Abstract
Several primary immunodeficiencies (PID) have recently been described which confer elevated risk of fungal infections as well as non-infectious cutaneous manifestations. In addition, immunological advances have provided new insights into our understanding of the pathophysiology of fungal infections in established PID. We reviewed PID that present with an eczematous dermatitis in Part I. In Part II, we will discuss updates on PID associated with fungal infections and their biological basis in PID as well as non-infectious cutaneous manifestations.
INTRODUCTION
In Part I of this two part continuing medical education series we discussed primary immunodeficiencies (PID) associated with eczematous dermatitis. In Part II we provide an update on other PID, including those associated with mucocutaneous candidiasis and PID with non-infectious skin manifestations.
Part II CME.
A 28 year-old female with a history of treatment refractory HPV infection of the hands, genitalia and face is evaluated for the first time in your office. She has a history of cervical dysplasia (Grade II) and cutaneous mycobacterial infection. Laboratory work-up reveals severe monocytopenia.
-
What additional clinical feature is most suggestive of a diagnosis of GATA2 deficiency?
Lymphedema
Joint hyperextensibility
High-arched palate
Scoliosis
Retained primary teeth
-
What malignancy is this patient at highest risk of developing?
Endometrial cancer
Glioblastoma multiforme
Breast cancer
Lung cancer
Acute myelogenous leukemia
A 7 year old boy with adenosine deaminase-associated severe combined immunodeficiency is seen in clinic for 3 depressed 3–6mm round brown plaques on the torso. The lesions have been slowly enlarging over the past 2 years.
-
What is the most likely diagnosis/next step in management?
Anetoderma/reassurance
Morphea/topical steroids
Dermatofibrosarcoma protuberans/biopsy
Tinea versicolor/topical antifungal therapy
Disseminated granuloma/biopsy
-
Biopsy reveals a spindle cell infiltrate in the reticular dermis extending into the fat. What immunohistochemical stain is most helpful to confirm the diagnosis?
CD34
CD20
CD68
Factor XIIIa
PAS
-
What is the molecular abnormality associated with this lesion?
c-kit
STAT1
STAT3
Ras
Col1A–PDGFR
NEW MUCOCUTANEOUS CANDIDIASIS SYNDROMES
Key points
Several new monogenic disorders have been associated with chronic mucocutaneous candidiasis (CMC)
Gain of function STAT1 mutations cause CMC with a variety of systemic manifestations
CARD9 mutations predispose to CMC, invasive fungal infections and deep dermatophytosis
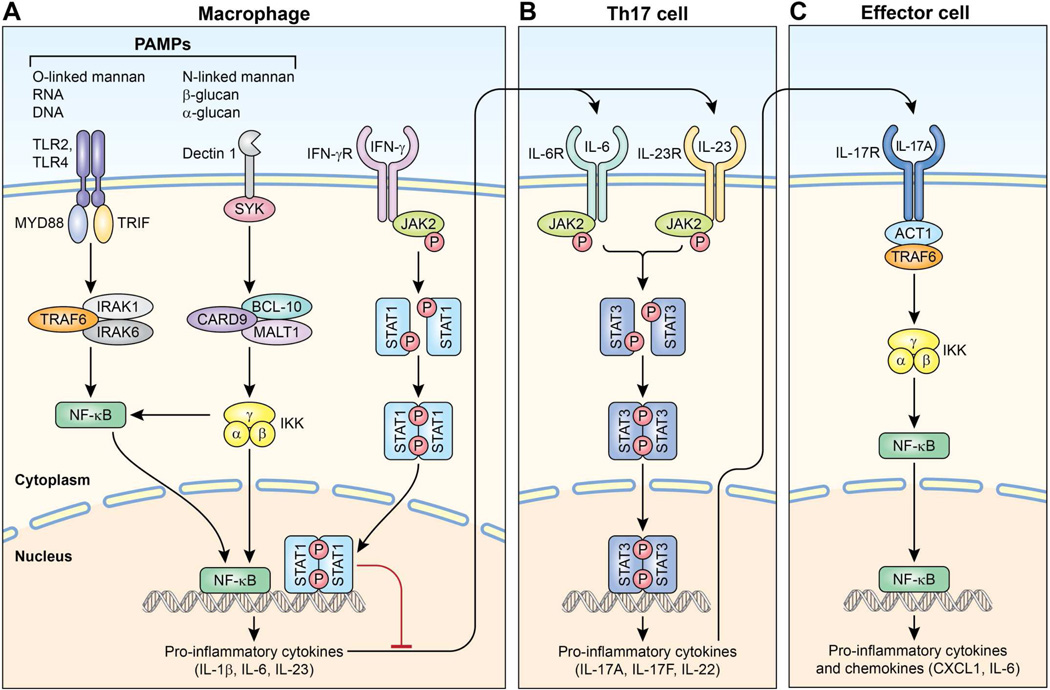
The innate immune response is the host’s first-line of defense against fungal infection (Fig 1). Pattern recognition receptors (PRR), such as toll-like receptors (TLR) and C-type lectin receptors, recognize components of pathogens, termed pathogen-associated molecular patterns (PAMPs), that are evolutionarily conserved. TLR2 and TLR4 recognize O-linked mannan on the fungal cell wall, and activate nuclear factor-kappa B (NF-κB) through the adaptor protein MyD88. Dectin-1, a C-type lectin receptor, recognizes β-glucans, leading to NF-κB induction through the adaptor protein caspase recruitment domain family, member 9 (CARD9). This results in transcription of proinflammatory cytokines which bind to receptors on Th17 cells. The discovery of Th17 cells in 2005 and, subsequently, mucocutaneous candidiasis syndromes associated with specific Th17 signaling defects, demonstrate the importance of this pathway in host defense to fungi.1, 2 This has also provided new insight into the pathogenesis of other established PIDs with CMC (Table 1).3
Figure 1.
Immune response to fungal infection. A. Pattern recognition receptors such as toll-like receptors (TLR) and C-type lectin receptors (e.g. Dectin-1) present on macrophages, dendritic cells, activate independent pathways. TLR2 and TLR4 use the adaptor protein MyD88 to activate nuclear factor kappa B (NF-κB). This transcription factor translocates into the nucleus and facilitates transcription of pro-inflammatory cytokines. Dectin-1 signals through the adaptor protein caspase recruitment domain family, member 9 (CARD-9), also activating NF-κB. Binding of interferon-γ (IFN) to its receptor on macrophages allows for homodimerization of signal transducer and activator of transcription 1 (STAT1). This transcription factor produces interferons and proinflammatory cytokines that inhibit Th17 cell development. B. Th17 cells, which produce cytokines critical for antifungal immunity, signal through STAT3. C. IL-17 receptors are expressed on numerous nonhematogenous cells. IL-17 binds its receptor allowing for activation of NF-κB through the adaptor protein ACT1, producing pro-inflammatory chemokines and cytokines that are important to the host defense against fungal organisms.
Table 1.
Primary immunodeficiencies associated with chronic mucocutaneous candidiasis
| PID (inheritance) | Infections (in addition to CMC) | Immunologic abnormalities | Other findings |
|---|---|---|---|
| STAT3/Autosomal dominant hyper-IgE syndrome (AD) |
Recurrent cold skin abscesses Sinopulmonary infections Lower respiratory tract infections Dermatomal herpes zoster |
Th17 cell deficiency Memory B and T cell differentiation impaired Elevated serum IgE level Eosinophilia |
Neonatal papulopustular eruption Eczematous dermatitis Non-Hodgkin’s lymphoma Craniofacial abnormalities Retained primary teeth Scoliosis Minimal trauma fracture Joint hyperextensibility Cerebral/coronary artery aneurysms |
| Gain of function STAT1 mutation (AD) |
Sinopulmonary infections Mycobacterial infections Herpesviridae family infections |
Diminished Th17 cells Enhanced response to Type I IFNs |
Insulin dependent DM Enteropathy Dental enamel abnormalities Hypothyroidism Hemolytic anemia Autoimmune hepatitis Cerebral aneurysm Oral, esophageal cancer |
| Dectin-1 mutation (AR) | Undetermined | Squamous cell carcinoma | |
| CARD9 deficiency (AR) | Invasive fungal infections Deep dermatophytosis |
Impaired Th17 differentiation | |
| IL-17RA deficiency (AR) | Superficial bacterial skin infections | Abolished response to IL-17A and IL-17F |
|
| IL-17F deficiency (AD) | Impaired IL-17 signaling | ||
| ACT1 deficiency (AR) | Superficial bacterial skin infections | Abolished response to IL-17A and IL-17F |
|
| APECED (AR) | Autoantibodies to IL-17, IL-22 Autoantibodies to Type I IFN |
Vitiligo Alopecia areata/ universalis Hypoparathyroidism Addison’s disease Hypothyroidism Autoimmune hepatitis Pernicious anemia |
AD – autosomal dominant, APECED – autoimmune polyendocrinopathy, candidiasis, and ectodermal dysplasia, AR – autosomal recessive, CMC – chronic mucocutaneous candidiasis, DM – diabetes mellitus, IFN – interferon, Ig – immunoglobulin, IL – interleukin, PID - primary immunodeficiency
Gain of function STAT1 mutations
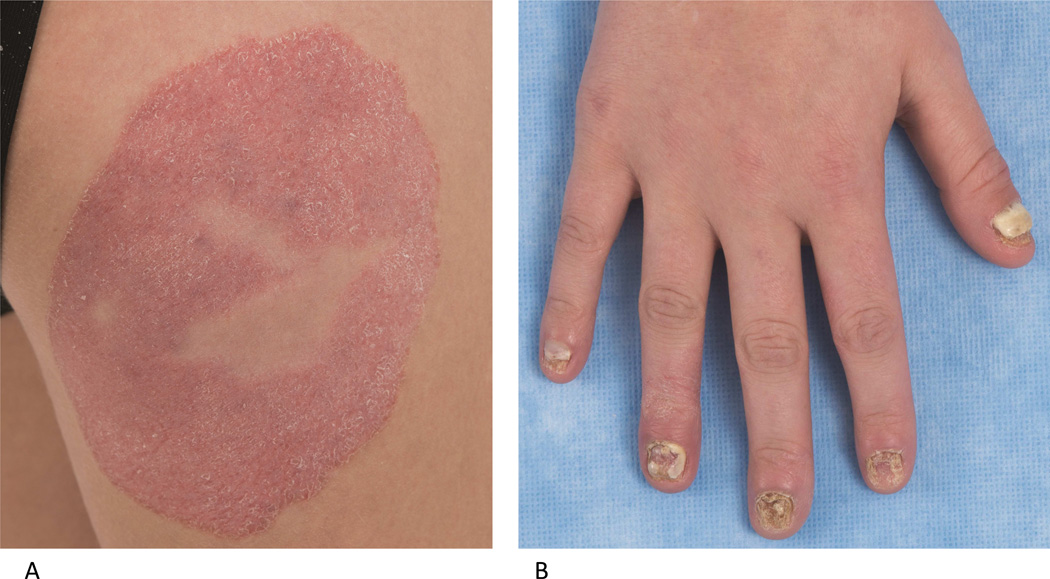
Gain of function (GOF) mutations in signal transducer and activator of transcription 1 (STAT1) are associated with autosomal dominant CMC, likely due to a STAT1-dependent increase in production of interferons (IFN) that inhibit Th17 development.3–5 GOF mutations in STAT1 result in diminished interleukin (IL)-17A and IL-22, and enhanced response to type I IFNs.4, 6 In addition to CMC, patients are at risk for other fungal infections (e.g. disseminated coccidioidomycosis and histoplasmosis), bacterial sinopulmonary infections, mycobacterial, and Herpesviridae family infections.7–9 The clinical severity of this syndrome is highly variable (Fig 2, A and B)--some patients manifest only CMC, whereas other patients develop multiple endocrine, dental, gastrointestinal and autoimmune abnormalities, including early onset diabetes, enteropathy, hypothyroidism, hemolytic anemia and autoimmune hepatitis.4, 10 Cerebral aneurysms and malignancy (oral and esophageal) have also been described.4, 11
Figure 2.
Multiple cutaneous fungal infections associated with STAT1 gain of function mutation. A. Trichophyton tonsurans dermatophyte infection on the thigh and extensive nail dystrophy (B) due to chronic fungal infection in an 18 year old female. Nail clippings identified septate hyphae.
Dectin-1 mutations
In 2009, Ferwerda et al.12 identified a family with autosomal recessive (AR) CMC associated with mutations in Dectin-1. Dectin-1, also known as C-type lectin domain family 7, member A (CLEC7A), is a PRR expressed by phagocytes that recognizes β-glucans on the fungal cell wall. This protein, along with CARD9, is vital to antifungal immunity via induction of the STAT3 pathway and release of Th17 differentiating cytokines.13, 14 Affected patients develop vulvovaginal candidiasis most commonly, followed by oral and esophageal candidiasis, but do not appear to be susceptible to invasive candidal infection. Variants in the Dectin-1 gene are fairly common; however, the functional significance of these polymorphisms remains unclear.
CARD9 deficiency
Mutations in CARD9 are also responsible for a new monogenic autosomal recessive CMC. However, unlike Dectin-1 deficiency, CARD9 deficiency is also associated with invasive fungal infection and deep dermatophytosis.15, 16 Neutrophil dysfunction may account for the invasive potential of fungal infections in CARD9 deficiency, particularly candidal meningitis.17, 18 Drewniak et al.17 demonstrated that production of IL-6 and IL-1β in response to Candida albicans is dependent on CARD9. Failure of monocytes or dendritic cells to produces these cytokines results in naïve T cells unable to differentiate into Th17 cells. Recommended therapy for superficial dermatophyte involvement is systemic terbinafine or posaconazole.19 The addition of GM-CSF to standard antifungal therapy may improve therapeutic response to invasive infections.20, 21
IL-17RA, IL-17, and ACT1 associated CMC
Three additional recently identified inborn errors of immunity associated with CMC further demonstrate the importance of Th17 cytokines in CMC: autosomal recessive mutations in the IL-17RA and ACT1 genes, and autosomal dominant mutations in the IL-17F gene. In 2011, Puel et al.22 described a boy born to consanguineous parents who developed CMC as well as superficial skin infection with S aureus in infancy. Gene sequencing identified a homozygous mutation in the IL17RA gene, which encodes the receptor IL-17RA. The same report described a second family with 5 affected family members who developed CMC due to a heterozygous missense mutation in the IL17F gene, which encodes IL-17F, one of 6 known IL-17 cytokines. Lastly, in 2013 a novel mutation was identified in ACT1 which results in CMC due to loss of response to IL-17.23 Two siblings were reported with oral thrush and onychomycosis from Candida as well as superficial S aureus skin infections. Genetic screening identified biallelic missense mutation in ACT1, also known as NF-κB activator 1 or TRAF3IP2, encoding an adaptor molecule that interacts with the IL-17 receptors to allow downstream activation of pathways including NF-κB.
AUTOIMMUNE POLYENDOCRINOPATHY, CANDIDIASIS, AND ECTODERMAL DYSPLASIA (APECED)
Key points
Autosomal recessive
Triad of hypoparathyroidism, Addison’s disease, and CMC
Autoimmune manifestations common due to loss of central tolerance
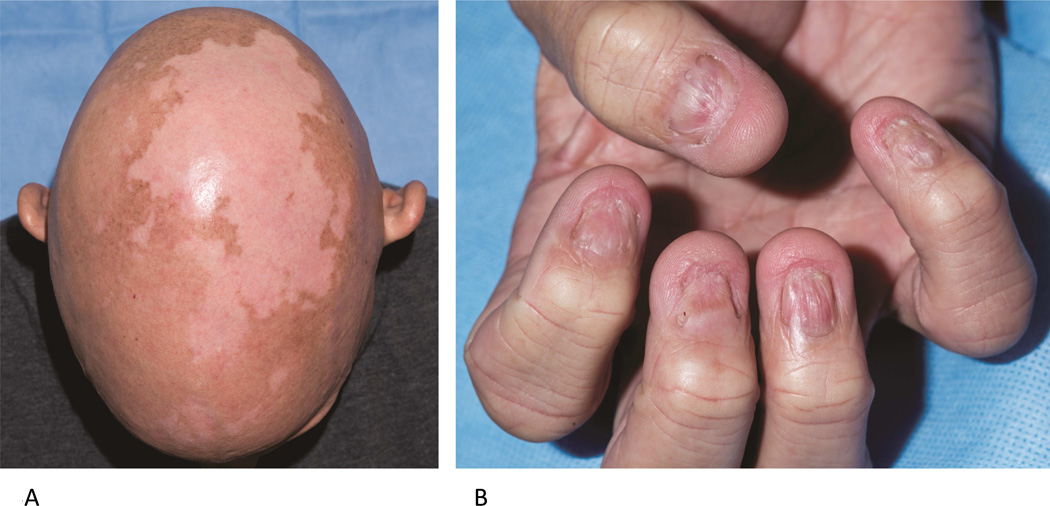
APECED is characterized by the triad of CMC, hypoparathyroidism, and Addison’s disease.14 Clinical diagnosis requires 2 of these 3 findings; however, additional autoimmune manifestations in the skin and other organs are common. This syndrome, also known as autoimmune polyendocrine syndrome type 1 (APS-1), is a rare autosomal recessive condition caused by biallelic mutations in the autoimmune regulator (AIRE) gene.24 AIRE is expressed in medullary thymic epithelial cells (mTECs) and is essential for deletion of autoreactive T cells. Mutations in AIRE render the AIRE protein undetectable in mTECs, and allow survival of autoreactive T cells.25, 26 APECED is associated with autoimmune dysfunction of the parathyroid and adrenal glands in early adolescence, hypothyroidism, autoimmune hepatitis, and pernicious anemia.27 Dermatologic manifestations of autoimmunity in APECED include vitiligo and alopecia areata/universalis (Fig 3, A). CMC, often present by age 6, is the primary infectious manifestation in APECED (Fig 3, B). In 2010, autoantibodies against Th17 cytokines (IL-17A, IL-17F, and IL-22) were discovered in patients with APECED, suggesting a causative link to CMC in these patients.26, 28, 29 A similar susceptibility to CMC due to autoantibodies to IL-17 and IL-22 has been described in patients with thymoma.26
Figure 3.
Autoimmune polyendocrinopathy candidiasis, and ectodermal dysplasia. A. 38 year old male with alopecia universalis, vitiligo and nail dystrophy (B) secondary to recurrent candidal infection beginning at age 3.
GRANULOMATOUS SKIN DISEASE IN PID
Noninfectious granulomatous skin inflammation is an important cutaneous clue to the diagnosis of PID. Phospholipase C, gamma 2 (PLCγ2)-associated antibody deficiency and immune dysregulation (PLAID) and severe combined immunodeficiency (SCID) due to hypomorphic recombination-activating gene (RAG) mutations, are recent additions to the differential diagnosis of PID associated with noninfectious granulomas (Table 2).
Table 2.
Primary immunodeficiencies associated with granuloma formation
| Common variable immunodeficiency |
| Chronic granulomatous disease |
| Nijmegen breakage syndrome |
| PLCγ2-associated antibody deficiency and immune dysregulation (PLAID) |
| Hypomorphic RAG mutation |
PLAID
Key points
Autosomal dominant
Evaporative cold urticaria in all patients, granulomas in a subset of patients
Neonatal nasal and acral inflammatory lesions
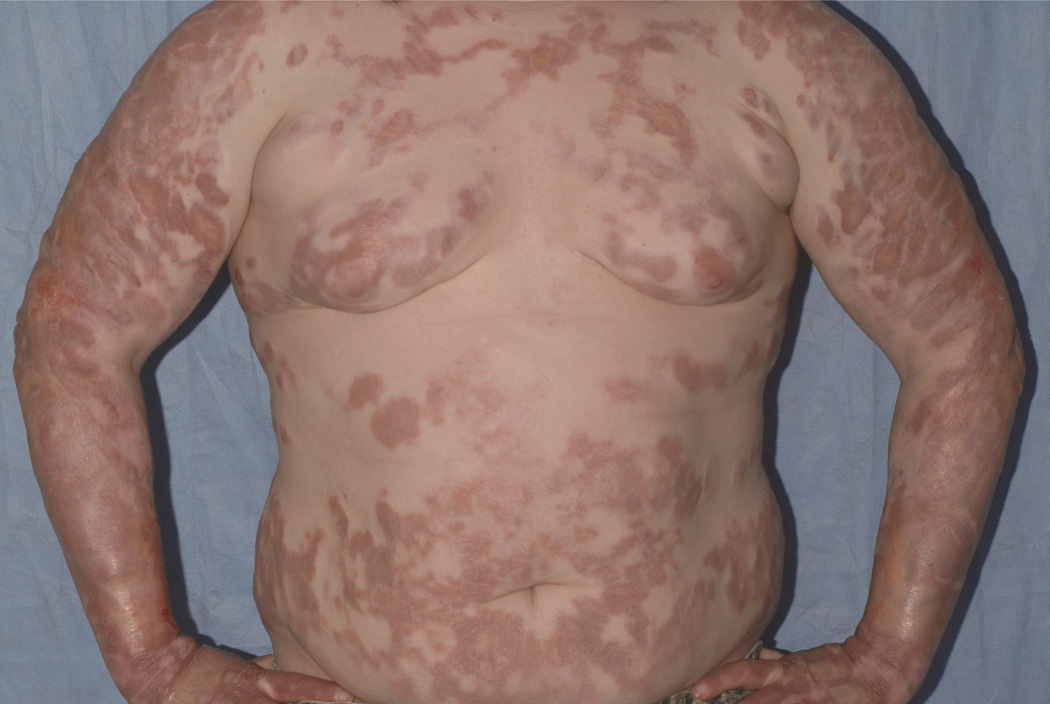
Familial atypical cold urticaria was first described in 2009 in three families.30 In 2012, three additional unrelated families with evaporative cold urticaria were identified and found to have a genomic deletion in PLCG2 leading to gain of function of the protein, PLCγ2, a syndrome termed PLAID.31 This is an autosomal dominant condition associated with urticaria induced by evaporative cooling or contact with cold air. Cold urticaria develops in early childhood and persists into adulthood. In this syndrome, PLCγ2 enzyme activity increases with cold temperature, leading to increased mast cell degranulation. A subset of patients with PLAID also develop neonatal skin lesions on the fingers, toes, and nasal tip. Although the nature of the lesions is not yet fully characterized, nasal lesions have progressed to nasal destruction in two cases.32 In addition, a minority of patients with neonatal skin lesions progress to granulomatous skin disease later in life (Fig 4). Patients also develop allergic disease (56%), recurrent sinopulmonary infections (44%), and autoimmune disease (26%) including vitiligo, thyroiditis and arthritis. Elevated ANA titers were found in 62% among patients with PLAID who were tested. Immune abnormalities in PLAID include elevated immunoglobulin (Ig)E, decreased IgA and IgM, and decreased circulating B cells and natural killer (NK) cells. B cells in PLAID have reduced class switched antibody production, but increased mature B cell compartment.31
Figure 4.
Granuloma formation in PLAID syndrome. Widespread granulomatous disease in 21 year old with PLAID syndrome. Histopathology demonstrated sarcoid-like granulomas without central necrosis as well as diffuse, superficial dermal granulomatous inflammation.
Autoinflammation and PLCγ2-associated antibody deficiency and immune dysregulation (APLAID), also reported in 2012, is associated with a missense mutation, rather than deletion, in the same gene, PLCG2.33 Patients with APLAID have a more pronounced inflammatory syndrome than PLAID, with recurrent sinopulmonary infections, interstitial pneumonitis and respiratory bronchiolitis, eye inflammation, colitis, and arthralgias. Skin features include epidermolysis bullosa-like blistering in infancy that develops into vesiculopustular lesions and erythematous plaques following heat exposure. The immunophenotype of APLAID demonstrates an absence of class switched memory B cells, as seen in PLAID, with normal naïve and memory T cells and NK cells.33
SCID
Key points
Most common cause is a mutation in IL-2Rγ
Hypomorphic RAG mutations are associated with granulomatous skin disease, autoimmunity, and a less severe infection profile than null RAG-associated SCID
Adenosine deaminase deficient SCID patients are at risk of single or multiple dermatofibrosarcoma protuberans (DFSP) tumors
SCID is a group of disorders characterized by B and T cell immunodeficiency and multiple known genetic defects. The most common form is X-linked recessive SCID, caused by mutations in the IL-2Rγ gene. This produces a T−B+NK− immunophenotype with early onset infection and risk of death; however, many other phenotypes have recently been described with variable presentations.
Null mutations in RAG1 or RAG2 are responsible for about 20% of cases of SCID. These genes play a critical role in recombination of V(D)J segments of immunoglobulins and T cell receptors. This is important both for antigen recognition as well as for maturation of lymphocytes.34 It has long been recognized that null mutations of this gene are responsible for classic autosomal recessive T−B−NK+ SCID. This leads to severe early-onset viral and bacterial infections, usually within the first month of life, requiring hematopoietic stem cell transplant (HSCT) for survival.
In contrast to patients with null mutations, patients with hypomorphic RAG mutations have residual V(D)J recombination with variable clinical presentations.35 The clinical spectrum of hypomorphic RAG SCID now includes (1) Omenn syndrome (OS), (2) early onset autoimmunity, (3) immunodeficiency with granulomas, (4) isolated CD4+ lymphopenia, and (5) cytomegalovirus infection with γδ T cell expansion.36–40 OS was the first characterized syndrome associated with hypomorphic RAG mutations, and presents similar to classic SCID with infections early in infancy. The immunophenotype is T+/−B−NK+. Patients develop erythroderma in infancy, lymphadenopathy, hepatosplenomegaly, and alopecia. Laboratory evaluation in OS shows eosinophilia, elevated serum IgE, an absence of B cells, and expansion of oligoclonal T cells that infiltrate organs.34, 36, 41 This condition may be fatal despite early HSCT.36
In 2008, Schuetz et al.36 reported a new presentation of hypomorphic mutations in RAG1 or RAG2, characterized by granulomatous skin disease early in life, but a less severe immunodeficiency than null mutation RAG SCID. The immunophenotype in these patients is T+/−B+/−NK+, with low lymphocyte counts and low immunoglobulin levels, including IgE.36 Destructive granulomas developed in the skin and visceral organs, and responded poorly to treatments including corticosteroids, antibiotics, and immunosuppressants.35, 36, 42, 43 It has been postulated that these destructive granulomas are a result of a dysregulated hyperinflammatory response to viral antigen.
Adenosine deaminase (ADA) deficiency is the most common cause of autosomal recessive SCID. The immunophenotype is notable for profound lymphopenia of T, B, and NK cells as well as impairment of both cellular and humoral immunity. ADA plays a key role in the purine salvage pathway, and ADA deficiency leads to accumulation of toxic metabolites and eventual chromosomal breakage.44 ADA-SCID is associated with high mortality and most patients present in the neonatal or infantile period with severe fungal, viral, and bacterial infections.45 However, the use of pegylated ADA enzyme replacement and gene therapy has dramatically altered the life expectancy in this severe immunodeficiency.
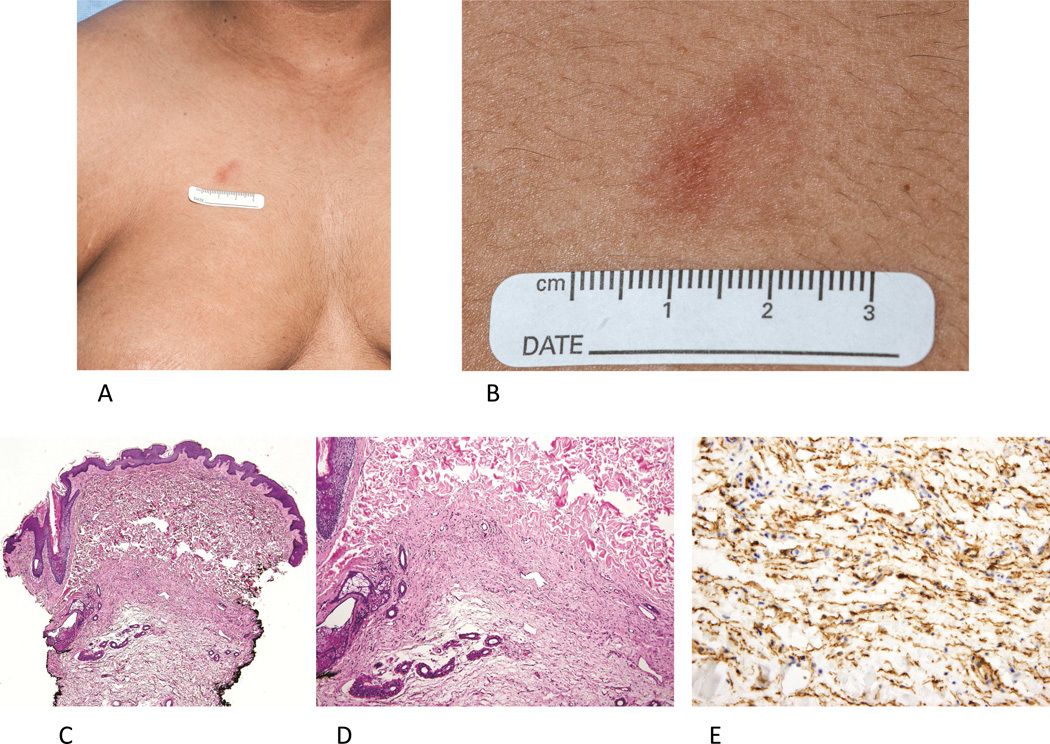
Recently, an association between ADA-SCID and dermatofibrosarcoma protuberans has been reported. Kesserwan et al.46 found that 8 of 12 patients with ADA-SCID followed at National Institutes of Health (NIH) had one or more DFSP lesions (including 15 lesions in one patient). The majority of tumors presented as atrophic plaques located on the trunk and extremities (Fig 5, A and B). Patient age at the time of biopsy ranged from 2–22 years. ADA-SCID associated DFSP lesions demonstrate a proliferation of CD34+ spindle cells but often lack the prototypic storiform histologic appearance (Fig 5, C-E). In this cohort, lesions were confirmed by molecular testing to harbor the characteristic collagen type I, alpha-1-platelet-derived growth factor subunit beta (COL1A1-PDGFB) fusion gene found in DFSP tumors. The natural history of ADA-SCID DFSP is unknown and the optimal management strategy is also unclear. Given the multiplicity of lesions in affected patients, wide excision of lesions would require numerous potentially disfiguring surgical procedures. The potential role of imatinib mesylate for management of ADA-SCID DFSPs has not been studied in this setting.
Figure 5.
Multiple dermatofibrosarcoma protuberans in ADA-SCID. A. Irregularly shaped red-brown plaque on the upper chest in 17 year old male with ADA-SCID. B. A depressed atrophic plaque with poorly-defined margins is seen on close examination. C. Histopathological analysis from 3 similar appearing lesions on this patient a revealed spindle-cell infiltrate, primarily in the reticular dermis (D). E. Immunohistochemistry demonstrates the spindle cell infiltrate strongly expresses CD34; RT-PCR testing from the specimen demonstrated the presence of COL1A1/PDGFB fusion transcripts. (C and D, Hematoxylin-eosin stain, original magnification: C, x40; D, x100; E, CD34 stain; original magnification x200.)
PID ASSOCIATED WITH HUMAN PAPILLOMAVIRUS
Cutaneous viral infections such as human papillomavirus (HPV) are a common presentation of numerous recently described PID: DOCK8 deficiency and MST1 deficiency (see Part I), GOF STAT1 mutation, as well as GATA2 deficiency and WHIM syndrome, which will be discussed below (Table 3). A history of widespread or treatment-refractory disease and careful family history can identify those patients at risk for an underlying PID who may require close surveillance for HPV-related dysplasia and malignancy.
Table 3.
New primary immunodeficiencies associated with viral infections
| Immunodeficiency | Non-infectious cutaneous manifestation |
Infection predisposition | Immunologic abnormalities |
Other associated findings |
|---|---|---|---|---|
| GATA2 deficiency | Panniculitis Congenital lymphedema Melanoma Nonmelanoma skin cancer |
HPV, HSV, VZV, NTM infection Severe C. difficile infection |
Monocytopenia B and NK cell lymphopenia Dendritic cell cytopenia Variable CD4 lymphopenia Variable neutropenia Normal immunoglobulins |
MDS Leukemia Thromboembolic disorder PAP Sensorineural hearing loss Thyroid disease |
| WHIM syndrome | Bacterial pneumonia, sinusitis, skin/soft tissue infection HPV, HSV, EBV, VZV |
Neutropenia B and T cell lymphopenia Decreased IgG and IgA Normal IgM and antibody response |
B cell lymphoma EBV-lymphoproliferative disorder Structural cardiac anomalies Upper limb anomalies |
|
| DOCK8 deficiency | Eczematous dermatitis Squamous cell carcinoma |
Bacterial pneumonia, sinusitis, skin/soft tissue infection HPV, HSV, MCV, VZV |
Variable B, T, and NK cell lymphopenia Eosinophilia Elevated IgE Normal/elevated IgG and IgA Decreased IgM Variable antibody response |
Asthma, allergies Lymphoma EBV-lymphoproliferative disorder |
| MST1 deficiency | Eczematous dermatitis |
Bacterial pneumonia, sinusitis, skin/soft tissue infection HPV, HSV, MCV, EBV, VZV |
Neutropenia B and T cell lymphopenia |
EBV-lymphoproliferative disorder Structural cardiac anomalies Autoimmunity |
| Gain of function STAT1 mutation |
Bacterial sinopulmonary Mycobacterial infection HSV, EBV, VZV, CMV Mucocutaneous candidiasis Disseminated fungal infection |
Diminished Th17 cells Enhanced response to Type I interferons |
Endocrine abnormalities Dental enamel abnormalities Autoimmunity Cerebral aneurysm Oral and esophageal malignancy |
CMV, cytomegalovirus; DOCK8, dedicator of cytokinesis 8; EBV, Epstein-Barr virus; GATA2, GATA-binding protein 2 HPV, human papillomavirus; HSV, herpes simplex virus; Ig, immunoglobulin; MCV, molluscum contagiosum virus; MDS, myelodysplastic syndrome; MST1, Mammalian sterile 20-like 1; NK, natural killer; NTM, nontuberculosis mycobacteria; PAP, pulmonary alveolar proteinosis; STAT1, signal transducer and activator of transcription 1; VZV, varicella zoster virus; WHIM, Warts, Hypogammaglobulinemia, Immunodeficiency and Myelokathexis
GATA2 deficiency
Key points
Autosomal dominant
HPV infection with frequent progression to dysplasia
Variable age of onset of infections
High risk of myelodysplasia/acute myeloid leukemia
In 2011, loss of function mutations in GATA-binding protein 2 (GATA2) were found to be responsible for four syndromes: (1) monocytopenia and mycobacterial infection (MonoMAC) syndrome, (2) dendritic cell, monocyte, B lymphocyte, and NK lymphocyte deficiency, (3) familial myelodysplastic syndrome (MDS)/acute myeloid leukemia (AML), and (4) Emberger syndrome (primary lymphedema and MDS) (Table 4).47–52 GATA2, one of six known mammalian GATA factors, is a zinc-finger transcription factor expressed in hematopoietic stem cells. The variety of functions of this gene, including regulation of other transcription factors, lymphatic development, endothelial cell activation, and proliferation, explains the diverse clinical findings of GATA2 deficiency (Fig 6).47 In 2014, a cohort of 57 patients with GATA2 deficiency was reported from the NIH, demonstrating remarkable disease heterogeneity in age of onset (early childhood to late adulthood) and disease severity.53 Kindred studies have shown that affected family members may manifest limited signs and symptoms. Therefore, genetic screening is recommended for all family members so that appropriate clinical screening can be performed.
Table 4.
Syndromes associated with GATA2 mutations
| GATA2-related syndrome | Immunologic findings | Infections | Other clinical findings |
|---|---|---|---|
| Monocytopenia and mycobacterial infection (MonoMac) syndrome |
Monocyte, B, and NK cell lymphopenia |
Mycobacterial Fungal Viral |
Pulmonary alveolar proteinosis Erythema nodosum Lymphedema Myelodysplasia Myeloid leukemia |
| Dendritic cell, monocyte, B, and NK lymphocyte (DCML) deficiency |
Dendritic cell, monocyte, B and NK cell lymphopenia |
Mycobacterial Viral | Pulmonary alveolar proteinosis Myelodysplasia Myeloid leukemia |
| Familial myelodysplastic syndrome/acute myeloid leukemia |
Multilineage cytopenia | Myelodysplasia Myeloid leukemia |
|
| Emberger syndrome | Variable multilineage cytopenia | Viral | Congenital sensorineural hearing loss Lower limb and genital primary lymphedema Anogenital dysplasia Myelodysplasia Myeloid leukemia |
DCML, dendritic cell, monocyte, B and NK lymphoid; MonoMac, monocytopenia and mycobacterial infection; NK, natural killer
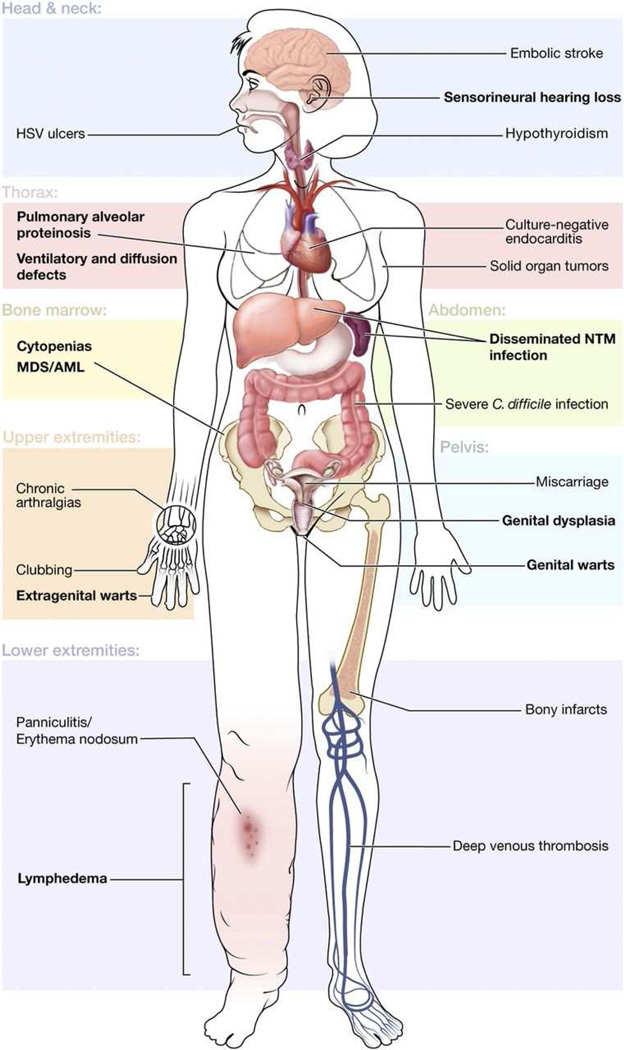
Figure 6.
Cutaneous and systemic findings in GATA2 syndrome. Reprinted, with permission, from Spinner MA et al.8
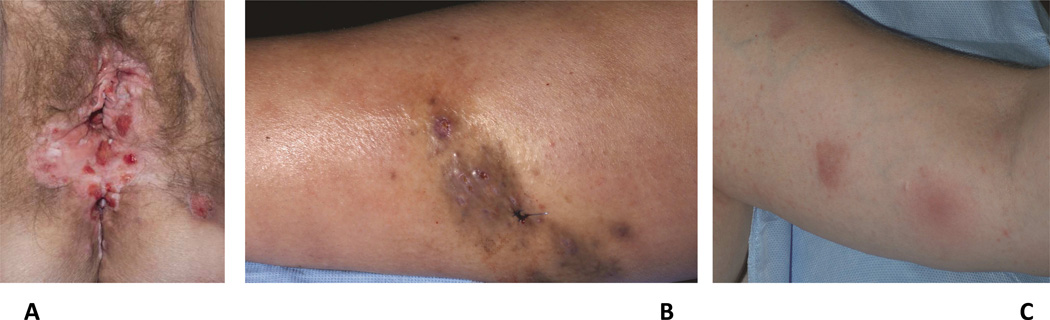
Infection and myelodysplasia are often the presenting signs of GATA2 deficiency and were found in 82 and 84% of the patients in the NIH cohort, respectively.53 The most common infection is HPV, reflecting the profound NK lymphocytopenia and dysfunction characteristic of GATA2 deficiency. HPV infection usually presents by the second decade of life and manifests as generalized verrucosis with persistent cutaneous and genital disease that can rapidly progress to cervical, vulvar, and anal dysplasia (Fig 7, A).49 Other common infections include disseminated nontuberculous mycobacteria (NTM) (Fig 7, B), severe cutaneous and systemic bacterial infections, Herpesviridae family infections, and fungal infections. Mycobacterial and fungal infections generally present in the third decade of life.49 HPV infection is often recalcitrant to treatment, but IFNα and IFNγ have been successful in several patients.54, 55 Azithromycin is recommended as prophylaxis against NTM infection and early HPV vaccination should be considered, although no studies on the efficacy of vaccination have been performed in the setting of GATA2 deficiency.53
Figure 7.
Cutaneous findings in GATA-2 deficiency. A. Extensive HPV infection with vulvar and anal intraepithelial neoplasia, cutaneous mycobacterial infection (B), and erythema nodosum-like panniculitis (C) in a 40 year old female with GATA2 deficiency.
Patients with GATA2 deficiency develop autoimmunity through mechanisms not yet fully understood. Autoimmune thyroid disease is most frequent, and was found in 8 of 57 patients followed at the NIH (14%).53 Decreased regulatory T (Treg) cells may promote autoimmunity. In addition, CD56bright NK cells play an immunoregulatory role, and therefore absence of this NK subtype may predispose patients with GATA2 deficiency to autoimmunity.55, 56
GATA2 deficiency is also associated with pulmonary alveolar proteinosis (18%), which can lead to pulmonary arterial hypertension (9%). Panniculitis, including erythema nodosum (Fig 7, C), develops in one-third of patients with GATA2 deficiency and is frequently associated with underlying infection, particularly mycobacterial infection. Eleven percent of patients in the NIH cohort also developed unilateral or bilateral lymphedema, and one-quarter developed venous thromboses.53
Malignancy is a major cause of morbidity and mortality in GATA2 deficiency. One third of patients develop HPV-related dysplasia and 11% of patients in the NIH cohort developed melanoma or nonmelanoma skin cancer.53 Breast cancer and other solid tumors were also identified in the NIH cohort; however, the greatest risk of death for patients with GATA2 deficiency is progression of MDS to AML and chronic myelomonocytic leukemia. Patients with GATA2 deficiency have approximately 50% risk of developing MDS or leukemia in early adulthood, so early diagnosis can be life saving.50, 57 Myelodysplasia, present in 84% of GATA2 patients in the NIH cohort, results in multilineage cytopenia of B cells, NK cells, monocytes, and dendritic cells. In addition, there is variable CD4+ lymphocytopenia and neutropenia.53, 58 In one recent study of patients carrying a clinical diagnosis of idiopathic cyclic neutropenia or CD4+ lymphocytopenia, GATA2 mutations were found in 6 of 14 patients screened.59 HSCT offers the only cure for GATA2 deficiency.53, 57
WILD syndrome, an acronym for a syndrome of disseminated warts, immunodeficiency, primary lymphedema, and anogenital dysplasia, was proposed by Kreuter et al60 in 2008. This group identified one previously reported case with similar findings to their patient.61 However, given the similarity between the clinical features between these presentations, it is suspected that the reported patients with WILD syndrome may have GATA2 deficiency.62
WHIM syndrome
Key points
Autosomal dominant
HPV infections with variable severity
Recurrent bacterial infections
Plerixafor offers a potential targeted therapy for WHIM syndrome
The syndrome of warts, hypogammaglobulinemia, immunodeficiency and myelokathexis (WHIM syndrome) is a rare autosomal dominant PID initially described in 1964 in a 10 year old girl with recurrent infections and granulocytopenia.63 This patient also had a characteristic bone marrow abnormality of WHIM syndrome, myelokathexis, which is retention of neutrophils in the marrow. In 2003, gain of function mutations in the gene encoding chemokine C-X-C motif receptor 4 (CXCR4) were found to be responsible for this condition.64 CXCR4 is the receptor for chemokine (C-X-C motif) ligand 12 (CXCL12), which regulates hematopoiesis and peripheral trafficking of neutrophils and lymphocyte subsets.64
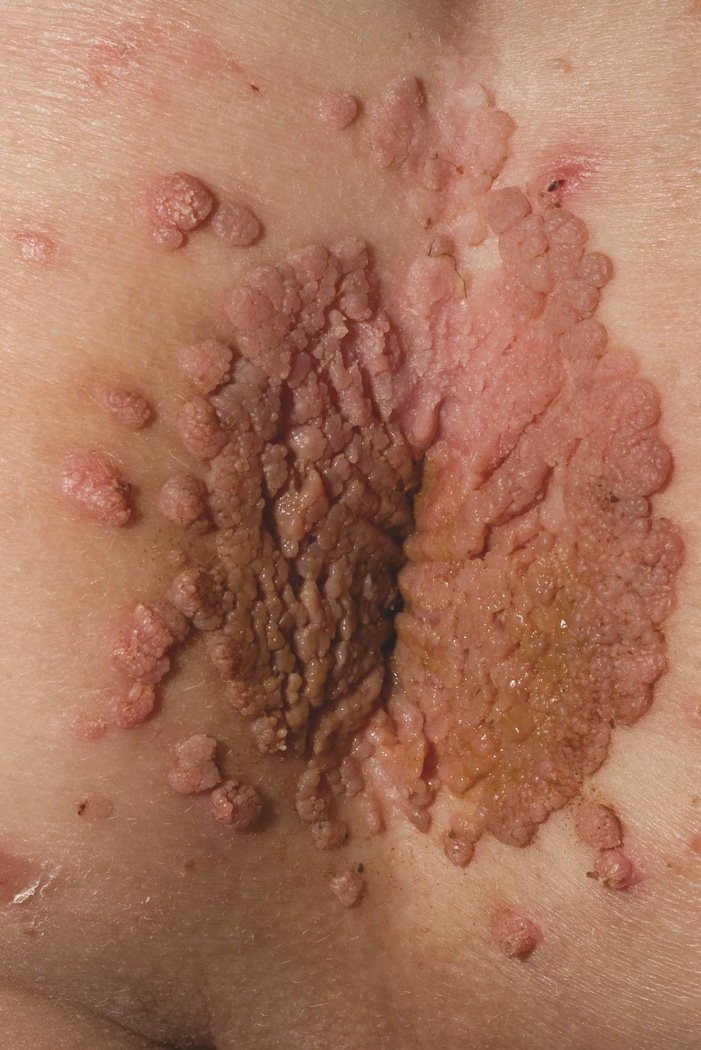
WHIM syndrome manifests with peripheral neutropenia, hypogammaglobulinemia, and HPV infection.65 HPV manifestations range from mild disease with multiple verrucae on the genitals, hands, and feet to severe involvement (Fig 8) with HPV-associated oral and cervical carcinoma. Despite peripheral neutropenia, there is appropriate release of neutrophils from the bone marrow in response to acute infection; therefore, serious bacterial infections are rare. However, hypogammaglobulinemia can lead to recurrent sinopulmonary infections. Unlike many primary immunodeficiencies, autoimmunity and malignancy are infrequent in WHIM syndrome and are limited to a few reports of B cell lymphoma, Epstein-Barr virus (EBV)-associated lymphoproliferative disorder, and cutaneous T cell lymphoma.66–68 Other reported systemic findings include tetralogy of fallot, double aortic arch, radius hypoplasia, and phalangeal dysgenesis.69
Figure 8.
WHIM syndrome. Confluent perianal condyloma and scattered verrucous papules on the buttock and perineum of a 4 year old with WHIM syndrome.
The immunodeficiency of WHIM syndrome is primarily a consequence of myelokathexis. Other immunologic abnormalities include lymphocytopenia, affecting B cells more than T cells, delayed antibody class switching to IgG, and hypogammaglobulinemia.70 Reduced plasmacytoid and myeloid dendritic cells at the site of HPV infection may result in reduction of IFNa production in response to viral infection in patients with WHIM syndrome.71, 72 CXCR4 is expressed by hematopoietic cells and has been extensively studied as a co-receptor for HIV entry. Both CXCR4 and its ligand, CXCL12 (also known as stromal cell-derived factor-1 or SDF-1) are necessary for the development and trafficking of myeloid cells and B lymphocytes.64, 71 Plerixafor is a small molecule antagonist of CXCR4 that is FDA-approved for hematopoietic stem cell mobilization in patients with non-Hodgkin lymphoma or multiple myeloma. In 2010, two Phase I trials demonstrated that plerixafor can effectively mobilize leukocytes from the bone marrow and increase the peripheral absolute lymphocyte, monocyte, and neutrophil counts in patients with WHIM syndrome.73, 74 These trials, as well as a more recent study in 2014, suggest that this targeted therapy may decrease bacterial infections and HPV burden in patients with WHIM syndrome.73–75
Prophylactic antibiotic coverage for encapsulated bacteria and Staphylococcus is recommended for patients with WHIM syndrome.71 The effectiveness of HPV vaccination in WHIM patients is unknown, but given the burden of HPV infection and risk of dysplasia, our recommendation is to offer HPV vaccination early. Replacement gammaglobulin may be needed as well as G-CSF for neutropenia. Although mortality in WHIM syndrome is lower than other primary immunodeficiencies, early diagnosis can lead to appropriate prophylactic and treatment strategies to minimize infections and HPV-related dysplasia and malignancy.
CONCLUSION
The use of powerful molecular tools has ushered in an exciting new era of understanding of the genetic basis of PID. This has allowed clear characterization of distinct disease phenotypes, including specific susceptibility to viral and fungal infections and the cutaneous manifestations of PID, both infectious and non-infectious. Hopefully, these advances will also continue to advance our ability to effectively manage these rare but potentially devastating conditions.
Learning objectives.
After completing this Journal CME activity, the learner should be able to differentiate primary immunodeficiencies that present with mucocuanteous candidiasis based on the other infectious and the noninfectious manifestations; identify primary immunodeficiencies that have noninfectious granulomas; identify the numerous presentations of GATA2 deficiency; identify the targeted therapy for WHIM syndrome
Acknowledgments
Funding/Support: This study was supported by the Intramural Research Program of the National Institutes of Health (NIH), Center for Cancer Research, National Cancer Institute.
ABBREVIATION AND ACRONYM LIST
- ADA
adenosine deaminase
- AIRE
autoimmune regulator
- AML
acute myeloid leukemia
- APECED
autoimmune polyendocrinopathy, candidiasis, and ectodermal dysplasia
- APLAID
autoinflammation and PLCγ2-associated antibody deficiency and immune dysregulation
- APS-1
autoimmune polyendocrine syndrome type 1
- AR
autosomal recessive
- CARD9
caspase-associated recruitment domain
- CLEC7A
C-type lectin domain family 7, member A
- CMC
chronic mucocutaneous candidiasis
- COL1A1-PDGFB
collagen type I, alpha-1-platelet-derived growth factor subunit beta
- CXCL12
chemokine CXC motif ligand 12
- CXCR4
chemokine CXC motif receptor 4
- DFSP
dermatofibrosarcoma protuberans
- DOCK8
dedicator of cytokinesis 8
- EBV
Epstein-Barr virus
- GATA2
GATA-binding protein 2
- GOF
gain of function
- HPV
human papillomavirus
- HSCT
hematopoietic stem cell transplant
- Ig
immunoglobulin
- IFN
interferon
- IL
interleukin
- MDS
myelodysplastic syndromes
- MonoMAC
monocytopenia and mycobacterial infection
- MST1
mammalian sterile 20-like 1
- mTECs
medullary thymic epithelial cells
- NIH
National Institutes of Health
- NF-κB
nuclear factor-kappa B
- NK
natural killer
- NTM
nontuberculous mycobacterial
- OS
Omenn syndrome
- PAMPs
pathogen-associated molecular patterns
- PID
primary immunodeficiency
- PLAID
PLCγ2-associated antibody deficiency and immune dysregulation
- PLCG2
phospholipase C, gamma 2
- PRR
pattern recognition receptor
- RAG
recombination activating gene
- SCID
severe combined immunodeficiency disease
- SDF-1
stromal cell-derived factor-1
- STAT3
signal transducer and activator of transcription 3
- Treg
T regulatory
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- VZV
varicella-zoster virus
- WHIM
warts, hypogammaglobulinemia, immunodeficiency and myelokathexis
- WILD
warts, immunodeficiency, primary lymphedema, and anogenital dysplasia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Public disclosure statement: The authors have no conflict of interest to declare
Disclosures
Editors
The editors involved with this CME activity and all content validation/peer reviewers of the journal-based CME activity have reported no relevant financial relationships with commercial interest(s).
Authors
The authors involved with this journal-based CME activity have reported no relevant financial relationships with commercial interest(s).
Planners
The planners involved with this journal-based CME activity have reported no relevant financial relationships with commercial interest(s). The editorial and education staff involved with this journal-based CME activity have reported no relevant financial relationships with commercial interest(s).
Contributor Information
Dominique C. Pichard, National Institutes of Health, National Cancer Institute, Bethesda, MD.
Alexandra F. Freeman, National Institutes of Health, National Institute of Allergy and Infectious Disease. Bethesda, MD.
Edward W. Cowen, National Institutes of Health, National Cancer Institute, Bethesda, MD.
REFERENCES
- 1.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature immunology. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature immunology. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 3.McDonald DR. TH17 deficiency in human disease. J Allergy Clin Immunol. 2012;129:1429–1435. doi: 10.1016/j.jaci.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van de Veerdonk FL, Plantinga TS, Hoischen A, Smeekens SP, Joosten LA, Gilissen C, et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med. 2011;365:54–61. doi: 10.1056/NEJMoa1100102. [DOI] [PubMed] [Google Scholar]
- 5.Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A, et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med. 2011;208:1635–1648. doi: 10.1084/jem.20110958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamazaki Y, Yamada M, Kawai T, Morio T, Onodera M, Ueki M, et al. Two Novel Gain-of-Function Mutations of STAT1 Responsible for Chronic Mucocutaneous Candidiasis Disease: Impaired Production of IL-17A and IL-22, and the Presence of Anti-IL-17F Autoantibody. J Immunol. 2014;193:4880–4887. doi: 10.4049/jimmunol.1401467. [DOI] [PubMed] [Google Scholar]
- 7.Sampaio EP, Bax HI, Hsu AP, Kristosturyan E, Pechacek J, Chandrasekaran P, et al. A novel STAT1 mutation associated with disseminated mycobacterial disease. J Clin Immunol. 2012;32:681–689. doi: 10.1007/s10875-012-9659-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toth B, Mehes L, Tasko S, Szalai Z, Tulassay Z, Cypowyj S, et al. Herpes in STAT1 gain-of-function mutation [corrected] Lancet. 2012;379:2500. doi: 10.1016/S0140-6736(12)60365-1. [DOI] [PubMed] [Google Scholar]
- 9.Uzel G, Sampaio EP, Lawrence MG, Hsu AP, Hackett M, Dorsey MJ, et al. Dominant gain-of-function STAT1 mutations in FOXP3 wild-type immune dysregulation-polyendocrinopathy-enteropathy-X-linked-like syndrome. J Allergy Clin Immunol. 2013;131:1611–1623. doi: 10.1016/j.jaci.2012.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frans G, Moens L, Schaballie H, Van Eyck L, Borgers H, Wuyts M, et al. Gain-of-function mutations in signal transducer and activator of transcription 1 (STAT1): Chronic mucocutaneous candidiasis accompanied by enamel defects and delayed dental shedding. J Allergy Clin Immunol. 2014;134:1209–1213. doi: 10.1016/j.jaci.2014.05.044. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soltesz B, Toth B, Shabashova N, Bondarenko A, Okada S, Cypowyj S, et al. New and recurrent gain-of-function STAT1 mutations in patients with chronic mucocutaneous candidiasis from Eastern and Central Europe. J Med Genet. 2013;50:567–578. doi: 10.1136/jmedgenet-2013-101570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361:1760–1767. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bishu S, Hernandez-Santos N, Simpson-Abelson MR, Huppler AR, Conti HR, Ghilardi N, et al. The adaptor CARD9 is required for adaptive but not innate immunity to oral mucosal Candida albicans infections. Infect Immun. 2014;82:1173–1180. doi: 10.1128/IAI.01335-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kisand K, Peterson P. Autoimmune polyendocrinopathy candidiasis ectodermal dystrophy and other primary immunodeficiency diseases help to resolve the nature of protective immunity against chronic mucocutaneous candidiasis. Curr Opin Pediatr. 2013:715–721. doi: 10.1097/MOP.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 15.Glocker EO, Hennigs A, Nabavi M, Schäffer AA, Woellner C, Salzer U, et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361:1727–1735. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lanternier F, Pathan S, Vincent QB, Liu L, Cypowyj S, Prando C, et al. Deep dermatophytosis and inherited CARD9 deficiency. N Engl J Med. 2013;369:1704–1714. doi: 10.1056/NEJMoa1208487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drewniak A, Gazendam RP, Tool AT, van Houdt M, Jansen MH, van Hamme JL, et al. Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood. 2013;121:2385–2392. doi: 10.1182/blood-2012-08-450551. [DOI] [PubMed] [Google Scholar]
- 18.Gazendam RP, van Hamme JL, Tool AT, van Houdt M, Verkuijlen PJ, Herbst M, et al. Two independent killing mechanisms of Candida albicans by human neutrophils: evidence from innate immunity defects. Blood. 2014;124:590–597. doi: 10.1182/blood-2014-01-551473. [DOI] [PubMed] [Google Scholar]
- 19.Jachiet M, Lanternier F, Rybojad M, Bagot M, Ibrahim L, Casanova JL, et al. Posaconazole Treatment of Extensive Skin and Nail Dermatophytosis Due to Autosomal Recessive Deficiency of CARD9. JAMA Dermatol. 2014 doi: 10.1001/jamadermatol.2014.2154. [DOI] [PubMed] [Google Scholar]
- 20.Gavino C, Cotter A, Lichtenstein D, Lejtenyi D, Fortin C, Legault C, et al. CARD9 Deficiency and Spontaneous Central Nervous System Candidiasis: Complete Clinical Remission With GM-CSF Therapy. Clin Infect Dis. 2014;59:81–84. doi: 10.1093/cid/ciu215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wildbaum G, Shahar E, Katz R, Karin N, Etzioni A, Pollack S. Continuous G-CSF therapy for isolated chronic mucocutaneous candidiasis: complete clinical remission with restoration of IL-17 secretion. J Allergy Clin Immunol. 2013;132:761–764. doi: 10.1016/j.jaci.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 22.Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boisson B, Wang C, Pedergnana V, Wu L, Cypowyj S, Rybojad M, et al. An ACT1 Mutation Selectively Abolishes Interleukin-17 Responses in Humans with Chronic Mucocutaneous Candidiasis. Immunity. 2013;39:676–686. doi: 10.1016/j.immuni.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Björses P, Aaltonen J, Horelli-Kuitunen N, Yaspo ML, Peltonen L. Gene defect behind APECED: a new clue to autoimmunity. Hum Mol Genet. 1998;7:1547–1553. doi: 10.1093/hmg/7.10.1547. [DOI] [PubMed] [Google Scholar]
- 25.Arstila TP, Jarva H. Human APECED; a Sick Thymus Syndrome? Front Immunol. 2013;4:313. doi: 10.3389/fimmu.2013.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kisand K, Lilic D, Casanova JL, Peterson P, Meager A, Willcox N. Mucocutaneous candidiasis and autoimmunity against cytokines in APECED and thymoma patients: clinical and pathogenetic implications. Eur J Immunol. 2011;41:1517–1527. doi: 10.1002/eji.201041253. [DOI] [PubMed] [Google Scholar]
- 27.Gupta S, Louis AG. Tolerance and autoimmunity in primary immunodeficiency disease: a comprehensive review. Clin Rev Allergy Immunol. 2013;45:162–169. doi: 10.1007/s12016-012-8345-8. [DOI] [PubMed] [Google Scholar]
- 28.Kisand K, Boe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med. 2010;207:299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207:291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gandhi C, Healy C, Wanderer AA, Hoffman HM. Familial atypical cold urticaria: description of a new hereditary disease. J Allergy Clin Immunol. 2009;124:1245–1250. doi: 10.1016/j.jaci.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ombrello MJ, Remmers EF, Sun G, Freeman AF, Datta S, Torabi-Parizi P, et al. Cold urticaria, immunodeficiency, and autoimmunity related to PLCG2 deletions. N Engl J Med. 2012;366:330–338. doi: 10.1056/NEJMoa1102140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aderibigbe OLPD, Kuhns DB, Cowen EW, Milner JD. Poster presented: 2014 Clinical Immunology Society Annual Meeting. Baltimore, MD: 2014. Distinct activation states of leukocytes may contribute to the cutaneous manifestations of PLAID. [Google Scholar]
- 33.Zhou Q, Lee GS, Brady J, Datta S, Katan M, Sheikh A, et al. A hypermorphic missense mutation in PLCG2, encoding phospholipase Cγ2, causes a dominantly inherited autoinflammatory disease with immunodeficiency. Am J Hum Genet. 2012;91:713–720. doi: 10.1016/j.ajhg.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niehues T, Perez-Becker R, Schuetz C. More than just SCID--the phenotypic range of combined immunodeficiencies associated with mutations in the recombinase activating genes (RAG) 1 and 2. Clin Immunol. 2010;135:183–192. doi: 10.1016/j.clim.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 35.Avila EM, Uzel G, Hsu A, Milner JD, Turner ML, Pittaluga S, et al. Highly variable clinical phenotypes of hypomorphic RAG1 mutations. Pediatrics. 2010;126:e1248–e1252. doi: 10.1542/peds.2009-3171. [DOI] [PubMed] [Google Scholar]
- 36.Schuetz C, Huck K, Gudowius S, Megahed M, Feyen O, Hubner B, et al. An immunodeficiency disease with RAG mutations and granulomas. N Engl J Med. 2008;358:2030–2038. doi: 10.1056/NEJMoa073966. [DOI] [PubMed] [Google Scholar]
- 37.de Villartay JP, Lim A, Al-Mousa H, Dupont S, Déchanet-Merville J, Coumau-Gatbois E, et al. A novel immunodeficiency associated with hypomorphic RAG1 mutations and CMV infection. J Clin Invest. 2005;115:3291–3299. doi: 10.1172/JCI25178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ehl S, Schwarz K, Enders A, Duffner U, Pannicke U, Kühr J, et al. A variant of SCID with specific immune responses and predominance of gamma delta T cells. J Clin Invest. 2005;115:3140–3148. doi: 10.1172/JCI25221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuijpers TW, Ijspeert H, van Leeuwen EM, Jansen MH, Hazenberg MD, Weijer KC, et al. Idiopathic CD4+ T lymphopenia without autoimmunity or granulomatous disease in the slipstream of RAG mutations. Blood. 2011;117:5892–5896. doi: 10.1182/blood-2011-01-329052. [DOI] [PubMed] [Google Scholar]
- 40.Henderson LA, Frugoni F, Hopkins G, de Boer H, Pai SY, Lee YN, et al. Expanding the spectrum of recombination-activating gene 1 deficiency: A family with early-onset autoimmunity. J Allergy Clin Immunol. 2013;132:969–971. doi: 10.1016/j.jaci.2013.06.032. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wada T, Toma T, Okamoto H, Kasahara Y, Koizumi S, Agematsu K, et al. Oligoclonal expansion of T lymphocytes with multiple second-site mutations leads to Omenn syndrome in a patient with RAG1-deficient severe combined immunodeficiency. Blood. 2005;106:2099–2101. doi: 10.1182/blood-2005-03-0936. [DOI] [PubMed] [Google Scholar]
- 42.Reiff A, Bassuk AG, Church JA, Campbell E, Bing X, Ferguson PJ. Exome sequencing reveals RAG1 mutations in a child with autoimmunity and sterile chronic multifocal osteomyelitis evolving into disseminated granulomatous disease. J Clin Immunol. 2013;33:1289–1292. doi: 10.1007/s10875-013-9953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Ravin SS, Cowen EW, Zarember KA, Whiting-Theobald NL, Kuhns DB, Sandler NG, et al. Hypomorphic Rag mutations can cause destructive midline granulomatous disease. Blood. 2010;116:1263–1271. doi: 10.1182/blood-2010-02-267583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirschhorn R. Overview of biochemical abnormalities and molecular genetics of adenosine deaminase deficiency. Pediatr Res. 1993;33:S35–S41. doi: 10.1203/00006450-199305001-00194. [DOI] [PubMed] [Google Scholar]
- 45.Sauer AV, Brigida I, Carriglio N, Aiuti A. Autoimmune dysregulation and purine metabolism in adenosine deaminase deficiency. Front Immunol. 2012;3:265. doi: 10.3389/fimmu.2012.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kesserwan C, Sokolic R, Cowen EW, Garabedian E, Heselmeyer-Haddad K, Lee CC, et al. Multicentric dermatofibrosarcoma protuberans in patients with adenosine deaminase-deficient severe combined immune deficiency. J Allergy Clin Immunol. 2012;129:762–769. doi: 10.1016/j.jaci.2011.10.028. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsu AP, Sampaio EP, Khan J, Calvo KR, Lemieux JE, Patel SY, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. 2011;118:2653–2655. doi: 10.1182/blood-2011-05-356352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dickinson RE, Griffin H, Bigley V, Reynard LN, Hussain R, Haniffa M, et al. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood. 2011;118:2656–2658. doi: 10.1182/blood-2011-06-360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dickinson RE, Milne P, Jardine L, Zandi S, Swierczek SI, McGovern N, et al. The evolution of cellular deficiency in GATA2 mutation. Blood. 2014;123:863–874. doi: 10.1182/blood-2013-07-517151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Camargo JF, Lobo SA, Hsu AP, Zerbe CS, Wormser GP, Holland SM. MonoMAC syndrome in a patient with a GATA2 mutation: case report and review of the literature. Clin Infect Dis. 2013;57:697–699. doi: 10.1093/cid/cit368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hahn CN, Chong CE, Carmichael CL, Wilkins EJ, Brautigan PJ, Li XC, et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet. 2011;43:1012–1017. doi: 10.1038/ng.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ostergaard P, Simpson MA, Connell FC, Steward CG, Brice G, Woollard WJ, et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome) Nat Genet. 2011;43:929–931. doi: 10.1038/ng.923. [DOI] [PubMed] [Google Scholar]
- 53.Spinner MA, Sanchez LA, Hsu AP, Shaw PA, Zerbe CS, Calvo KR, et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123:809–821. doi: 10.1182/blood-2013-07-515528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.West ES, Kingsbery MY, Mintz EM, Hsu AP, Holland SM, Rady PL, et al. Generalized verrucosis in a patient with GATA2 deficiency. Br J Dermatol. 2014;170:1182–1186. doi: 10.1111/bjd.12794. [DOI] [PubMed] [Google Scholar]
- 55.Mace EM, Hsu AP, Monaco-Shawver L, Makedonas G, Rosen JB, Dropulic L, et al. Mutations in GATA2 cause human NK cell deficiency with specific loss of the CD56(bright) subset. Blood. 2013;121:2669–2677. doi: 10.1182/blood-2012-09-453969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poli A, Michel T, Thérésine M, Andrès E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126:458–465. doi: 10.1111/j.1365-2567.2008.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cuellar-Rodriguez J, Gea-Banacloche J, Freeman AF, Hsu AP, Zerbe CS, Calvo KR, et al. Successful allogeneic hematopoietic stem cell transplantation for GATA2 deficiency. Blood. 2011;118:3715–3720. doi: 10.1182/blood-2011-06-365049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vinh DC, Patel SY, Uzel G, Anderson VL, Freeman AF, Olivier KN, et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood. 2010;115:1519–1529. doi: 10.1182/blood-2009-03-208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pasquet M, Bellanné-Chantelot C, Tavitian S, Prade N, Beaupain B, Larochelle O, et al. High frequency of GATA2 mutations in patients with mild chronic neutropenia evolving to MonoMac syndrome, myelodysplasia, and acute myeloid leukemia. Blood. 2013;121:822–829. doi: 10.1182/blood-2012-08-447367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kreuter A, Hochdorfer B, Brockmeyer NH, Altmeyer P, Pfister H, Wieland U, et al. A human papillomavirus-associated disease with disseminated warts, depressed cell-mediated immunity, primary lymphedema, and anogenital dysplasia: WILD syndrome. Arch Dermatol. 2008;144:366–372. doi: 10.1001/archderm.144.3.366. [DOI] [PubMed] [Google Scholar]
- 61.Ostrow RS, Bender M, Niimura M, Seki T, Kawashima M, Pass F, et al. Human papillomavirus DNA in cutaneous primary and metastasized squamous cell carcinomas from patients with epidermodysplasia verruciformis. Proc Natl Acad Sci U S A. 1982;79:1634–1638. doi: 10.1073/pnas.79.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.West ES, Kingsbery MY, Mintz EM, Hsu AP, Holland SM, Rady PL, et al. Generalized verrucosis in a patient with GATA2 deficiency. Br J Dermatol. 2014;170:1182–1186. doi: 10.1111/bjd.12794. [DOI] [PubMed] [Google Scholar]
- 63.Zuelzer W. “Myelokathexis”--A new form of chronic granulocytopenia. Report of a case. N Engl J Med. 1964;270:699–704. doi: 10.1056/NEJM196404022701402. [DOI] [PubMed] [Google Scholar]
- 64.Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F, et al. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet. 2003;34:70–74. doi: 10.1038/ng1149. [DOI] [PubMed] [Google Scholar]
- 65.Wetzler M, Talpaz M, Kleinerman ES, King A, Huh YO, Gutterman JU, et al. A new familial immunodeficiency disorder characterized by severe neutropenia, a defective marrow release mechanism, and hypogammaglobulinemia. Am J Med. 1990;89:663–672. doi: 10.1016/0002-9343(90)90187-i. [DOI] [PubMed] [Google Scholar]
- 66.Imashuku S, Miyagawa A, Chiyonobu T, Ishida H, Yoshihara T, Teramura T, et al. Epstein-Barr virus-associated T-lymphoproliferative disease with hemophagocytic syndrome, followed by fatal intestinal B lymphoma in a young adult female with WHIM syndrome. Warts, hypogammaglobulinemia, infections, and myelokathexis. Ann Hematol. 2002;81:470–473. doi: 10.1007/s00277-002-0489-9. [DOI] [PubMed] [Google Scholar]
- 67.Chae KM, Ertle JO, Tharp MD. B-cell lymphoma in a patient with WHIM syndrome. J Am Acad Dermatol. 2001;44:124–128. doi: 10.1067/mjd.2001.111337. [DOI] [PubMed] [Google Scholar]
- 68.Beaussant Cohen S, Fenneteau O, Plouvier E, Rohrlich PS, Daltroff G, Plantier I, et al. Description and outcome of a cohort of 8 patients with WHIM syndrome from the French Severe Chronic Neutropenia Registry. Orphanet J Rare Dis. 2012;7:71. doi: 10.1186/1750-1172-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Badolato R, Dotta L, Tassone L, Amendola G, Porta F, Locatelli F, et al. Tetralogy of fallot is an uncommon manifestation of warts, hypogammaglobulinemia, infections, and myelokathexis syndrome. J Pediatr. 2012;161:763–765. doi: 10.1016/j.jpeds.2012.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mc Guire PJ, Cunningham-Rundles C, Ochs H, Diaz GA. Oligoclonality, impaired class switch and B-cell memory responses in WHIM syndrome. Clin Immunol. 2010;135:412–421. doi: 10.1016/j.clim.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al Ustwani O, Kurzrock R, Wetzler M. Genetics on a WHIM. Br J Haematol. 2014;164:15–23. doi: 10.1111/bjh.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tassone L, Moratto D, Vermi W, De Francesco M, Notarangelo LD, Porta F, et al. Defect of plasmacytoid dendritic cells in warts, hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome patients. Blood. 2010;116:4870–4873. doi: 10.1182/blood-2010-03-272096. [DOI] [PubMed] [Google Scholar]
- 73.McDermott DH, Liu Q, Ulrick J, Kwatemaa N, Anaya-O'Brien S, Penzak SR, et al. The CXCR4 antagonist plerixafor corrects panleukopenia in patients with WHIM syndrome. Blood. 2011;118:4957–4962. doi: 10.1182/blood-2011-07-368084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dale DC, Bolyard AA, Kelley ML, Westrup EC, Makaryan V, Aprikyan A, et al. The CXCR4 antagonist plerixafor is a potential therapy for myelokathexis, WHIM syndrome. Blood. 2011;118:4963–4966. doi: 10.1182/blood-2011-06-360586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McDermott DH, Liu Q, Velez D, Lopez L, Anaya-O'Brien S, Ulrick J, et al. A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood. 2014;123:2308–2216. doi: 10.1182/blood-2013-09-527226. [DOI] [PMC free article] [PubMed] [Google Scholar]