Abstract
Objective:
The nonlesioned motor cortex (M1NL) is thought to be hyperexcitable in patients with subacute or chronic stroke and offers a promising therapeutic target. However, whether M1NL excitability behaves the same for subcortical and cortical strokes is unknown. The aim of the present study was to determine whether cortical, or purely subcortical, strokes have a different effect on M1NL excitability.
Methods:
We looked for correlations between the Fugl-Meyer (FM) score and M1NL resting motor threshold (RMTNL) in 34 stroke survivors classified according to lesion location (cortico-subcortical or purely subcortical). In addition to the FM, the Wolf Motor Score and motor power were measured.
Results:
FM correlated with RMTNL for subcortical (r = 0.82; p = 0.001) but not for cortical strokes (r = 0.11; p = 0.62). Likewise, Wolf Motor Score (r = −0.62; p = 0.03) and motor power (r = 0.64; p = 0.023) were correlated with RMTNL for the subcortical group, but not for the cortical group.
Conclusion:
We show that the impact on M1NL depends on lesion location and conclude that protocols aimed at reducing M1NL cortical excitability may be worth exploring for subcortical but not for cortical stroke.
Physical rehabilitation in the subacute or chronic phase after stroke can draw on a number of tactics, one of which is to target motor areas of the nonlesioned hemisphere, including primary motor cortex (M1NL). An example is the use of noninvasive brain stimulation to reduce M1NL excitability.1–3
Evidence supporting this notion comes from transcranial magnetic stimulation studies of intracortical and interhemispheric inhibition that show that (1) M1NL can be overactive or disinhibited and exert an excessive inhibitory drive to the lesioned motor cortex (M1L),4 and (2) noninvasive brain stimulation of M1NL downregulating excitability can improve motor outcome.1,3 Furthermore, this hyperactivity is less apparent in chronic strokes exhibiting good motor recovery.5 It is thought that interhemispheric cortico-cortical connectivity underlies these effects.6
However, given stroke variability, it is also accepted that a “one size fits all” approach to stroke rehabilitation is not likely to be optimal.3 Accordingly, there is interest in identifying markers to stratify therapeutic approaches after stroke.
In the present study, we investigated the relationship between M1NL excitability and paretic hand function in patients with strokes that involved motor cortex compared with purely subcortical strokes. We hypothesized that this relationship may differ between groups because of differential effects of lesion location on intra- and interhemispheric circuits.
METHODS
We studied 34 stroke survivors satisfying the following criteria: right hand dominant; first-ever middle cerebral artery ischemic stroke affecting the right upper limb; upper extremity Fugl-Meyer (FM) 7–58; ability to follow instructions; no fixed joint contracture at the upper limb; no history of seizures; and no other neurologic conditions or implanted devices. In addition to the FM, the Wolf Motor Score (WMS) and motor power (MP) (Medical Research Council) were measured in all participants. Stroke subtypes were classified according to TOAST (Trial of Org 10172 in Acute Stroke Treatment) criteria.7
Participants were classified into 2 groups according to neuroimaging as read by a clinical neuroradiologist. The cortico-subcortical (Cx) group had lesions involving primary motor cortex that could extend to underlying white matter; the subcortical (SC) group had deeper infarcts of the basal ganglia and internal capsule (excluding cerebral cortex, brainstem, and cerebellum).
Nonlesioned (right hemisphere) resting motor threshold (RMTNL) and resting motor evoked potential (MEPNL) amplitude were measured from the left flexor carpi radialis muscle using transcranial magnetic stimulation (MagPro X100) and a figure-8 coil placed over the optimal stimulus site from initial exploration. RMTNL was defined as the intensity (2% steps of maximum stimulator output [MSO], ascending and descending order) that elicited a response with >50 μV amplitude (peak-peak) in at least 5 of 10 stimuli. Resting MEPNL amplitude was averaged from 10 stimuli delivered at 120% RMTNL. In all participants, we also explored the lesioned hemisphere to establish whether a MEP could be recorded from the right flexor carpi radialis, in which case RMTL was measured.
Between-group characteristics were compared with Student t tests and a χ2. We modeled the data with FM as the dependent outcome, and site of lesion and RMTNL and the interaction term between these 2 variables as independent variables. We conducted post hoc correlational analysis (Pearson product-moment) between clinical scores and neurophysiologic measures separately for Cx and SC groups. Data are presented as mean ± SD.
Standard protocol approvals, registrations, and patient consents.
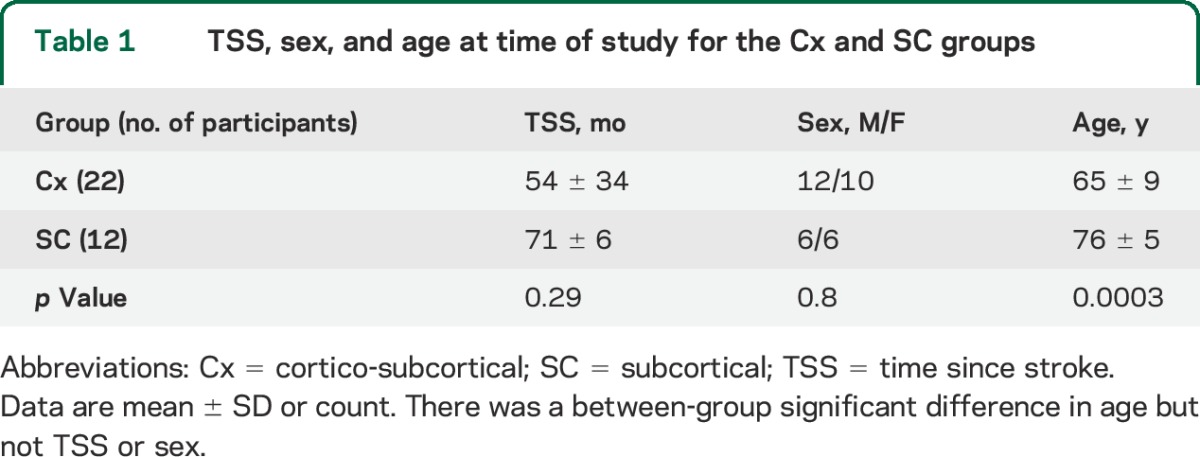
All patients gave written informed consent to the study, which was approved by the institutional review board of the Burke Rehabilitation Hospital. See table 1 and table e-1 on the Neurology® Web site at Neurology.org.
Table 1.
TSS, sex, and age at time of study for the Cx and SC groups
RESULTS
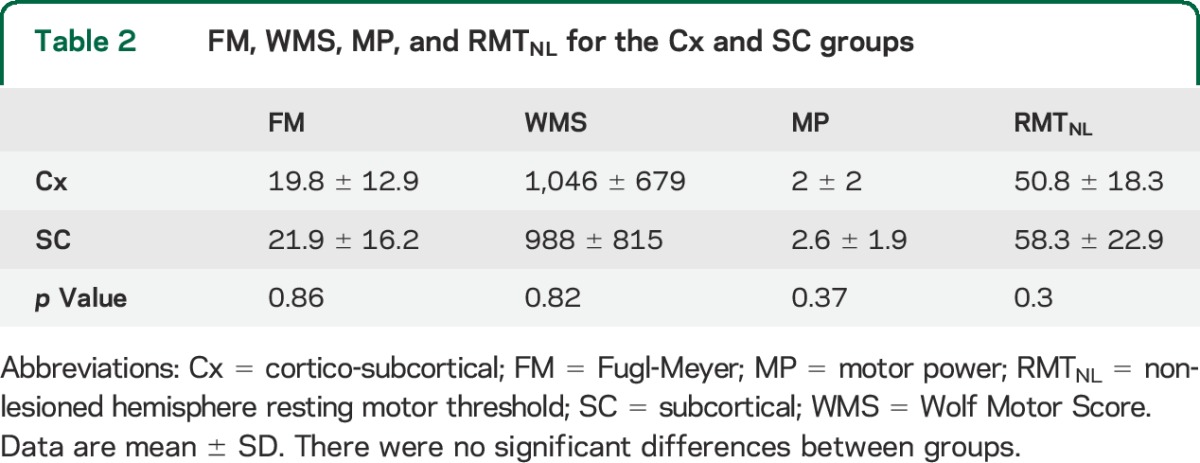
Of the 34 participants who met the inclusion criteria, 22 were categorized as Cx and 12 as SC. Approximately half of the strokes (53%) had a TOAST classification of 3 (small artery occlusion), 18% 2 (cardioembolism), and 18% 1 (large artery atherosclerosis) (see table e-1). There was no between-group difference in time since stroke (4.5 ± 2.8 vs 6 ± 0.5 years) or sex; however, the mean age of the SC group was greater than for the Cx group (76 ± 5 vs 65 ± 9 years of age; p = 0.0003; table 1). Clinical scores (FM, WMS, MP) and RMTNL were not significantly different between groups (table 2).
Table 2.
FM, WMS, MP, and RMTNL for the Cx and SC groups
The between-group FM and RMTNL interaction term was significant (r = 0.25; p = 0.024). The interaction remained significant (p = 0.028) when age was added to the model to allow for the between-group age difference.
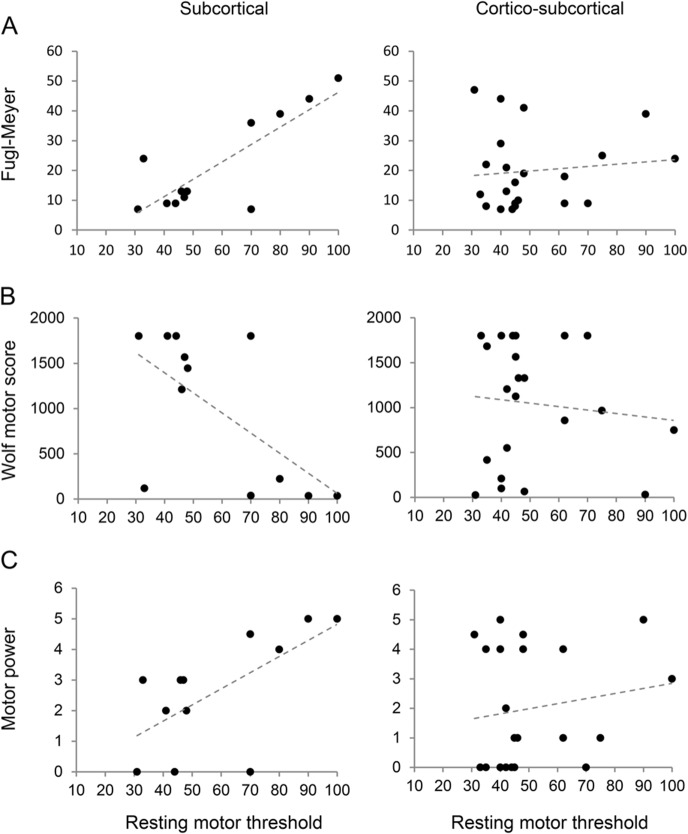
We investigated the interaction further by correlation analysis. There was a significant correlation between FM and RMTNL for the SC group (r = 0.82; p = 0.001) but not for the Cx group (r = 0.11; p = 0.62; figure, A).
Figure. Relationship between paretic upper limb clinical grades.
(A) Fugl-Meyer; (B) Wolf Motor Score; (C) motor power, and resting motor threshold measured over the nonlesioned hemisphere for each participant with subcortical and cortico-subcortical strokes. There were significant correlations between the clinical grades and resting motor threshold for subcortical stroke but not for cortico-subcortical stroke.
We examined the relationship between RMTNL and the remaining clinical scores in an exploratory manner. Both WMS (r = −0.62; p = 0.03) and MP (r = 0.64; p = 0.023) were correlated with RMTNL for the SC group but not for the Cx group (figure, B and C).
There was no significant interaction or correlation between MEPNL and clinical variables for either the SC or the Cx group. For the lesioned hemisphere, RMTL could not be determined (i.e., >MSO) in the majority of participants in both groups (16, Cx; 11, SC).
DISCUSSION
There was no significant difference in M1NL excitability or clinical scales between Cx and SC strokes; rather, it was the relationship between these parameters that was strikingly different between groups and correlated only for SC. Increased impairment (as determined by FM), decreased function (WMS), and reduced power (MP) were associated with reduced RMTNL and thus greater M1NL excitability for patients with SC. This relationship was not observed for lesions involving motor cortex. The results indicate that the excitability of the nonlesioned hemisphere after stroke is related to the anatomical level of the lesion and to the degree of paretic motor function or impairment.
Interhemispheric excitability effects are widely attributed to reciprocal transcallosal inhibition (TCI) between motor cortices.2 Strokes involving motor cortex could disrupt TCI depending on how the lesion affects intra- or perilesional connections or callosal fibers.8 However, cortical lesions extending into underlying white matter could differentially affect descending corticospinal conduction compared with transcallosal conduction. In this situation, M1NL excitability would not necessarily be related to paretic hand function, which is in keeping with our findings, although we cannot rule out that there may be a subgroup of cortical strokes (e.g., sparing transcallosal conduction) in which this relationship holds.
While lesions in the SC group did not extend to M1L, they could still exert a remote effect, for example, secondary to reduced sensorimotor afference or alterations in corticomotor drive, while leaving TCI functionally and anatomically intact. Our results in the SC group can be explained if the effect of the lesion is to downregulate M1L excitability and if a greater impairment results in a greater reduction. The ensuing withdrawal of inhibitory drive from M1L to M1NL could thus result in an increase in excitability that is in proportion to the impairment. This explanation is in keeping with recent models of functional diaschisis.9
While clinical scales correlated with RMTNL for SC stroke, there was no correlation in either group with MEPNL, presumably because stimulus intensity was threshold-adjusted (120% RMTNL) for recording the MEP, thus partially correcting for excitability differences. We did not attempt to record interhemispheric effects with paired-pulse protocols8 because RMTL was greater than MSO in the majority of participants (27/34).
Detailed tractography was beyond the scope of the present study; however, it is likely that Cx lesions resulting in hand paresis involved M1 and therefore the gray matter origin of the transcallosal pathway and could also extend into underlying white matter to disrupt transcallosal axons. It might have been expected that damage to transcallosal conduction in Cx compared with SC patients would then lead to a between-group difference in RMTNL. It is possible that the dispersion in RMTNL between patients has obscured an intergroup difference or that factors in addition to transcallosal disinhibition contribute to RMTNL. The only significant intergroup difference was age, which could potentially modulate corticomotor excitability. However, we found that the relationship between FM and RMTNL remained significant when age was added to the model.
The close relationship between excitability and impairment for SC strokes suggests that patients with SC strokes may be the best candidates for therapeutic approaches aimed at reducing M1NL excitability. However, it is also possible that M1NL hyperexcitability is secondary to the impairment and not a driving force, and that as recovery proceeds, excitability correspondingly normalizes.5 In this case, there may be little to be gained by targeting M1NL. Given these considerations, clinical trials may want to stratify by lesion location and consider patients with Cx and SC strokes separately.10,11
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Michael Reding and Dr. Alejandra Climent-Perin for their assistance with the manuscript.
GLOSSARY
- Cx
cortico-subcortical
- FM
Fugl-Meyer
- M1L
lesioned motor cortex
- M1NL
nonlesioned primary motor cortex
- MEPNL
nonlesioned motor evoked potential
- MP
motor power
- MSO
maximum stimulator output
- RMTL
lesioned hemisphere resting motor threshold
- RMTNL
nonlesioned hemisphere resting motor threshold
- SC
subcortical
- TCI
transcallosal inhibition
- TOAST
Trial of Org 10172 in Acute Stroke Treatment
- WMS
Wolf Motor Score
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Gary Thickbroom: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval. Mar Cortes: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data, obtaining funding. Avrielle Rykman: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data, statistical analysis, study supervision. Bruce Volpe: drafting/revising the manuscript, study concept or design, accepts responsibility for conduct of research and will give final approval. Felipe Fregni: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, statistical analysis. Igo Krebs: study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data, statistical analysis, study supervision. Alvaro Pascual-Leone: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, statistical analysis, study supervision, obtaining funding. Dylan Edwards: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data, study supervision, obtaining funding.
STUDY FUNDING
This study was supported by NICHD of the NIH, under award number R01HD069776. A.P.-L. is supported in part by grants from the Sidney R. Baer Jr. Foundation, the NIH (R01HD069776, R01NS073601, R21 MH099196, R21 NS082870, R21 NS085491, R21 HD07616), Harvard Catalyst | The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH, UL1 RR025758). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the NIH, or the Sidney R. Baer Jr. Foundation.
DISCLOSURE
G. Thickbroom, M. Cortes, A. Rykman, B. Volpe, F. Fregni, and H. Igo Krebs report no disclosures relevant to the manuscript. A. Pascual-Leone serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Neuroelectrics, Axilum Robotics, Magstim Inc., and Neosync; and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation with EEG and MRI. D. Edwards reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Fregni F, Boggio PS, Valle AC, et al. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke 2006;37:2115–2122. [DOI] [PubMed] [Google Scholar]
- 2.Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol 2006;5:708–712. [DOI] [PubMed] [Google Scholar]
- 3.Bradnam LV, Stinear CM, Barber PA, Byblow WD. Contralesional hemisphere control of the proximal paretic upper limb following stroke. Cereb Cortex 2012;22:2662–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liepert J, Hamzei F, Weiller C. Motor cortex disinhibition of the unaffected hemisphere after acute stroke. Muscle Nerve 2000;23:1761–1763. [DOI] [PubMed] [Google Scholar]
- 5.Rossini PM, Calautti C, Pauri F, Baron JC. Post-stroke plastic reorganisation in the adult brain. Lancet Neurol 2003;2:493–502. [DOI] [PubMed] [Google Scholar]
- 6.Boroojerdi B, Diefenbach K, Ferbert A. Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. J Neurol Sci 1996;144:160–170. [DOI] [PubMed] [Google Scholar]
- 7.Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 8.Butefisch CM, Wessling M, Netz J, Seitz RJ, Homberg V. Relationship between interhemispheric inhibition and motor cortex excitability in subacute stroke patients. Neurorehabil Neural Repair 2008;22:4–21. [DOI] [PubMed] [Google Scholar]
- 9.Carrera E, Tononi G. Diaschisis: past, present, future. Brain 2014;137:2408–2422. [DOI] [PubMed] [Google Scholar]
- 10.Raffin E, Siebner HR. Transcranial brain stimulation to promote functional recovery after stroke. Curr Opin Neurol 2014;27:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyai I, Blau AD, Reding MJ, Volpe BT. Patients with stroke confined to basal ganglia have diminished response to rehabilitation efforts. Neurology 1997;48:95–101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.