Abstract
Background
Achieving persistent expression is a prerequisite for genetic therapies for inherited metabolic enzymopathies. Such disorders potentially could be treated with gene therapy shortly after birth to prevent pathology. However, rapid cell turnover leads to hepatic episomal vector loss, which diminishes effectiveness. The current studies assessed whether tolerance to transgene proteins expressed in the neonatal period is durable and if the expression may be augmented with subsequent adeno-associated virus (AAV) administration.
Methods
AAV was administered to mice on day two with re-injection at 14 or at 14 and 42 days with examination of changes in hepatic copies and B and T cell-mediated immune responses.
Results
Immune responses to the transgene protein and AAV were absent after neonatal administration. Re-injection at 14 or at 14 and 42 days resulted in augmented expression with greater hepatic genome copies. Unlike controls, immune responses to transgene proteins were not detected in animals injected as neonates and subsequently. However, while no immune response developed after neonatal administration, anticapsid immune responses developed with further injections suggesting immunological ignorance was the initial mechanism of unresponsiveness.
Conclusions
Persistence of transgene protein allows for tolerance induction permitting readministration of AAV to re-establish protein levels that decline with growth.
Introduction
In individuals with genetic diseases of abnormal protein synthesis, the normal protein may be recognized as a neoantigen leading to a potential immune reaction with the early introduction and expression by gene transfer (1-3). The likelihood of an immune response to an expressed protein is influenced by several factors including the specific host, the underlying mutation in the protein, the type of gene delivery vector, and the route by which the vector is administered (3). In animal models, xenogenic homologous proteins are more immunogenic than are proteins from the same species (2-4). In addition, the tissue in which genes are expressed may affect the likelihood of eliciting immune responses (3, 5).
We have demonstrated that early expression is detected in neonatal mice with different AAV serotypes; some, such as serotype 9 and rh10 have improved vector properties such as higher transduction efficiencies (6-7). Such early administration after birth results in persistent gene expression that can be achieved after a single dose (6-8). The serotype and cell cycle of the tissue of interest (e.g. liver vs. muscle (8)) may determine whether substantial persistent expression remains as cells and tissues grow and divide in this period of rapid cellular proliferation of the neonate; hepatic loss of episomal AAV results in a substantial expression decline in mice during the first several weeks of life (6) and this loss can affect the efficacy of therapy (7, 9). Such findings demonstrate the challenges that rapid cellular proliferation adds to treatment initiated early in life with episomally-located vector genomes.
In adult mammals, re-administration of the same serotype of AAV is generally not successful due to neutralizing antibody responses to the viral capsid proteins (10-13) that develop after the initial administration. However, delivery of gene-expression vectors in a mammal where the immune system is immature may facilitate the development of tolerance to therapeutic proteins (14). In utero and neonatal gene transfer has the potential for preventing the development of disease and may allow for transduction of expanding stem cell populations or organ systems that may not be accessible postnatally (15-16). In previous studies, we have been able to administer AAV expressing factor VIII during the neonatal period (7). This led to operational tolerance to this antigen. However, the decline in transgene-encoded protein expression, particularly during the early rapid growth phase of dividing tissues of neonatal and juvenile mice, remains a substantial problem that affects the long-term high-level protein expression that may be necessary for correcting certain genetic disorders affecting the liver (8-9). Similar growth, albeit at a slower rate, over a longer period of time is present in humans. Newborns typically double their body weight in the first months of life and triple it within the first year (17); the human liver has similar increases in size: first doubling by 3 months, a second doubling by 10 months, and a doubling again by about year 5 (18). The focus of the present studies was to assess the durability of operational tolerance with neonatal delivery of AAV and expression of a xenogenic transgene-encoded protein and if augmentation of hepatic expression and genome copy number was possible with subsequent AAV administration.
Results
Augmenting Expression with Postnatal Doses of AAV
In these experiments, all mice were administered 3×1012 gc/kg of AAV on the second day of life (Figure 1A). The first group of animals (n=5 per time point) received a vector injection as a single dose. Subsequently, a second group of mice (n=5 per time point) received the same serotype vector, rh10, on day 2 and day 14 of life with genome copy number of AAV and expression along with immune responses examined. A final group (n=5 per time point) received AAV serotype rh10 on the second day of life and at two weeks and then received a further augmenting dose at 6 weeks of life; this time with serotype switched AAV9. All animals received AAV with the CBA promoter/CMV enhancer and firefly luciferase. To determine an optimal time for readministration of vector, the kinetics of murine liver growth were examined (Figure 1B). By 6 weeks, the liver had undergone nearly 4 doublings since birth with the mouse itself reaching nearly adult size.
Figure 1.
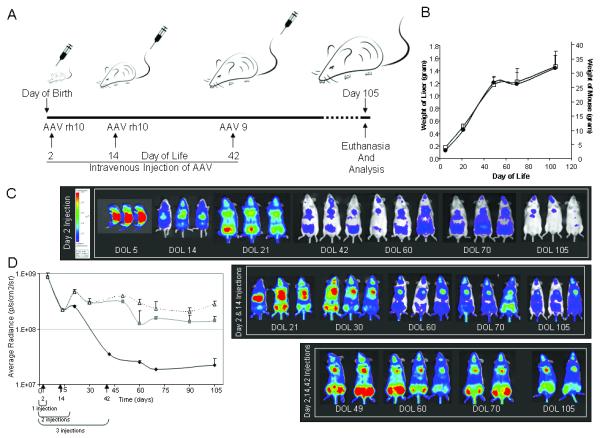
A) Schematic of experiments. B) Increase in weight of mouse and liver with time.
Mice grow most rapidly during the first 5-6 weeks of life after which the rate
of weight gain slows. During this period mouse and liver weight increases 4-fold
(● liver weight; □ animal weight). C) In vivo
imaging of firefly luciferase after intravenous injection of AAV on the 2nd day
of life and additional injections with subsequent photon diffusion patterns.
1st Group: Neonatal mice were injected a single time with rh10
serotype AAV. 2nd Group: Mice were injected twice with rh10 serotype
AAV, first as neonates on the 2nd day of life, followed by an intravenous
injection on day 14. 3rd Group: Mice were injected three times with
AAV with rh10 on the second day of life and day 14 followed by AAV9 on day 42.
All mice were followed for 15 weeks. The same pseudocolor scale was used for
each of the animals, with blue indicating lower levels and red indicating higher
levels of luciferase activity; thus direct comparison can be made. (D)
Quantitation of photon emission as luciferase gene expression ◆ 1
injection,  2 injections, △ 3 injections).
(Data is expressed as mean + SD.)
2 injections, △ 3 injections).
(Data is expressed as mean + SD.)
Administration of AAV to neonatal mice was well tolerated and resulted in early expression of luciferase; peak expression was detected at the first time point, 72 hours after vector administration (Figure 1C, day of life 5). Mice were followed longitudinally using bioluminescent imaging. Mice that received the single dose of AAV as a neonate had a substantial loss of expression during the 15 weeks of study (Figure 1C and D). Expression was widely distributed with use of the CBA promoter/CMV enhancer up to week 3 of life, but then expression markedly declined with residual expression primarily in the heart and lungs at the end of the study (Figure 2). This corresponded with a marked decline of AAV genome copies in the liver by nearly 3 logs to about 0.2% of the copy number 15 weeks after vector administration in the neonatal period (Table 1).
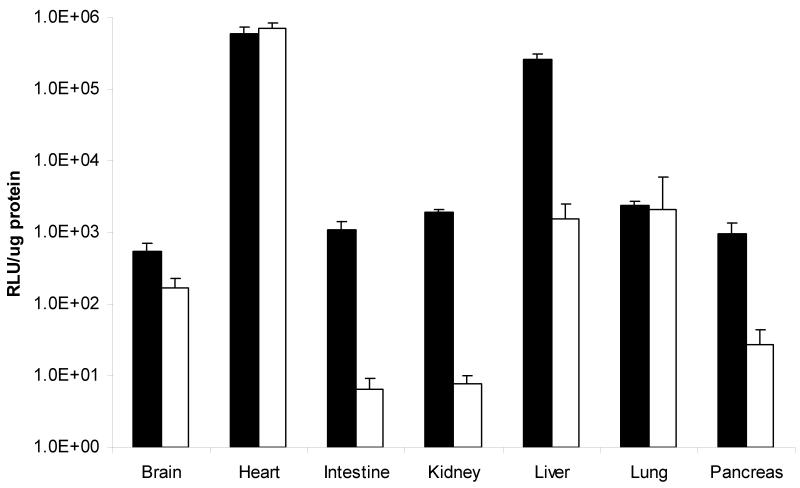
Figure 2.
Levels of AAV-mediated tissue gene expression decline with animal growth. Tissues were removed from animals on day 5 (■) and day 105 (□) and gene expression levels were measured and normalized to protein content. All tissues examined demonstrated declines in expression except for the heart. (Data is expressed as mean + SEM.)
Table 1. Change in Genome Copy Number in Liver With Injection Regimens.
| DOL 5 | DOL 21 | DOL 49 | DOL 70 | DOL 105* | |
|---|---|---|---|---|---|
| Day 2 | 663.8 ± 377.1 | 5.6 ± 0.9 | 2.0 ± 1.4 | 1.9 ± 0.8 | 1.3 ± 0.4 |
| Day 2 & 14 | 256.5 ± 114.7 | 45.9 ± 0.8 | 18.6 ± 2.1 | 10.4 ± 9.2 | |
| Day 2, 14 & 42 | 70.3 ± 32.0 | 24.3 ± 21.6 | 29.3 ± 14.3 |
Numbers are expressed as genome copies per nanogram DNA.
P = 0.003
Because of the vector copy number decline, we decided to initially administer vector at day 2 of life followed by an augmenting dose at 2 weeks of age, suspecting there would still be substantial loss of AAV vector genomes due to continued hepatocellular division. After the augmenting dose of AAV at 2 weeks, expression was maintained at higher levels longer than the group of mice that received only one dose of AAV on day 2 (Figure 1C, day 2 and 14 injections). In the augmented group higher residual expression was found at week 15 in multiple tissues: the heart, liver, and lung (Figure 1 C). With the single administration of AAV on the second day of life, there were 1.3 gc/nanogram DNA at 15 weeks in the liver (Table 1). While there is variability due to some differences in size of animals at the 2 week injection, with this second dose the residual AAV hepatic copies at 15 weeks had increased on average eight times to 10.4 gc/nanogram DNA.
Because the adult size of the liver is nearly attained by 6 weeks of age, we decided to give an additional dose of AAV at that time to determine if expression could be further stabilized. Administration of this third dose of vector resulted in higher levels of expression in multiple tissues (Figure 1C, day 2, 14, and 42 injections) and with higher sustained expression at these levels to at least 15 weeks of life. Hepatic genome copy numbers, again affected by some variability of sizes when injected, were higher at 15 weeks (29.3 gc/nanogram DNA): on average 22.5 times that of the single dose at day 2 and 2.8 times that of mice having received AAV on the second and fourteenth days of life.
The Role of Innate Immunity in Viral Copy Number Decline
While the decline of copies of AAV with hepatocellular division has been previously described by our group (6, 8) and others (19-20), the mechanism is not completely understood. To determine if the innate immune system has any role in this decline, we performed studies in the MyD88 knockout mouse. Toll-like receptor (TLR)-mediated responses are important in the innate immune response to certain viral infections. The MyD88 gene is essential for their control and the maturation and activation of virus-specific CD8+ (21) and the regulation of virus-specific CD4+ (22) T cells. We hypothesized that if innate immunity had a role in the decline of AAV copy number in the liver then we would have higher numbers of AAV in hepatocytes of the MyD88 knockout animals after the administration of AAV on the second day of life.
MyD88 (n=4) and control mice (n=5) received identical doses of AAVrh10 on the second day of life. Both groups were euthanized at 35 days. Livers were removed and viral DNA was quantified using qPCR to determine total viral copy number per genomic DNA. At day 35, both MyD88 and wild type mice demonstrated similar residual genome copy numbers suggesting that innate immunity and activation of virus-specific T cells are likely not involved in the decline in copy number with hepatocellular division (Figure 3). Differences in copy numbers are likely due to strain differences. The MyD88 mice were obtained on the C57Bl/6 background and thus control studies were also performed with C57Bl/6; FVB/N mice were used for all other studies.
Figure 3.
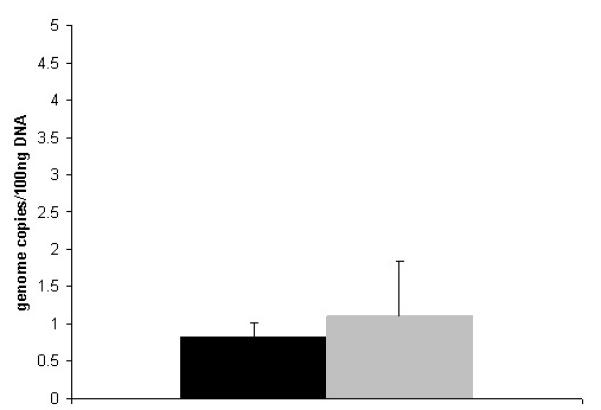
Genome copy number decline is not affected by innate immunity. Vector copy number
was measured in the liver of MyD88 mice by quantitative PCR at day 35 after AAV
administration (3×1012 gc/kg rh10 luciferase) on day 2 of
life. (Data is expressed at mean + SD.) ( designates wild type liver and ■ designates liver from MyD88
knockout.) († p = 0.5)
designates wild type liver and ■ designates liver from MyD88
knockout.) († p = 0.5)
Cellular Immune Responses to Transgene-Encoded Protein
To examine if cellular immunity develops to transgene-encoded proteins when expression begins in the neonatal period, we employed an enzyme-linked immunospot (ELISPOT) assay (Figure 4). The ELISPOT technique is useful to both qualitatively and quantitatively monitor cell-mediated immunity as it is sensitive and accurate in the detection of rare antigen-specific T cells. In these studies, IL-2 (involved in adaptive immunity by augmenting T cell proliferation, survival, and effector differentiation (23)) and IFN-γ (a Th1 cytokine where T lymphocytes are the major source in the adaptive immune response (24)) were examined.
Figure 4.
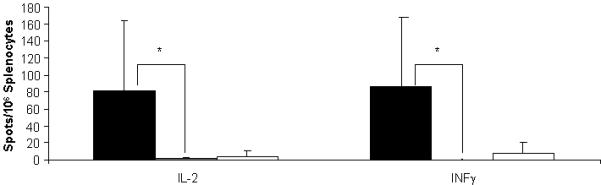
ELISpot data demonstrate an absence of cell-mediated immune responses against
luciferase after neonatal injection. Adult splenocytes were stimulated with
luciferase to examine for a proliferative response after neonatal injection of
AAVrh10-luciferase. Negative controls (□) (DOL 2 saline → DOL 35
saline) included neonatal animals not exposed to luciferase protein in
vivo. Positive controls (■) were animals that received
saline only as a neonate followed by luciferase/adjuvant at 5 weeks.
Experimental animals ( ) received AAV-luciferase as a neonate
followed by luciferase/adjuvant at 5 weeks (Data are presented as mean + SD) (*
p ≤ 0.05)
) received AAV-luciferase as a neonate
followed by luciferase/adjuvant at 5 weeks (Data are presented as mean + SD) (*
p ≤ 0.05)
Three groups of mice were studied: 1) positive controls (n=4) that received saline intravenously on the second day of life followed by luciferase with adjuvant at day 35; 2) negative controls that received saline intravenously on day 2 and on day 35; and 3) an experimental group (n=4) that received AAV expressing luciferase on day 2 followed by luciferase with adjuvant on day 35. Splenocytes were collected after the vaccinating dose and examined by ELISPOT stimulated with recombinant luciferase.
Cells were plated and spots were examined with recombinant luciferase used at 0.5 μg per well and the number of IL-2 and IFN-γ spots elicited from splenocytes were studied. While all groups did respond to ConA, negative control animals (those that received saline neonatally and no recombinant luciferase/adjuvant postnatally) as expected did not substantially demonstrate either IL-2-secreting or IFN-γ-secreting lymphocytes. Also as expected, the positive control animals, which were naïve to luciferase before vaccination, had development of both IL-2- and IFN-γ-secreting splenocytes. The experimental group had results similar to that of the negative controls; that is adult mice that were administered AAV-expressing luciferase in the neonatal period did not have production of IL-2 or IFN-γ from splenocytes after vaccination.
Humoral Immune Responses to AAV Capsid Proteins
We have previously shown that humoral immune responses do not develop to AAV capsid proteins after a neonatal dose of IV administered AAV. The durability of this unresponsiveness has not been clear. In these studies we examined the humoral immune response to AAV with subsequent vector administration in mice that received AAV in the neonatal period.
Using a serotype specific ELISA, plasma of mice were examined at day 14 after day 2 administration of AAVrh10. As previously demonstrated (7) there was no development of antibody to serotype rh10 (Figure 5A). Because of the lack of development of anti-capsid humoral immunity, administration of a second dose of AAVrh10 on day 14 was possible. However, when measured at day 42, high levels of antibody to serotype rh10 developed 4 weeks after this second administration (gray bar, Figure 5A), suggesting that operational tolerance did not develop to AAVrh10 capsid (n=3-5 per group).
Figure 5.
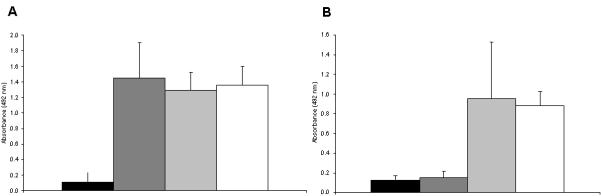
Absence of antibody-mediated responses against AAV after neonatal administration.
A) Antibody titers to AAV rh10 capsid proteins were measured (as adults) by
ELISA after neonatal injection of AAVrh10 at day 2 of life (■), at day 2
and day 14 ( ), and at day 2 and day 14 with rh10 and
with AAV serotype 9 at day 42 (
), and at day 2 and day 14 with rh10 and
with AAV serotype 9 at day 42 ( ). Positive control was demonstrated by administering AAVrh10 on day 14 and
AAV 9 on day 42 (□). B) Antibody titers to AAV 9 capsid proteins were
measured by ELISA after neonatal injection of AAVrh10 at day 2 of life
(■), at day 2 and day 14 (
). Positive control was demonstrated by administering AAVrh10 on day 14 and
AAV 9 on day 42 (□). B) Antibody titers to AAV 9 capsid proteins were
measured by ELISA after neonatal injection of AAVrh10 at day 2 of life
(■), at day 2 and day 14 ( ), and at day 2 and day 14 with rh10 and with AAV serotype 9 at day 42
(
), and at day 2 and day 14 with rh10 and with AAV serotype 9 at day 42
( ). Positive control was demonstrated by
administering AAVrh10 on day 14 and AAV 9 on day 42 (□). Plasma was
collected at least 14 days after AAV administration for both sets of studies.
(Data are presented as mean + SD.)
). Positive control was demonstrated by
administering AAVrh10 on day 14 and AAV 9 on day 42 (□). Plasma was
collected at least 14 days after AAV administration for both sets of studies.
(Data are presented as mean + SD.)
The humoral immunity that developed was found to be capsid specific. After two injections (day 2 and day 14) of AAVrh10, antibody did not develop to AAV9 (Figure 5B, black and dark gray bars). However, after the administration of AAV9 at 6 weeks, antigen specific humoral immunity to AAV9 also developed (Figure 5B, light gray bar) (n=3-5 per group).
In control naïve juvenile or adult animals when AAVrh10 was administered at 2 weeks and AAV9 was administered at 6 weeks, capsid-specific antibodies developed to both AAVrh10 and AAV9 (Figure 5A and B white bars) (n=5). However, in the experimental mice, antigen-specific operational tolerance to AAVrh10 administered in the neonatal period did not result. While a second dose at day 14 did allow for repeat serotype rh10 transduction, serotype switching for further transgene augmentation was necessary at 6 weeks due to the interval development of capsid-specific humoral immunity to serotype rh10 (n=5 per group).
Humoral Immune Response to Transgene-Encoded Protein
We have previously demonstrated that a humoral immune response to transgene-encoded proteins could be avoided with a single neonatally administered dose of AAV expressing a transgene in mice (7). The durability of this lack of immune response has not been previously evaluated to immunogenic and xenogenic proteins such as luciferase. Neonatal mice on day two of life were administered AAVrh10 expressing luciferase (n=5 per group). Subsequent injections were given at two weeks and at 6 weeks. In both cases, antibodies did not develop to the luciferase protein (Figure 6, first 3 bars). Conversely, when naïve animals were administered AAV expressing luciferase at two weeks and six weeks of life, a strong antigen-specific humoral immune response did develop (Figure 6, 4th bar).
Figure 6.
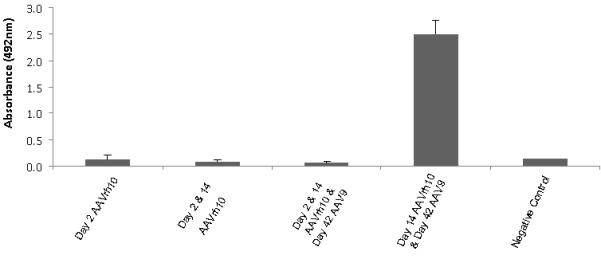
Absence of antibody-mediated responses against luciferase after neonatal and subsequent injections for augmentation of expression and hepatic copy number. Antibody titers to luciferase were measured by ELISA after neonatal injection of AAVrh10-luciferase at day 2, after injections at day 2 and day 14, and with a subsequent dose at 42 days. Control studies included animals where AAV expressing luciferase was administered at 14 days and 42 days. (Data are presented as mean + SD.)
To further test the durability of this operational tolerance, vaccination was performed with luciferase and adjuvant. Experimental mice were administered AAV rh10 expressing luciferase on the second day of life while control animals received saline. Plasma was collected on day 34 from both groups followed by vaccination the next day. Plasma was then collected on day 70 from both groups and analyzed by a luciferase-specific ELISA. While control animals, as expected, demonstrated high titers of undilutable antibody to at least 1:2560 (Figure 7, dotted bars), the experimental animals had a substantially blunted humoral immune response that was reduced with dilution (Figure 7, dotted black bars).
Figure 7.
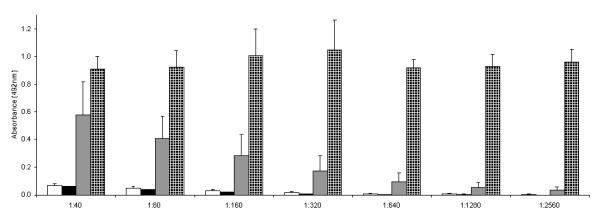
Blunted humoral immune response to luciferase in neonatally injected animals
after stimulating adult animals with purified luciferase and adjuvant.
35-day-old mice were administered luciferase/adjuvant after having received
AAVrh10-luciferase (■) or saline (□) as neonates. Plasma was
examined for anti-luciferase antibodies at day 34 (before adult injection) and
at day 70 (after luciferase and adjuvant injection IP) in mice that received AAV
as neonates ( ) and those that received saline as
(
) and those that received saline as
( ) neonates The x-axis indicates plasma
dilution; the y-axis demonstrates the optical density of samples analyzed by
spectrophotometry. (Data are presented as mean + SD.)
) neonates The x-axis indicates plasma
dilution; the y-axis demonstrates the optical density of samples analyzed by
spectrophotometry. (Data are presented as mean + SD.)
Discussion
The present studies demonstrate that a) neonatal delivery of AAV produces long-term transgene-encoded protein expression without the development of cellular or humoral immunity to either virus or gene product (suggesting that operational tolerance to the stably expressed foreign protein, but not to transiently present capsid, can be achieved); b) delivery of a second postnatal dose of AAV results in a humoral immune response to AAV capsid proteins, which suggests that immunologic ignorance was the mechanism responsible for the unresponsiveness seen after neonatal administration; c) the innate immune system does not appear to influence the decline in AAV copy number in hepatocytes after neonatal administration; and d) transgene expression and AAV hepatic copy number can be augmented postnatally by re-administration of the same or a different serotype AAV vector since operational tolerance was established to the transgene-encoded protein.
There are two major mechanisms reported to prevent the reactivity of CD8+ T cells: ignorance and tolerance. When ignorance is operative, naïve autoreactive CD8+ T cells ignore antigens and recirculate without causing damage. In the case of tolerance, CD8+ T cells are deleted if the mechanism is centrally mediated or controlled by T regulatory cells if the mechanism is peripheral. Which factors contribute to each particular outcome is only partly known. When antigen is expressed and/or cross-presented at concentrations too low to stimulate T cells, peripheral T cells can remain ‘ignorant’ of the antigen (25). Thus ignorant T cells, unlike tolerant T cells, are not rendered dysfunctional from future antigen encounters but remain antigen inexperienced: they persist as naïve but potentially functional and can be activated by external stimuli.
A number of studies have suggested that the relative immunological immaturity of the fetus (and likely the neonatal mouse) may contribute to diminished immune responses or induction of immune tolerance (14). Before thymic processing of lymphocytes in early immunologic development, induction of tolerance in the fetus to foreign proteins can occur. In the neonatal mouse the mechanisms may be different. Neonatal mice have a decreased frequency of professional antigen presenting cells (APC) including activated macrophages, B cells, and dendritic cells and murine neonates contain fewer T cells in their spleens (26-27); their T cells are functionally deficient in both in vivo and in vitro standard activation conditions. In addition, TH1 and cytotoxic T lymphocyte functions are poor with TH2 responses predominating(26). Other neonatal mouse studies suggest the expression of CD40 ligand is reduced in T cells (28) and the diversity of T cell receptors is restricted (29). Additional evidence suggests that dendritic cell function may be immature resulting in a bias towards TH2 rather than TH1 responses (3, (30). These differences and the nature of the APC itself may determine whether the outcome of antigen presentation is neonatal tolerance or immunization (28).
In the studies conducted here, operational tolerance does not develop to AAV capsid protein. As the AAV vectors administered are replication incompetent, there are no coding genes for the capsid proteins. Thus there is a transient presence of capsid that is lost after cellular transduction. However with subsequent administration of AAV, anticapsid humoral immunity develops as it would in a naive immune system thus demonstrating the functionality of these cells. This lack of an immune response with initial AAV exposure appears to be consistent with immunological ignorance as functional immune cells were activated with later antigen exposure.
A number of studies have demonstrated that continual antigen exposure is required to maintain tolerance (31-33). In our present neonatal and prior in utero studies, luciferase expression was detected within 3 days of AAV administration and persisted for the lifetime of the animals (7, 34) without anti-AAV or anti-luciferase humoral immunity. Because of the lack of an immune response to the transgene-encoded protein and viral vector, AAV in the present studies could be readministered and transgene-encoded protein expression, in this case luciferase, could be augmented as well as the genome copy number per hepatocyte. The establishment of operational tolerance allowed for the achievement of greater AAV copy number in juvenile and adult mice. Unlike neonates, where the rate of hepatocellular proliferation is much higher and affects episomal vector genomes (20), rapid cellular proliferation in adults is uncommon as individual hepatocytes in the adult mouse liver are replaced once every 180 to 400 days (35-36) suggesting that once an adult sized liver is attained, the hepatic copy number would be relatively stable with a slow decline over years. Thus, subsequent administration is most effective when adult liver size is reached but augmenting doses during the juvenile period are also of benefit. These doses could address the loss of hepatocyte copy numbers in infants treated with AAV for inherited metabolic disorders of the liver as they grow into childhood, adolescence and later adulthood.
In utero injection of recombinant adenoviral vectors to murine fetuses also have not been found to elicit immune responses to adenovirus or luciferase in animals examined postnatally (37); however, gene expression was transient and no longer detected 4 weeks after birth. Thus while subsequent readministration of adenovirus in adult animals resulted in brisk humoral immune responses to adenoviral capsid proteins as expected, the animals also developed a strong humoral immune response to luciferase. It appears that this loss of transgene-encoded protein expression after neonatal administration resulted in the transgene-encoded protein subsequently being detected as a neoantigen by the immune system when later expression occurred with adult adenoviral vector delivery.
The ability to re-administer AAV in mice injected with vector in the first few days of life appears to be due to ignorance, which allows for augmentation of expression postnatally. This could be important in certain disorders (e.g. hemophilia, Pompe disease) if therapeutic levels of protein expression were not maintained after neonatal administration. Alternatively it could allow for the exogenous administration of recombinant protein (e.g. factor VIII or factor IX) without the development of inhibitory antibodies as operational tolerance has been achieved. The efficacy and safety of AAV delivered neonatally in mice provides an opportunity to develop strategies for the induction of tolerance to therapeutic proteins in humans. Furthermore in disorders where treatment with AAV was initiated in the neonatal period, repeated AAV dosing provides a method to augment the number of AAV vector genomes in hepatocytes that would otherwise be subject to permanent genome copy loss with hepatocellular cytokinesis.
Materials and Methods
Preparation of Recombinant Adeno-Associated Viral Vectors
AAVrh10- and AAV9-luciferase are serotype rh10 and 9 vectors containing the firefly luciferase reporter gene and the chicken β-actin promoter/CMV enhancer promoter that have been previously described in our laboratory (8). AAV was prepared by triple transfection of 293 cells as described previously (6). Viral titer was determined by quantitative real time PCR.
Animal Procedures
Procedures were approved by the University of California, Los Angeles Committee on Animal Research. FVB/N female and male mice were purchased from Charles River Breeding Laboratories (Wilmington, MA). MyD88 knockout mice and C57BL/6 controls were from Jackson Laboratories (Bar Harbor, ME). FVB/N mice were used for all studies otherwise. At birth, an intravenous injection of 3×1012 AAV genome copies (gc)/kg in 50 μl of normal saline was performed as previously described (6). Adult mice received alum/luciferase by intraperitoneal (IP) injection to the right lower abdomen. AAV was delivered in adults as 3×1012 gc/kg in 200 μl of normal saline by tail vein injection.
Recombinant luciferase (Promega, Madison, WI) was mixed with a pre-formulated aqueous solution of aluminum hydroxide and magnesium hydroxide (Imject Alum, Pierce, Rockford, IL) in a 1:1 ratio according to the manufacturer’s instructions. A volume of 200 μl (including 1 μg of recombinant luciferase) was administered IP to each mouse.
In vivo Bioluminescent Imaging (BLI) and Tissue Luminometry
Mice were anesthetized injected intraperitoneally with an aqueous solution of luciferin substrate and imaged as previously described (6). Tissue luminometry and normalization to protein concentrations were performed as previously described (38).
ELISpot for IFN-γ and IL-2
Mice were euthanized, spleens harvested aseptically, finely minced in 10 ml RPMI, and filtered through a 40 μm cell strainer to remove debris. The cell suspension was transferred to a 15 ml tube and centrifuged at 200 × g for 5 minutes. Cells were resuspended at 5×106 viable cells/ml in RPMI with 10% FBS and penicillin and streptomycin. Concavalin A (ConA), a non-specific mitogen, was used as a positive control for the proliferative ability of splenocytes in the assay. Samples were set up in triplicate in a 96-well plate with 1 μg recombinant luciferase (Promega) or ConA (1 μg), 100 μl of growth medium, and 100 μl of spleen cell suspension. Antibody pairs were used and performed per manufacturer instructions of analysis of interleuken-2 (IL-2) and interferon-γ (IFN-γ) (MabTech Inc., Cincinnati, OH). Spots were analyzed by using a ImmunoSpot/BioSpot UV Analyzer (CTL Analyzers, Shaker Heights, OH). Change as compared to unstimulated negative control cells was plotted.
ELISA Assays
1) ELISA for anti-luciferase antibodies was performed as previously described (37). Positive control sera were obtained from serum samples of adult mice that had been injected with AAV-luciferase and had previously anti-luciferase antibody levels (4). Animals injected neonatally with AAV-luciferase (n=5) were tested at each time point and the 1:20 dilution is presented in figure 5.
2) ELISA for anti-AAV9 and rh10 antibodies. Ninety-six well plates were coated overnight at 4°C with 1×109 gc of AAVrh10 or AAV9 vector preparations per well in PBS. An ELISA was then performed as outlined (37). Positive control sera were obtained from AAV-luciferase-injected adult mice with established anti-AAV antibody titers. AAV-injected (n=5) and control animals (n=5) were tested at each time point and the 1:5 dilution is presented in figure 4.
Genome Copy Number Determination
At regular intervals, mice were euthanized and liver tissue was removed. Genomic DNA was extracted using a DNAEasy Kit (Qiagen) and quantitated by nanodrop (Implen, Westlake Village, CA) and real-time quantitative PCR performed as described (6).
Statistical Calculations
Mean, standard deviation, standard error of the mean, and Student’s T-test were calculated using standard formulae. T test was used for paired comparisons while the comparison of three groups was performed using analysis of variance (ANOVA). P values of <0.05 were considered significant.
Acknowledgements
Mouse drawing was purchased from Fotosearch (johny007pan www.fotosearch.com Stock Photography) as was the syringe (soleilc www.fotosearch.com stock photography).
This work was supported by grants to G.S.L. from the National Institutes of Health (5K08HD057555-05, 1R01NS071076-05A1, and 1R01NS071076-04S1). D.S.T. received funding from the Society of University Surgeons and was a recipient of a NIGMS Medical Genetics NIH T32 (GM008243). The Immuno/BioSpot Core is supported by UCLA CFAR grant 5P30 AI028697 and the UCLA AIDS Institute. It is also supported by a grant from the James B. Pendleton Charitable Trust and a donation from the UCLA Children’s Discovery and Innovation Institute.
Footnotes
I confirm that the data in the manuscript is original and the manuscript is not under consideration elsewhere. In addition, none of the manuscript contents has been previously published. All authors have approved the manuscript, its content, and its submission to the Pediatric Research and none have declared a conflict of interest regarding this work.
References
- 1.Saint-Remy JM. Immunology of factor VIII inhibitors. Semin Thromb Hemost. 2002;28:265–268. doi: 10.1055/s-2002-32660. [DOI] [PubMed] [Google Scholar]
- 2.Fields PA, Arruda VR, Armstrong E, Chu K, Mingozzi F, Hagstrom JN, Herzog RW, High KA. Risk and prevention of anti-factor IX formation in AAV-mediated gene transfer in the context of a large deletion of F9. Mol Ther. 2001;4:201–210. doi: 10.1006/mthe.2001.0441. [DOI] [PubMed] [Google Scholar]
- 3.Herzog RW, Mount JD, Arruda VR, High KA, Lothrop CD., Jr. Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol Ther. 2001;4:192–200. doi: 10.1006/mthe.2001.0442. [DOI] [PubMed] [Google Scholar]
- 4.Fields PA, Kowalczyk DW, Arruda VR, Armstrong E, McCleland ML, Hagstrom JN, Pasi KJ, Ertl HC, Herzog RW, High KA. Role of vector in activation of T cell subsets in immune responses against the secreted transgene product factor IX. Mol Ther. 2000;1:225–235. doi: 10.1006/mthe.2000.0032. [DOI] [PubMed] [Google Scholar]
- 5.Nathwani AC, Davidoff A, Hanawa H, Zhou JF, Vanin EF, Nienhuis AW. Factors influencing in vivo transduction by recombinant adeno-associated viral vectors expressing the human factor IX cDNA. Blood. 2001;97:1258–1265. doi: 10.1182/blood.v97.5.1258. [DOI] [PubMed] [Google Scholar]
- 6.Hu C, Busuttil RW, Lipshutz GS. RH10 provides superior transgene expression in mice when compared with natural AAV serotypes for neonatal gene therapy. J Gene Med. 2010;12:766–778. doi: 10.1002/jgm.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu C, Lipshutz GS. AAV-based neonatal gene therapy for hemophilia A: long-term correction and avoidance of immune responses in mice. Gene Ther. 2012;19:1166–1176. doi: 10.1038/gt.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu C, Kasten J, Park H, Bhargava R, Tai DS, Grody WW, Nguyen QG, Hauschka SD, Cederbaum SD, Lipshutz GS. Myocyte-mediated arginase expression controls hyperargininemia but not hyperammonemia in arginase-deficient mice. Mol Ther. 2014;22:1792–1802. doi: 10.1038/mt.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kok CY, Cunningham SC, Carpenter KH, Dane AP, Siew SM, Logan GJ, Kuchel PW, Alexander IE. Adeno-associated virus-mediated rescue of neonatal lethality in argininosuccinate synthetase-deficient mice. Mol Ther. 2013;21:1823–1831. doi: 10.1038/mt.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chirmule N, Xiao W, Truneh A, Schnell MA, Hughes JV, Zoltick P, Wilson JM. Humoral immunity to adeno-associated virus type 2 vectors following administration to murine and nonhuman primate muscle. J Virol. 2000;74:2420–2425. doi: 10.1128/jvi.74.5.2420-2425.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moskalenko M, Chen L, van Roey M, Donahue BA, Snyder RO, McArthur JG, Patel SD. Epitope mapping of human anti-adeno-associated virus type 2 neutralizing antibodies: implications for gene therapy and virus structure. J Virol. 2000;74:1761–1766. doi: 10.1128/jvi.74.4.1761-1766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halbert CL, Standaert TA, Wilson CB, Miller AD. Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J Virol. 1998;72:9795–9805. doi: 10.1128/jvi.72.12.9795-9805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manning WC, Zhou S, Bland MP, Escobedo JA, Dwarki V. Transient immunosuppression allows transgene expression following readministration of adeno-associated viral vectors. Hum Gene Ther. 1998;9:477–485. doi: 10.1089/hum.1998.9.4-477. [DOI] [PubMed] [Google Scholar]
- 14.Iwamoto HS. The window of opportunity for fetal gene therapy. Mol Hum Reprod. 1996;2:472–474. doi: 10.1093/molehr/2.7.472. [DOI] [PubMed] [Google Scholar]
- 15.Lipshutz GS, Sarkar R, Flebbe-Rehwaldt L, Kazazian H, Gaensler KM. Short-term correction of factor VIII deficiency in a murine model of hemophilia A after delivery of adenovirus murine factor VIII in utero. Proc Natl Acad Sci U S A. 1999;96:13324–13329. doi: 10.1073/pnas.96.23.13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell M, Jerebtsova M, Batshaw ML, Newman K, Ye X. Long-term gene transfer to mouse fetuses with recombinant adenovirus and adeno-associated virus (AAV) vectors. Gene Ther. 2000;7:1986–1992. doi: 10.1038/sj.gt.3301332. [DOI] [PubMed] [Google Scholar]
- 17.Nelson WE. Growth and Development in the Infant and Child. In: Nelson WE, editor. Textbook of Pediatrics. W.B Saunders Company; Philadelphia: 1964. p. 21. [Google Scholar]
- 18.Coppoletta JM, Wolbach SB. Body Length and Organ Weights of Infants and Children: A Study of the Body Length and Normal Weights of the More Important Vital Organs of the Body between Birth and Twelve Years of Age. Am J Pathol. 1933;9:55–70. [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Bell P, Lin J, Calcedo R, Tarantal AF, Wilson JM. AAV8-mediated hepatic gene transfer in infant rhesus monkeys (Macaca mulatta) Mol Ther. 2011;19:2012–2020. doi: 10.1038/mt.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunningham SC, Dane AP, Spinoulas A, Logan GJ, Alexander IE. Gene delivery to the juvenile mouse liver using AAV2/8 vectors. Mol Ther. 2008;16:1081–1088. doi: 10.1038/mt.2008.72. [DOI] [PubMed] [Google Scholar]
- 21.Zhou S, Kurt-Jones EA, Mandell L, Cerny A, Chan M, Golenbock DT, Finberg RW. MyD88 is critical for the development of innate and adaptive immunity during acute lymphocytic choriomeningitis virus infection. Eur J Immunol. 2005;35:822–830. doi: 10.1002/eji.200425730. [DOI] [PubMed] [Google Scholar]
- 22.Zhou S, Kurt-Jones EA, Cerny AM, Chan M, Bronson RT, Finberg RW. MyD88 intrinsically regulates CD4 T-cell responses. J Virol. 2009;83:1625–1634. doi: 10.1128/JVI.01770-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240:1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 24.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 25.Ohashi PS, Oehen S, Buerki K, Pircher H, Ohashi CT, Odermatt B, Malissen B, Zinkernagel RM, Hengartner H. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991;65:305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- 26.Adkins B. T-cell function in newborn mice and humans. Immunol Today. 1999;20:330–335. doi: 10.1016/s0167-5699(99)01473-5. [DOI] [PubMed] [Google Scholar]
- 27.Piguet PF, Irle C, Kollatte E, Vassalli P. Post-thymic T lymphocyte maturation during ontogenesis. J Exp Med. 1981;154:581–593. doi: 10.1084/jem.154.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 29.Bogue M, Candeias S, Benoist C, Mathis D. A special repertoire of alpha:beta T cells in neonatal mice. Embo J. 1991;10:3647–3654. doi: 10.1002/j.1460-2075.1991.tb04931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forsthuber T, Yip HC, Lehmann PV. Induction of TH1 and TH2 immunity in neonatal mice. Science. 1996;271:1728–1730. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 31.Garza KM, Agersborg SS, Baker E, Tung KS. Persistence of physiological self antigen is required for the regulation of self tolerance. J Immunol. 2000;164:3982–3989. doi: 10.4049/jimmunol.164.8.3982. [DOI] [PubMed] [Google Scholar]
- 32.Rocha B, Tanchot C, Von Boehmer H. Clonal anergy blocks in vivo growth of mature T cells and can be reversed in the absence of antigen. J Exp Med. 1993;177:1517–1521. doi: 10.1084/jem.177.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Triplett EL. On the mechanism of immunological self recognition. J. Immunol. 1962;89:505. [PubMed] [Google Scholar]
- 34.Lipshutz GS, Gruber CA, Cao Y, Hardy J, Contag CH, Gaensler KM. In utero delivery of adeno-associated viral vectors: intraperitoneal gene transfer produces long-term expression. Mol Ther. 2001;3:284–292. doi: 10.1006/mthe.2001.0267. [DOI] [PubMed] [Google Scholar]
- 35.Fausto N, Campbell JS. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev. 2003;120:117–130. doi: 10.1016/s0925-4773(02)00338-6. [DOI] [PubMed] [Google Scholar]
- 36.Magami Y, Azuma T, Inokuchi H, Kokuno S, Moriyasu F, Kawai K, Hattori T. Cell proliferation and renewal of normal hepatocytes and bile duct cells in adult mouse liver. Liver. 2002;22:419–425. doi: 10.1034/j.1600-0676.2002.01702.x. [DOI] [PubMed] [Google Scholar]
- 37.Lipshutz GS, Flebbe-Rehwaldt L, Gaensler KM. Reexpression following readministration of an adenoviral vector in adult mice after initial in utero adenoviral administration. Mol Ther. 2000;2:374–380. doi: 10.1006/mthe.2000.0136. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen AT, Dow AC, Kupiec-Weglinski J, Busuttil RW, Lipshutz GS. Evaluation of gene promoters for liver expression by hydrodynamic gene transfer. J Surg Res. 2008;148:60–66. doi: 10.1016/j.jss.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu C, Cela RG, Suzuki M, Lee B, Lipshutz GS. Neonatal helper-dependent adenoviral vector gene therapy mediates correction of hemophilia A and tolerance to human factor VIII. Proc Natl Acad Sci U S A. 2011;108:2082–2087. doi: 10.1073/pnas.1015571108. [DOI] [PMC free article] [PubMed] [Google Scholar]