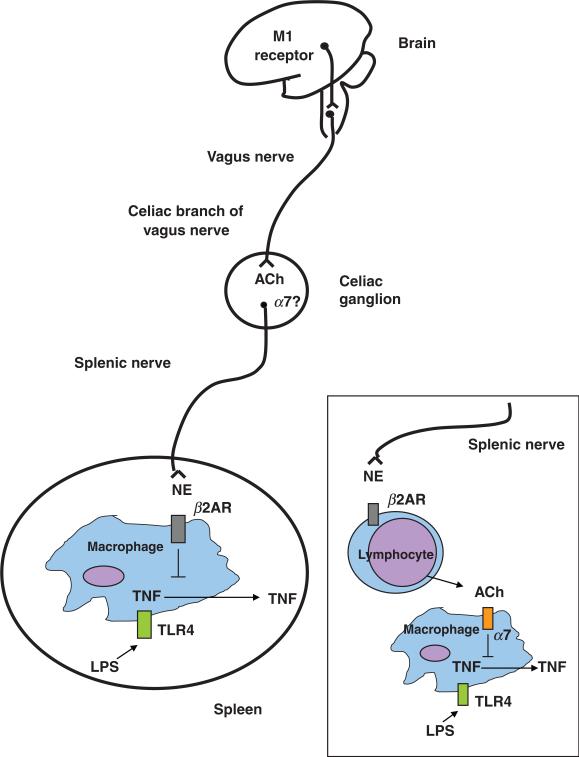
The requirement of the spleen in the cholinergic anti-inflammatory pathway was perhaps surprising because the celiac branches of the vagus terminate in the celiac-superior mesenteric plexus [21] and not in the spleen [22]. Recent experimental evidence indicates that the splenic nerve is required for attenuation of serum TNF by vagus nerve stimulation [23]. The spleen is innervated by the splenic nerve, which originates in celiac-superior mesenteric plexus [24–26]. Thus, the neural pathway that allows control of systemic cytokine production by vagus nerve stimulation is composed of two neurons connected in series: one originating in the dorsal motor nucleus that travels through the vagus nerve and a second originating in the celiac-superior mesenteric plexus embodied in the splenic nerve, which terminates in the spleen.
The splenic nerve consists mainly of catecholaminergic fibres [27], which terminate in close apposition to immune cells in the white pulp, the marginal zone and red pulp areas of the spleen [28]. Catecholamine depletion by reserpine ablates the TNF-suppressive effect of vagus nerve stimulation [23], suggesting that attenuation of TNF production by spleen macrophages induced by vagus nerve stimulation is mediated by norepinephrine released from splenic nerve endings. It has been observed in perfused rat spleens that electrical stimulation of the splenic nerve induces release of norepinephrine and attenuation of LPS-induced TNF production, an effect that is dependent on β-adrenergic receptors [29]. Catecholamines enhance or attenuate pro-inflammatory cytokine production by macrophages depending on whether they act on α- or α-adrenergic receptors respectively [30, 31]. It is possible that stimulation of the vagus nerve induces release of norepinephrine by the splenic nerve, which in turn acts on b-adrenergic receptors expressed on macrophages to attenuate TNF production. But, this possibility does not explain the previously described role of α7 in attenuating TNF levels by vagus nerve stimulation. The α7 subunit is expressed in autonomic ganglia where it mediates fast synaptic transmission [32, 33]. Mice lacking α7 present a smaller increase in heart rate compared to wildtype mice when infused with the vasodilator nitroprusside, supporting its role in regulating autonomic function [34]. It is theoretically possible that acetylcholine released by the vagus nerve acting upon α7 expressed in neurons of the celiac-superior mesenteric plexus elicits norepinephrine release from splenic nerve endings. The spleen contains acetylcholine and releases it upon electrical stimulation of the splenic nerve [35–37], and acetylcholine attenuates TNF production in spleen cell suspensions in vitro through an α7-dependent mechanism [19]. Therefore, it is plausible that cholinergic signalling directly attenuates cytokine production by splenic macrophages (Fig. 2).
Fig. 2.
The cholinergic anti-inflammatory pathway, the efferent arm of the inflammatory reflex, is composed of the vagus nerve and its major neurotransmitter, acetylcholine. Electrical stimulation of the cervical vagus nerve attenuates systemic TNF through a pathway that requires the α7 subunit of the nicotinic acetylcholine receptor. Administration of α7 agonists or activation of a brain cholinergic network that depends on M1 muscarinic receptors and increases vagus nerve activity, attenuate systemic TNF levels. Two-neuron model of vagus nerve modulation of cytokine production via the splenic nerve: the preganglionic neuron, originates in the dorsal motor nucleus of the vagus; the postganglionic neuron, located in ganglia of the celiac-superior mesenteric plexus, reaches the spleen through the splenic nerve. In this model, electrical stimulation of the cervical vagus nerve attenuates systemic TNF through a pathway that requires the α7 subunit of the nicotinic acetylcholine receptor, the splenic nerve and catecholamines. Vagus nerve firing would modulate norepinephrine release by the splenic nerve. In this scenario, release of norepinephrine by the splenic nerve would act on β2-adrenergic receptors expressed on macrophages to attenuate TNF, and α7 expressed on neurons of the celiac/superior mesenteric plexus would convey signals between the vagus and the splenic nerve. An alternate possibility is that norepinephrine originating from splenic nerve terminals induces release of acetylcholine from cell sources other than neurons (e.g. lymphocytes), which would then act on α7 expressed on macrophages to attenuate TNF. NE, norepinephrine; β2AR, beta2-adrenergic receptor; ACh, acetylcholine.
The functional connection between the central nervous system and immune cells in the spleen through the splenic nerve has now been characterized. Electrical stimulation of the hypothalamus or intraventricular administration of angiotensin and IFNα has been shown to enhance the activity of the splenic nerve and modify in vitro responses of NK cells and T lymphocytes obtained from the spleen [38–40]. Until now, these effects have been ascribed to the sympathetic nervous system, because they are mediated by the greater splanchnic nerve, which originates in the intermediolateral column of the spinal cord, and the splenic nerve. As the neuro-chemical anatomy of the celiac-superior plexus is not fully elucidated, it is plausible to consider that the vagus nerve and the greater splanchnic nerve provide input to second neurons that modify immune function in spleen.
Other cells of the innate and adaptive immune system, including dendritic cells and lymphocytes, reside in the spleen. The functional connection between the vagus nerve and the spleen mediated through the splenic nerve, puts forth the possibility of using vagus nerve stimulation to clinically modulate other immune functions such as antibody production.
Vagus nerve-based and cholinergic drug therapeutic approach to inflammatory disease
Further insight into the physiology and therapeutic potential of the cholinergic anti-inflammatory pathway has been obtained by characterizing the role of the vagus nerve or its stimulation on cytokine-mediated tissue injury in various models of local and systemic inflammation. Similarly, nicotine and selective α7 agonists have been tested in several models of inflammation providing additional insight into the functional biology of α7. Table 1 summarizes the effect of vagus nerve stimulation and cholinergic agonists in experimental models of inflammation.
Table 1.
Effect of vagus nerve stimulation and selective α7 agonists in experimental models of inflammatory disease
| Model | Vagus nerve stimulation (VNS) or vagotomy | α7 Agonist or antagonist |
|---|---|---|
| Endotoxaemia | VNS: Reduced liver and serum TNF prevented shock [15] | Nicotine patch: Attenuated fever and increased mean arterial pressure in humans injected with endotoxin. No significant effect on circulating TNF, IL-6, IL-8 nor soluble E-selectin. Increased circulating IL-10 and cortisol [123] GTS-21: Improved survival. Reduced serum TNF [46] GTS-21: Reduced serum TNF. Decreased neutrophil recruitment to peritoneum through an effect independent of TNF, KC and MIP-2. Did not alter serum IL-10 [47] |
| VNS: Reduced TNF in heart [122] VNS: Decreased mRNA TNF in spleen. Reduced liver and spleen TNF [19] |
||
| Vagotomy: Increased serum and liver TNF [15] Transcutaneous VNS: Reduced serum TNF [17] VNS: Reduced procoagulant response and fibrinolytic response. Reduced serum TNF and IL-6. Reduced TNF, IL-1β and IL-6 in spleen. No effect on serum and spleen IL-10 [44] |
||
| Sepsis (cecal ligation and puncture) | Transcutaneous VNS: Reduced serum HMGB1 levels and improved survival [17] | Nicotine: Attenuated serum HMGB1. Improved survival [45] GTS-21: Improved survival. Reduced serum HMGB1 [46] AChE inhibitors: Improved survival (physostigmine and neostigmine). Reduced serum TNF, IL-1β and IL-6 (physostigmine) [101] |
| Sepsis (intraperitoneal injection of E. coli) | Vagotomy: Increased TNF, IL-1β and IL-6 in serum and peritoneum. Increased granulocyte and macrophage counts in peritoneum [41] | Nicotine: Reduced serum TNF, IL-1β and IL-6 in serum and peritoneum. Reduced granulocyte and macrophage counts in peritoneum. Reduced serum ALT and AST. Facilitated E. coli growth in peritoneal lavage fluid, blood and liver and accelerated mortality [41] |
| Sepsis (ascendent colon stent peritonitis) | Vagotomy: Increased mortality. Increased serum TNF, IL-6, IL-10 and MCP-1 [42] | |
| Postoperative ileus | VNS: Prevented gastroparesis induced by intestinal manipulation. Reduced Ccl 2 and Ccl 3 mRNA in muscularis tissue. Reduced TNF, IL-6, MIP-2 and MIP-1α concentration in the peritoneal cavity. Reduced inflammatory cell recruitment to the intestinal muscularis. Activated STAT3 in resident macrophages of the intestinal muscularis [61] | Nicotine: Reduced TNF and IL-6 production by peritoneal macrophages. AR-R17779: Improved gastric emptying. Reduced inflammatory cell recruitment to intestinal muscle [62] |
| Pancreatitis | Vagotomy: Increased plasma amylase and lipase. Increased pancreatitis severity [49] | Mecamylamine: Increased pancreatitis severity GTS-21: Attenuated pancreatitis severity [49] |
| Schwartzman reaction | Nicotine: Reduced VCAM and E-selectin mRNA [63] | |
| Carrageenan air pouch model | Nicotine: Reduced inflammatory cell infiltration into the pouch [63] | Nicotine: Reduced inflammatory cell infiltration into the pouch. Reduced TNF and MCP-1 content in the pouch [63] |
| Haemorrhagic shock | VNS: Attenuated NF-κB activation and prevented IκBα loss in the liver. Decreased TNF mRNA and serum TNF. Reverted hypotension and prolonged survival time [53] ACTH-mediated activation of vagus nerve: Decreased NF-κB activity and TNF mRNA in liver. Decreased serum TNF. Improved cardiovascular and pulmonary function and increased survival [56] High-fat diet-induced activation of vagus nerve: Decreased serum TNF and IL-6. Preserved intestinal barrier function [57] |
Chlorisondamine: Reverted the effects of vagus nerve stimulation [53] Atropine sulphate: Reverted the effects of ACTH-mediated activation of the vagus nerve [56] Clorisondamine: Reverted the effects of high-fat diet-induced activation of vagus nerve [57] |
| Ischaemia/reperfusion | VNS: Attenuated TNF in serum, heart and liver. Prevented the development of hypotension [55] | |
| Myocardial ischaemia/reperfusion | VNS: Decreased frequency of severe arrhythmias. Decreased free radical levels in blood. Ameliorated histopathological changes in the left ventricle and enhanced ERK1/2 activation. Reduced lethality [124] | Atropine methylbromide: Abolished the effect of vagus nerve stimulation [124] |
| Renal ischaemia/reperfusion | Nicotine and GTS-21: Improved renal function and decreased tubular necrosis and renal TNF [58]. Nicotine: Reduced renal TNF, KC and HMGB1. Improved renal function and reduced tubular damage [59] |
|
| Splanchnic artery occlusion shock | VNS: Decreased NF-κB activity and prevented IκBα loss in the liver. Attenuated TNF mRNA in liver and serum TNF. Decreased leukocyte infiltration to the ileum and lung. Reverted hypotension and increased survival rate [54] | Chlorisondamine: Reverted the effects of vagus nerve stimulation [54] |
| Inflammatory bowel disease | Vagotomy: Increased disease activity index, macroscopic and histology scores, myeloperoxidase activity. Augmented IL-1β, IL-6 and TNF in colonic tissue [108] | Nicotine reduced disease activity index in vagotomized rats but not in sham-operated rats. Hexamethonium (nicotinic antagonist) worsened disease activity index in sham-operated rats [108] |
| Others | Nicotine, GTS and CAP55: Reduced TNF and IL-6 production by LPS-stimulated placenta cells. Possible role in preeclampsia [93] |
Sepsis
In addition to its anti-inflammatory effect in endotoxaemia, vagus nerve stimulation downregulates proinflammatory cytokine production in other models of sepsis. Vagus nerve stimulation attenuated serum levels of HMGB1, a late mediator of sepsis lethality, and improved survival in cecal ligation and puncture, a preclinical standardized model of septic peritonitis. The vagus nerve has intrinsic anti-inflammatory activity in microbial peritonitis models because unilateral cervical [41] or subdiaphragmatic vagotomy [42] increase serum TNF, IL-1β and IL-6; worsen liver damage, augment peritoneal infiltration of neutrophils and macrophages [41]; and increase mortality [42]. In rats subjected to polymicrobial peritonitis, serum TNF was increased by bilateral cervical vagotomy, whereas electrical stimulation of the vagus nerve attenuated it and prevented hypotension [43]. Sepsis-induced organ dysfunction is associated with abnormalities in the coagulatory system, which can lead to disseminated intravascular coagulation [2]. Vagus nerve stimulation modulates coagulation activation and fibrinolysis and thus can alter haemostatic responses through as yet uncharacterized mechanisms [44].
The therapeutic potential of cholinergic agonists to treat disorders characterized by cytokine dysregulation has been demonstrated recently by the protective effect of nicotine and more selective α7 agonists in sepsis. Administration of nicotine reduced serum HMGB1 and improved survival in endotoxaemia [45]. The selective α7 agonist GTS-21 improved survival and reduced serum TNF [46]; decreased neutrophil recruitment into the peritoneal cavity in endotoxaemia [47]; attenuated serum HMGB1 levels; and improved survival in cecal ligation and puncture [46].
Pancreatitis
Pancreatitis is a sterile inflammatory process of the pancreas associated with systemic elevation of serum cytokines and inflammatory cell activation; it can develop to multiple organ failure and death [48]. Cervical unilateral vagotomy increased local and systemic markers of inflammation and worsened clinical evolution in a rodent model of acute pancreatitis, indicating that the vagus nerve exerts a tonic anti-inflammatory effect. Further, activation of the cholinergic anti-inflammatory pathway by administration of GTS-21 reduced pancreatitis severity [49].
Haemorrhagic shock and ischaemia/reperfusion
The morbidity and mortality associated with severe haemorrhage and organ ischaemia is attributed, at least in part, to endothelial dysfunction mediated by cytokines [50–52]. Vagus nerve stimulation decreased hepatic TNF mRNA levels, attenuated serum TNF and improved survival in rat a model of haemorrhagic shock [53]. Electrical stimulation of the vagus nerve attenuated serum TNF, reversed hypotension and improved survival in a model of ischaemia/reperfusion [54, 55], an effect that was abrogated by the nicotinic receptor antagonist clorisondamine [54]. Activation of brain melanocortin MC4 receptors by the melanocortin peptide ACTH-(1-24) increased vagus nerve activity, decreased liver and serum TNF and reversed hypo-tension in haemorrhagic shock [56]. This protective effect was impeded by bilateral cervical vagotomy, administration of clorisondamine or pretreatment with atropine methylsulphate, a muscarinic receptor antagonist that crosses the brain blood barrier. Considered together, these findings support the conclusion that a central cholinergic system, dependent on muscarinic receptors, signals through the vagus nerve to attenuate systemic inflammation. Activation of the afferent vagus nerve by cholecystokinin induced by high-fat diet reduced intestinal permeability and serum pro-inflammatory cytokines by a vagus nerve-dependent mechanism in a model of haemorrhagic shock [57]. It has been proposed that this functional inhibitory loop involving the vagus nerve and the gut participates in maintenance of immune tolerance to normal gut flora and dietary antigens [57].
Nicotinic agonists have also been used to prevent renal dysfunction resulting from ischaemia-reperfusion. Treatment with nicotine and GTS-21 improved renal function, reduced renal tubular damage and renal TNF and decreased neutrophil infiltration to the kidneys in rats [58]. Signalling via α7 is required for this protective effect, because nicotine failed to ameliorate renal injury in α7 knockout mice [59]. Administration of selective nicotinic agonists is a potential therapeutic strategy against renal failure induced by ischaemia and reperfusion inherent to kidney transplantation and major cardiovascular surgery [58, 59].
Postoperative ileus
Postoperative ileus is an impairment of peristalsis caused by leukocyte infiltration into the muscular layer of the intestinal wall after abdominal surgery [60]. Vagus nerve stimulation increased gut motility after intestinal manipulation, decreased peritoneal IL-6 concentration and reduced inflammatory cell recruitment in a murine model of postoperative ileus [61]. This effect is attributed to inactivation of local macrophages residing in the intestinal wall located in close proximity to cholinergic terminals of the myenteric plexus [61]. Likewise, intraperitoneal administration of AR-R17779, an α7 selective agonist, improved gastric motility and reduced inflammatory cell infiltration to the muscular layer of the small intestine [62].
Endothelial cell activation
Expression of adhesion molecules on endothelial cells is essential for recruitment and extravasation of blood leukocytes to the injured tissue. Nicotinic agonists modulate inflammation by regulating expression of adhesion molecules on endothelial cells. Nicotine and the selective α7 agonist CAP55 reduced endothelial cell associated VCAM-1 and E-selectin expression and impaired leukocyte recruitment in the Schwartz-man reaction and the carrageenan air pouch model, respectively, two experimental models of local inflammation [63]. In vitro, nicotine reduced TNF-induced secretion of chemokines and adhesion molecule expression by human endothelial cells [63].
Control of inflammation through α7 signalling
Acetylcholine receptors are classified as muscarinic and nicotinic based on pharmacology and function. Nicotinic receptors are ligand-gated ion channels organized as hetero- or homo-pentamers by the combination of 17 different subunits (α1-10, β1-4, γ, δ, ε) [64]. In neurons, nicotinic receptors containing α7 subunits are homo-pentameric calcium channels that modulate neurotransmitter release in presynaptic nerve terminals and induce excitatory impulses in postsynaptic neurons. Signalling through α7 in the central nervous system is associated with neuronal plasticity and cell survival [65, 66]. Early events during α7 signalling in neurons include phosphorylation of α7 by Src, which negatively regulates α7 activity [67]; increased ERK1/2 activity [68]; and phosphorylation of PI3K, Akt, and Bcl-2 [69]. Gene transcription induced by α7 is mediated by long-term activation of CREB and occurs at a later time-point [65]. Discovery of the cholinergic anti-inflammatory pathway has prompted the characterization of the intracellular mechanism that mediates inhibition of cytokine production via α7 and its expression in immune cells.
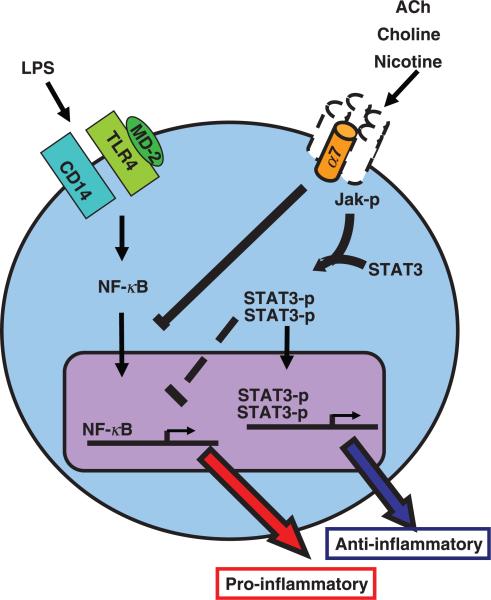
Stimulation of α7 in murine macrophage cell lines by cholinergic agonists like nicotine, GTS-21 and choline results in inhibition of LPS-induced TNF and HMGB1 release [45, 46, 70]. The transcription factor NF-κB is a key mediator of inflammatory response to cytokines and bacterial products. Stimulation of endotoxin activates a signalling cascade that induces phosphorylation and proteasome degradation of IκB, which retains NF-κB in cytoplasm under resting conditions; IκB degradation ultimately leads to NF-κB translocation into the cell nucleus and initiation of gene transcription [71, 72]. In human endothelial cells, nicotine upregulates protein levels of IkBα and IkBε and prevents activation of NF-κB [63]. Activation of α7 in human monocytes inhibits IkB phosphorylation and NF-κB activation, but does not alter IkB protein expression [73].
Jak/STAT is a signalling pathway common to class I and II cytokine receptor families [74]. Receptor binding triggers Jak-mediated receptor phosphorylation, leading to STAT recruitment to the receptor complex, and STAT phophorylation by Jak. Phosphorylated STAT then forms dimers that translocate into the cell nucleus and activate gene transcription [75]. Signalling through α7 induces recruitment to and phosphor-ylation of Jak2 by α7 and subsequent Jak2-induced activation of STAT3 [61]. In peritoneal macrophages, nicotine-mediated suppression of TNF production via α7 is dependent on phosphorylated STAT3 and its capacity to bind DNA [61]. Considering that STAT3 does not regulate TNF transcription directly [76] and that NF-κB recruits and associates with STAT3 [77], it is proposed that attenuation of cytokine production through α7 may implicate the collaboration of NF-κB and Jak/STAT pathways [78].
Intracellular pathways triggered by vagus nerve stimulation have also been object of study. In rats, vagus nerve stimulation reversed the haemorrhagic shock-induced degradation of liver IκBα, resulting in reduced NF-κB nuclear translocation [53]. Vagus nerve stimulation failed to attenuate intestinal inflammation in mice whose macrophages lack STAT3 [61]. Thus, activation of α7 in macrophages and other inflammatory cells in vitro and in vivo attenuates proinflammatory cytokine release by inhibition of NF-κB activation and Jak/STAT signalling.
Neuronal α7 homo-pentamers function as calcium channels [79], but it is not clear whether attenuation of cytokine production by macrophages via α7 activation is dependent on calcium. Nicotine releases calcium from intracellular stores through a mechanism dependent on a functional TCR/CD3 complex and Lck (leukocyte-specific tyrosine kinase) in T lymphocytes, where α7 co-immunoprecipitates with CD3zeta [80], suggesting that activated α7 interacts with TCR signalling to increase intracellular calcium. Further studies are required to fully elucidate α7-induced cytokine regulation in macrophages and other immune cells (Fig. 3).
Fig. 3.
Cholinergic signalling through α7. Activation of α7 in endotoxin-stimulated macrophages leads to reduced proinflammatory cytokine production and decreased translocation of NF-κB into the cell nucleus. In peritoneal macrophages, activation of α7 leads to recruitment and activation of Jak2 with subsequent STAT3 activation. Whether these pathways converge or function independently to attenuate pro-inflammatory cytokine production is not known. Also unknown is the subunit composition of α7-containing nicotinic acetylcholine receptors (homomer versus heteromer) in macrophages and whether they function as calcium channels. Dotted lines represent unknown α7 signalling components or events.
Acetylcholine and its role in inflammation
Acetylcholine is synthesized by preganglionic fibres of the sympathetic and parasympathetic autonomic nervous system and by postganglionic parasympathetic fibres. Until recently, neurons were the only identified source of acetylcholine. It is now known that cells other than neurons express the proteins required for acetylcholine metabolism. Acetylcholine is synthesized, amongst others, by immune cells (lymphocytes, dendritic cells, neutrophils) [81–83], keratinocytes [84], endothelial cells [85], and epithelial cells of placenta [86], urinary bladder [87] and airways [88]. Acting through muscarinic and nicotinic receptors, acetylcholine participates in a wide range of physiological processes including keratinocyte proliferation and differentiation, ciliary beat frequency in airway epithelial cells and relaxation of smooth muscle in vessels [89].
It is possible that acetylcholine derived from these sources is involved in modulation of local inflamma-tory processes. For example, placenta endothelial cells synthesize acetylcholine [90] and express the α7 subunit of the nicotinic acetylcholine receptor [91–93]. Nicotine and the α7 selective agonist GTS-21 attenuate LPS-induced TNF production by placental cells [93]. Thus, acetylcholine released by placenta endothelial cells could regulate local cytokine production through α7. This anti-inflammatory effect could be relevant in preeclampsia, a pathological condition characterized by systemic inflammation induced by production of cytokines and other soluble factors by placenta cells [94, 95]. For instance, placentas of patients with severe preeclampsia expressed higher α7 protein levels in comparison to placentas of healthy subjects, suggesting a possible role of α7 and acetylcholine in the pathogenesis of this disease [91]. Similarly, endothelial cell-derived acetylcholine may act in a paracrine way to modulate leukocyte trafficking through regulation of adhesion molecule expression. Finally, inhibition of acetylcholinesterase attenuates TNF production in spleen cell suspensions stimulated with endotoxin, strongly suggesting that spleen cells are an endogenous source of acetylcholine with the capacity to regulate inflammatory cytokine production.
Besides macrophages, other cells of the immune system express nicotinic and muscarinic receptors, and acetylcholine derived from lymphocytes or endothelial cells, acting in an autocrine/paracrine way, could modify immune responses including antigen presentation or antibody production. Release of acetylcholine from these sources is regarded as nerve-independent, which raises the possibility of immune cell-derived cholinergic activity that can modulate inflammation; this could be relevant in inflammatory conditions occurring with decreased activity of the vagus nerve.
Acetylcholine is detectable in blood of several animal species [96]. In humans, the mean concentration of acetylcholine in plasma is approximately 3 nmol L−1 (or 456 pg mL−1, range 151–1312 pg mL−1) [97, 98]. Sixty per cent of the total acetylcholine in human blood is contained in mononuclear leukocytes and the rest is found in plasma [98]. Nicotine elevates serum acetylcholine levels and reduces acetylcholine content in blood leukocytes, supporting the hypothesis of an inducible pool of acetylcholine contained in circulating blood cells [99]. Contribution of cholinergic nerves to circulating acetylcholine is also possible as the gastroprokinetic agent KW-5092, which inhibits acetylcholinesterase activity and facilitates acetylcho-line release from nerve endings, increases plasma levels of acetylcholine [100]. It is not clear whether circulating acetylcholine levels are physiologically relevant. The effect of circulating acetylcholine is likely to be determined by its concentration in the immediate vicinity of the target cell, which in turn is a function of acetylcholinesterase activity in the milieu.
Acetylcholine is rapidly hydrolyzed by acetylcholinesterase in neural synapses and the motor endplate. Considering the inflammatory suppressive effect of acetylcholine, it is conceivable that acetylcholinesterase activity is an intrinsic regulator of inflammation. Indeed, peritoneal injection of acetylcholinesterase inhibitors reduce serum pro-inflammatory cytokine levels and improve survival in a murine model of sepsis [101]; intravenous acetylcholinesterase inhibitors reduce IL-1β in brain and blood and decrease serum acetylcholinesterase activity in mice [102]; and basal acetylcholinesterase activity in circulation is inversely related to serum IL-6 levels induced by endotoxin in humans [103]. These observations highlight the pivotal role of acetylcholine in inflammation and put forward the therapeutic potential of cholinesterase inhibitors, already approved for the treatment of Alzheimer's diseases, as part of the arsenal against inflammatory disease.
Clinical implications
Characterization of the cholinergic anti-inflammatory pathway has provided new grounds for understanding and treating inflammatory diseases. Nicotine has been used to treat ulcerative colitis, a disease characterized by inflammation in the large intestine. In a randomized double-blind study, patients given nicotine patches in addition to conventional therapy presented milder symptoms compared to patients receiving conventional therapy and placebo patches, but showed more side effects [104]. The development of selective α7 agonists devoid of the secondary effects of nicotine will warrant the use of these compounds in future clinical trials against sepsis and other conditions characterized by acute or chronic inflammation, including autoimmune diseases such as rheumatoid arthritis.
Electrical stimulation of the vagus nerve is clinically approved and has been used to treat drug-resistant epilepsy and depression [105–107] and only relatively minor effects have been reported [106]. Unlike administration of cholinergic agonists, stimulation of the vagus nerve might be a more precise therapeutic approach to regulate inflammatory disease because it offers an amenable technique that takes advantage of the anatomical distribution of nerve fibres to reach specific organs and cell targets. Treatment of diseases characterized by uncontrolled inflammation in organs innervated by the vagus nerve, such as the gut in ulcerative colitis where the beneficial effect of nicotine has been overshadowed by its secondary side effects, could take advantage of this principle. The vagus nerve has been recently shown to be a tonic regulator of gut inflammation in a mouse model of acute colitis [108]. Importantly, in animal models, decreased activity of the vagus nerve has been implicated in the inflammatory outbursts of colitis induced by depression, and the beneficial effect of tricyclic anti-depressants on inflammatory bowel markers is blunted by vagotomy [109]. These findings support a brain-to-gut anti-inflammatory pathway mediated by the vagus nerve, and highlight the underlying interdependency of the nervous and immune systems whereby, brain networks affect inflammatory responses and contribute to homeostasis.
Suppression of inflammation in the brain and in the periphery can be achieved by enhancing cholinergic signalling by administration of acetylcholinesterase inhibitors [102]. The acetylcholinesterase inhibitor galantamine, acting through a central mechanism, has been shown to attenuate serum TNF and IL-6 and improve survival in a murine model of endotoxaemia [110]. Together with the central anti-inflammatory effect of muscarinic receptor agonists [18], these experimental approaches give proof of concept that cholinergic enhancing compounds act centrally to attenuate inflammation. The use of galantamine is approved for the treatment of mild to moderate Alzheimer's disease [111] and clinical trials using galantamine to inhibit cytokine production in inflammatory diseases are thus feasible.
Choline, the byproduct of acetylcholine hydrolysis, is an endogenous and selective α7 agonist [112, 113]. Treatment with choline, either as a choline-rich diet in rats [114] or intravenous injection in dogs [115] confers protection against endotoxin-induced shock. Insight into choline's anti-inflammatory properties comes from experiments in which intraperitoneal administration of choline attenuated serum TNF in endotoxaemic mice but failed to do so in α7 knockout mice [70]. In vitro, choline attenuates LPS-induced production of TNF by peritoneal macrophages in an α7-dependent manner and inhibits NF-κB activation [70]. These findings support further study of the use of choline as an anti-inflammatory compound and indicate that its cytokine inhibiting effect is mediated, at least in part, via α7 signalling. This mechanism could explain the association between high choline dietary intake with reduced pro-inflammatory markers in serum [116].
Immune responses during stress have been widely studied in the context of the HPA axis and the sympathetic nervous system activation. Tonic control of cytokine production and leukocyte trafficking by the vagus nerve [117, 118] indicate that the parasympathetic branch of the autonomic nervous system is an important mechanism that contributes to homeostasis. Altered mental states (i.e. depression or anxiety) or other conditions associated with autonomic dysfunction could result in loss of homeostasis and disease by perturbing inflammatory balance. Conversely, biofeedback training and other techniques that allow voluntary control of autonomic output already used to treat asthma [119], hypertension [120] and migraine [121] amongst others, could be used to restore homeostasis in inflammatory diseases.
Conclusion
The central nervous system, through humoral and neural pathways, regulates immune function. The hardwired nature of neural pathways allows for integrated responses to peripheral stimuli in real-time and in a localized fashion. Identification of α7 as an essential modulator of inflammation has rendered additional insight into the molecular basis of the complex and dynamic interaction between the immune and the nervous system, whilst offering a rationale for novel therapeutic strategies. Discovery of the cholinergic anti-inflammatory pathway, a mechanism by which the efferent vagus nerve modulates inflammation, has provided additional anatomical and functional evidence supporting a nervous-to-immune system connection. It has also offered a basis for regarding inflammation as a highly integrated physiological response involving various body systems previously thought to function independently. Consideration of this interdependency when interpreting results and designing new therapeutic strategies can only lead to a more comprehensive approach to understand and treat inflammatory disease. This framework has opened new avenues of inquiry that lead to several intriguing questions. What is the contribution of cholinergic signalling to diseases characterized by autonomic dysfunction such as diabetes, rheumatoid arthritis or atherosclerosis? Can modulation of vagus nerve activity ameliorate these illnesses? How do different mind states affect immune function? Is immune function subject to voluntary control? Some of these questions can now be addressed; their answers will help unravel the intricate relationship between the nervous and immune systems in health and disease.
Acknowledgement
We thank Sangeeta Chavan and Valentin Pavlov for critical reading of the manuscript.
Footnotes
Conflict of interest statement
The authors are inventors on technology related to the topic.
References
- 1.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–7. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 2.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517–24. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 3.Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qin S, Wang H, Yuan R, et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med. 2006;203:1637–42. doi: 10.1084/jem.20052203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 6.Agostoni E, Chinnock JE, De Daly MB, Murray JG. Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. J Physiol. 1957;135:182–205. doi: 10.1113/jphysiol.1957.sp005703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 8.Goehler LE, Gaykema RP, Hammack SE, Maier SF, Watkins LR. Interleukin-1 induces c-Fos immunoreactivity in primary afferent neurons of the vagus nerve. Brain Res. 1998;804:306–10. doi: 10.1016/s0006-8993(98)00685-4. [DOI] [PubMed] [Google Scholar]
- 9.Goehler LE, Relton JK, Dripps D, et al. Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: a possible mechanism for immune-to-brain communication. Brain Res Bull. 1997;43:357–64. doi: 10.1016/s0361-9230(97)00020-8. [DOI] [PubMed] [Google Scholar]
- 10.Maier SF, Goehler LE, Fleshner M, Watkins LR. The role of the vagus nerve in cytokine-to-brain communication. Ann N Y Acad Sci. 1998;840:289–300. doi: 10.1111/j.1749-6632.1998.tb09569.x. [DOI] [PubMed] [Google Scholar]
- 11.Blalock JE. The immune system as a sensory organ. J Immunol. 1984;132:1067–70. [PubMed] [Google Scholar]
- 12.Blalock JE. The immune system as the sixth sense. J Intern Med. 2005;257:126–38. doi: 10.1111/j.1365-2796.2004.01441.x. [DOI] [PubMed] [Google Scholar]
- 13.Mascarucci P, Perego C, Terrazzino S, De Simoni MG. Gluta-mate release in the nucleus tractus solitarius induced by peripheral lipopolysaccharide and interleukin-1 beta. Neuroscience. 1998;86:1285–90. doi: 10.1016/s0306-4522(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 14.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–9. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 15.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–8. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 17.Huston JM, Gallowitsch-Puerta M, Ochani M, et al. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit Care Med. 2007;35:2762–8. doi: 10.1097/01.CCM.0000288102.15975.BA. [DOI] [PubMed] [Google Scholar]
- 18.Pavlov VA, Ochani M, Gallowitsch-Puerta M, et al. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc Natl Acad Sci USA. 2006;103:5219–23. doi: 10.1073/pnas.0600506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huston JM, Ochani M, Rosas-Ballina M, et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–8. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mignini F, Streccioni V, Amenta F. Autonomic innervation of immune organs and neuroimmune modulation. Auton Autacoid Pharmacol. 2003;23:1–25. doi: 10.1046/j.1474-8673.2003.00280.x. [DOI] [PubMed] [Google Scholar]
- 21.Berthoud HR, Powley TL. Interaction between parasympathetic and sympathetic nerves in prevertebral ganglia: morphological evidence for vagal efferent innervation of ganglion cells in the rat. Microsc Res Tech. 1996;35:80–6. doi: 10.1002/(SICI)1097-0029(19960901)35:1<80::AID-JEMT7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 22.Bellinger DL, Lorton D, Hamill RW, Felten SY, Felten DL. Acetylcholinesterase staining and choline acetyltransferase activity in the young adult rat spleen: lack of evidence for cholinergic innervation. Brain Behav Immun. 1993;7:191–204. doi: 10.1006/brbi.1993.1021. [DOI] [PubMed] [Google Scholar]
- 23.Rosas-Ballina M, Ochani M, Parrish WR, et al. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Sci USA. 2008;105:11008–13. doi: 10.1073/pnas.0803237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellinger DL, Felten SY, Lorton D, Felten DL. Origin of noradrenergic innervation of the spleen in rats. Brain Behav Immun. 1989;3:291–311. doi: 10.1016/0889-1591(89)90029-9. [DOI] [PubMed] [Google Scholar]
- 25.Nance DM, Burns J. Innervation of the spleen in the rat: evidence for absence of afferent innervation. Brain Behav Immun. 1989;3:281–90. doi: 10.1016/0889-1591(89)90028-7. [DOI] [PubMed] [Google Scholar]
- 26.Cano G, Sved AF, Rinaman L, Rabin BS, Card JP. Characterization of the central nervous system innervation of the rat spleen using viral transneuronal tracing. J Comp Neurol. 2001;439:1–18. doi: 10.1002/cne.1331. [DOI] [PubMed] [Google Scholar]
- 27.Klein RL, Wilson SP, Dzielak DJ, Yang WH, Viveros OH. Opioid peptides and noradrenaline co-exist in large dense-cored vesicles from sympathetic nerve. Neuroscience. 1982;7:2255–61. doi: 10.1016/0306-4522(82)90135-x. [DOI] [PubMed] [Google Scholar]
- 28.Felten DL, Ackerman KD, Wiegand SJ, Felten SY. Noradrenergic sympathetic innervation of the spleen: I. Nerve fibers associate with lymphocytes and macrophages in specific compartments of the splenic white pulp. J Neurosci Res. 1987;18:28–36. doi: 10.1002/jnr.490180107. [DOI] [PubMed] [Google Scholar]
- 29.Kees MG, Pongratz G, Kees F, Scholmerich J, Straub RH. Via beta-adrenoceptors, stimulation of extrasplenic sympathetic nerve fibers inhibits lipopolysaccharide-induced TNF secretion in perfused rat spleen. J Neuroimmunol. 2003;145:77–85. doi: 10.1016/j.jneuroim.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Spengler RN, Allen RM, Remick DG, Strieter RM, Kunkel SL. Stimulation of alpha-adrenergic receptor augments the production of macrophage-derived tumor necrosis factor. J Immunol. 1990;145:1430–4. [PubMed] [Google Scholar]
- 31.Deng J, Muthu K, Gamelli R, Shankar R, Jones SB. Adrenergic modulation of splenic macrophage cytokine release in polymicrobial sepsis. Am J Physiol Cell Physiol. 2004;287:C730–6. doi: 10.1152/ajpcell.00562.2003. [DOI] [PubMed] [Google Scholar]
- 32.Lips KS, Konig P, Schatzle K, et al. Coexpression and spatial association of nicotinic acetylcholine receptor subunits alpha7 and alpha10 in rat sympathetic neurons. J Mol Neurosci. 2006;30:15–6. doi: 10.1385/JMN:30:1:15. [DOI] [PubMed] [Google Scholar]
- 33.Skok MV, Voitenko LP, Voitenko SV, et al. Alpha subunit composition of nicotinic acetylcholine receptors in the rat autonomic ganglia neurons as determined with subunit-specific anti-alpha(181-192) peptide antibodies. Neuroscience. 1999;93:1427–36. doi: 10.1016/s0306-4522(99)00160-8. [DOI] [PubMed] [Google Scholar]
- 34.Franceschini D, Orr-Urtreger A, Yu W, et al. Altered baroreflex responses in alpha7 deficient mice. Behav Brain Res. 2000;113:3–10. doi: 10.1016/s0166-4328(00)00195-9. [DOI] [PubMed] [Google Scholar]
- 35.Brandon KW, Rand MJ. Acetylcholine and the sympathetic innervation of the spleen. J Physiol. 1961;157:18–32. doi: 10.1113/jphysiol.1961.sp006702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bulloch K, Damavandy T, Badamchian M. Characterization of choline O-acetyltransferase (ChAT) in the BALB/C mouse spleen. Int J Neurosci. 1994;76:141–9. doi: 10.3109/00207459408985999. [DOI] [PubMed] [Google Scholar]
- 37.Rinner I, Kawashima K, Schauenstein K. Rat lymphocytes produce and secrete acetylcholine in dependence of differentiation and activation. J Neuroimmunol. 1998;81:31–7. doi: 10.1016/s0165-5728(97)00155-0. [DOI] [PubMed] [Google Scholar]
- 38.Okamoto S, Ibaraki K, Hayashi S, Saito M. Ventromedial hypothalamus suppresses splenic lymphocyte activity through sympathetic innervation. Brain Res. 1996;739:308–13. doi: 10.1016/s0006-8993(96)00840-2. [DOI] [PubMed] [Google Scholar]
- 39.Ganta CK, Lu N, Helwig BG, et al. Central angiotensin II-enhanced splenic cytokine gene expression is mediated by the sympathetic nervous system. Am J Physiol Heart Circ Physiol. 2005;289:H1683–91. doi: 10.1152/ajpheart.00125.2005. [DOI] [PubMed] [Google Scholar]
- 40.Katafuchi T, Take S, Hori T. Roles of sympathetic nervous system in the suppression of cytotoxicity of splenic natural killer cells in the rat. J Physiol. 1993;465:343–57. doi: 10.1113/jphysiol.1993.sp019680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Westerloo DJ, Giebelen IA, Florquin S, et al. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J Infect Dis. 2005;191:2138–48. doi: 10.1086/430323. [DOI] [PubMed] [Google Scholar]
- 42.Kessler W, Traeger T, Westerholt A, et al. The vagal nerve as a link between the nervous and immune system in the instance of polymicrobial sepsis. Langenbecks Arch Surg. 2006;391:83–7. doi: 10.1007/s00423-006-0031-y. [DOI] [PubMed] [Google Scholar]
- 43.Song XM, Li JG, Wang YL, et al. The protective effect of the cholinergic anti-inflammatory pathway against septic shock in rats. Shock. 2008;30:468–72. doi: 10.1097/SHK.0b013e31816d5e49. [DOI] [PubMed] [Google Scholar]
- 44.van Westerloo DJ, Giebelen IA, Meijers JC, et al. Vagus nerve stimulation inhibits activation of coagulation and fibrinolysis during endotoxemia in rats. J Thromb Haemost. 2006;4:1997–2002. doi: 10.1111/j.1538-7836.2006.02112.x. [DOI] [PubMed] [Google Scholar]
- 45.Wang H, Liao H, Ochani M, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–21. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 46.Pavlov VA, Ochani M, Yang LH, et al. Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med. 2007;35:1139–44. doi: 10.1097/01.CCM.0000259381.56526.96. [DOI] [PubMed] [Google Scholar]
- 47.Giebelen IA, van Westerloo DJ, Larosa GJ, de Vos AF, van der PT. Stimulation of alpha 7 cholinergic receptors inhibits lipopolysaccharide-induced neutrophil recruitment by a tumor necrosis factor alpha-independent mechanism. Shock. 2007;27:443–7. doi: 10.1097/01.shk.0000245016.78493.bb. [DOI] [PubMed] [Google Scholar]
- 48.Rakonczay Z, Jr, Hegyi P, Takacs T, McCarroll J, Saluja AK. The role of NF-kappaB activation in the pathogenesis of acute pancreatitis. Gut. 2008;57:259–67. doi: 10.1136/gut.2007.124115. [DOI] [PubMed] [Google Scholar]
- 49.van Westerloo DJ, Giebelen IA, Florquin S, et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130:1822–30. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 50.Zingarelli B, Squadrito F, Altavilla D, Calapai G, Di Rosa M, Caputi AP. Role of tumor necrosis factor-alpha in acute hypovolemic hemorrhagic shock in rats. Am J Physiol. 1994;266:H1512–5. doi: 10.1152/ajpheart.1994.266.4.H1512. [DOI] [PubMed] [Google Scholar]
- 51.Squadrito F, Altavilla D, Canale P, et al. Participation of tumour necrosis factor and nitric oxide in the mediation of vascular dysfunction in splanchnic artery occlusion shock. Br J Pharmacol. 1994;113:1153–8. doi: 10.1111/j.1476-5381.1994.tb17118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Squadrito F, Altavilla D, Canale P, et al. Contribution of inter-cellular adhesion molecule 1 (ICAM-1) to the pathogenesis of splanchnic artery occlusion shock in the rat. Br J Pharmacol. 1994;113:912–6. doi: 10.1111/j.1476-5381.1994.tb17079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guarini S, Altavilla D, Cainazzo MM, et al. Efferent vagal fibre stimulation blunts nuclear factor-kappaB activation and protects against hypovolemic hemorrhagic shock. Circulation. 2003;107:1189–94. doi: 10.1161/01.cir.0000050627.90734.ed. [DOI] [PubMed] [Google Scholar]
- 54.Altavilla D, Guarini S, Bitto A, et al. Activation of the cholinergic anti-inflammatory pathway reduces NF-kappab activation, blunts TNF-alpha production, and protects againts splanchic artery occlusion shock. Shock. 2006;25:500–6. doi: 10.1097/01.shk.0000209539.91553.82. [DOI] [PubMed] [Google Scholar]
- 55.Bernik TR, Friedman SG, Ochani M, et al. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J Vasc Surg. 2002;36:1231–6. doi: 10.1067/mva.2002.129643. [DOI] [PubMed] [Google Scholar]
- 56.Guarini S, Cainazzo MM, Giuliani D, et al. Adrenocorticotropin reverses hemorrhagic shock in anesthetized rats through the rapid activation of a vagal anti-inflammatory pathway. Cardiovasc Res. 2004;63:357–65. doi: 10.1016/j.cardiores.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 57.Luyer MD, Greve JW, Hadfoune M, Jacobs JA, Dejong CH, Buurman WA. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J Exp Med. 2005;202:1023–9. doi: 10.1084/jem.20042397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yeboah MM, Xue X, Duan B, et al. Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney Int. 2008;74:62–9. doi: 10.1038/ki.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sadis C, Teske G, Stokman G, et al. Nicotine protects kidney from renal ischemia/reperfusion injury through the cholinergic anti-inflammatory pathway. PLoS ONE. 2007;2:e469. doi: 10.1371/journal.pone.0000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalff JC, Schraut WH, Simmons RL, Bauer AJ. Surgical manipulation of the gut elicits an intestinal muscularis inflam matory response resulting in postsurgical ileus. Ann Surg. 1998;228:652–63. doi: 10.1097/00000658-199811000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Jonge WJ, van der Zanden EP, The FO, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–51. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 62.The FO, Boeckxstaens GE, Snoek SA, et al. Activation of the cholinergic anti-inflammatory pathway ameliorates postoperative ileus in mice. Gastroenterology. 2007;133:1219–28. doi: 10.1053/j.gastro.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 63.Saeed RW, Varma S, Peng-Nemeroff T, et al. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005;201:1113–23. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Millar NS. Assembly and subunit diversity of nicotinic acetylcholine receptors. Biochem Soc Trans. 2003;31:869–74. doi: 10.1042/bst0310869. [DOI] [PubMed] [Google Scholar]
- 65.Berg DK, Conroy WG. Nicotinic alpha 7 receptors: synaptic options and downstream signaling in neurons. J Neurobiol. 2002;53:512–23. doi: 10.1002/neu.10116. [DOI] [PubMed] [Google Scholar]
- 66.Drisdel RC, Green WN. Neuronal alpha-bungarotoxin receptors are alpha7 subunit homomers. J Neurosci. 2000;20:133–9. doi: 10.1523/JNEUROSCI.20-01-00133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Charpantier E, Wiesner A, Huh KH, et al. Alpha7 neuronal nicotinic acetylcholine receptors are negatively regulated by tyrosine phosphorylation and Src-family kinases. J Neurosci. 2005;25:9836–49. doi: 10.1523/JNEUROSCI.3497-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dajas-Bailador FA, Soliakov L, Wonnacott S. Nicotine activates the extracellular signal-regulated kinase 1/2 via the alpha7 nicotinic acetylcholine receptor and protein kinase A, in SH-SY5Y cells and hippocampal neurones. J Neurochem. 2002;80:520–30. doi: 10.1046/j.0022-3042.2001.00725.x. [DOI] [PubMed] [Google Scholar]
- 69.Kihara T, Shimohama S, Sawada H, et al. Alpha 7 nicotinic receptor transduces signals to phosphatidylinositol 3-kinase to block A beta-amyloid-induced neurotoxicity. J Biol Chem. 2001;276:13541–6. doi: 10.1074/jbc.M008035200. [DOI] [PubMed] [Google Scholar]
- 70.Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, et al. Modulation of TNF release by choline requires alpha7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol Med. 2008;14:567–74. doi: 10.2119/2008-00079.Parrish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 72.Cheong R, Hoffmann A, Levchenko A. Understanding NF-kappaB signaling via mathematical modeling. Mol Syst Biol. 2008;4:192. doi: 10.1038/msb.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoshikawa H, Kurokawa M, Ozaki N, et al. Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-kappaB phosphorylation and nuclear factor-kappaB transcriptional activity through nicotinic acetylcholine receptor alpha7. Clin Exp Immunol. 2006;146:116–23. doi: 10.1111/j.1365-2249.2006.03169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O'Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477–87. doi: 10.1016/j.immuni.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–9. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 76.Murray PJ. The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proc Natl Acad Sci USA. 2005;102:8686–91. doi: 10.1073/pnas.0500419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoshida Y, Kumar A, Koyama Y, et al. Interleukin 1 activates STAT3/nuclear factor-kappaB cross-talk via a unique T. J Biol Chem. 2004;279:1768–76. doi: 10.1074/jbc.M311498200. [DOI] [PubMed] [Google Scholar]
- 78.de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. 2007;151:915–29. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Broide RS, Leslie FM. The alpha7 nicotinic acetylcholine receptor in neuronal plasticity. Mol Neurobiol. 1999;20:1–16. doi: 10.1007/BF02741361. [DOI] [PubMed] [Google Scholar]
- 80.Razani-Boroujerdi S, Boyd RT, Davila-Garcia MI, et al. T cells express alpha7-nicotinic acetylcholine receptor subunits that require a functional TCR and leukocyte-specific protein tyro-sine kinase for nicotine-induced Ca2+ response. J Immunol. 2007;179:2889–98. doi: 10.4049/jimmunol.179.5.2889. [DOI] [PubMed] [Google Scholar]
- 81.Kawashima K, Fujii T, Watanabe Y, Misawa H. Acetylcholine synthesis and muscarinic receptor subtype mRNA expression in T-lymphocytes. Life Sci. 1998;62:1701–5. doi: 10.1016/s0024-3205(98)00131-3. [DOI] [PubMed] [Google Scholar]
- 82.Kawashima K, Yoshikawa K, Fujii YX, Moriwaki Y, Misawa H. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci. 2007;80:2314–9. doi: 10.1016/j.lfs.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 83.Neumann S, Razen M, Habermehl P, et al. The non-neuronal cholinergic system in peripheral blood cells: effects of nicotinic and muscarinic receptor antagonists on phagocytosis, respiratory burst and migration. Life Sci. 2007;80:2361–4. doi: 10.1016/j.lfs.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 84.Grando SA, Kist DA, Qi M, Dahl MV. Human keratinocytes synthesize, secrete, and degrade acetylcholine. J Invest Dermatol. 1993;101:32–6. doi: 10.1111/1523-1747.ep12358588. [DOI] [PubMed] [Google Scholar]
- 85.Kawashima K, Watanabe N, Oohata H, et al. Synthesis and release of acetylcholine by cultured bovine arterial endothelial cells. Neurosci Lett. 1990;119:156–8. doi: 10.1016/0304-3940(90)90822-q. [DOI] [PubMed] [Google Scholar]
- 86.Wessler I, Herschel S, Bittinger F, Kirkpatrick CJ. Release of non-neuronal acetylcholine from the isolated human placenta is affected by antidepressants. Life Sci. 2007;80:2210–3. doi: 10.1016/j.lfs.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 87.Yoshida M, Inadome A, Maeda Y, et al. Non-neuronal cholinergic system in human bladder urothelium. Urology. 2006;67:425–30. doi: 10.1016/j.urology.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 88.Wessler IK, Kirkpatrick CJ. The Non-neuronal cholinergic system: an emerging drug target in the airways. Pulm Pharmacol Ther. 2001;14:423–34. doi: 10.1006/pupt.2001.0313. [DOI] [PubMed] [Google Scholar]
- 89.Kawashima K, Fujii T. Basic and clinical aspects of non-neuronal acetylcholine: overview of non-neuronal cholinergic systems and their biological significance. J Pharmacol Sci. 2008;106:167–73. doi: 10.1254/jphs.fm0070073. [DOI] [PubMed] [Google Scholar]
- 90.Wessler I, Kilbinger H, Bittinger F, Unger R, Kirkpatrick CJ. The non-neuronal cholinergic system in humans: expression, function and pathophysiology. Life Sci. 2003;72:2055–61. doi: 10.1016/s0024-3205(03)00083-3. [DOI] [PubMed] [Google Scholar]
- 91.Kwon JY, Kim YH, Kim SH, et al. Difference in the expression of alpha 7 nicotinic receptors in the placenta in normal versus severe preeclampsia pregnancies. Eur J Obstet Gynecol Reprod Biol. 2007;132:35–9. doi: 10.1016/j.ejogrb.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 92.Lips KS, Bruggmann D, Pfeil U, Vollerthun R, Grando SA, Kummer W. Nicotinic acetylcholine receptors in rat and human placenta. Placenta. 2005;26:735–46. doi: 10.1016/j.placenta.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 93.Dowling O, Rochelson B, Way K, Al Abed Y, Metz CN. Nicotine inhibits cytokine production by placenta cells via NFkappaB: potential role in pregnancy-induced hypertension. Mol Med. 2007;13:576–83. doi: 10.2119/2007-00067.Dowling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rusterholz C, Hahn S, Holzgreve W. Role of placentally produced inflammatory and regulatory cytokines in pregnancy and the etiology of preeclampsia. Semin Immunopathol. 2007;29:151–62. doi: 10.1007/s00281-007-0071-6. [DOI] [PubMed] [Google Scholar]
- 95.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;294:H541–50. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 96.Fujii T, Yamada S, Yamaguchi N, Fujimoto K, Suzuki T, Kawashima K. Species differences in the concentration of acetylcholine, a neurotransmitter, in whole blood and plasma. Neurosci Lett. 1995;201:207–10. doi: 10.1016/0304-3940(95)12180-3. [DOI] [PubMed] [Google Scholar]
- 97.Kawashima K, Oohata H, Fujimoto K, Suzuki T. Plasma concentration of acetylcholine in young women. Neurosci Lett. 1987;80:339–42. doi: 10.1016/0304-3940(87)90478-2. [DOI] [PubMed] [Google Scholar]
- 98.Kawashima K, Fujii T. Extraneuronal cholinergic system in lymphocytes. Pharmacol Ther. 2000;86:29–48. doi: 10.1016/s0163-7258(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 99.Kawashima K, Oohata H, Fujimoto K, Suzuki T. Extraneuronal localization of acetylcholine and its release upon nicotinic stimulation in rabbits. Neurosci Lett. 1989;104:336–9. doi: 10.1016/0304-3940(89)90599-5. [DOI] [PubMed] [Google Scholar]
- 100.Yamada S, Fujii T, Kawashima K. Oral administration of KW-5092, a novel gastroprokinetic agent with acetylcholinesterase inhibitory and acetylcholine release enhancing activities, causes a dose-dependent increase in the blood acetylcholine content of beagle dogs. Neurosci Lett. 1997;225:25–8. doi: 10.1016/s0304-3940(97)00188-2. [DOI] [PubMed] [Google Scholar]
- 101.Hofer S, Eisenbach C, Lukic IK, et al. Pharmacologic cholinesterase inhibition improves survival in experimental sepsis. Crit Care Med. 2008;36:404–8. doi: 10.1097/01.CCM.0B013E31816208B3. [DOI] [PubMed] [Google Scholar]
- 102.Pollak Y, Gilboa A, Ben Menachem O, Ben Hur T, Soreq H, Yirmiya R. Acetylcholinesterase inhibitors reduce brain and blood interleukin-1beta production. Ann Neurol. 2005;57:741–5. doi: 10.1002/ana.20454. [DOI] [PubMed] [Google Scholar]
- 103.Ofek K, Krabbe KS, Evron T, et al. Cholinergic status modulations in human volunteers under acute inflammation. J Mol Med. 2007;85:1239–51. doi: 10.1007/s00109-007-0226-x. [DOI] [PubMed] [Google Scholar]
- 104.Pullan RD, Rhodes J, Ganesh S, et al. Transdermal nicotine for active ulcerative colitis. N Engl J Med. 1994;330:811–5. doi: 10.1056/NEJM199403243301202. [DOI] [PubMed] [Google Scholar]
- 105.Oommen J, Morrell M, Fisher RS. Experimental electrical stimulation therapy for epilepsy. Curr Treat Options Neurol. 2005;7:261–71. doi: 10.1007/s11940-005-0036-9. [DOI] [PubMed] [Google Scholar]
- 106.Tecoma ES, Iragui VJ. Vagus nerve stimulation use and effect in epilepsy: what have we learned? Epilepsy Behav. 2006;8:127–36. doi: 10.1016/j.yebeh.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 107.Walsh SP, Kling MA. VNS and depression: current status and future directions. Expert Rev Med Devices. 2004;1:155–60. doi: 10.1586/17434440.1.1.155. [DOI] [PubMed] [Google Scholar]
- 108.Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006;131:1122–30. doi: 10.1053/j.gastro.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 109.Ghia JE, Blennerhassett P, Collins SM. Impaired parasympathetic function increases susceptibility to inflammatory bowel disease in a mouse model of depression. J Clin Invest. 2008;118:2209–18. doi: 10.1172/JCI32849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pavlov VA, Parrish WR, Rosas-Ballina M, et al. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun. 2008;23:41–5. doi: 10.1016/j.bbi.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt HP, van den BH. Cholinesterase inhibitors for patients with Alzheimer's disease: systematic review of randomised clinical trials. BMJ. 2005;331:321–7. doi: 10.1136/bmj.331.7512.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci. 1997;9:2734–42. doi: 10.1111/j.1460-9568.1997.tb01702.x. [DOI] [PubMed] [Google Scholar]
- 113.Papke RL, Bencherif M, Lippiello P. An evaluation of neuronal nicotinic acetylcholine receptor activation by quaternary nitrogen compounds indicates that choline is selective for the alpha 7 subtype. Neurosci Lett. 1996;213:201–4. doi: 10.1016/0304-3940(96)12889-5. [DOI] [PubMed] [Google Scholar]
- 114.Rivera CA, Wheeler MD, Enomoto N, Thurman RG. A cho-line-rich diet improves survival in a rat model of endotoxin shock. Am J Physiol. 1998;275:G862–7. doi: 10.1152/ajpgi.1998.275.4.G862. [DOI] [PubMed] [Google Scholar]
- 115.Ilcol YO, Yilmaz Z, Ulus IH. Endotoxin alters serum-free cho-line and phospholipid-bound choline concentrations, and cho-line administration attenuates endotoxin-induced organ injury in dogs. Shock. 2005;24:288–93. doi: 10.1097/01.shk.0000174018.02688.4b. [DOI] [PubMed] [Google Scholar]
- 116.Detopoulou P, Panagiotakos DB, Antonopoulou S, Pitsavos C, Stefanadis C. Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in healthy adults: the ATTICA study. Am J Clin Nutr. 2008;87:424–30. doi: 10.1093/ajcn/87.2.424. [DOI] [PubMed] [Google Scholar]
- 117.Antonica A, Magni F, Mearini L, Paolocci N. Vagal control of lymphocyte release from rat thymus. J Auton Nerv Syst. 1994;48:187–97. doi: 10.1016/0165-1838(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 118.Antonica A, Ayroldi E, Magni F, Paolocci N. Lymphocyte traffic changes induced by monolateral vagal denervation in mouse thymus and peripheral lymphoid organs. J Neuroimmunol. 1996;64:115–22. doi: 10.1016/0165-5728(95)00157-3. [DOI] [PubMed] [Google Scholar]
- 119.Lehrer P, Vaschillo E, Lu SE, et al. Heart rate variability biofeedback: effects of age on heart rate variability, baroreflex gain, and asthma. Chest. 2006;129:278–84. doi: 10.1378/chest.129.2.278. [DOI] [PubMed] [Google Scholar]
- 120.Nakao M, Yano E, Nomura S, Kuboki T. Blood pressure-lowering effects of biofeedback treatment in hypertension: a meta-analysis of randomized controlled trials. Hypertens Res. 2003;26:37–46. doi: 10.1291/hypres.26.37. [DOI] [PubMed] [Google Scholar]
- 121.Kaushik R, Kaushik RM, Mahajan SK, Rajesh V. Biofeedback assisted diaphragmatic breathing and systematic relaxation versus propranolol in long term prophylaxis of migraine. Complement Ther Med. 2005;13:165–74. doi: 10.1016/j.ctim.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 122.Bernik TR, Friedman SG, Ochani M, et al. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med. 2002;195:781–8. doi: 10.1084/jem.20011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wittebole X, Hahm S, Coyle SM, Kumar A, Calvano SE, Lowry SF. Nicotine exposure alters in vivo human responses to endotoxin. Clin Exp Immunol. 2007;147:28–34. doi: 10.1111/j.1365-2249.2006.03248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mioni C, Bazzani C, Giuliani D, et al. Activation of an efferent cholinergic pathway produces strong protection against myocardial ischemia/reperfusion injury in rats. Crit Care Med. 2005;33:2621–8. doi: 10.1097/01.ccm.0000186762.05301.13. [DOI] [PubMed] [Google Scholar]