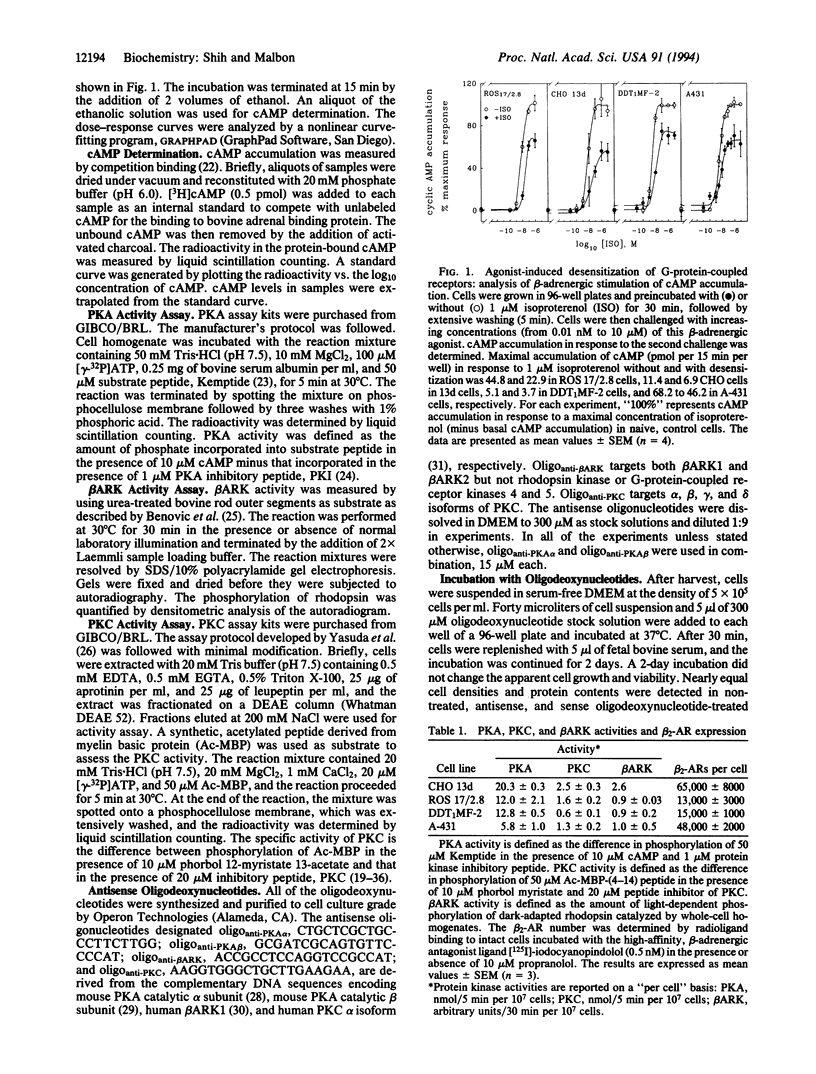
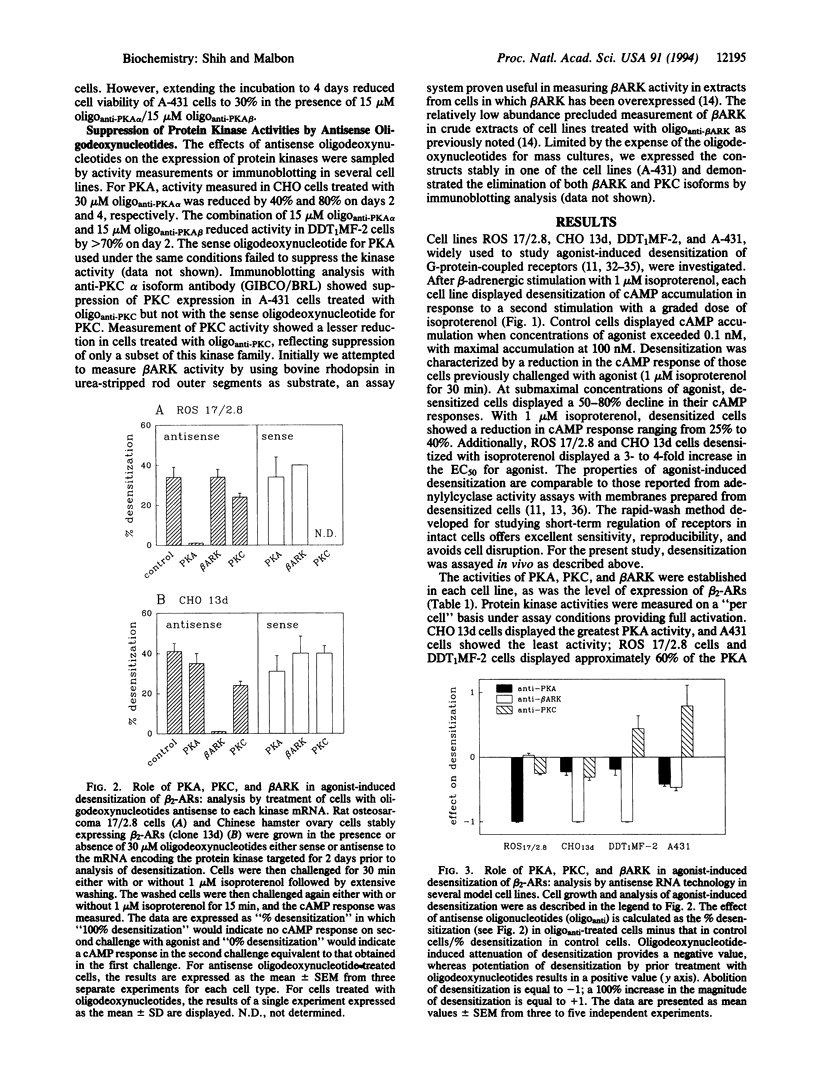
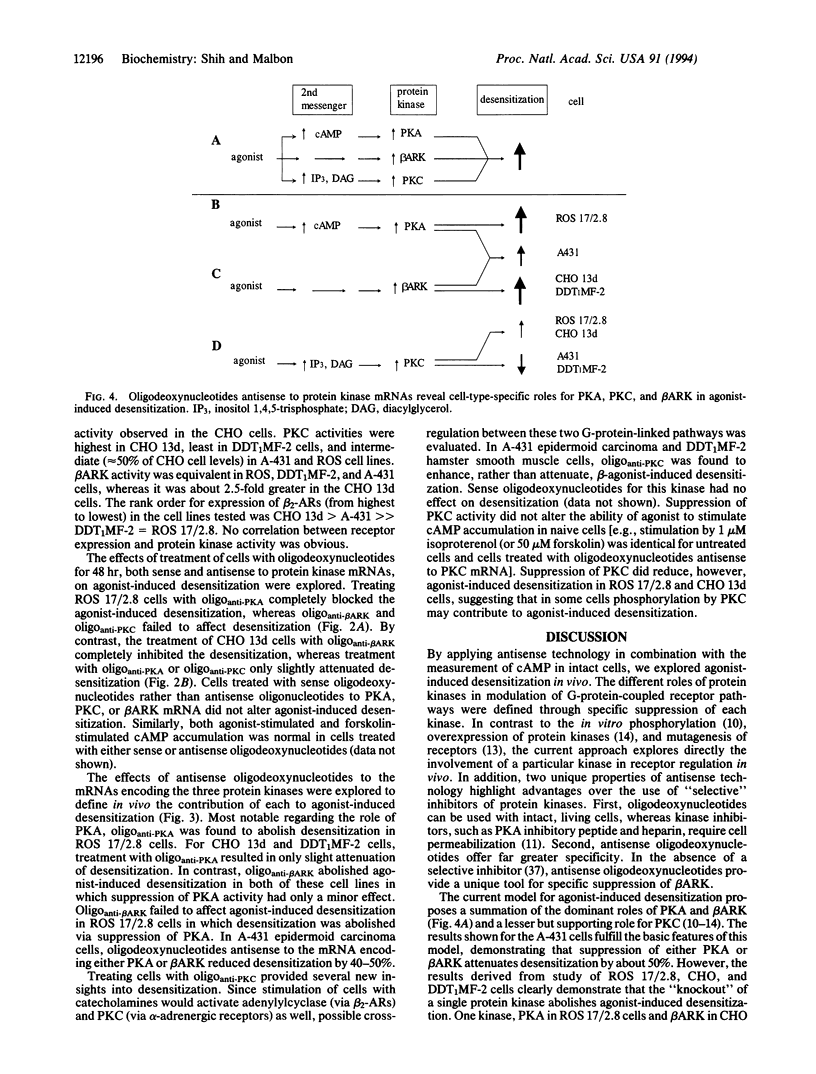
Abstract
The roles of three protein kinases, cyclic AMP-dependent protein kinase (protein kinase A), protein kinase C, and beta-adrenergic receptor kinase (beta ARK), implicated in agonist-induced desensitization of guanine nucleotide-binding protein (G-protein)-coupled receptors were explored in four different cell lines after 48 hr of incubation with oligodeoxynucleotides antisense to the mRNA encoding each kinase. Desensitization of beta 2-adrenergic receptors was analyzed in cell types in which the activities of the endogenous complement of protein kinases A and C and beta ARK were distinctly different. Protein kinase A was necessary for desensitization of rat osteosarcoma cells (ROS 17/2.8), whereas the contribution of beta ARK to desensitization was insignificant. In Chinese hamster ovary cells that stably express beta 2-adrenergic receptors and in smooth muscle cells (DDT1MF-2), oligodeoxynucleotides antisense to beta ARK mRNA nearly abolished desensitization, whereas oligodeoxynucleotides antisense to protein kinase A mRNA attenuated desensitization to a lesser extent. In human epidermoid carcinoma cells (A-431), oligodeoxynucleotides antisense to either protein kinase A mRNA or beta ARK mRNA attenuated agonist-induced desensitization, providing a third scenario in which two kinases constitute the basis for agonist-induced desensitization. In sharp contrast, oligodeoxynucleotides antisense to protein kinase C mRNA were found to enhance rather than attenuate desensitization in DDT1MF-2 and A-431 cell lines, demonstrating counterregulation between prominent protein kinases in desensitization. Using antisense oligodeoxynucleotides to "knock out" target protein kinases in vivo, we reveal distinctive cell-type-specific roles of protein kinase A, protein kinase C, and beta ARK in agonist-induced desensitization.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benovic J. L., DeBlasi A., Stone W. C., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptor kinase: primary structure delineates a multigene family. Science. 1989 Oct 13;246(4927):235–240. doi: 10.1126/science.2552582. [DOI] [PubMed] [Google Scholar]
- Benovic J. L., Onorato J. J., Arriza J. L., Stone W. C., Lohse M., Jenkins N. A., Gilbert D. J., Copeland N. G., Caron M. G., Lefkowitz R. J. Cloning, expression, and chromosomal localization of beta-adrenergic receptor kinase 2. A new member of the receptor kinase family. J Biol Chem. 1991 Aug 15;266(23):14939–14946. [PubMed] [Google Scholar]
- Benovic J. L. Purification and characterization of beta-adrenergic receptor kinase. Methods Enzymol. 1991;200:351–362. doi: 10.1016/0076-6879(91)00152-m. [DOI] [PubMed] [Google Scholar]
- Benovic J. L., Stone W. C., Caron M. G., Lefkowitz R. J. Inhibition of the beta-adrenergic receptor kinase by polyanions. J Biol Chem. 1989 Apr 25;264(12):6707–6710. [PubMed] [Google Scholar]
- Brown B. L., Albano J. D., Ekins R. P., Sgherzi A. M. A simple and sensitive saturation assay method for the measurement of adenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Feb;121(3):561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang T. T., Sallese M., Ambrosini G., Parruti G., De Blasi A. High expression of beta-adrenergic receptor kinase in human peripheral blood leukocytes. Isoproterenol and platelet activating factor can induce kinase translocation. J Biol Chem. 1992 Apr 5;267(10):6886–6892. [PubMed] [Google Scholar]
- Finkenzeller G., Marmé D., Hug H. Sequence of human protein kinase C alpha. Nucleic Acids Res. 1990 Apr 25;18(8):2183–2183. doi: 10.1093/nar/18.8.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S. T., Berrios M., Hadcock J. R., Wang H. Y., Malbon C. C. Receptor density and cAMP accumulation: analysis in CHO cells exhibiting stable expression of a cDNA that encodes the beta 2-adrenergic receptor. Biochem Biophys Res Commun. 1988 Jan 29;150(2):665–672. doi: 10.1016/0006-291x(88)90443-3. [DOI] [PubMed] [Google Scholar]
- Glass D. B., Cheng H. C., Mende-Mueller L., Reed J., Walsh D. A. Primary structural determinants essential for potent inhibition of cAMP-dependent protein kinase by inhibitory peptides corresponding to the active portion of the heat-stable inhibitor protein. J Biol Chem. 1989 May 25;264(15):8802–8810. [PubMed] [Google Scholar]
- Hadcock J. R., Port J. D., Gelman M. S., Malbon C. C. Cross-talk between tyrosine kinase and G-protein-linked receptors. Phosphorylation of beta 2-adrenergic receptors in response to insulin. J Biol Chem. 1992 Dec 25;267(36):26017–26022. [PubMed] [Google Scholar]
- Haga K., Haga T. Activation by G protein beta gamma subunits of agonist- or light-dependent phosphorylation of muscarinic acetylcholine receptors and rhodopsin. J Biol Chem. 1992 Feb 5;267(4):2222–2227. [PubMed] [Google Scholar]
- Haga K., Haga T. Dual regulation by G proteins of agonist-dependent phosphorylation of muscarinic acetylcholine receptors. FEBS Lett. 1990 Jul 30;268(1):43–47. doi: 10.1016/0014-5793(90)80968-o. [DOI] [PubMed] [Google Scholar]
- Hausdorff W. P., Bouvier M., O'Dowd B. F., Irons G. P., Caron M. G., Lefkowitz R. J. Phosphorylation sites on two domains of the beta 2-adrenergic receptor are involved in distinct pathways of receptor desensitization. J Biol Chem. 1989 Jul 25;264(21):12657–12665. [PubMed] [Google Scholar]
- Hausdorff W. P., Campbell P. T., Ostrowski J., Yu S. S., Caron M. G., Lefkowitz R. J. A small region of the beta-adrenergic receptor is selectively involved in its rapid regulation. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):2979–2983. doi: 10.1073/pnas.88.8.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff W. P., Caron M. G., Lefkowitz R. J. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990 Aug;4(11):2881–2889. [PubMed] [Google Scholar]
- House C., Kemp B. E. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987 Dec 18;238(4834):1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- Imai Y., Rodan S. B., Rodan G. A. Effects of retinoic acid on alkaline phosphatase messenger ribonucleic acid, catecholamine receptors, and G proteins in ROS 17/2.8 cells. Endocrinology. 1988 Feb;122(2):456–463. doi: 10.1210/endo-122-2-456. [DOI] [PubMed] [Google Scholar]
- Jaskulski D., deRiel J. K., Mercer W. E., Calabretta B., Baserga R. Inhibition of cellular proliferation by antisense oligodeoxynucleotides to PCNA cyclin. Science. 1988 Jun 10;240(4858):1544–1546. doi: 10.1126/science.2897717. [DOI] [PubMed] [Google Scholar]
- Kleuss C., Scherübl H., Hescheler J., Schultz G., Wittig B. Selectivity in signal transduction determined by gamma subunits of heterotrimeric G proteins. Science. 1993 Feb 5;259(5096):832–834. doi: 10.1126/science.8094261. [DOI] [PubMed] [Google Scholar]
- Kong G., Penn R., Benovic J. L. A beta-adrenergic receptor kinase dominant negative mutant attenuates desensitization of the beta 2-adrenergic receptor. J Biol Chem. 1994 May 6;269(18):13084–13087. [PubMed] [Google Scholar]
- Kunapuli P., Benovic J. L. Cloning and expression of GRK5: a member of the G protein-coupled receptor kinase family. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5588–5592. doi: 10.1073/pnas.90.12.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggett S. B., Bouvier M., Hausdorff W. P., O'Dowd B., Caron M. G., Lefkowitz R. J. Altered patterns of agonist-stimulated cAMP accumulation in cells expressing mutant beta 2-adrenergic receptors lacking phosphorylation sites. Mol Pharmacol. 1989 Oct;36(4):641–646. [PubMed] [Google Scholar]
- Listerud M., Brussaard A. B., Devay P., Colman D. R., Role L. W. Functional contribution of neuronal AChR subunits revealed by antisense oligonucleotides. Science. 1991 Dec 6;254(5037):1518–1521. doi: 10.1126/science.1720573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse M. J., Benovic J. L., Caron M. G., Lefkowitz R. J. Multiple pathways of rapid beta 2-adrenergic receptor desensitization. Delineation with specific inhibitors. J Biol Chem. 1990 Feb 25;265(6):3202–3211. [PubMed] [Google Scholar]
- Lorenz W., Inglese J., Palczewski K., Onorato J. J., Caron M. G., Lefkowitz R. J. The receptor kinase family: primary structure of rhodopsin kinase reveals similarities to the beta-adrenergic receptor kinase. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8715–8719. doi: 10.1073/pnas.88.19.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton A. C., Williams D. S. Rhodopsin is the major in situ substrate of protein kinase C in rod outer segments of photoreceptors. J Biol Chem. 1993 Aug 25;268(24):18181–18186. [PubMed] [Google Scholar]
- Palczewski K., Benovic J. L. G-protein-coupled receptor kinases. Trends Biochem Sci. 1991 Oct;16(10):387–391. doi: 10.1016/0968-0004(91)90157-q. [DOI] [PubMed] [Google Scholar]
- Pippig S., Andexinger S., Daniel K., Puzicha M., Caron M. G., Lefkowitz R. J., Lohse M. J. Overexpression of beta-arrestin and beta-adrenergic receptor kinase augment desensitization of beta 2-adrenergic receptors. J Biol Chem. 1993 Feb 15;268(5):3201–3208. [PubMed] [Google Scholar]
- Pitcher J., Lohse M. J., Codina J., Caron M. G., Lefkowitz R. J. Desensitization of the isolated beta 2-adrenergic receptor by beta-adrenergic receptor kinase, cAMP-dependent protein kinase, and protein kinase C occurs via distinct molecular mechanisms. Biochemistry. 1992 Mar 31;31(12):3193–3197. doi: 10.1021/bi00127a021. [DOI] [PubMed] [Google Scholar]
- Potts J. D., Dagle J. M., Walder J. A., Weeks D. L., Runyan R. B. Epithelial-mesenchymal transformation of embryonic cardiac endothelial cells is inhibited by a modified antisense oligodeoxynucleotide to transforming growth factor beta 3. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1516–1520. doi: 10.1073/pnas.88.4.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont R. T., Koch W. J., Inglese J., Lefkowitz R. J. Identification, purification, and characterization of GRK5, a member of the family of G protein-coupled receptor kinases. J Biol Chem. 1994 Mar 4;269(9):6832–6841. [PubMed] [Google Scholar]
- Richardson R. M., Kim C., Benovic J. L., Hosey M. M. Phosphorylation and desensitization of human m2 muscarinic cholinergic receptors by two isoforms of the beta-adrenergic receptor kinase. J Biol Chem. 1993 Jun 25;268(18):13650–13656. [PubMed] [Google Scholar]
- Samama P., Cotecchia S., Costa T., Lefkowitz R. J. A mutation-induced activated state of the beta 2-adrenergic receptor. Extending the ternary complex model. J Biol Chem. 1993 Mar 5;268(7):4625–4636. [PubMed] [Google Scholar]
- Sho K. M., Okajima F., Abdul Majid M., Kondo Y. Reciprocal modulation of thyrotropin actions by P1-purinergic agonists in FRTL-5 thyroid cells. Inhibition of cAMP pathway and stimulation of phospholipase C-Ca2+ pathway. J Biol Chem. 1991 Jul 5;266(19):12180–12184. [PubMed] [Google Scholar]
- Strasser R. H., Benovic J. L., Caron M. G., Lefkowitz R. J. Beta-agonist- and prostaglandin E1-induced translocation of the beta-adrenergic receptor kinase: evidence that the kinase may act on multiple adenylate cyclase-coupled receptors. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6362–6366. doi: 10.1073/pnas.83.17.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassheim D., Malbon C. C. Phosphorylation of Gi alpha 2 attenuates inhibitory adenylyl cyclase in neuroblastoma/glioma hybrid (NG-108-15) cells. J Biol Chem. 1994 May 13;269(19):14307–14313. [PubMed] [Google Scholar]
- Uhler M. D., Carmichael D. F., Lee D. C., Chrivia J. C., Krebs E. G., McKnight G. S. Isolation of cDNA clones coding for the catalytic subunit of mouse cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1300–1304. doi: 10.1073/pnas.83.5.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhler M. D., Chrivia J. C., McKnight G. S. Evidence for a second isoform of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1986 Nov 25;261(33):15360–15363. [PubMed] [Google Scholar]
- Wang H. Y., Watkins D. C., Malbon C. C. Antisense oligodeoxynucleotides to GS protein alpha-subunit sequence accelerate differentiation of fibroblasts to adipocytes. Nature. 1992 Jul 23;358(6384):334–337. doi: 10.1038/358334a0. [DOI] [PubMed] [Google Scholar]
- Whitehouse S., Feramisco J. R., Casnellie J. E., Krebs E. G., Walsh D. A. Studies on the kinetic mechanism of the catalytic subunit of the cAMP-dependent protein kinase. J Biol Chem. 1983 Mar 25;258(6):3693–3701. [PubMed] [Google Scholar]
- Wickstrom E. L., Bacon T. A., Gonzalez A., Freeman D. L., Lyman G. H., Wickstrom E. Human promyelocytic leukemia HL-60 cell proliferation and c-myc protein expression are inhibited by an antisense pentadecadeoxynucleotide targeted against c-myc mRNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1028–1032. doi: 10.1073/pnas.85.4.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda I., Kishimoto A., Tanaka S., Tominaga M., Sakurai A., Nishizuka Y. A synthetic peptide substrate for selective assay of protein kinase C. Biochem Biophys Res Commun. 1990 Feb 14;166(3):1220–1227. doi: 10.1016/0006-291x(90)90996-z. [DOI] [PubMed] [Google Scholar]


