Abstract
Hypericin is a naturally occurring photosensitizer that displays potent antiviral activity in the presence of light. The absence of light in many regions of the body may preclude the use of hypericin and other photosensitizers as therapeutic compounds for the treatment of viral infections in vivo. The chemiluminescent oxidation of luciferin by the luciferase from the North American firefly Photinus pyralis was found to generate sufficiently intense and long-lived emission to induce antiviral activity of hypericin. Light-induced virucidal activity of hypericin was demonstrated against equine infectious anemia virus, a lentivirus structurally, genetically, and antigenically related to the human immunodeficiency virus. The implications for exploiting chemiluminescence as a "molecular flashlight" for effecting photodynamic therapy against virus-infected cells and tumor cells are discussed.
Full text
PDF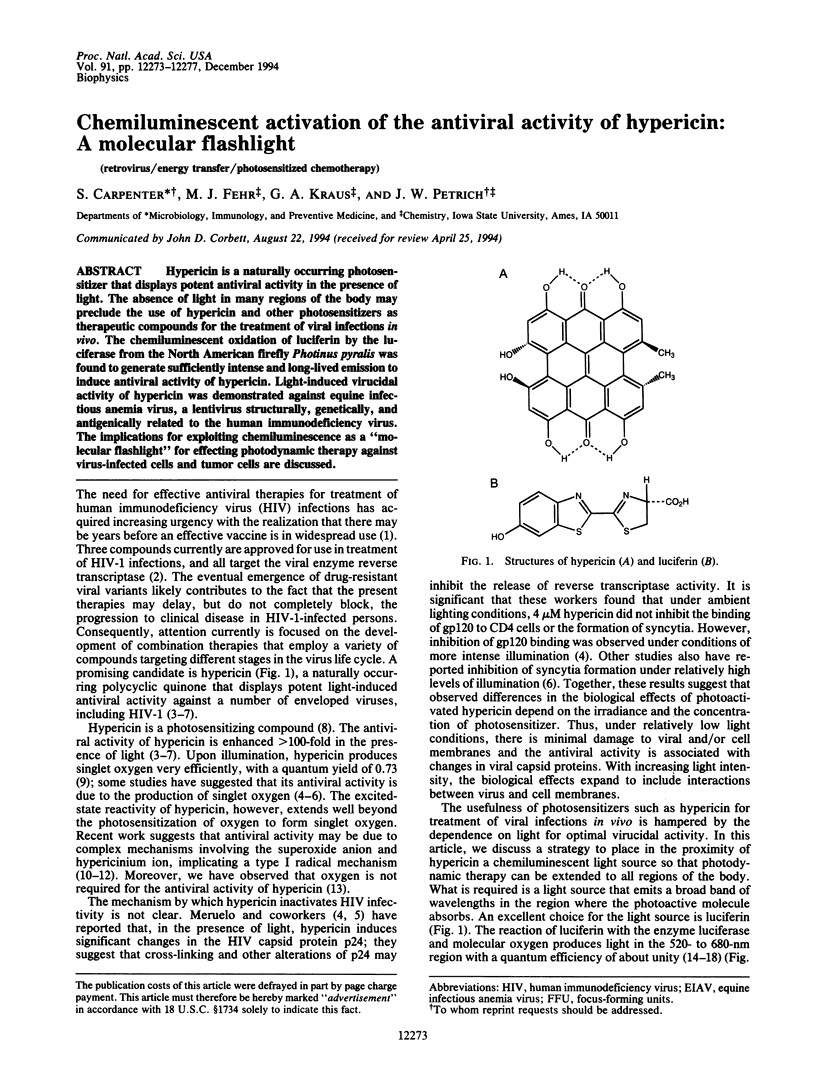


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpenter S., Evans L. H., Sevoian M., Chesebro B. Role of the host immune response in selection of equine infectious anemia virus variants. J Virol. 1987 Dec;61(12):3783–3789. doi: 10.1128/jvi.61.12.3783-3789.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter S., Kraus G. A. Photosensitization is required for inactivation of equine infectious anemia virus by hypericin. Photochem Photobiol. 1991 Feb;53(2):169–174. doi: 10.1111/j.1751-1097.1991.tb03919.x. [DOI] [PubMed] [Google Scholar]
- Degar S., Prince A. M., Pascual D., Lavie G., Levin B., Mazur Y., Lavie D., Ehrlich L. S., Carter C., Meruelo D. Inactivation of the human immunodeficiency virus by hypericin: evidence for photochemical alterations of p24 and a block in uncoating. AIDS Res Hum Retroviruses. 1992 Nov;8(11):1929–1936. doi: 10.1089/aid.1992.8.1929. [DOI] [PubMed] [Google Scholar]
- Diwu Z., Lown J. W. Photosensitization with anticancer agents. 17. EPR studies of photodynamic action of hypericin: formation of semiquinone radical and activated oxygen species on illumination. Free Radic Biol Med. 1993 Feb;14(2):209–215. doi: 10.1016/0891-5849(93)90012-j. [DOI] [PubMed] [Google Scholar]
- Durán N., Song P. S. Hypericin and its photodynamic action. Photochem Photobiol. 1986 Jun;43(6):677–680. doi: 10.1111/j.1751-1097.1986.tb05646.x. [DOI] [PubMed] [Google Scholar]
- Haynes B. F. Scientific and social issues of human immunodeficiency virus vaccine development. Science. 1993 May 28;260(5112):1279–1286. doi: 10.1126/science.8493572. [DOI] [PubMed] [Google Scholar]
- Hopkins T. A., Seliger H. H., White E. H., Cass M. H. The chemiluminescence of firefly luciferin. A model for the bioluminescent reaction and identification of the product excited state. J Am Chem Soc. 1967 Dec 20;89(26):7148–7150. doi: 10.1021/ja01002a076. [DOI] [PubMed] [Google Scholar]
- Hudson J. B., Lopez-Bazzocchi I., Towers G. H. Antiviral activities of hypericin. Antiviral Res. 1991 Feb;15(2):101–112. doi: 10.1016/0166-3542(91)90028-p. [DOI] [PubMed] [Google Scholar]
- Johnston M. I., Hoth D. F. Present status and future prospects for HIV therapies. Science. 1993 May 28;260(5112):1286–1293. doi: 10.1126/science.7684163. [DOI] [PubMed] [Google Scholar]
- Koo J. A., Schmidt S. P., Schuster G. B. Bioluminescence of the firefly: key steps in the formation of the electronically excited state for model systems. Proc Natl Acad Sci U S A. 1978 Jan;75(1):30–33. doi: 10.1073/pnas.75.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus G. A., Pratt D., Tossberg J., Carpenter S. Antiretroviral activity of synthetic hypericin and related analogs. Biochem Biophys Res Commun. 1990 Oct 15;172(1):149–153. doi: 10.1016/s0006-291x(05)80185-8. [DOI] [PubMed] [Google Scholar]
- Lenard J., Rabson A., Vanderoef R. Photodynamic inactivation of infectivity of human immunodeficiency virus and other enveloped viruses using hypericin and rose bengal: inhibition of fusion and syncytia formation. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):158–162. doi: 10.1073/pnas.90.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meruelo D., Lavie G., Lavie D. Therapeutic agents with dramatic antiretroviral activity and little toxicity at effective doses: aromatic polycyclic diones hypericin and pseudohypericin. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5230–5234. doi: 10.1073/pnas.85.14.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L. H., Holsinger L. J., Lamb R. A. Influenza virus M2 protein has ion channel activity. Cell. 1992 May 1;69(3):517–528. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- Redepenning J., Tao N. Measurement of formal potentials for hypericin in dimethylsulfoxide. Photochem Photobiol. 1993 Oct;58(4):532–535. doi: 10.1111/j.1751-1097.1993.tb04927.x. [DOI] [PubMed] [Google Scholar]
- SELIGER H. H., McELROY W. D. Spectral emission and quantum yield of firefly bioluminescence. Arch Biochem Biophys. 1960 May;88:136–141. doi: 10.1016/0003-9861(60)90208-3. [DOI] [PubMed] [Google Scholar]
- Song P. S., Walker E. B., Auerbach R. A., Robinson G. W. Proton release from Stentor photoreceptors in the excited states. Biophys J. 1981 Aug;35(2):551–555. doi: 10.1016/S0006-3495(81)84811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C., Pardini R. S. Oxygen dependence of hypericin-induced phototoxicity to EMT6 mouse mammary carcinoma cells. Photochem Photobiol. 1992 Jun;55(6):831–837. doi: 10.1111/j.1751-1097.1992.tb08531.x. [DOI] [PubMed] [Google Scholar]
- Walker E. B., Lee T. Y., Song P. S. Spectroscopic characterization of the Stentor photoreceptor. Biochim Biophys Acta. 1979 Sep 20;587(1):129–144. doi: 10.1016/0304-4165(79)90227-7. [DOI] [PubMed] [Google Scholar]
- Yang K. C., Prusti R. K., Walker E. B., Song P. S., Watanabe M., Furuya M. Photodynamic action in Stentor coeruleus sensitized by endogenous pigment stentorin. Photochem Photobiol. 1986 Mar;43(3):305–310. doi: 10.1111/j.1751-1097.1986.tb05609.x. [DOI] [PubMed] [Google Scholar]
- Zhirnov O. P. Solubilization of matrix protein M1/M from virions occurs at different pH for orthomyxo- and paramyxoviruses. Virology. 1990 May;176(1):274–279. doi: 10.1016/0042-6822(90)90253-n. [DOI] [PubMed] [Google Scholar]


