Abstract
Progesterone (P4) and its metabolites, rapidly facilitate lordosis of rats partly through actions in the ventral tegmental area (VTA). The study of membrane progestin receptors (mPRs), of the Progestin and AdipoQ Receptor (PAQR) superfamily, has been limited to expression and regulation, instead of function. We hypothesized that if mPRs are required for progestin-facilitated lordosis in the VTA, then mPRs will be expressed in this region and knockdown will attenuate lordosis. First, expression of mPR was examined by reverse-transcriptase polymerase chain reaction (RT-PCR) in brain and peripheral tissues of proestrous Long–Evans rats. Expression of mPRα (paqr7) was observed in peripheral tissues and brain areas, including hypothalamus and midbrain. Expression of mPRβ (paqr8) was observed in brain tissues and was abundant in the midbrain and hypothalamus. Second, ovariectomized rats were estrogen (E2; 0.09 mg/kg, SC), and P4 (4 mg/kg, SC) or vehicle-primed, and infused with antisense oligodeoxynucleotides (AS-ODNs) targeted against mPRα and/or mPRβ intracerebroventricularly or to the VTA. Rats were assessed for motor (open field), anxiety (elevated plus maze), social (social interaction), and sexual (lordosis) behavior. P4-facilitated lordosis was significantly reduced with administration of AS-ODNs for mPRα, mPRβ, or co-administration of mPRα and mPRβ to the lateral ventricle, compared to vehicle. P4-facilitated lordosis was reduced, compared to vehicle, by administration of mPRβ AS-ODNs, or co-administration of mPRα and mPRβ AS-ODNs, but not mPRα AS-ODNs alone, to the VTA. No differences were observed for motor, anxiety, or social behaviors. Thus, mPRs in the VTA are targets of progestin-facilitated lordosis of rats.
Keywords: Lordosis, Progesterone, Neurosteroids, Progestin and AdipoQ Receptor (PAQR), Ventromedial hypothalamus, Ventral tegmental area
Introduction
Steroid signaling is typically considered to be mediated through nuclear steroid receptors, which regulate gene transcription and translation. This classical “genomic” mechanism of steroid action involves binding of steroid receptors directly to DNA, altering transcription and synthesis of proteins. Genomic signaling mechanisms for steroid-mediated behaviors, such as mating, have been well-characterized, and can even be considered dogma in biological fields, resulting from decades of studies. Genomic steroid action is a relatively slow process that can take hours to days to elicit a biological response. However, unlike this classical steroid mechanism, many actions of steroids can occur much more rapidly and in the presence of inhibitors of transcription and/or translation. These rapid, “non-genomic,” or non-classical, steroid actions have been demonstrated for all major classes of steroids, but the identity of these targets has been hotly contested (Lösel et al., 2003; Norman et al., 2004; Pietras and Szego, 1975; Thomas, 2008; Zhu et al., 2008). The onset and duration of reproductive behavior induced by estradiol (E2) and progesterone (P4) at least in part are mediated by this rapid non-genomic progestin signaling in female rodents (Caldwell, 2002; Delville, 1991; Frye, 2009). A research interest has been on the receptor targets for these effects.
In E2-primed rodents, P4 has classical and non-classical actions in the ventromedial hypothalamus (VMH) and midbrain ventral tegmental area (VTA) to mediate mating. Briefly, in the VMH, P4's actions via nuclear progestin receptors (nPRs) and induction of gene transcription reflect the classical actions of ovarian steroids' modulation of reproductive responses (reviewed in Blaustein, 2003). P4's actions in the VTA, an area of the brain with few E2 induced nPRs, influence the intensity and duration of sexual receptivity of rodents exclusively through non-classical, rapid actions at neuronal membranes (reviewed in Frye, 2001a,b, 2009). In support, free P4 and P4 bound to large macromolecules, such as P4:BSA, P4:HRP conjugates, that are impermeable have similar effects to rapidly enhance lordosis when applied to the VTA (Frye and DeBold, 1993; Frye and Gardiner, 1996; Frye et al., 1992), suggesting that P4 does not have to diffuse through the cell membrane to have its actions in the VTA. When administered directly to the VTA, P4 increases cell firing in the VTA within 60 s, and facilitates lordosis within 5 min (Frye and Bayon, 1999). These effects are considered to occur in a shorter timeframe than is necessary for binding to nPRs and altering gene transcription (Pfaff and McEwen, 1983). Prior investigations have supported the notion that P4's actions in the VTA for lordosis were through membrane receptors, such as GABAA, dopamine type 1-like receptors or glutamatergic receptors, and their downstream signal transduction processes, rather than nPRs (see Frye and Walf, 2008 for a review). Of interest, and investigated in the current project, was whether some of the non-genomic actions of P4 in the VTA may occur through the membrane progestin receptors (mPRs), of the Progestin and AdipoQ Receptor (PAQR) super-family.
In the present study, the hypothesis that mPRα (PAQR7) and mPRβ (PAQR8), two of the most common variants of mPRs, are targets of P4 for mating and reproduction-related behavior was tested. First, whether there was expression of mPRα and mPRβ in peripheral tissues (spleen, heart, lungs, kidney, liver, intestines) and different brain regions (prefrontal cortex, hippocampus, amygdala, hypothalamus, and midbrain) of sexually-receptive rats was examined. Second, whether sexual receptivity (as defined by lordosis), and other behaviors (exploration, anxiety, social interaction), will be affected when expression of mPRs is altered following intracerebroventricular (ICV) or VTA infusions of mPRα and/or mPRβ antisense deoxynucleotides (AS-ODN) was examined. We predicted that if mPRs in the VTA are required for progestin-facilitated lordosis, then mPRs will be expressed in this region and knockdown will attenuate lordosis. Furthermore, if these effects are progestin-dependent, we expect to see a different pattern of results among rats that were E2-primed alone, compared to those that were E2- and P4-primed.
Materials and methods
These methods were approved by the Institutional Animal Care and Use Committee at The University at Albany-SUNY and were conducted in accordance with ethical guidelines defined by the National Institutes of Health (NIH Publication No. 85-23).
Animal housing
Adult (55–60 days old), Long–Evans female rats (N = 167) were bred in the Life Sciences Laboratory Animal Care Facility at The University at Albany-SUNY (original stock obtained from Taconic, Germantown, NY, USA). Rats were housed in polycarbonate cages with woodchip bedding (45 × 24 × 21 cm) in a temperature-controlled room (21 ± 1 °C) and were maintained on a 12:12 h reversed light cycle (lights off at 08:00 h). Rats had continuous access to Purina Rat Chow and tap water in their home cages.
Experiment 1—mPR expression
Determination of estrous cycle stage
Adult, female rats had estrous cycle stage determined by assessment of vaginal cytology. The presence of nucleated and cornified cells was used to identify rats in proestrus, and the presence of heterogeneous cell types in smears taken was used to identify rats in diestrus. Rats had tissues collected when they were sexually-receptive in proestrus, which is an estrus stage associated with high E2 and P4 levels.
Tissue collection and RT-PCR
Whole brains and peripheral tissues were collected and expression of mPRα and mPRβ was determined with reverse transcriptase polymerase chain reaction (RT-PCR). The expression of mPRα and mPRβ was determined by RT-PCR for whole brain, spleen, heart, lungs, kidney, liver, and intestines, as well as in grossly-dissected brain regions (prefrontal cortex, hippocampus, amygdala, hypothalamus, and midbrain). These tissues were frozen immediately following their collection and dissection. Total RNA was extracted from snap-frozen tissue samples with TRIzol reagent (Invitrogen), homogenized using a sonicator (Sonic Dismembrator Model 100; Fisher Scientific), and purified following the manufacturer's instructions. Then, total RNA (1 μg) of each sample was reverse transcribed into cDNA in a 10-μl reaction using Superscript III (Invitrogen). As a negative control, samples were prepared using the same procedure except without Superscript III (RT minus). The PCR was conducted on the cDNA template for 25 or 30 cycles with an annealing temperature of 55 °C using mPRα- or mPRβ-specific primer pairs (mPRα forward: 5′-CTGCCCCTCTTCATCATTGT-3′; mPRα reverse: 5′-GAAAACCACCTGGCACTTGT-3′; mPRβ forward: 5′-TTGTTTGCAGAGACCCTGTG-3′, mPRβ reverse: 5′-GAGGCTGCAGGTGAGGTAAG-3′). The PCR products were run on a 2% agarose gel and imaged using a Fluor Chem 8900 imaging station (Alpha Innotech, Santa Clara, CA).
Experiments 2 and 3—mPR knockdown and behavioral testing
Surgical protocol
Rats were administered xylazine (12 mg/kg) and ketamine (80 mg/kg) anesthesia for placement of bilateral guide cannulae aimed at the lateral ventricle (from bregma: AP −1.0, ML ± 1.0, DV –2.0, Exp 2) or VTA (from bregma: AP −5.3, ML ± 0.4, DV −7.0, Exp 3). Guide cannulae consisted of 23-gauge stainless steel needles with 30-gauge removable inserts. Immediately after stereotaxic surgery, rats were ovariectomized (ovx). Following surgery, rats were neurologically evaluated daily for their ability to right themselves, cage-climb, have proper muscle tone and reflexive responses to hind limb extension. Rats were also evaluated for weight gain after surgery. Only rats that passed neurological evaluations and gained weight following surgery were continued in the experiment. Rats were administered post-operative analgesic for 5 days following surgery.
Hormone-priming
In Experiment 2, using ICV administration of AS-ODNs, rats were E2-primed with subcutaneous (SC) injections (0.09 mg/kg; in vegetable oil vehicle) 44–48 h prior to behavioral testing and then SC administered P4 (4 mg/kg; in vegetable oil vehicle) 4–6 h before behavioral testing. Hormones were purchased from Steraloids (Newport, RI). In Experiment 3 using intra-VTA administration of AS-ODNs, rats were SC primed with E2 and P4, as described above, or SC primed with E2 only.
Infusion condition
Rats were infused 44 h, 24 h, and immediately before behavioral testing. Infusions were saline control, mPRα AS-ODN, mPRβ AS-ODN, or mPRαβ AS-ODN to the lateral ventricle (Exp 2) or the VTA (Exp 3). Rats were tested once after receiving the three infusions over 44 h. The timing of these infusions was based upon the hormone-priming protocol that was utilized to mimic proestrous-increases in E2 and P4 and to counter the known instability of AS-ODNs so that these targets were knocked down during behavioral testing and hormone-priming. Full phosphorothioate AS-ODNs were synthesized, such that S-oligonucleotides were capped and remaining links were unmodified, purified by HPLC, and desalted by Invitrogen Life Technologies (Carlsbad, CA). The sequence for the mPRα AS-ODN was: 5′-CGCTCTTCTGGAAGCCGTACATCTATG-3′. The sequence for the mPRβ AS-ODN was: 5-GACTGGAAAGTAAGTAGGTGGCTGGCTGGTCCTC-3′.
Behavioral tasks
Rats were tested sequentially in the following tasks (open field, elevated plus maze, social interaction, and paced mating), in the order listed. For behavioral testing, assessments of each subject were made by an experienced experimenter with the Any-Maze video-tracking system (Stoelting, Wood Dale, IL). Rats were tested in smaller cohorts, with different experienced experimenters collecting the data; the concordance rate between experimenters and the video-tracking system was high (95%), with no evidence to suggest that there were large differences between experimenters' assessments. Experimenters were not fully knowledgeable about the conditions of rats, or hypothesized outcome of these studies.
For the open field testing, rats were placed in the open field, observed for 5 min, while the number spent in central and peripheral squares (summed for total) was recorded (Frye et al., 2000; McCarthy et al., 1995). The open field (76 × 57 × 35 cm) has a 48-square grid floor (6 × 8 squares, 9.5 cm/side), and the central squares (all but the 24 perimeter squares) are illuminated from overhead.
For the elevated plus maze, rats were placed at the junction of the open and closed arms, and the number of entries, and amount of time spent on the open and closed arms, were recorded for 5 min (Frye et al., 2000, 2008; Walf and Frye, 2007). The elevated plus maze consists of 2 arms (49 cm long, 10 cm wide), enclosed by walls 30 cm high, and 2 exposed arms, elevated 50 cm off the ground.
For the social interaction task, experimental and conspecific rats are placed in opposite corners of the open field (described above). An ovariectomized rat was utilized as the conspecific in this task. Time spent by the experimental rat engaging in social interaction (crawling over and under partner, sniffing, following with contact, anogenital investigation, tumbling, boxing and grooming) with the conspecific was recorded for 5 min (Frye et al., 2000, 2008).
In the paced mating task, the female's pacing of male's contacts for an ejaculatory series was evaluated in a chamber with a partition, which divides the chamber equally (Frye and Erskine, 1990; Frye and Orecki, 2002a,b). Males are relegated to one side and the frequency of lordosis, proceptivity, and aggression as well as a qualitative measure of lordosis (lordosis ratings (LRs) on a 3 point scale according to Hardy and DeBold, 1971) are calculated (Frye and Erskine, 1990; Frye et al., 1998). Lordosis quotients (LQs) were determined by calculating the total number of lordosis responses / total number of mounts by the male. Proceptivity quotients (PQs) were determined by calculating the incidence of proceptive behaviors (hopping, darting, and ear wiggling) / total number of mounts by the male. Aggression quotients (AQs) were determined by calculating the incidence of aggression / rejection behaviors / total number of mounts by the male.
Tissue collection and measurement of plasma E2 and P4 levels
Immediately after testing, rats were rapidly decapitated. Trunk blood and whole brain were collected to measure hormone levels and examine the expression of mPRα and mPRβ, respectively. Plasma levels of E2 and P4 were determined with radioimmunoassay using previously described methods (Frye et al., 2009) to validate that physiological levels were achieved with the systemic dosing of E2 and P4 that were utilized. We found that there were proestrous-like levels of E2 in plasma of ovx rats administered E2 (52.5 ± 4.9 pg/ml) or E2 and P4 (51.0 ± 4.5 pg/ml). As expected, levels of P4 were higher in plasma of rats administered systemic P4 (24.0 ± 2.8 ng/ml) and reached proestrous-like levels, compared to rats that were administered E2 alone (11.9 ± 2.0 ng/ml).
Infusion placements and site-specificity
Frozen brains were sliced at the level of the midbrain and cut in 100 micron slices. These slices were inspected visually using a dissecting microscope as previously described (Frye et al., 2009). All rats with ICV infusions were found to have infusions to the ventricle. Eleven of the rats with intended infusions to the VTA had infusions to other sites. Data from rats that had infusions to sites other than the VTA did not show the same pattern of behavior as did rats with infusions to the VTA (see Table 1): their data were excluded from the overall analyses. To determine efficacy of AS-ODNs, mPRα and mPRβ expression was examined in micropunches from the VTA which were taken from the slices and in some cases included additional tissues from the surrounding midbrain. Tissues were placed in RNAlater (Qiagen) until homogenizations and extractions to prevent degradation and were used for determining mPRα and mPRβ expression with quantitative PCR (qPCR). Expression resulting from ICV and VTA AS-ODN infusions was determined in 6–9 and 4–6 rats per condition, respectively.
Table 1.
Missed sites/infusions outside the VTA: Behavioral measures in the open field, elevated plus maze, and social interaction task, and effects on reproductive behaviors (lordosis ratings, proceptivity and aggression quotients) of rats that were infused with antisense oligonucleotides (AS-ODNs) outside of the ventral tegmental area (missed sites).
| Brain infusion |
Infusions to sites other than the VTA |
|||
|---|---|---|---|---|
| Condition |
Veh |
mPRα AS-ODN |
mPRβ AS-ODN |
mPRαβ, AS-ODN |
| n | 0 | 2 | 5 | 4 |
| Total entries (#) | - | 284 ± 68 | 234 ± 20 | 287 ± 19 |
| Central entries (#) | - | 72 ± 21 | 55 ± 20 | 80 ± 13 |
| Open arm time (s) | - | 23 ± 6 | 5±3 | 22 ± 5 |
| Social interaction (s) | - | 51 ± 8 | 73 ± 18 | 57 ± 9 |
| Lordosis quotient | - | 0±0 | 85 ± 10 | 86 ± 11 |
| Lordosis rating | - | 0±0 | 1.8 ± 0.2 | 2.0 ± 0.3 |
| Proceptivity quotient | - | 0±0 | 79 ± 8 | 78±14 |
| Aggression quotient | - | 35 ± 35 | 28 ± 19 | 33 ± 4 |
qPCR
Standard qPCR methods were utilized (Zhu et al., 2003a). For extractions, total RNA was isolated from tissue using the Qiagen RNeasy Micro Kit (Valencia, CA) according to the manufacturer's protocol. Reverse transcription was carried out using Oligo(dT)20 and the Superscript III First-Strand Synthesis System for RT-PCR from Invitrogen (Carlsbad, CA). qPCR was performed using Bio-Rad SYBR Green Supermix (Hercules, CA) and the following gene-specific primers (Integrated DNA Technologies, Coralville, IA): β-actin forward (5′-GCTCGTCGTCGACAACGGCT-3′), β-actin reverse (5′-CAAACATGATCTGGGTCATCTTCTC-3′), mPRα forward (5′-TGTGGCCGTGTACCAGTTT-3′) and reverse (5′-TGATACAGAAGGGCGGGAT-3′) and mPRβ forward (5′-GAGTGGACTGCACCTCTGTC-3′) and reverse (5′-CTCCTCGGGGTTCAGTAGGA-3′). Reactions were run on an Applied Biosystems 7900HT and analyzed using the comparative cycle time (DeltaDeltaCT) method (Applied Biosystems, Foster City, CA). The fold change in comparison to vehicle controls of the delta CT values of mPR versus actin is depicted for the differences in the number of cycles for expression as observed from subjects in each condition (Livak and Schmittgen, 2001; Schmittgen and Livak, 2008).
Statistical analyses
One-way analyses of variances (ANOVAs) were used to examine effects of infusion condition (control, mPRα, β, αβ AS-ODN groups) on behavior and mPR RNA expression. When the α level for statistical significance was reached p ≤ 0.05, or a trend was observed p < 0.10, post hoc tests were used to examine group differences.
Results
Experiment 1—brain and periphery expression patterns of mPRα and mPRβ
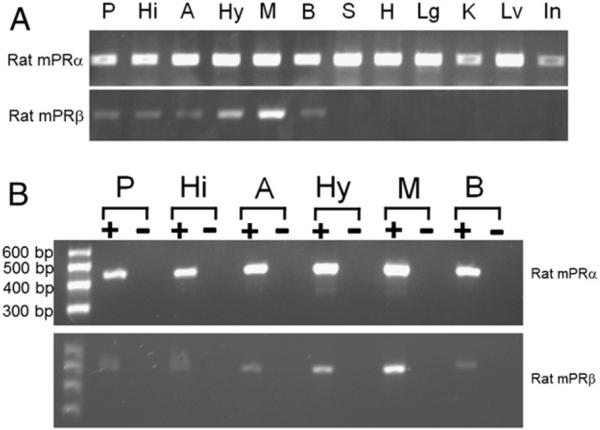
Analyses of samples from proestrous rats demonstrated that mPRβ was expressed in the brain, but was undetectable in the peripheral tissues (spleen, heart, lung, kidney, liver, intestines) of rats (Fig. 1). The expression of mPRα was observed in all tissues of proestrous rats at 25 PCR cycles, and the expression of mPRβ was observed at 30 cycles. Further characterization of expression of mPRs in the central nervous system (prefrontal cortex, hippocampus, amygdala, hypothalamus, midbrain) was completed using RT-PCR (Fig. 1). The expression patterns of both receptors were strong in the midbrain region.
Fig. 1.
Expression of membrane progestin receptor α (mPRα, Paqr7) and mPRβ (Paqr8) transcripts in proestrous rat peripheral tissues and brain regions, as analyzed by RT-PCR. P: prefrontal cortex; Hi; hippocampus; A: amygdale; Hy: hypothalamus; M: midbrain; B: brain; S: spleen; H: heart; Lg: lungs; K: kidney; Lv: liver; and In: intestines. Samples were prepared for all tissues samples using same procedure with (+, indicated in Panel B) or without Superscript III (−, indicated in Panel B). Specific PCR products amplified from the transcripts with expected size were observed. There was no evidence of genomic DNA contamination.
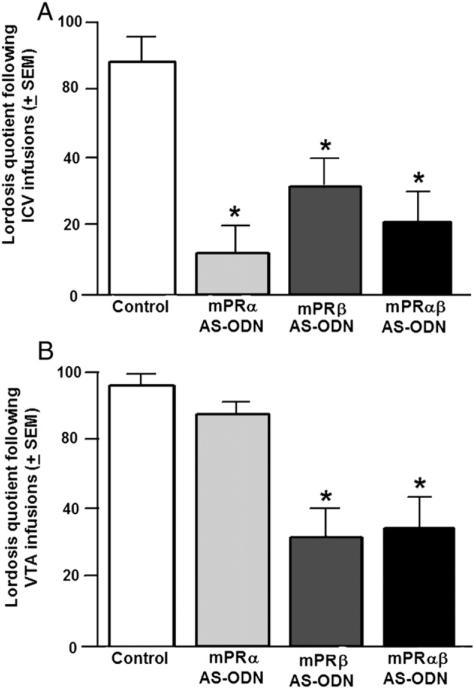
Experiment 2: ICV infusions of mPRβ, mPRα or mPRαβ AS-ODNs attenuate sexual receptivity
Although AS-ODN infusions did not significantly alter behavior in the open field, social interaction or elevated plus maze (see Table 2, top), there were significant effects of ICV AS-ODN infusions on reproductive behaviors. As Fig. 2 (top) illustrates, infusions of mPRα, mPRβ, and mPRαβ AS-ODNs significantly reduced incidence of lordosis (F(3, 32) = 11.85, p < 0.01). Other parameters of reproductive behaviors (see Table 2, middle), such as intensity of lordosis (F(3, 32) = 3.09, p < 0.01) and solicitation behaviors (F(3, 32) = 6.81 p < 0.01) were reduced by AS-ODN infusions, and incidence of aggression was increased by AS-ODN infusion (F(3, 32) = 5.69, p < 0.01), compared to control infusions. Rats infused ICV with mPRα, mPRβ, or both AS-ODNs had lower mPRα expression in the midbrain compared to those in the control condition (F(3, 26) = 4.56, p < 0.01) (see Table 2, bottom). ICV infusions of mPRβ AS-ODNs also reduced mPRβ expression. In contrast, ICV infusions of mPRα ODN increased the expression of mPRβ in the midbrain (Table 2, bottom).
Table 2.
Infusions to the lateral ventricle: Behavioral measures in the open field, elevated plus maze, and social interaction task, and effects on reproductive behaviors (lordosis ratings, proceptivity and aggression quotients) and fold changes in midbrain mPR expression of ovx, E2- and P4-primed rats infused with mPRα, mPRβ and mPRαβ antisense oligonucleotides (AS-ODN) or vehicle to the lateral ventricle. Average fold changes of mPR transcripts were obtained using 2–ΔΔCT (Schmittgen and Livak, 2008). Minus (–) before the number indicates average fold decrease compared to the control.
| Brain infusion |
Brain infusion-ICV infusion condition |
|||
|---|---|---|---|---|
| Condition |
Veh |
mPRα AS-ODN |
mPRβ AS-ODN |
mPRαβ AS-ODN |
| n | 8 | 9 | 9 | 10 |
| Total entries (#) | 148 ± 29 | 200 ± 20 | 183 ± 18 | 167 ± 20 |
| Central entries (#) | 33 ± 7 | 47 ± 6 | 41 ± 8 | 41 ± 8 |
| Open arm time (s) | 34 ± 11 | 48 ± 14 | 31 ± 12 | 17 ± 6 |
| Social interaction (s) | 75 ± 8 | 41 ± 10 | 64 ± 11 | 77 ± 15 |
| Lordosis rating | 1.8 ± 0.3 | 0.5 ± 0.3* | 0.7 ± 0.3* | 0.6 ± 0.2* |
| Proceptivity quotient | 68.5 ± 15.6 | 7.5 ± 5.3* | 18.4 ± 10.6* | 15.8 ± 8.0* |
| Aggression quotient | 15.3 ± 10.9 | 32.0 ± 14.2 | 79.8 ± 10.9* | 66.6 ± 12.3* |
| MPRα fold change | - | –1.5 ± 0.3* | –1.5 ± 0.2* | –1.4 ± 0.1* |
| MPRβ fold change | - | 2.1 ± 1.9 | –1.3 ± 1.0 | 1.1 ± 1.6 |
Indicates a statistically significant effect compared to control group (p < 0.05).
Fig. 2.
Mean (±SEM) lordosis quotients (LQs) of E2- and P4-primed rats infused with vehicle (control), mPRα antisense oligonucleotides (AS-ODNs), mPRβ AS-ODNs, or both mPRα/β AS-ODNs intracerebroventricularly (top panel A) or to the VTA (bottom panel B). *Indicates significant difference from control group (p < 0.05).
Experiment 3: VTA infusions of mPRβ or mPRαβ, but not mPRα, AS-ODNs attenuate sexual receptivity
Infusion to the VTA of AS-ODN did not significantly alter behavior in the open field, social interaction or elevated plus maze (see Table 3, top). There was a significant effect of VTA infusions on LQs (F(3, 54) = 20.05, p < 0.01). Rats infused with mPRβ or mPRαβ had significantly lower LQs compared to the control infusion condition (see Fig. 2, bottom). Infusions of mPRβ or mPRαβ to the VTA significantly reduced LRs (F(3, 54) = 14.25, p < 0.01), PQs (F(3, 54) = 14.62, p < 0.01), and increased AQs (F(3, 54) = 4.49, p < 0.01) compared to control (see Table 3, middle). Rats infused with mPRα, mPRβ, and mPRαβ AS-ODNs had significantly reduced mPRα expression in the midbrain (F(3, 24) = 13.22, p < 0.01) compared to vehicle. Rats infused with mPRβ or mPRαβ AS-ODNs compared to control had significantly lower mPRβ (F(3, 15) = 4.59, p < 0.01) expression in the midbrain, while mPRα AS-ODNs increased mPRβ expression (see Table 3, bottom). Moreover, the effects on sexual behavior were not observed among rats that were primed only with E2 when mPR AS-ODNs significantly downregulated the expression of mPRs (Table 4).
Table 3.
Infusions to the VTA, E2- and P4-priming: Behavioral measures in the open field, elevated plus maze, and social interaction task, and effects on reproductive behaviors (lordosis ratings, proceptivity and aggression quotients) and fold changes in mibrain mPR expression of ovx, E2- and P4-primed rats infused with mPRα, mPRβ and mPRαβ anti-sense oligonucleotide (ODN) or vehicle to the VTA. Average fold changes of mPR transcripts were obtained using 2–ΔΔCT (Schmittgen and Livak, 2008). Minus (–) before the number indicates average fold decrease compared to the control.
| Brain infusion |
Effects of AS-ODN infusions to the VTA |
|||
|---|---|---|---|---|
| Condition |
Veh |
mPRα AS-ODN |
mPRβ AS-ODN |
mPRαβ AS-ODN |
| n | 16 | 14 | 13 | 13 |
| Total entries (#) | 245 ± 17 | 264 ± 17 | 229 ± 20 | 254 ± 24 |
| Central entries (#) | 60 ± 8 | 64 ± 9 | 47 ± 7 | 56 ± 8 |
| Open arm time (s) | 12 ± 3 | 10 ± 3 | 10 ± 3 | 23 ± 9 |
| Social interaction (s) | 71 ± 6 | 70 ± 7 | 84 ± 7 | 80 ± 6 |
| Lordosis rating | 2.3 ± 0.1 | 1.7 ± 0.2 | 0.7 ± 1.0* | 0.7 ± 0.2* |
| Proceptivity quotient | 96 ± 3 | 68 ± 10* | 25 ± 11* | 30 ± 11* |
| Aggression quotient | 3±2 | 9 ± 4* | 30 ± 11* | 35 ± 11* |
| MPRα fold change | - | –1.0 ± 0.2* | –1.0 ± 0.1* | –1.4 ± 0.1* |
| MPRβ fold change | - | 2.6 ± 1.8 | –2.9 ± 1.6* | –1.3 ± 0.2* |
Indicates a statistically significant effect compared to control group (p < 0.05).
Table 4.
Infusions to the VTA, E2-only priming. Behavioral measures in the open field, elevated plus maze, and social interaction task, and effects on reproductive behaviors (lordosis ratings, proceptivity and aggression quotients) and fold changes in mibrain mPR expression of ovx, E2-primed rats infused with AS-ODN to the VTA. Average fold changes of mPR transcripts were obtained using 2–ΔΔCT (Schmittgen and Livak, 2008). Minus (–) before the number indicates average fold decrease compared to the control.
| Brain infusion |
Effects of AS-ODN infusions to the VTA |
|||
|---|---|---|---|---|
| Condition |
Veh |
mPRα AS-ODN |
mPRβ AS-ODN |
mPRαβ AS-ODN |
| n | 16 | 13 | 13 | 10 |
| Total entries (#) | 255 ± 20 | 262 ± 16 | 280 ± 19 | 265 ± 30 |
| Central entries (#) | 58 ± 6 | 65 ± 8 | 70 ± 11 | 48 ± 10 |
| Open arm time (s) | 8±3 | 19 ± 5 | 17 ± 5 | 10±5 |
| Social interaction (s) | 88 ± 9 | 89 ± 12 | 90 ± 11 | 98 ± 9 |
| Lordosis quotient | 17 ± 5 | 10 ± 7 | 15 ± 5 | 15±7 |
| Lordosis rating | 0.3 ± 0.1 | 0.2 ± 0.2 | 0.5 ± 0.2 | 0.3 ± 0.1 |
| Proceptivity quotient | 3±2 | 7±7 | 1±1 | 1±1 |
| Aggression quotient | 38 ± 11 | 33 ± 12 | 58 ± 11 | 41 ± 11 |
| MPRα fold change | - | –1.4 ± 0.2* | –1.0 ± 0.2* | –1.8 ± 0.3* |
| MPRβ fold change | - | –2.2 ± 0.5 | –5.9 ± 2.0 | –2.1 ± 0.4 |
Indicates a statistically significant effect compared to control group (p < 0.05).
Discussion
The hypothesis that mPRs are involved in P4's actions in the VTA, relevant for reproductive behavior of E2-primed rats, was supported. First, mPRα and mPRβ were expressed in the midbrain and knockdown was observed with AS-ODN infusions to the lateral ventricle and VTA. Second, reductions in incidence and intensity of lordosis behavior were observed with lower expression of mPRβ, either alone or in conjunction with mPRα in the midbrain. Third, these effects were site-, behavior-, and hormone-specific. Site-specificity is demonstrated by more circumspect effects observed with VTA, compared to ICV, infusions and the different patterns of effects observed with infusions to sites outside the VTA. There were no salient effects of AS-ODN infusions on open field, social interaction or elevated plus maze behavior, albeit the expression of mPRs in hippocampus, a brain region that may be central for mediating behavior in these tasks, was not examined. As well, no effects of AS-ODN infusions were observed without P4 priming. Together, these findings suggest that mPRs in VTA may be specific targets in the VTA for membrane actions of P4 to facilitate lordosis.
The present data confirm and extend the literature on the role of non-genomic effects of progestins in the VTA for lordosis. The previously published studies of mPRs were almost exclusively focused on the functions and signaling of mPRα. The unique functions of other mPR isoforms, particular mPRβ, have not been clearly demonstrated. We believe that our study is the first to report that mPRβ may play a major role in progestin-facilitated lordosis in a rodent model. We have previously reported that traditional signaling via nPRs in this region is not required for P4's actions. First, there are very few nPRs in the VTA of adult rodents and those that are localized to the VTA are not E2-induced (Blaustein, 2003; Munn et al., 1983). Second, actions of P4 at the few nPRs in the VTA are not required for facilitation of lordosis. P4 enhances lordosis when applied to the VTA of mutant mice lacking nPRs, or of rats that have nPRs knocked down with AS-ODNs (Frye and Vongher, 2001; Frye et al., 2000). Third, the rapidity of effects of progestins applied directly to the VTA suggests that these actions do not require genomic signaling of nPRs. Other groups have also shown how important this brain region is for natural rewards, like mating, as well as the effects of drugs of abuse for sexual behavior (Pitchers et al., 2010). Some of these effects in our model, and others, involve signal transduction pathways associated with G-protein coupled receptors in the VTA (reviewed in Frye and Walf, 2008; Pitchers et al., 2010). As such, mPRs, which are G-protein coupled receptors, as putative membrane targets in the VTA, were investigated here.
There is some evidence for the role of ovarian steroids for mPR expression. In support, among ovx and E2-primed rats, expression of mPRβ, as measured by in situ hybridization and histochemistry, was greater than mPRα in the hippocampus, lateral and medial septum, thalamus, and regions of the midbrain (Intlekofer and Petersen, 2011). Similar results were shown in a recent study comparing expression of mPRα and mPRβ in the hypothalamus, midbrain and forebrain. E2-treatment decreased mPRβ expression in the medial septum (Zuloaga et al., 2012). Both mPRα and mPRβ were increased during proestrus, compared to diestrus, of rats in the mediobasal hypothalamus (Liu and Arbogast, 2009). In the present study, we saw that reduction of mPRβ in the midbrain was particularly effective in attenuating lordosis of rats. This occurred with ICV infusions, which knocked down mPRα and mPRβ in the VTA, or intra-VTA infusions of AS-ODNs, which only knocked down mPRβ. Another interesting pattern was observed with mPRα AS-ODNs ICV or to the VTA increasing expression of mPRβ in the midbrain. We cannot rule out the importance of mPRs in other brain regions in mediating reproductive and other behaviors. For example, mPRs may also be expressed in regions nearby the VTA that were not investigated in this study, such as the midbrain central gray (a brain structure, which has also previously been implicated in the hormonal regulation of lordosis behavior in female rats; McCarthy et al., 1995). The present findings provide strong support for a role of mPRβ in the VTA in mediating lordosis and may even suggest that mPRα may influence mPRβ signaling to mediate the expression of progestin-facilitated lordosis in this region.
In addition to the midbrain VTA, the hypothalamus is an important brain region in mediating progestin-facilitated lordosis. The hypothalamus has high expression of classic targets of progestins, nPRs, which are involved in reproductive behaviors of female rodents. Additionally, there is some evidence that P4 may also have actions independent of nPRs, involving non-classical targets, such as neurotransmitters (e.g. GABA, glutamate), and signal transduction pathways, in the VMH for lordosis (Balasubramanian et al., 2008a,b; Frye, 2001c; Georgescu and Pfaus, 2006a,b; González-Flores et al., 2010; Hoffman et al., 2002). A question not addressed in the present study was the role of mPRs in the VMH as a membrane target for progestins' actions for lordosis. Perhaps hormone-primed rats had attenuated lordosis responding following lateral ventricle infusions of mPRα and/or mPRβ AS-ODNs coincident with reduction in mPRs in the hypothalamus. Indeed, both VTA and ICV infusions of mPRα AS-ODNs resulted in an increased expression of mPRβ in the midbrain, but only ICV infusions of mPRα AS-ODNs reduced lordosis. This suggests to us that mPRα in the hypothalamus and mPRβ in the VTA may be important for progestin-facilitated lordosis. A limitation of this study was that we were unable to systematically examine expression of hypothalamic mPRs. As such, a question for further research is the complementary roles of mPRα in the hypothalamus and mPRβ in the VTA underlying rapid actions of progestins for lordosis.
In the present study, there were no differences in behaviors other than reproductive behavior. Given these null effects, and that we do not have data on mPR knock down in other regions, we cannot rule out the possibility that mPRs may play a role in hippocampally-mediated behaviors. Indeed, there is considerable clinical evidence that supports the need for further understanding progestin-sensitive behaviors (Girdler et al., 2012). The selective expression of mPRβ in the brain, but not in the body, implies that it may be possible in the future to target these receptors without the liability of trophic effects in peripheral reproductive tissues. As such, future studies manipulating mPRs in regions, such as the hippocampus, are of considerable interest. In these experiments, we tried to verify our knock down of mPR following lateral ventricle and midbrain VTA infusions of AS-ODNs in experimental rats using qPCR. The approach that we utilized for taking tissue punches of these regions in a subset of subjects and extracting RNA for qPCR precluded examination of mPR proteins. It is notable that we saw modest changes in RNA expression following AS-ODN infusions; yet, robust behavioral changes. We hope to address the shortcoming of these approaches in our future work.
Currently, strong evidence exists for two main groups of PRs mediating non-genomic progestin actions. One candidate is the nPRs acting through non-genomic signaling pathways (Bayaa et al., 2000; Tian et al., 2000). The nPR, despite having well-defined transcriptional activity (Schwartz et al., 1977), is also thought to have a separate non-genomic signaling function. The nPRs have non-genomic function in breast cancer signaling and Xenopus oocyte maturation (Bayaa et al., 2000; Boonyaratanakornkit et al., 2001, 2007; Faivre et al., 2005), but these non-genomic effects of nPRs in the brain for behavior are still being elucidated. Another candidate is the mPRs, which were characterized a decade ago (Zhu et al., 2003a,b) and investigated here. mPRs have been grouped into a unique receptor class called the PAQR family, which contains 5 unique mPR members (α ~ ε, class II receptors), 4 adiponectin receptor like (class I receptors), and 2 hemolysin-like receptors (Smith et al., 2008; Tang et al., 2005; Thomas et al., 2007). The mPRα and mPRβ are the most well-studied of the mPRs (Dosiou et al., 2008; Dressing and Thomas, 2007; Hanna et al., 2006; Karteris et al., 2006; Thomas et al., 2005), and expression/localization and functional effects of mPRγ, mPRδ, and mPRε are still relatively unknown (Nutu et al., 2007; Zhu et al., 2003a). In support, the mPRα was first characterized in spotted seatrout and studied most extensively of all mPRs. Biochemical analyses have shown that the transcripts and proteins of mPRα are expressed abundantly in the brain, spinal cord, testis, kidney, uterus, and ovary in vertebrates including fish, mice and human (Hanna et al., 2006; Labombarda et al., 2010; Lemale et al., 2008; Sleiter et al., 2009; Zhu et al., 2003b). The potential functions of mPRα have been suggested to mediate rapid progestin signaling in final oocyte maturation in fish oocytes (Thomas et al., 2005; Tokumoto et al., 2006; Zhu et al., 2003b), sperm motility in fish (Tubbs and Thomas, 2009), P4 signaling in human myometrial cells, breast cancer cells and lymphocytes (Dosiou et al., 2008; Dressing and Thomas, 2007; Karteris et al., 2006; Thomas et al., 2007), reproductive activity in rat and sheep reproductive tissues (Ashley et al., 2006; Cai and Stocco, 2005), and rapid gonadotropin releasing hormone secretion among mice (Sleiter et al., 2009). As well, mPRβ has been proposed to be involved in the controlled beating of cilia of the fallopian tubes of mice and humans (Nutu et al., 2009). A question that had yet to be addressed was the functional role of mPRs in a whole rodent model. The present data suggest that mPRβ may be the membrane target for these rapid effects of P4 in the VTA. These findings extend what has previously been reported in the literature regarding mPR expression, and suggest a functional role of mPRβ for a hard-wired behavior in a brain region that is conserved across species, the midbrain. Of interest, are the relative roles of nPRs and mPRs for their involvement in rapid functional effects of progestins. Moreover, the extent to which there may be conserved functional effects in other species, such as mice, is the subject of ongoing investigation.
Acknowledgments
This research was funded by NSF (IOS-0957148). Technical assistance provided by Carolyn Koonce, Danielle Llaneza, Jacob Martinez, Julianne Power, Anthony Santarelli, Jennifer Torgersen, and Zhenhong Zhao is greatly appreciated.
References
- Ashley RL, Clay CM, Farmerie TA, Niswender GD, Nett TM. Cloning and characterization of an ovine intracellular seven transmembrane receptor for progesterone that mediates calcium mobilization. Endocrinology. 2006;147:4151–4159. doi: 10.1210/en.2006-0002. [DOI] [PubMed] [Google Scholar]
- Balasubramanian B, Portillo W, Reyna A, Chen JZ, Moore AN, Dash PK, Mani SK. Nonclassical mechanisms of progesterone action in the brain: I. Protein kinase C activation in the hypothalamus of female rats. Endocrinology. 2008a;149:5509–5517. doi: 10.1210/en.2008-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian B, Portillo W, Reyna A, Chen JZ, Moore AN, Dash PK, Mani SK. Nonclassical mechanisms of progesterone action in the brain: II. Role of calmodulin-dependent protein kinase II in progesterone-mediated signaling in the hypothalamus of female rats. Endocrinology. 2008b;149:5518–5526. doi: 10.1210/en.2008-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayaa M, Booth RA, Sheng Y, Liu XJ. The classical progesterone receptor mediates Xenopus oocyte maturation through a nongenomic mechanism. Proc. Natl. Acad. Sci. U. S. A. 2000;97:12607–12612. doi: 10.1073/pnas.220302597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein JD. Progestin receptors: neuronal integrators of hormonal and environmental stimulation. Ann. N. Y. Acad. Sci. 2003;1007:238–250. doi: 10.1196/annals.1286.023. [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol. Cell. 2001;8:269–280. doi: 10.1016/s1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, McGowan E, Sherman L, Mancini MA, Cheskis BJ, Edwards DP. The role of extranuclear signaling actions of progesterone receptor in mediating progesterone regulation of gene expression and the cell cycle. Mol. Endocrinol. 2007;21:359–375. doi: 10.1210/me.2006-0337. [DOI] [PubMed] [Google Scholar]
- Cai Z, Stocco C. Expression and regulation of progestin membrane receptors in the rat corpus luteum. Endocrinology. 2005;146:5522–5532. doi: 10.1210/en.2005-0759. [DOI] [PubMed] [Google Scholar]
- Caldwell JD. A sexual arousability model involving steroid effects at the plasma membrane. Neurosci. Biobehav. Rev. 2002;26:13–30. doi: 10.1016/s0149-7634(01)00035-5. [DOI] [PubMed] [Google Scholar]
- Delville Y. Progesterone-facilitated sexual receptivity: a review of arguments supporting a nongenomic mechanism. Neurosci. Biobehav. Rev. 1991;15:407–414. doi: 10.1016/s0149-7634(05)80033-8. [DOI] [PubMed] [Google Scholar]
- Dosiou C, Hamilton AE, Pang Y, Overgaard MT, Tulac S, Dong J, Thomas P, Giudice LC. Expression of membrane progesterone receptors on human T lymphocytes and Jurkat cells and activation of G-proteins by progesterone. J. Endocrinol. 2008;196:67–77. doi: 10.1677/JOE-07-0317. [DOI] [PubMed] [Google Scholar]
- Dressing GE, Thomas P. Identification of membrane progestin receptors in human breast cancer cell lines and biopsies and their potential involvement in breast cancer. Steroids. 2007;72:111–116. doi: 10.1016/j.steroids.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Faivre E, Skildum A, Pierson-Mullany L, Lange CA. Integration of progesterone receptor mediated rapid signaling and nuclear actions in breast cancer cell models: role of mitogen-activated protein kinases and cell cycle regulators. Steroids. 2005;70:418–426. doi: 10.1016/j.steroids.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Frye CA. The role of neurosteroids and nongenomic effects of progestins in the ventral tegmental area in mediating sexual receptivity of rodents. Horm. Behav. 2001a;40:226–233. doi: 10.1006/hbeh.2001.1674. [DOI] [PubMed] [Google Scholar]
- Frye CA. The role of neurosteroids and non-genomic effects of progestins and androgens in mediating sexual receptivity of rodents. Brain Res. Rev. 2001b;37:201–222. doi: 10.1016/s0165-0173(01)00119-9. [DOI] [PubMed] [Google Scholar]
- Frye CA. Inhibition of 5alpha-reductase enzyme or GABA(A) receptors in the VMH and the VTA attenuates progesterone-induced sexual behavior in rats and hamsters. J. Endocrinol. Invest. 2001c;24:399–407. [PubMed] [Google Scholar]
- Frye CA. Neurosteroids’ effects and mechanisms for social, cognitive, emotional, and physical functions. Psychoneuroendocrinology. 2009;34:S143–S161. doi: 10.1016/j.psyneuen.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Bayon LE. Mating stimuli influence endogenous variations in the neurosteroids 3alpha-diol. J. Neuroendocrinol. 1999;11:839–847. doi: 10.1046/j.1365-2826.1999.00379.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, DeBold JF. 3alpha-OH-DHP and 5alpha-THDOC implants to the ventral tegmental area facilitate sexual receptivity in hamsters after progesterone priming to the ventral medial hypothalamus. Brain Res. 1993;612:130–137. doi: 10.1016/0006-8993(93)91653-a. [DOI] [PubMed] [Google Scholar]
- Frye CA, Erskine MS. Influence of time of mating and paced copulation on induction of pseudopregnancy in cyclic female rats. J. Reprod. Fertil. 1990;90:375–385. doi: 10.1530/jrf.0.0900375. [DOI] [PubMed] [Google Scholar]
- Frye CA, Gardiner SG. Progestins can have a membrane-mediated action in rat midbrain for facilitation of sexual receptivity. Horm. Behav. 1996;30:682–691. doi: 10.1006/hbeh.1996.0069. [DOI] [PubMed] [Google Scholar]
- Frye CA, Orecki ZA. Prenatal stress alters reproductive responses of rats in behavioral estrus and paced mating of hormone-primed rats. Horm. Behav. 2002a;42:472–483. doi: 10.1006/hbeh.2002.1834. [DOI] [PubMed] [Google Scholar]
- Frye CA, Orecki ZA. Prenatal stress produces deficits in socio-sexual behavior of cycling, but not hormone-primed, Long–Evans rats. Pharmacol. Biochem. Behav. 2002b;73:53–56. doi: 10.1016/s0091-3057(02)00759-1. [DOI] [PubMed] [Google Scholar]
- Frye CA, Vongher JM. Progesterone and 3α,5α-THP enhance sexual receptivity in mice. Behav. Neurosci. 2001;115:1118–1128. [PubMed] [Google Scholar]
- Frye CA, Walf AA. Membrane actions of progestins at dopamine type 1-like and GABAA receptors involve downstream signal transduction pathways. Steroids. 2008;73:906–913. doi: 10.1016/j.steroids.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Mermelstein PG, DeBold JF. Evidence for a non-genomic action of progestins on sexual receptivity in hamster ventral tegmental area but not hypothalamus. Brain Res. 1992;578:87–93. doi: 10.1016/0006-8993(92)90233-y. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE, Pursnani NK, Purdy RH. The neurosteroids, progesterone and 3alpha,5alpha-THP, enhance sexual motivation, receptivity, and proceptivity in female rats. Brain Res. 1998;808:72–83. doi: 10.1016/s0006-8993(98)00764-1. [DOI] [PubMed] [Google Scholar]
- Frye CA, Murphy RE, Platek SM. Anti-sense oligonucleotides, for progestin receptors in the VMH and glutamic acid decarboxylase in the VTA, attenuate progesterone-induced lordosis in hamsters and rats. Behav. Brain Res. 2000;115:55–64. doi: 10.1016/s0166-4328(00)00242-4. [DOI] [PubMed] [Google Scholar]
- Frye CA, Paris JJ, Rhodes ME. Estrogen is necessary for 5alpha-pregnan-3alpha-ol-20-one (3alpha,5alpha-THP) infusion to the ventral tegmental area to facilitate social and sexual, but neither exploratory nor affective behavior of ovariectomized rats. Pharmacol. Biochem. Behav. 2008;91:261–270. doi: 10.1016/j.pbb.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Paris JJ, Rhodes ME. Increasing 3α,5α-THP following inhibition of neurosteroid biosynthesis in the ventral tegmental area reinstates anti-anxiety, social, and sexual behavior of naturally receptive rats. Reproduction. 2009;137:119–128. doi: 10.1530/REP-08-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu M, Pfaus JG. Role of glutamate receptors in the ventromedial hypothalamus in the regulation of female rat sexual behaviors. I. Behavioral effects of glutamate and its selective receptor agonists AMPA, NMDA, and kainate. Pharmacol. Biochem. Behav. 2006a;83:322–332. doi: 10.1016/j.pbb.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Georgescu M, Pfaus JG. Role of glutamate receptors in the ventromedial hypothalamus in the regulation of female rat sexual behaviors. II. Behavioral effects of selective glutamate receptor antagonists AP-5, CNQX, and DNQX. Pharmacol. Biochem. Behav. 2006b;83:333–341. doi: 10.1016/j.pbb.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Lindgren M, Porcu P, Rubinow DR, Johnson JL, Morrow AL. A history of depression in women is associated with an altered GABAergic neuroactive steroid profile. Psychoneuroendocrinology. 2012;37:543–553. doi: 10.1016/j.psyneuen.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Flores O, Beyer C, Gómora-Arrati P, García-Juárez M, Lima-Hernández FJ, Soto-Sánchez A, Etgen AM. A role for Src kinase in progestin facilitation of estrous behavior in estradiol-primed female rats. Horm. Behav. 2010;58:223–229. doi: 10.1016/j.yhbeh.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Hanna R, Pang Y, Thomas P, Zhu Y. Cell-surface expression, progestin binding, and rapid nongenomic signaling of zebrafish membrane progestin receptors alpha and beta in transfected cells. J. Endocrinol. 2006;190:247–260. doi: 10.1677/joe.1.06694. [DOI] [PubMed] [Google Scholar]
- Hardy DF, DeBold JF. Effects of mounts without intromission upon the behavior of female rats during the onset of estrogen-induced heat. Physiol. Behav. 1971;7:643–645. doi: 10.1016/0031-9384(71)90120-x. [DOI] [PubMed] [Google Scholar]
- Hoffman CS, Westin TM, Miner HM, Johnson PL, Summers CH, Renner KJ. GABAergic drugs alter hypothalamic serotonin release and lordosis in estrogen-primed rats. Brain Res. 2002;946:96–103. doi: 10.1016/s0006-8993(02)02867-6. [DOI] [PubMed] [Google Scholar]
- Intlekofer KA, Petersen SL. Distribution of mRNAs encoding classical progestin receptor, progesterone membrane components 1 and 2, serpine mRNA binding protein 1, and progestin and ADIPOQ receptor family members 7 and 8 in rat fore-brain. Neuroscience. 2011;172:55–65. doi: 10.1016/j.neuroscience.2010.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karteris E, Zervou S, Pang Y, Dong J, Hillhouse EW, Randeva HS, Thomas P. Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: potential role in functional progesterone withdrawal at term. Mol. Endocrinol. 2006;20:1519–1534. doi: 10.1210/me.2005-0243. [DOI] [PubMed] [Google Scholar]
- Labombarda F, Meffre D, Delespierre B, Krivokapic-Blondiaux S, Chastre A, Thomas P, Pang Y, Lydon JP, Gonzalez SL, De Nicola AF, Schumacher M, Guennoun R. Membrane progesterone receptors localization in the mouse spinal cord. Neuroscience. 2010;166:94–106. doi: 10.1016/j.neuroscience.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Lemale J, Bloch-Faure M, Grimont A, El Abida B, Imbert-Teboul M, Crambert G. Membrane progestin receptors alpha and gamma in renal epithelium. Biochim. Biophys. Acta. 2008;1783:2234–2240. doi: 10.1016/j.bbamcr.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Liu B, Arbogast LA. Gene expression profiles of intracellular and membrane progesterone receptor isoforms in the mediobasal hypothalamus during prooestrus. J. Neuroendocrinol. 2009;21:993–1000. doi: 10.1111/j.1365-2826.2009.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–Delta Delta C(T)). Method. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lösel RM, Falkenstein E, Feuring M, Schultz A, Tillmann HC, Rossol-Haseroth K, Wehling M. Nongenomic steroid action: controversies, questions, and answers. Physiol. Rev. 2003;83:965–1016. doi: 10.1152/physrev.00003.2003. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Felzenberg E, Robbins A, Pfaff DW, Schwartz-Giblin S. Infusions of diazepam and allopregnanolone into the midbrain central gray facilitate open-field behavior and sexual receptivity in female rats. Horm. Behav. 1995;29:279–295. doi: 10.1006/hbeh.1995.1020. [DOI] [PubMed] [Google Scholar]
- Munn AR, III, Sar M, Stumpf WE. Topographic distribution of progestin target cells in hamster brain and pituitary after injection of [3H]R5020. Brain Res. 1983;274:1–10. doi: 10.1016/0006-8993(83)90515-2. [DOI] [PubMed] [Google Scholar]
- Norman AW, Mizwicki MT, Norman DP. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat. Rev. Drug Discov. 2004;3:27–41. doi: 10.1038/nrd1283. [DOI] [PubMed] [Google Scholar]
- Nutu M, Weijdegård B, Thomas P, Bergh C, Thurin-Kjellberg A, Pang Y, Billig H, Larsson DG. Membrane progesterone receptor gamma: tissue distribution and expression in ciliated cells in the fallopian tube. Mol. Reprod. Dev. 2007;74:843–850. doi: 10.1002/mrd.20685. [DOI] [PubMed] [Google Scholar]
- Nutu M, Weijdegård B, Thomas P, Thurin-Kjellberg A, Billig H, Larsson DG. Distribution and hormonal regulation of membrane progesterone receptors beta and gamma in ciliated epithelial cells of mouse and human fallopian tubes. Reprod. Biol. Endocrinol. 2009;7:89. doi: 10.1186/1477-7827-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW, McEwen BS. Actions of estrogens and progestins on nerve cells. Science. 1983;219:808–814. doi: 10.1126/science.6297008. [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Szego CM. Endometrial cell calcium and oestrogen action. Nature. 1975;253:357–359. doi: 10.1038/253357a0. [DOI] [PubMed] [Google Scholar]
- Pitchers KK, Balfour ME, Lehman MN, Richtand NM, Yu L, Coolen LM. Neuroplasticity in the mesolimbic system induced by natural reward and subsequent reward abstinence. Biol. Psychiatry. 2010;67:872–879. doi: 10.1016/j.biopsych.2009.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schwartz RJ, Chang C, Schrader WT, O'Malley BW. Effect of progesterone receptors on transcription. Ann. N. Y. Acad. Sci. 1977;286:147–160. doi: 10.1111/j.1749-6632.1977.tb29413.x. [DOI] [PubMed] [Google Scholar]
- Sleiter N, Pang Y, Park C, Horton TH, Dong J, Thomas P, Levine JE. Progesterone receptor A (PRA) and PRB-independent effects of progesterone on GnRH release. Endocrinology. 2009;150:3833–3844. doi: 10.1210/en.2008-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Kupchak BR, Garitaonandia I, Hoang LK, Maina AS, Regalla LM, Lyons TJ. Heterologous expression of human mPRα, mPRβ and mPRγ in yeast confirms their ability to function as membrane progesterone receptors. Steroids. 2008;73:1160–1173. doi: 10.1016/j.steroids.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YT, Hu T, Arterburn M, Boyle B, Bright JM, Emtage PC, Funk WD. PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J. Mol. Evol. 2005;61:372–380. doi: 10.1007/s00239-004-0375-2. [DOI] [PubMed] [Google Scholar]
- Thomas P. Characteristics of membrane progestin receptor alpha (mPRα) and progesterone membrane receptor component one (PGMRC1) and their roles in mediating rapid progestin actions. Front. Neuroendocrinol. 2008;29:292–312. doi: 10.1016/j.yfrne.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Tubbs C, Detweiler C, Das S, Ford L, Breckenridge-Miller D. Binding characteristics, hormonal regulation and identity of the sperm membrane progestin receptor in Atlantic croaker. Steroids. 2005;70:427–433. doi: 10.1016/j.steroids.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Dong J, Groenen P, Kelder J, de Vlieg J, Zhu Y, Tubbs C. Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor alpha subtypes and their evolutionary origins. Endocrinology. 2007;148:705–718. doi: 10.1210/en.2006-0974. [DOI] [PubMed] [Google Scholar]
- Tian J, Kim S, Heilig E, Ruderman JV. Identification of XPR-1, a progesterone receptor required for Xenopus oocyte activation. Proc. Natl. Acad. Sci. U. S. A. 2000;97:14358–14363. doi: 10.1073/pnas.250492197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumoto M, Nagahama Y, Thomas P, Tokumoto T. Cloning and identification of a membrane progestin receptor in goldfish ovaries and evidence it is an intermediary in oocyte meiotic maturation. Gen. Comp. Endocrinol. 2006;145:101–108. doi: 10.1016/j.ygcen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Tubbs C, Thomas P. Progestin signaling through an olfactory G protein and membrane progestin receptor-alpha in Atlantic croaker sperm: potential role in induction of sperm hypermotility. Endocrinology. 2009;150:473–484. doi: 10.1210/en.2008-0512. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc. Natl. Acad. Sci. U. S. A. 2003a;100:2237–2242. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc. Natl. Acad. Sci. U. S. A. 2003b;100:2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Hanna RN, Schaaf MJ, Spaink HP, Thomas P. Candidates for membrane progestin receptors—past approaches and future challenges. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008;148:381–389. doi: 10.1016/j.cbpc.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Yahn SL, Pang Y, Quihuis AM, Oyola MG, Reyna A, Thomas P, Handa RJ, Mani SK. Distribution and estrogen regulation of membrane progesterone receptor-β in the female rat brain. Endocrinology. 2012;153:4432–4443. doi: 10.1210/en.2012-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]