Abstract
Objective:
To compare the burden of neuropathology in black and white participants with clinical Alzheimer disease (AD).
Methods:
Participants included 122 persons enrolled in the Rush Alzheimer's Disease Clinical Core, a prospective cohort study of AD. Forty-one black decedents were matched two-to-one to 81 white decedents according to age at death, sex, years of education, and cognition proximate to death. We examined common brain pathologies related to dementia (AD, Lewy body, and macroscopic and microinfarct pathology) and arteriolar sclerosis and atherosclerosis. We calculated the frequency of each dementia pathology both alone and in combination (mixed pathologies). Racial differences in the odds of a single pathology vs mixed pathologies, and in the odds of vessel disease and its severity, were examined using logistic regression analyses.
Results:
AD pathology was confirmed in >93% of both black and white decedents with AD dementia. However, black decedents were less likely to have Alzheimer pathology as a single dementia pathology than white decedents (19.5% vs 42.0%), and were more likely to have AD mixed with an additional pathology (70.7% vs 50.6%), particularly Alzheimer pathology and Lewy bodies, and Alzheimer pathology, Lewy bodies, and infarcts. Black decedents also had more severe arteriolar sclerosis and atherosclerosis.
Conclusion:
Black decedents with AD dementia are more likely to have mixed brain pathologies compared with age-, sex-, education-, and cognition-matched white decedents with AD dementia.
Alzheimer disease (AD) dementia, a devastating and common neurodegenerative disease, is a leading cause of disability in our aging society. It is increasingly recognized that the underlying pathologic basis of AD dementia is heterogeneous and frequently mixed. Many cases of AD dementia demonstrate an underlying AD pathology, yet other dementia-related pathologies including Lewy bodies and infarcts frequently coexist with AD pathology and add to the likelihood of clinically symptomatic AD.1,2
Knowledge about the neuropathologic basis of AD dementia has been based almost exclusively on autopsy studies of white persons. There is a dearth of neuropathologic information available in older black persons with AD dementia despite the fact that they may have a higher prevalence and incidence of AD dementia than white persons,3,4 as well as a disproportionate burden of vascular disease.5,6 In the current study, we tested the hypothesis that mixed pathologies, in particular AD pathology and infarcts, are more common in black than white participants with AD dementia.
METHODS
Participants.
Participants included 122 deceased persons with AD dementia from the Rush Alzheimer's Disease Clinical Core7: 41 consecutive autopsied black decedents matched to 81 white decedents. All participants were evaluated for possible dementia at either the Rush Memory Clinic or a neighboring memory assessment clinic at a county hospital in the Illinois Medical District. At the initial evaluation, the patient and caregiver were presented with study information, which required that the person be community-dwelling and live within the Chicago area. Those who expressed interest were enrolled and signed informed consent at the first visit. Eligibility for inclusion in the present analysis required a clinical diagnosis of probable or possible dementia due to AD, using criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association.8 Through July 25, 2014, 573 participants agreed to autopsy and 487 received an autopsy. Of those, 42 were black and had dementia, and complete neuropathologic data were available on AD pathology, Lewy body pathology, and macroscopic and microinfarcts. One black participant was excluded from the analysis because there was no final clinical diagnosis of possible or probable AD. A sample size of 41 black participants remained. Black decedents were matched to white decedents on age, sex, education, and Mini-Mental State Examination (MMSE) with a goal of 2 white participants to every 1 black participant. For 1 black participant, only 1 white participant had the same age, education, and cognition combination. Thus, the final sample size for white participants was 81 persons with possible or probable AD dementia.
Standard protocol approvals, registrations, and patient consents.
Written informed consent was obtained from the participant or from a legally authorized representative. The study was approved by the Institutional Review Board of Rush University Medical Center. Following death, a legal representative or next of kin provided consent for the autopsy as previously described.9
Clinical assessment.
Clinical evaluations were uniform and structured and included procedures used by the Consortium to Establish a Registry for Alzheimer's Disease (CERAD),10 as previously reported.7,9 Briefly, the evaluation included a medical history, neurologic examination, cognitive function testing, a brief psychiatric evaluation, and an interview with a knowledgeable informant. After death, a neurologist, blinded to the neuropathologic findings, reviewed all of the available clinical data, and rendered a summary diagnostic opinion.
Neuropathologic evaluation.
Brains were removed and weighed in a standard fashion, and the brainstem and cerebellum were removed.11 The hemispheres were cut coronally into 1-cm slabs in a Plexiglas jig. All fresh slabs were photographed and examined for visible pathology. One hemisphere (without visualized pathology) was frozen. The other hemisphere and slabs with visible pathology were fixed for at least 3 days in 4% paraformaldehyde. A macroscopic review (including assessment of macroscopic infarcts) was subsequently conducted and diagnostic blocks (midfrontal, middle temporal, inferior parietal, anterior cingulate, entorhinal cortex, hippocampus, basal ganglia, thalamus, and midbrain with substantia nigra) were dissected. Blocks were then embedded in paraffin, cut into 6-μm sections, and mounted on glass slides.9
Pathologic diagnoses.
Board-certified neuropathologists blinded to age, race, and clinical data diagnosed 3 age- and dementia-related brain pathologies: AD pathology, Lewy bodies, and chronic infarcts (macroscopic and microscopic). Severity of arteriolar sclerosis and atherosclerosis was also graded. For the pathologic diagnosis of AD, Bielschowsky silver stain was used to visualize neuritic plaques and neurofibrillary tangles in the frontal, temporal, parietal, entorhinal, and hippocampal cortices, as previously described.9 A neuropathologic diagnosis of no AD, low likelihood AD, intermediate likelihood AD, or high likelihood AD was rendered based on semiquantitative estimates of neuritic plaque density (CERAD and Braak score).12,13 A final pathologic diagnosis of AD required either intermediate or high likelihood AD by National Institute on Aging–Reagan criteria, and at least moderate neuritic plaques in neocortical regions and minimum of Braak III–IV (limbic stage tangles). α-Synuclein immunohistochemistry (Zymed, 1:100; or Wako #015–25191; 1:20,000) was used to detect Lewy bodies in the substantia nigra, entorhinal, cingulate, midfrontal, middle temporal, and inferior parietal cortex.9 All suspected gross infarcts were blocked and microscopically examined for confirmation. For chronic microscopic infarcts, we examined a minimum of 6 cortical regions, 2 subcortical regions, and 1 brainstem region (midfrontal, middle temporal, inferior parietal, anterior cingulate, entorhinal cortex, hippocampus, basal ganglia, thalamus, and midbrain with substantia nigra), and they were then summarized as present or absent. Microscopic infarcts were labeled as chronic, cavitated, or incomplete, with few remaining macrophages or fibrillary gliosis.
Finally, arteriolar sclerosis was graded subjectively on a semiquantitative scale, and based on concentric hyalinized thickening (with consequent narrowing of the lumen) of the walls of small arterioles in anterior basal ganglia. Thickening with less than double wall thickness was considered mild; double wall thickness was moderate; and over double the thickness was considered severe. The degree of atherosclerosis of the circle of Willis vessels was also recorded on a semiquantitative scale consisting of the following categories: none (0), mild (1), moderate (2), and severe (3), as previously described.14
Statistical analysis.
Descriptive statistical analyses were conducted to characterize differences between black and white decedents on postmortem interval (PMI) and clinical interval (number of months between last clinical examination and death). Extent of arteriolar sclerosis and atherosclerosis was compared via Mann-Whitney nonparametric tests. Next, we calculated the frequency of each dementia pathology both alone and in combination (mixed pathologies). Finally, the effect of being black on the different pathologic markers is presented as an odds ratio (OR), the ratio of the odds of the finding in black participants to the odds for white participants. Similar analyses were separately conducted for arteriolar sclerosis and atherosclerosis. The distributions of other findings among those with AD dementia were compared via Fisher exact test. Statistical significance was set at α = 0.05.
RESULTS
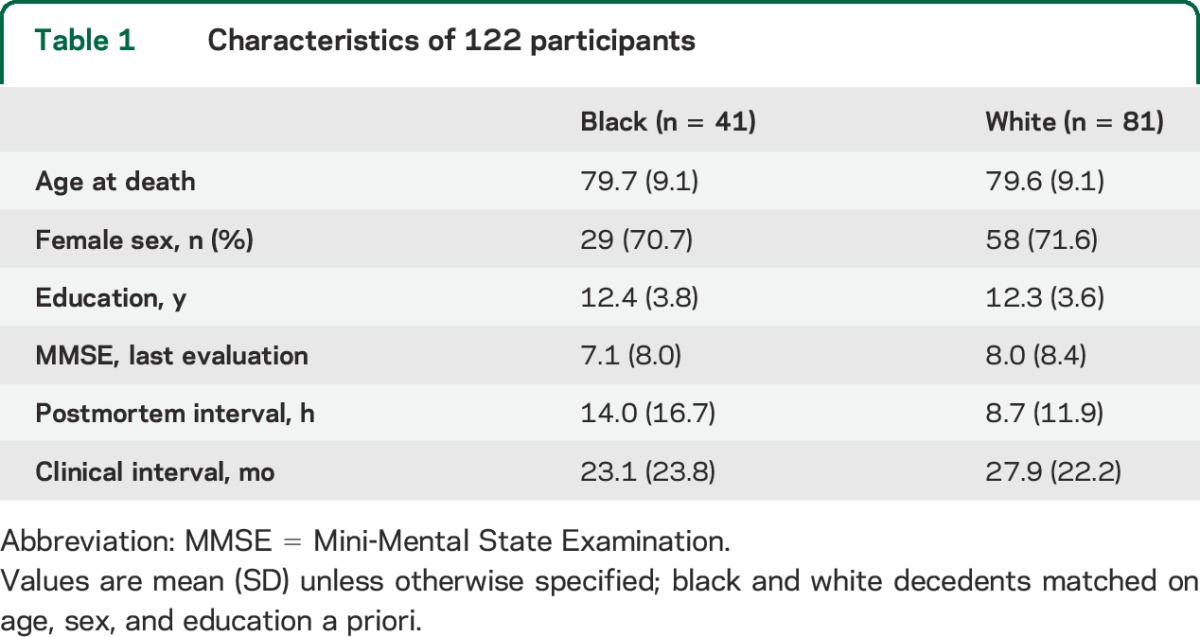
The average age at death was approximately 80 years. Participants had an average educational level of 12 years, and 71% were female. Demographic characteristics are listed in table 1. There were no racial differences in clinical intervals (p = 0.14), but black decedents had a significantly longer PMI than white decedents (p < 0.01).
Table 1.
Characteristics of 122 participants
Overall, more than 93% of both black and white decedents had one or more of the major dementia neuropathologies under investigation; only 5% (2 black and 5 white participants) had no AD, Lewy body, or infarct pathology to account for dementia. The pathologic diagnosis for dementia in the 2 black participants was hippocampal sclerosis, and the other was diffuse leukoencephalopathy with neuronal axonal spheroids. Three of the white participants had frontal temporal lobar degeneration, and the other 2 had a nonspecific pathologic diagnosis (1 with AD pathology not sufficient for AD diagnosis and the other with severe nigral degeneration and mild AD pathology not sufficient for a pathologic diagnosis of AD). As shown in table 2, AD was pathologically confirmed, with or without another contributing pathology, in the majority of both black and white decedents. For black participants, the next most common pathology was Lewy bodies (with or without another contributing pathology), which was more than twice as common as in white decedents. Chronic macroscopic and microscopic infarcts were also more common among black than white decedents, with macroscopic infarcts being more common than microscopic infarcts.
Table 2.
Pattern of pathology in black and white decedents
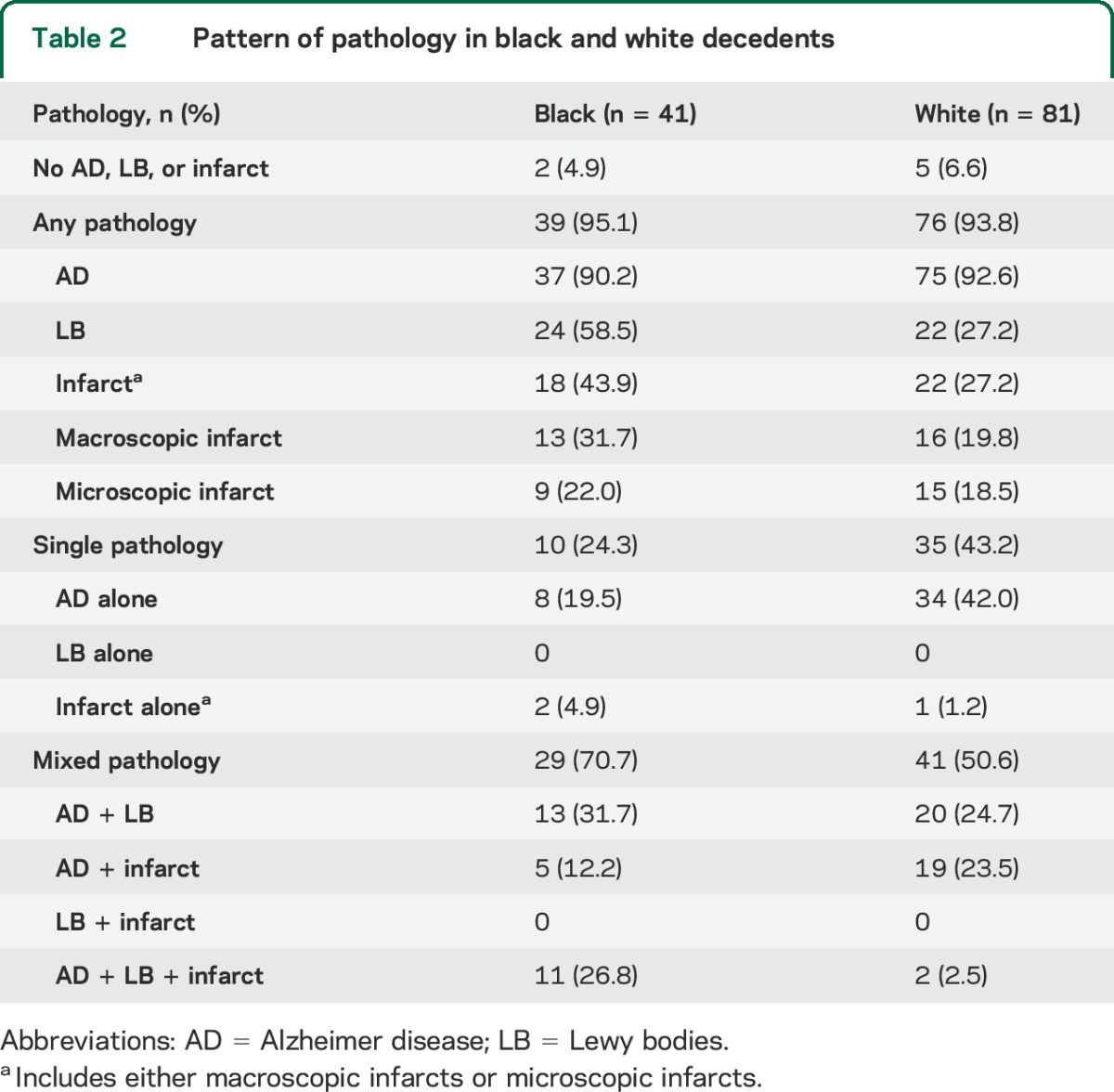
Examination of single pathologies (alone with no additional contributing pathology) revealed different patterns by race. White participants were almost twice as likely as black participants to have a single pathology. In both black and white decedents, the most common single pathology was AD pathology. No decedent had Lewy bodies without coexisting AD pathology. Chronic infarcts (either macroscopic or microscopic) as a single pathology underlying a clinical diagnosis of AD was also rare in both racial groups.
Although mixed pathologies were common in both black and white decedents, patterns differed by race. Overall, black participants were more likely to have mixed pathologies compared with white participants (70.7% vs 50.6%). The most common mixed pathology for black participants was Alzheimer pathology with Lewy bodies, followed closely by Alzheimer pathology with both Lewy bodies and infarcts. By contrast, about half of the white participants with mixed pathology had AD and infarcts with the other half having AD and Lewy body pathology. Both black and white decedents with mixed pathology had a similar percentage of cases with AD and infarcts (with or without Lewy bodies). On the other hand, black participants were much more likely to have all 3 pathologies compared to white participants (table 2). No one in either group had Lewy bodies with infarcts without Alzheimer pathology (figure).
Figure. Racial differences in mixed pathology.
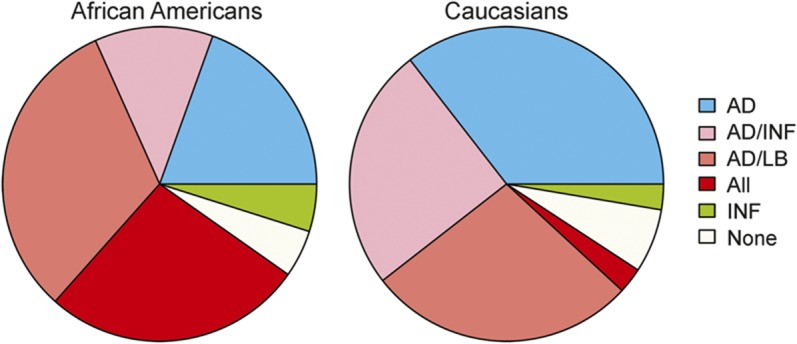
Pie chart shows proportions of individual and mixed pathologies in black and white decedents with Alzheimer disease (AD) dementia. INF = infarcts; LB = Lewy bodies.
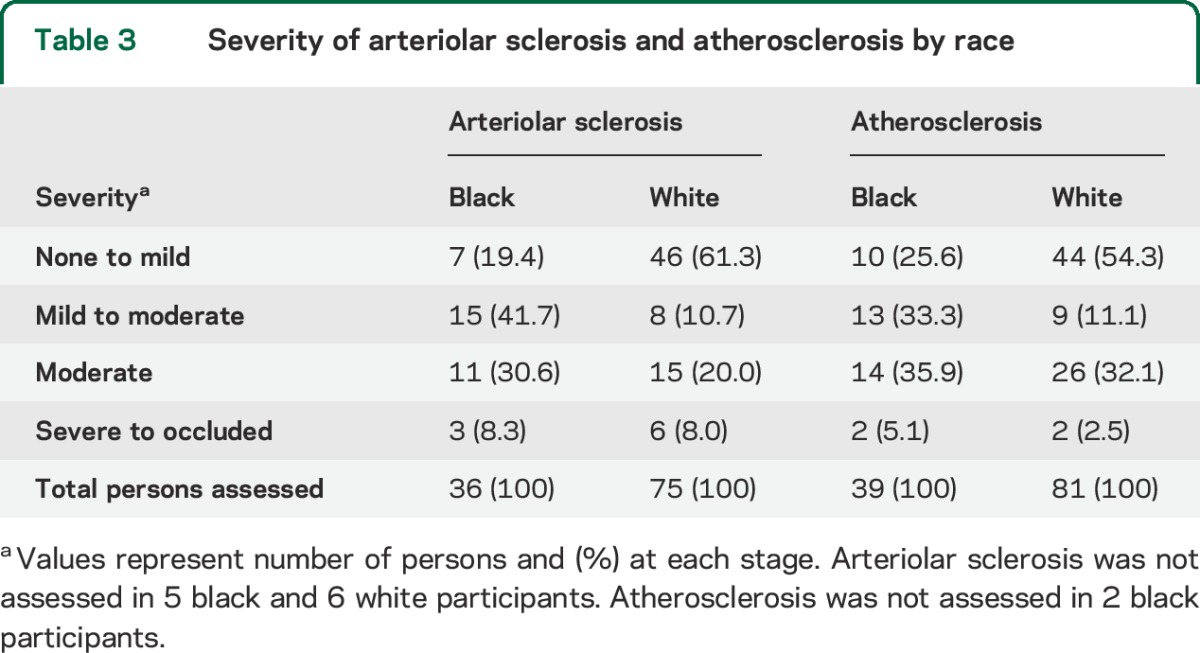
The severity of arteriolar sclerosis was greater in black than white decedents (p = 0.0032, Mann-Whitney with correction for ties). Of those with assessments of arteriolar sclerosis, 80.6% of black decedents were rated as mild to severe compared with only 38.7% of white decedents (table 3). The severity of atherosclerosis was also greater in black participants than white participants (p < 0.01). Black decedents were more likely to have ratings of mild to severe (74%) than were white participants (46%) (table 3).
Table 3.
Severity of arteriolar sclerosis and atherosclerosis by race
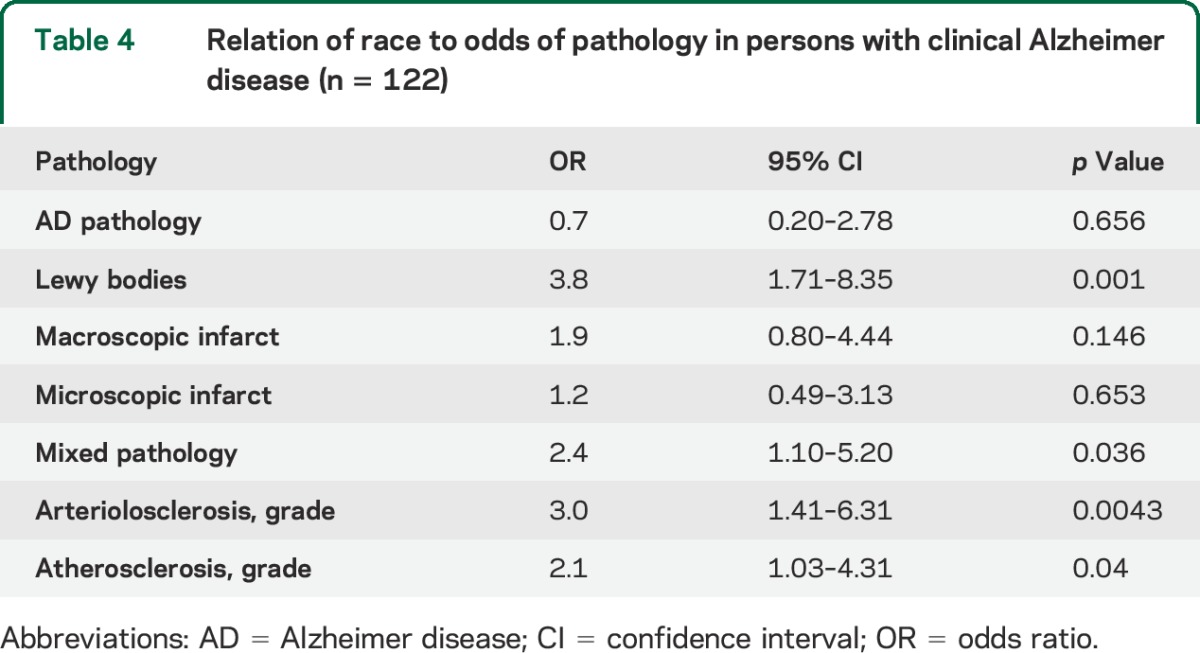
Next, we obtained the ratio of the odds of each individual pathology and any of the mixed pathologies in black relative to white participants. Because black and white decedents were matched on age, education, sex, and MMSE, models were conducted without adjustments. We first fit separate models with each pathology as the outcome variable. We then fit a model with mixed pathology (any 2 or more of the individual pathologies) as the outcome variable. Finally, we fit a model with arteriolar sclerosis as an ordinal outcome variable (mild-moderate vs moderate and severe), and another model with atherosclerosis as the outcome. As shown in table 4, black decedents were more likely to have Lewy body pathology and mixed pathology. Though the ORs for macroscopic and microinfarcts infarcts were greater than one, they did not reach statistical significance. However, black decedents had a greater odds of having vessel disease. They were more likely to have more severe arteriolar sclerosis as well as more severe atherosclerosis compared with white decedents.
Table 4.
Relation of race to odds of pathology in persons with clinical Alzheimer disease (n = 122)
DISCUSSION
In this study of patients with AD dementia diagnosed in a clinic setting and matched on demographics and cognition, we examined racial differences in underlying dementia-related neuropathologies. AD pathology was confirmed in the majority of both races; however, mixed pathologies were far more common in black decedents. However, contrary to our hypothesis that black decedents would have more mixed pathology consisting of AD pathology and infarcts, we found that black decedents with AD dementia were more likely to have AD mixed with Lewy body pathology and AD mixed with Lewy bodies and infarcts compared with white decedents with dementia.
Previous studies of racial differences in pathology have been autopsy series from medical examiners in which premortem status is generally unknown or extracted from medical records. One such study reported that the frequency of AD and Parkinson disease–related pathology was higher in white decedents than black decedents while cerebrovascular disease pathology was more frequent in black decedents.15 Three other autopsy series reported no racial differences in the frequency of AD pathology.16–18 To our knowledge, only one previous study of well-characterized black and white decedents directly compared underlying pathologies in persons who died with AD dementia. That study also found no differences in AD pathology between black and white decedents, but with only 10 black decedents and 10 matched white decedents, and they could not report on mixed pathologies.19
Previous studies with white decedents have demonstrated that the most common mixed pathology in AD dementia is AD with infarcts.20,21 We are aware of only one study of 13 autopsied brains from well-characterized black participants with a clinical diagnosis of AD that reported a wide spectrum of vascular disease and AD pathology, with more than half having a mixture of AD pathology and infarcts.22 Our results are not entirely consistent with this finding. We found that the most frequent mixed pathology among black decedents with AD dementia was AD with Lewy bodies, followed closely by AD, Lewy bodies, and infarcts. The basis for an increase in Lewy bodies in black decedents is uncertain. One study in Brazil examined ancestry and neuropathology in an admixed sample of 202 brains. While the primary result was that African ancestry is protective of AD pathology, there was also a tendency of higher odds of Lewy body pathology in persons of African ancestry; however, it did not reach significance and was not a major focus of the article.23 The high frequency of Lewy body pathology among black decedents in our sample may reflect a selection bias for clinic samples. Lewy body disease, with and without coexisting AD pathology, is associated more with behavioral abnormalities, such as hallucinations and REM sleep behavior disorder, than clinical AD. It is possible that African Americans are more likely to seek medical attention for behavioral issues, which are often more distressing for caregivers,24,25 than for memory complaints. In fact, previous studies have noted that African Americans often do not seek medical attention for memory complaints, or present at a later stage of the disease, presumably because of cultural perceptions that memory loss is a normal part of aging.24,26,27 Our data do not allow us to evaluate these potential explanations. Studies of neuropathology in black decedents followed longitudinally and without AD dementia are needed to determine whether the high frequency of Lewy bodies among black decedents in this study is an artifact of a selection bias.
Interestingly, AD mixed with infarcts alone was not as common in black compared to white decedents. Nonetheless, overall mixed pathologies with infarcts (with or without Lewy bodies) was present in about half of both black and white decedents. These patterns are consistent with a previous clinical-pathologic study that found mixed pathologies with infarcts were less common in clinic than community samples, again suggesting a potential selection bias for persons presenting to memory disorders clinics.9
Infarcts were common in black decedents with AD dementia, present in over 40%, compared to fewer than 30% of white decedents; however, the difference did not reach statistical significance in adjusted models. It is interesting to note that while the proportion of infarcts did not significantly differ in black vs white participants, black participants had more severe small and large vessel disease (arteriolar sclerosis and atherosclerosis) compared with white participants. A couple of explanations may explain this apparent discrepancy. First, given that vessel disease is commonly associated with the same risk factors as stroke, namely diabetes and hypertension, conditions that have consistently been found to be more prevalent in the black than the white population,28,29 it is possible that there was insufficient power, and that larger numbers of subjects are needed to confirm the difference in frequency of infarcts. Alternatively, it is also possible that persons with clinical stroke are more likely to be evaluated at a stroke center than a memory disorder clinic and the discrepancy reflects a selection bias, similar to that proposed for Lewy body disease. Further study of infarcts in community-dwelling African Americans is warranted.
Black participants had a significantly longer PMI, on average about 6 hours, than white participants. Although difference in PMI did not influence the patterns of pathology observed in this study, the longer interval for black participants reflects the challenges in obtaining autopsy in this population. While significant variability exists among families as far as end-of-life preferences,30 it is possible that certain traditions associated with black culture, such as family-centered decision-making styles and mistrust of the health care system,31,32 which is known to be a factor in the lower rates of organ donation, and the lack of knowledge regarding organ donation in general among the black population,33 may create barriers to rapid autopsy in this population. The data from the current study support the difficulty in obtaining autopsy but also underscore the dangers in generalizing from studies in the white population.
The greater burden and differing profile of mixed pathologies in black participants has far-reaching implications. First, it suggests that response to therapeutic agents targeting AD pathology may be diminished in African Americans, and underscores the need to include more African Americans in clinical trials to ensure that findings are generalizable across race. Indeed, African Americans have typically been underrepresented in studies of cholinesterase inhibitors. Second, given that most current therapeutic strategies focus primarily on the modification of amyloid, a central AD pathology, it will be important to develop new treatments that target other common pathologies, particularly in African Americans, to lessen the burden of AD dementia. Ultimately, the differing profiles of mixed pathologies in African Americans in the clinic may suggest a need for different therapeutic regimens in black compared to white participants. Third, the preventive strategies for AD dementia should target not only risk factors for amyloid and tau, but also risk factors for other common age-related pathologies, particularly in African Americans. Because recommendations for prevention could differ according to the burden of mixed pathologies, further clinical-pathologic studies of black participants without AD dementia are needed to determine whether the spectrum and profiles in the clinic reflect the more general black population. Finally, in order to better study the profiles, risks, and impact of mixed pathologies both in the preclinical and clinical phases of disease, it will be important to generate and confirm biomarkers of both AD and non-AD pathologies in black participants.
The study has limitations. Participants are from a clinic and are therefore a select sample that may not represent the general population with AD dementia, particularly older African Americans who are less likely to come to medical attention.26 Although this study is the largest clinical-pathologic study of racial differences in mixed pathology of which we are aware, the number of brains from African Americans was relatively small, limiting our ability to detect subtle differences in mixed pathology across race. We did not have measures of other pathologies like TDP43, which has recently been shown to coexist with AD pathology.34 We also did not have data on vascular risk factors, parkinsonism, motor signs, or neuropsychiatric features, which limits our ability to interpret clinical correlates of racial differences in the underlying pathology.
The study also had strengths. We measured the 3 most common pathologies using validated techniques in both black and white decedents. In addition, black and white decedents were matched on important demographic variables that are associated with AD dementia, race, and pathology, lending confidence that differences in pathology are not due to the potentially confounding effects of age, sex, education, or cognition. Finally, participants from both racial groups had pathology evaluations conducted at the same center and by neuropathologists who were blind to race, age, and clinical measures, reducing the chance of rater differences in counts and measurement of various pathologies.
ACKNOWLEDGMENT
The authors thank the participants of the Alzheimer's Disease Core Center Clinical Core for their contributions; Theresa Jenkins, Barbara Eubeler, Karen Lowe-Graham, MS, and Karen Skish, MS, for study recruitment and coordination; John Gibbons, MS, and Greg Klein, MS, for data management; Wenqing Fan, MS, for data analysis; and the staff of the Rush Alzheimer's Disease Center.
GLOSSARY
- AD
Alzheimer disease
- CERAD
Consortium to Establish a Registry for Alzheimer's Disease
- MMSE
Mini-Mental State Examination
- OR
odds ratio
- PMI
postmortem interval
AUTHOR CONTRIBUTIONS
Lisa L. Barnes: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, statistical analysis, study supervision. Sue Leurgans: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, statistical analysis. Neelum T. Aggarwal: drafting/revising the manuscript, study concept or design, accepts responsibility for conduct of research and final approval, acquisition of data, study supervision. Raj C. Shah: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval. Zoe Arvanitakis: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, obtaining funding. Bryan James: drafting/revising the manuscript, accepts responsibility for conduct of research and final approval. Aron S. Buchman: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval. David A. Bennett: drafting/revising the manuscript, accepts responsibility for conduct of research and final approval, acquisition of data, study supervision, obtaining funding. Julie A. Schneider: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, study supervision.
STUDY FUNDING
Supported by NIH grant P30AG10161 and the Illinois Department of Public Health.
DISCLOSURE
L. Barnes reports no relevant disclosures for this manuscript. This work was supported in part by NIH grant P30AG10161 and the Illinois Department of Public Health. S. Leurgans reports no relevant disclosures for this manuscript. This work was supported in part by NIH grant P30AG10161 and the Illinois Department of Public Health. N. Aggarwal reports no relevant disclosures for this manuscript. This work was supported by NIH grant P30AG10161. R. Shah reports no relevant disclosures for this manuscript. This work was supported in part by NIH grant P30AG10161 and the Illinois Department of Public Health. Z. Arvanitakis reports no relevant disclosures for this manuscript. This work was supported in part by NIH grants P30AG10161, R01NS084965, and R01AG040039. B. James consults for the Alzheimer's Association and Partners Healthcare. This work was supported by NIH grant P30AG10161. A. Buchman reports no relevant disclosures for this manuscript. This work was supported in part by NIH grants P30AG10161, R01AG043379, and R01NS078009. D. Bennett serves on the editorial board of Neurology®; has received honoraria for non-industry-sponsored lectures; has served as a consultant to Danone, Inc., Wilmar Schwabe GmbH & Co., Eli Lilly, Inc., Schlesinger Associates, and Geson Lehrman Group; and receives research support for NIH grants P30AG010161, R01AG015819, R01AG017917, R01AG036042, U01AG046152, R01AG039478, R01AG040039, R01NS084965, R01AG022018, P20MD006886, R01AG043617, R01NS078009, R01AG036836, R01NS082416, R01AG038651, R01NS086736, R01AG041797, P01AG014449, U18NS082140, U01AG032984, R01AG042210, R01AG043975, and R01AG034119, and research support from Zinfandel. This work was supported in part by NIH grants P30AG10161 and R01AG15819 and the Illinois Department of Public Health. J. Schneider serves on the editorial board of Journal of Neuropathology and Experimental Neurology and Journal of Histochemistry and Cytochemistry and has consulting/advisory relationships with the following companies: AVID Radiopharmaceuticals, Navidea Biopharmaceuticals, Eli Lily Inc., and Genentech USA, and receives research support for NIH grants P30AG010161, R01AG015819, R01AG017917, R01AG042210, R01AG039478, R01AG022018, R01AG036042, and R01AG036836. This work was supported in part by NIH grant P30AG10161 and the Illinois Department of Public Health. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Lo RY, Jagust WJ. Vascular burden and Alzheimer disease pathologic progression. Neurology 2012;79:1349–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matthews FE, Brayne C, Lowe J, McKeith I, Wharton SB, Ince P. Epidemiological pathology of dementia: attributable risks at death in the Medical Research Council Cognitive Function and Ageing Study. PLoS Med 2009;6:e1000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shadlen MF, Siscovick D, Fitzpatrick AL, Dulberg C, Kuller LH, Jackson S. Education, cognitive test scores, and black-white differences in dementia risk. J Am Geriatr Soc 2006;54:898–905. [DOI] [PubMed] [Google Scholar]
- 4.Tang MX, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology 2001;56:49–56. [DOI] [PubMed] [Google Scholar]
- 5.Keil JE, Sutherland SE, Knapp RG, Lackland DT, Gazes PC, Tyroler HA. Mortality rates and risk factors for coronary disease in black as compared with white men and women. N Engl J Med 1993;329:73–78. [DOI] [PubMed] [Google Scholar]
- 6.Kuller LH. Cardiovascular diseases and stroke in African-Americans and other racial minorities in the United States: a statement for health professionals: introduction. Circulation 1991;83:1463–1465. [DOI] [PubMed] [Google Scholar]
- 7.Bennett DA, Schneider JA, Aggarwal NT, et al. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology 2006;27:169–176. [DOI] [PubMed] [Google Scholar]
- 8.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 9.Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis 2009;18:691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD): part I: clinical and neuropsychological assessment of Alzheimer's disease. Neurology 1989;39:1159–1165. [DOI] [PubMed] [Google Scholar]
- 11.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 2006;66:1837–1844. [DOI] [PubMed] [Google Scholar]
- 12.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–259. [DOI] [PubMed] [Google Scholar]
- 13.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD): part II: standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991;41:479–486. [DOI] [PubMed] [Google Scholar]
- 14.Nag S, Yu L, Capuano AW, et al. Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer's disease. Ann Neurol 2015;77:942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de la Monte SM, Hutchins GM, Moore GW. Racial differences in the etiology of dementia and frequency of Alzheimer lesions in the brain. J Natl Med Assoc 1989;81:644–652. [PMC free article] [PubMed] [Google Scholar]
- 16.Miller FD, Hicks SP, D'Amato CJ, Landis JR. A descriptive study of neuritic plaques and neurofibrillary tangles in an autopsy population. Am J Epidemiol 1984;120:331–341. [DOI] [PubMed] [Google Scholar]
- 17.Riudavets MA, Rubio A, Cox C, Rudow G, Fowler D, Troncoso JC. The prevalence of Alzheimer neuropathologic lesions is similar in blacks and whites. J Neuropathol Exp Neurol 2006;65:1143–1148. [DOI] [PubMed] [Google Scholar]
- 18.Sandberg G, Stewart W, Smialek J, Troncoso JC. The prevalence of the neuropathological lesions of Alzheimer's disease is independent of race and gender. Neurobiol Aging 2001;22:169–175. [DOI] [PubMed] [Google Scholar]
- 19.Wilkins CH, Grant EA, Schmitt SE, McKeel DW, Morris JC. The neuropathology of Alzheimer disease in African American and white individuals. Arch Neurol 2006;63:87–90. [DOI] [PubMed] [Google Scholar]
- 20.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007;69:2197–2204. [DOI] [PubMed] [Google Scholar]
- 21.White L, Small BJ, Petrovitch H, et al. Recent clinical-pathologic research on the causes of dementia in late life: update from the Honolulu-Asia Aging Study. J Geriatr Psychiatry Neurol 2005;18:224–227. [DOI] [PubMed] [Google Scholar]
- 22.Pytel P, Cochran EJ, Bonner G, Nyenhuis DL, Thomas C, Gorelick PB. Vascular and Alzheimer-type pathology in an autopsy study of African-Americans. Neurology 2006;66:433–435. [DOI] [PubMed] [Google Scholar]
- 23.Schlesinger D, Grinberg LT, Alba JG, et al. African ancestry protects against Alzheimer's disease-related neuropathology. Mol Psychiatry 2013;18:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohamed S, Rosenheck R, Lyketsos CG, Schneider LS. Caregiver burden in Alzheimer disease: cross-sectional and longitudinal patient correlates. Am J Geriatr Psychiatry 2010;18:917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ornstein KA, Gaugler JE, Devanand DP, Scarmeas N, Zhu CW, Stern Y. Are there sensitive time periods for dementia caregivers? The occurrence of behavioral and psychological symptoms in the early stages of dementia. Int Psychogeriatr 2013;25:1453–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes LL, Bennett DA. Alzheimer's Disease in African Americans: risk factors and challenges for the future. Health Aff 2014;33:580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chui HC, Gatz M. Cultural diversity in Alzheimer disease: the interface between biology, belief, and behavior. Alzheimer Dis Assoc Disord 2005;19:250–255. [DOI] [PubMed] [Google Scholar]
- 28.Lackland DT. Racial differences in hypertension: implications for high blood pressure management. Am J Med Sci 2014;348:135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenstock S, Whitman S, West JF, Balkin M. Racial disparities in diabetes mortality in the 50 most populous US cities. J Urban Health 2014;91:873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blackhall LJ, Frank G, Murphy ST, Michel V, Palmer JM, Azen SP. Ethnicity and attitudes towards life sustaining technology. Soc Sci Med 1999;48:1779–1789. [DOI] [PubMed] [Google Scholar]
- 31.Shavers VL, Lynch CF, Burmeister LF. Factors that influence African-Americans' willingness to participate in medical research studies. Cancer 2001;91:233–236. [DOI] [PubMed] [Google Scholar]
- 32.Shavers VL, Lynch CF, Burmeister LF. Racial differences in factors that influence the willingness to participate in medical research studies. Ann Epidemiol 2002;12:248–256. [DOI] [PubMed] [Google Scholar]
- 33.Bratton C, Chavin K, Baliga P. Racial disparities in organ donation and why. Curr Opin Organ Transplant 2011;16:243–249. [DOI] [PubMed] [Google Scholar]
- 34.Wilson RS, Yu L, Trojanowski JQ, et al. TDP-43 pathology, cognitive decline, and dementia in old age. JAMA Neurol 2013;70:1418–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]


