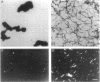
Abstract
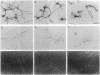
beta-Amyloid (beta A) is normally produced as a nontoxic soluble peptide. In Alzheimer disease, beta A aggregates and accumulates in the brain as inert diffuse plaques or compact plaques associated with neurodegenerative changes. To determine the relationship of neurotoxicity to the physical state of beta A, we created (i) nonamyloidogenic amorphous aggregates of beta A [amorphous beta A (Am-beta A)] analogous to diffuse plaques and (ii) amyloidogenic fibrils of beta A [fibrillar beta A (Fib-beta A)] analogous to compact plaques. In primary rat hippocampal culture, Fib-beta A was neurotoxic, whereas Am-beta A was not toxic. Fib-beta A caused significant loss of synapses in viable neurons, while Am-beta A had no effect on synapse number. The amyloid fibril-binding dye Congo red inhibited Fib-beta A neurotoxicity by inhibiting fibril formation or by binding to preformed fibrils. Congo red also inhibited the pancreatic islet cell toxicity of diabetes-associated amylin, another type of amyloid fibril. These results indicate that beta A neurotoxicity requires fibril formation. These findings and our previous demonstration that amylin fibrils are toxic suggest that a common cytopathic effect of amyloid fibrils may contribute to the pathogenesis of Alzheimer disease and other amyloidoses.
Full text
PDF

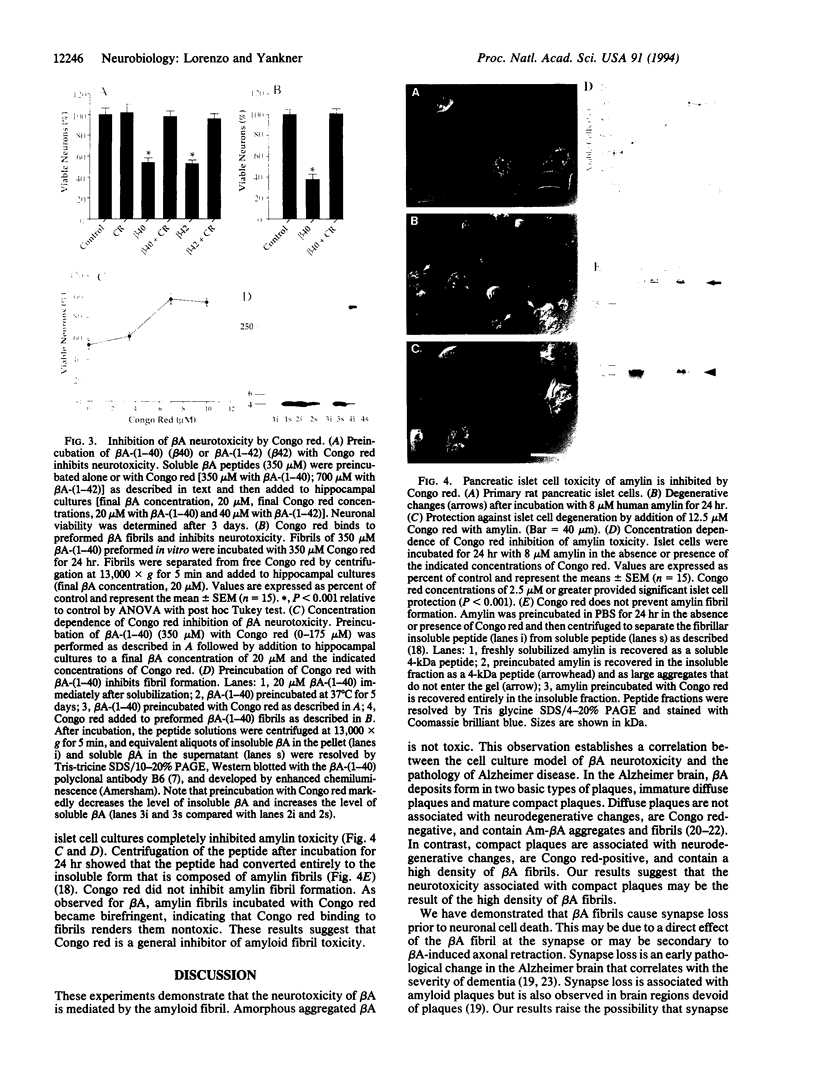

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Busciglio J., Gabuzda D. H., Matsudaira P., Yankner B. A. Generation of beta-amyloid in the secretory pathway in neuronal and nonneuronal cells. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2092–2096. doi: 10.1073/pnas.90.5.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busciglio J., Lorenzo A., Yankner B. A. Methodological variables in the assessment of beta amyloid neurotoxicity. Neurobiol Aging. 1992 Sep-Oct;13(5):609–612. doi: 10.1016/0197-4580(92)90065-6. [DOI] [PubMed] [Google Scholar]
- Caughey B., Brown K., Raymond G. J., Katzenstein G. E., Thresher W. Binding of the protease-sensitive form of PrP (prion protein) to sulfated glycosaminoglycan and congo red [corrected]. J Virol. 1994 Apr;68(4):2135–2141. doi: 10.1128/jvi.68.4.2135-2141.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey B., Ernst D., Race R. E. Congo red inhibition of scrapie agent replication. J Virol. 1993 Oct;67(10):6270–6272. doi: 10.1128/jvi.67.10.6270-6272.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey B., Race R. E. Potent inhibition of scrapie-associated PrP accumulation by congo red. J Neurochem. 1992 Aug;59(2):768–771. doi: 10.1111/j.1471-4159.1992.tb09437.x. [DOI] [PubMed] [Google Scholar]
- Davies C. A., Mann D. M. Is the "preamyloid" of diffuse plaques in Alzheimer's disease really nonfibrillar? Am J Pathol. 1993 Dec;143(6):1594–1605. [PMC free article] [PubMed] [Google Scholar]
- Forloni G., Angeretti N., Chiesa R., Monzani E., Salmona M., Bugiani O., Tagliavini F. Neurotoxicity of a prion protein fragment. Nature. 1993 Apr 8;362(6420):543–546. doi: 10.1038/362543a0. [DOI] [PubMed] [Google Scholar]
- Frautschy S. A., Baird A., Cole G. M. Effects of injected Alzheimer beta-amyloid cores in rat brain. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8362–8366. doi: 10.1073/pnas.88.19.8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Goldfarb L. G., Brown P., Haltia M., Ghiso J., Frangione B., Gajdusek D. C. Synthetic peptides corresponding to different mutated regions of the amyloid gene in familial Creutzfeldt-Jakob disease show enhanced in vitro formation of morphologically different amyloid fibrils. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4451–4454. doi: 10.1073/pnas.90.10.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992 Sep 24;359(6393):322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kowall N. W., Beal M. F., Busciglio J., Duffy L. K., Yankner B. A. An in vivo model for the neurodegenerative effects of beta amyloid and protection by substance P. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7247–7251. doi: 10.1073/pnas.88.16.7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo A., Razzaboni B., Weir G. C., Yankner B. A. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994 Apr 21;368(6473):756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- Masliah E., Mallory M., Hansen L., DeTeresa R., Alford M., Terry R. Synaptic and neuritic alterations during the progression of Alzheimer's disease. Neurosci Lett. 1994 Jun 6;174(1):67–72. doi: 10.1016/0304-3940(94)90121-x. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Multhaup G., Simms G., Pottgiesser J., Martins R. N., Beyreuther K. Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer's disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J. 1985 Nov;4(11):2757–2763. doi: 10.1002/j.1460-2075.1985.tb04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M. P., Tomaselli K. J., Rydel R. E. Calcium-destabilizing and neurodegenerative effects of aggregated beta-amyloid peptide are attenuated by basic FGF. Brain Res. 1993 Sep 3;621(1):35–49. doi: 10.1016/0006-8993(93)90295-x. [DOI] [PubMed] [Google Scholar]
- May P. C., Boggs L. N., Fuson K. S. Neurotoxicity of human amylin in rat primary hippocampal cultures: similarity to Alzheimer's disease amyloid-beta neurotoxicity. J Neurochem. 1993 Dec;61(6):2330–2333. doi: 10.1111/j.1471-4159.1993.tb07480.x. [DOI] [PubMed] [Google Scholar]
- Pike C. J., Burdick D., Walencewicz A. J., Glabe C. G., Cotman C. W. Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci. 1993 Apr;13(4):1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike C. J., Walencewicz A. J., Glabe C. G., Cotman C. W. In vitro aging of beta-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res. 1991 Nov 1;563(1-2):311–314. doi: 10.1016/0006-8993(91)91553-d. [DOI] [PubMed] [Google Scholar]
- Roher A. E., Ball M. J., Bhave S. V., Wakade A. R. Beta-amyloid from Alzheimer disease brains inhibits sprouting and survival of sympathetic neurons. Biochem Biophys Res Commun. 1991 Jan 31;174(2):572–579. doi: 10.1016/0006-291x(91)91455-l. [DOI] [PubMed] [Google Scholar]
- Roher A. E., Lowenson J. D., Clarke S., Wolkow C., Wang R., Cotter R. J., Reardon I. M., Zürcher-Neely H. A., Heinrikson R. L., Ball M. J. Structural alterations in the peptide backbone of beta-amyloid core protein may account for its deposition and stability in Alzheimer's disease. J Biol Chem. 1993 Feb 15;268(5):3072–3083. [PubMed] [Google Scholar]
- Seubert P., Vigo-Pelfrey C., Esch F., Lee M., Dovey H., Davis D., Sinha S., Schlossmacher M., Whaley J., Swindlehurst C. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992 Sep 24;359(6393):325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Shoji M., Golde T. E., Ghiso J., Cheung T. T., Estus S., Shaffer L. M., Cai X. D., McKay D. M., Tintner R., Frangione B. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992 Oct 2;258(5079):126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- Simmons L. K., May P. C., Tomaselli K. J., Rydel R. E., Fuson K. S., Brigham E. F., Wright S., Lieberburg I., Becker G. W., Brems D. N. Secondary structure of amyloid beta peptide correlates with neurotoxic activity in vitro. Mol Pharmacol. 1994 Mar;45(3):373–379. [PubMed] [Google Scholar]
- Terry R. D., Masliah E., Salmon D. P., Butters N., DeTeresa R., Hill R., Hansen L. A., Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991 Oct;30(4):572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Verga L., Frangione B., Tagliavini F., Giaccone G., Migheli A., Bugiani O. Alzheimer patients and Down patients: cerebral preamyloid deposits differ ultrastructurally and histochemically from the amyloid of senile plaques. Neurosci Lett. 1989 Nov 6;105(3):294–299. doi: 10.1016/0304-3940(89)90636-8. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Nakazato Y., Shoji M., Takatama M., Hirai S. Ultrastructure of diffuse plaques in senile dementia of the Alzheimer type: comparison with primitive plaques. Acta Neuropathol. 1991;82(1):13–20. doi: 10.1007/BF00310918. [DOI] [PubMed] [Google Scholar]
- Yankner B. A. Commentary and perspective on studies of beta amyloid neurotoxicity. Neurobiol Aging. 1992 Sep-Oct;13(5):615–616. doi: 10.1016/0197-4580(92)90067-8. [DOI] [PubMed] [Google Scholar]
- Yankner B. A., Duffy L. K., Kirschner D. A. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990 Oct 12;250(4978):279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- Yankner B. A., Mesulam M. M. Seminars in medicine of the Beth Israel Hospital, Boston. beta-Amyloid and the pathogenesis of Alzheimer's disease. N Engl J Med. 1991 Dec 26;325(26):1849–1857. doi: 10.1056/NEJM199112263252605. [DOI] [PubMed] [Google Scholar]