Abstract
The menopause transition is marked by increased prevalence in disturbed sleep and insomnia, present in 40–60% of women, but evidence for a physiological basis for their sleep complaints is lacking. We aimed to quantify sleep disturbance and the underlying contribution of objective hot flashes in 72 women (age range: 43–57 years) who had (38 women), compared to those who had not (34 women), developed clinical insomnia in association with the menopause transition. Sleep quality was assessed with two weeks of sleep diaries and one laboratory polysomnographic (PSG) recording. In multiple regression models controlling for menopause transition stage, menstrual cycle phase, depression symptoms, and presence of objective hot flashes, a diagnosis of insomnia predicted PSG-measured total sleep time (p<0.01), sleep efficiency (p=0.01) and wakefulness after sleep onset (WASO) (p=0.01). Women with insomnia had, on average, 43.5 minutes less PSG-measured sleep time (p<0.001). There was little evidence of cortical EEG hyperarousal in insomniacs apart from elevated beta EEG power during REM sleep. Estradiol and follicle stimulating hormone levels were unrelated to beta EEG power but were associated with the frequency of hot flashes. Insomniacs were more likely to have physiological hot flashes, and the presence of hot flashes predicted the number of PSG-awakenings per hour of sleep (p=0.03). From diaries, women with insomnia reported more WASO (p=0.002), more night-to-night variability in WASO (p<0.002) and more hot flashes (p=0.012) compared with controls. Women who develop insomnia in the approach to menopause have a measurable sleep deficit, with almost 50% of the sample having less than 6 hours of sleep. Compromised sleep that develops in the context of the menopause transition should be addressed, taking into account unique aspects of menopause like hot flashes, to avoid the known negative health consequences associated with insufficient sleep and insomnia in midlife women.
Keywords: menopause, sleep, insomnia, hot flashes, midlife women, health, estradiol
1. Introduction
Sleep difficulties, particularly night-time awakenings, are a major complaint in the approach to menopause, being present in 40–60% of women (Joffe et al., 2010; Polo-Kantola, 2011) and are one of the top health issues in peri- and postmenopausal women (van Dijk et al., 2015). Together with hot flashes/night sweats, feeling tired and weight gain, sleep problems are the most common symptoms women discuss with their healthcare providers (Williams et al., 2007). In 26% of perimenopausal women (a prevalence that is higher than in premenopausal or postmenopausal women), sleep disturbances cause significant distress and impact women’s daytime functioning qualifying them for a diagnosis of insomnia disorder (Ohayon, 2006).
Untreated insomnia is associated with several adverse physical (e.g. hypertension, stroke, diabetes) and psychological (e.g. depression) consequences (Buysse, 2013; Irwin, 2015), yet it is under-appreciated and under-treated, possibly due to its subjective diagnostic nature and multifactorial etiology making it difficult to recognize and treat. The economic burden of insomnia is substantial, with decreased productivity and increased healthcare utilization (Buysse, 2013). The impact of insomnia is particularly relevant in midlife women; in the United States, an estimated 2 million women reach menopause every year (The North American Menopause Society, 2010) and by 2030 more than 1.2 billion women will be 50 years or older.
There is overwhelming evidence of an increase in perceived sleep difficulties as women approach menopause from several large longitudinal studies like the Study of Women’s Health Across the Nation (SWAN) (Kravitz and Joffe, 2011), the Australian Longitudinal Study on Women’s Health (Berecki-Gisolf et al., 2009), and the Seattle Midlife Women’s Health study (Woods and Mitchell, 2010). However, polysomnographic (PSG) evidence of poorer sleep during this stage is sparse (Shaver et al., 1988; Young et al., 2003; Kalleinen et al., 2008), with a large epidemiological study showing even better PSG sleep in peri- and postmenopausal women, despite less sleep satisfaction, compared with premenopausal women (Young et al., 2003). Similarly, while there is substantial evidence associating self-reported hot flashes and self-reported sleep disturbances in peri- and post-menopausal women, there are conflicting data about a link between menopausal hot flashes and PSG-defined measures of sleep quality (Joffe et al., 2010). The discordance between subjective perceptions and “objective” PSG and hot flash findings has led to a lack of clarity in the medical field about the nature of sleep problems in midlife women and how to treat them most effectively.
Conflicting results, both when considering subjective versus objective measurement of sleep as well as across PSG studies, could be due to several factors, including the possibility that subjective and objective sleep assessments tap into different constructs, different analytical approaches, and poor characterization of sub-groups of women with and without clinically-significant sleep disturbances. The presence of insomnia disorder, which reflects a more severe form of interference of poor sleep on a woman’s quality of life and daytime functioning, has not been previously considered.
Here, the major aim was to determine whether there is physiological evidence of disturbed sleep, based on PSG and spectral EEG analysis, in women who developed DSM-IV insomnia in the context of the menopause transition (with no past history of insomnia disorder) compared with age-matched women in the menopause transition without insomnia. We also investigated the contribution of physiological night-time hot flashes to physiological sleep disturbance. We hypothesized that women with insomnia would have more hot flashes and that a diagnosis of insomnia would be associated with more indications of difficulty maintaining sleep (e.g., more awakenings and more wakefulness within the sleep period, more high frequency EEG power) controlling for effects of menopausal stage, menstrual cycle phase, presence of hot flashes, and depression symptoms.
2. Method
2.1. Participants and Procedure
The study was reviewed and approved by SRI International’s Institutional Review Board. Participants were recruited from the San Francisco Bay Area community area through flyers, announcements, advertisements, or word of mouth. 72 participants gave written informed consent and received compensation for participation. Sample characteristics and screening procedures are fully described in Sassoon et al. (2014). Briefly, all women had to be in the menopause transition (menstrual cycle lengths that differed by more than 7 days from normal, early menopausal transition; occurrence of amenorrhea of 60 days or longer, late menopausal transition), according to Stages of Reproductive Aging Workshop (STRAW) criteria (Soules et al., 2001), have an intact uterus and at least one ovary, and a body mass index (BMI) of 32 kg.m−2 or lower. None of the women had ≥ 12 months of amenorrhea. Exclusion criteria were use of hormone therapy, severe medical conditions (e.g., hypertension or diabetes), or current sleep medication and/or antidepressant use.
All women underwent a structured clinical interview (SCID) (First et al., 1998) including a customized module querying DSM-IV criteria for insomnia (Morin and Espie, 2003). Thirty-eight women (27 Caucasian) met criteria for an insomnia disorder, presenting with a prominent self-described disturbance in sleep characterized by difficulty falling asleep (23.7%), difficulty maintaining sleep (92.1%), and/or early morning awakening (60.5%), evident at least three times per week for at least a month, and associated with clinically significant distress/impairment. Sleep disturbance needed to be coincident with the onset of the menopause transition; none of the participants had a lifetime history of DSM-IV insomnia, or current other Axis-I disorders (except nicotine dependence, in 4 controls and 5 insomniacs). Thirty-four women (26 Caucasian) without sleep complaints were included as controls.
According to STRAW criteria, 20 women with insomnia and 27 controls were in the early menopause transition while the remaining women were in the late menopause transition (7 controls and 18 insomniacs). All women had a clinical/adaptation in-lab PSG assessment, to confirm absence of sleep-disordered breathing (apnea-hypopnea index >5) and/or periodic limb movement disorder (periodic limb movement index >10). Women self-selected their bedtimes and wake-up times and slept in temperature-controlled, sound-attenuated bedrooms.
A recording PSG assessment was scheduled after the clinical/adaptation night, at which time a blood sample was collected. Serum samples were frozen at −70°C before analysis with standard immunoassay kits for follicle-stimulating hormone (FSH, IU) (Siemens Healthcare Diagnostics, Los Angeles, CA, USA; intraassay and interassay coefficients of variations were 2.6% and 5.5%, respectively) and estradiol (pg.mol−1) (Beckman Coulter Inc., Fullerton, CA, USA; intraassay and interassay coefficients of variations were 6.7% and 7.6%, respectively). Blood samples were not obtained from 2 controls and one insomniac. Thirty-five women had recordings in the follicular phase of their menstrual cycle (3–11 days after the first day of their period; 16 controls and 19 insomniacs), 11 women had recordings in the luteal phase of the menstrual cycle (progesterone ≥ 3 ng.ml−1; 8 controls and 3 insomniacs). 23 women had irregular, non–ovulatory cycles and were assessed on a random day (8 controls and 15 insomniacs). For the purposes of analysis, women were grouped into two groups (luteal and non-luteal).
2.2. Main Outcomes
Severity of psychological, somatic, and vasomotor symptoms was assessed with the Greene Climacteric Scale (GCS) (Greene, 1998). The Beck Depression Inventory (BDI-II) (Beck et al., 1996) was used to assess depression symptoms. Women completed sleep diaries (Monk et al., 1994) about timing and duration of sleep, awakenings and number of hot flashes. Sleep diary data were collected before PSG recordings. Only diaries with at least 10 days of reliable data within a 2-week period were included in analyses (six insomniacs and five controls were excluded). Variables were averaged and standard deviations were calculated as a measure of within-participant night-to-night variability (Buysse et al., 2010).
Electroencephalographic (EEG), electrooculographic and electromyographic signals were acquired using Compumedics amplifiers (Compumedics, Abbotsford, Victoria, Australia) according to American Academy of Sleep Medicine (AASM) criteria (Iber, 2007). EEG signals were sampled at 256Hz and band-pass filtered (0.03–35Hz). Sleep architecture was scored according to AASM rules (Iber, 2007). Upon awakening, women completed a brief questionnaire about their sleep (Monk et al., 1994).
EEG spectral analysis was conducted using EEGLAB (Delorme and Makeig, 2004). For each 30-sec epoch of artifact-free N2, N3 and rapid-eye-movement (REM) sleep stages, frequency power was calculated from C4, referenced to linked-mastoids, using a sliding 4096ms Hanning window. Absolute power (μV2.Hz−1) was calculated for each epoch in delta (0.3-<4Hz), theta (4-<8Hz), alpha (8-<12Hz), sigma (12-<15Hz) and beta (15-<30Hz) bands.
Nocturnal hot flashes were measured using a BioDerm Skin Conductance Meter (model 2701; UFI, Morro Bay, CA). Two 1.5 cm in diameter Ag/AgCl electrodes filled with 0.05 M potassium chloride Velvachol/glycol gel were placed on either side of the sternum and a 0.5-V constant voltage circuit was maintained between them. The Bioderm was connected via an optically isolated DC input to the Compumedics amplifiers and the skin conductance signal was sampled at 16 Hz and co-registered online as a polysomnographic channel. Hot flashes were manually evaluated by two scorers (F.C.B. and M.d.Z.), blinded to PSG scoring, for fluctuations meeting accepted standard criteria for physiological hot flashes, i.e. an increase of 2 micro Siemens (μmho) within 30-s (Freedman, 1989). Number of hot flashes was calculated. Skin conductance measures were missing in 2 insomniacs and 3 controls.
2.3. Statistical Analysis
Menopausal symptoms, BDI-II scores, serum hormone levels, and variables derived from sleep diaries were compared between groups using independent t-tests.
Multiple Regression models were used to investigate predictors of polysomnographic and morning self-report sleep variables, and power in EEG bands using Group (insomniacs and non-insomniacs), Menopause transition stage (early or late), Phase of the menstrual cycle (luteal or non-luteal), Presence of objective hot flashes (yes or no) and Depression (BDI-II scores) as independent factors entered in each model.
We also ran exploratory Pearson’s correlations separately for women with and without insomnia to investigate the association between hormone levels (estradiol or FSH) and beta EEG power, an indicator of arousal, shown by others to be influenced by menopausal status (Campbell et al., 2011). In addition, we explored the association between number of objective hot flashes and FSH and estradiol levels using Spearman’s Rank correlations.
FSH and estradiol levels and EEG power were log-transformed before analysis. Chi-squared (χ2) tests were used to compare the number of women in each group with short PSG sleep duration (<6 hours) or objective hot flashes. Mean ±SD and ±95%CI are provided for each of the variables analyzed. P-values of less than 0.05 were considered statistically significant.
3. Results
Groups did not differ in age, BMI, FSH, or estradiol levels (Table 1). Compared with controls, women with insomnia reported more severe psychological, somatic, and vasomotor symptoms, and had higher scores on the BDI-II (p<0.05).
Table 1.
Age, body mass index, menopausal symptoms, Beck Depression Inventory-II scores, and hormone levels in women in the menopause transition with and without insomnia.
| Women with insomnia (Mean ± SD, [95%CI]) |
Women without insomnia (Mean ± SD, [95%CI]) |
t | p | ||
|---|---|---|---|---|---|
| Sample (No.) | 38 | 34 | – | – | |
| Age (years) | 50.0 ± 3.0 [49.0–51.0] | 49.3 ± 2.6 [48.4–50.2] | −1.10 | 0.274 | |
| BMI (kg.m−2) | 24.3 ± 3.5 [23.1–25.4] | 24.7 ± 4.1 [23.3–26.2] | 0.53 | 0.600 | |
| GCS-psychological | 6.6 ± 3.1 [5.5–7.6] | 2.9 ± 2.2 [2.1–3.6] | −5.76 | <0.001 | ◄ |
| GCS-somatic | 2.5 ± 2.2 [1.8–3.2] | 1.5 ± 1.6 [0.9–2.0] | −2.21 | 0.031 | ◄ |
| GCS-vasomotor | 2.3 ± 1.3 [1.9–2.7] | 1.1 ± 1.0 [0.7–1.4] | −4.64 | <0.001 | ◄ |
| BDI-II scores | 7.8 ± 5.4 [6.0–9.5] | 2.9 ± 2.9 [1.9–3.9] | −4.64 | <0.001 | ◄ |
| FSH (IU)a | 40.0 ± 41.3 [26.2–53.8] | 22.7 ± 17.1 [16.5–28.8] | −1.40 | 0.181 | |
| Estradiol (pg.mol−1)a | 50.8 ± 38.5 [38.0–63.7] | 64.9 ± 47.5 [47.7–82.0] | 1.35 | 0.141 |
p<0.05
analysis performed on 37 insomniacs and 32 controls on log-transformed data; BMI = body mass index; BDI = Beck Depression Inventory; FSH = Follicle Stimulating Hormone; GCS = Greene Climacteric Scale
Overnight polysomnographic and self-report sleep assessments
Variables derived from the in-lab PSG assessment are shown in Table 2. Multiple regression models were significant for total sleep duration (R2=0.28, F5,66=5.04, p<0.001) and sleep efficiency (R2=0.18, F5,66=2.80, p=0.024) with Group as the only significant factor in each model. Women with insomnia had shorter sleep duration (t=−3.35, p=0.001) and a lower sleep efficiency (t=−2.49, p=0.015) compared with non-insomniacs. Follow-up analysis showed that more insomniacs (18 of 38) than controls (3 of 34) had a sleep duration less than six hours (χ2=12.90, p<0.001) (Figure 1). The model for wakefulness after sleep onset (WASO) (R2=0.19, F5,66=3.02, p=0.016) was also significant, with Group and Phase of the menstrual cycle as significant factors. Women with insomnia had a higher percentage of WASO than non-insomniacs (t=2.41, p=0.019). Women recorded in the luteal phase had more WASO compared to women recorded outside the luteal phase (t=2.12, p=0.038).
Table 2.
Objective and subjective sleep as assessed by polysomnography and morning sleep questionnaires in women in the menopause transition with and without insomnia.
| Women with insomnia (Mean ± SD, [95%CI]) |
Women without insomnia (Mean ± SD, [95%CI]) |
||
|---|---|---|---|
| Polysomnographic sleep | |||
| Sample (No.) | 38 | 34 | |
| Lights-out (hh:mm) | 23:11 ± 00:46 [22:56–23:26] | 22:54 ± 00:45 [22:39–23:09] | |
| Wake-up (hh:mm) | 06:21 ± 00:43 [06:07–06:35] | 06:24 ± 00:53 [06:07–06:35] | |
| TIB (min) | 428.1 ± 51.8 [411.0–445.1] | 452.1 ± 41.7 [437.6–466.7] | |
| TST (min) | 364.3 ± 37.4 [352.0–376.6] | 406.8 ± 37.2 [393.8–419.8] | ◄ |
| SE (%)c | 85.6 ± 7.5 [83.2–88.1] | 90.1 ± 4.7 [88.5–91.7] | ◄ |
| SOL (min) | 11.0 ± 8.9 [8.1–14.0] | 9.5 ± 8.5 [6.5–12.5] | |
| REML (min)a | 86.3 ± 34.2 [74.9–97.7] | 89.2 ± 26.7 [79.9–98.5] | |
| Arousal index (No/h) | 8.7 ± 3.3 [7.6–9.8] | 8.1 ± 3.0 [7.0–9.1] | |
| Awakening index (No/h) | 3.7 ± 1.4 [3.2–4.2] | 3.2 ± 1.2 [2.8–3.6] | |
| WASO (%)c | 11.9 ± 7.1 [9.6–14.2] | 7.8 ± 4.1 [6.4–9.2] | ◄ (†) |
| Time in N1 (%)c | 7.7 ± 3.5 [6.6–8.9] | 7.3 ± 2.9 [6.3–8.3] | |
| Time in N2 (%)c | 46.2 ± 6.7 [43.9–48.3] | 49.8 ± 6.6 [47.5–52.1] | |
| Time in N3 (%)c | 12.1 ± 6.8 [9.8–14.3] | 12.6 ± 4.5 [11.1–14.2] | |
| Time in REM (%)c | 19.5 ± 5.0 [17.9–21.1] | 20.5 ± 4.8 [18.8–22.1] | |
| Morning self-report sleep evaluation | |||
| TST (min)b | 371.8 ± 56.0 [352.8–390.7] | 414.2 ± 42.9 [399.0–429.4] | ◄ |
| SOL (min)b | 22.5 ± 29.9 [12.4–32.6] | 15.4 ± 13.7 [10.6–20.1] | |
| WASO (min)b | 32.6 ± 32.4 [21.8–43.4] | 21.8 ± 22.2 [13.9–29.6] | |
significant Multiple Regression Models with group as a significant factor
significant Multiple Regression Models with luteal phase as a significant factor
one insomniac has been removed from the analysis (>3SD outside the mean)
one control and one insomniac did not complete the morning questionnaire
calculated as percentage of time spent in bed; TIB = Time in Bed; TST = Total Sleep Time; SE = Sleep Efficiency; SOL = Sleep Onset Latency; REM = Rapid Eye Movement (L, Latency); WASO = Wake After Sleep Onset
Figure 1.
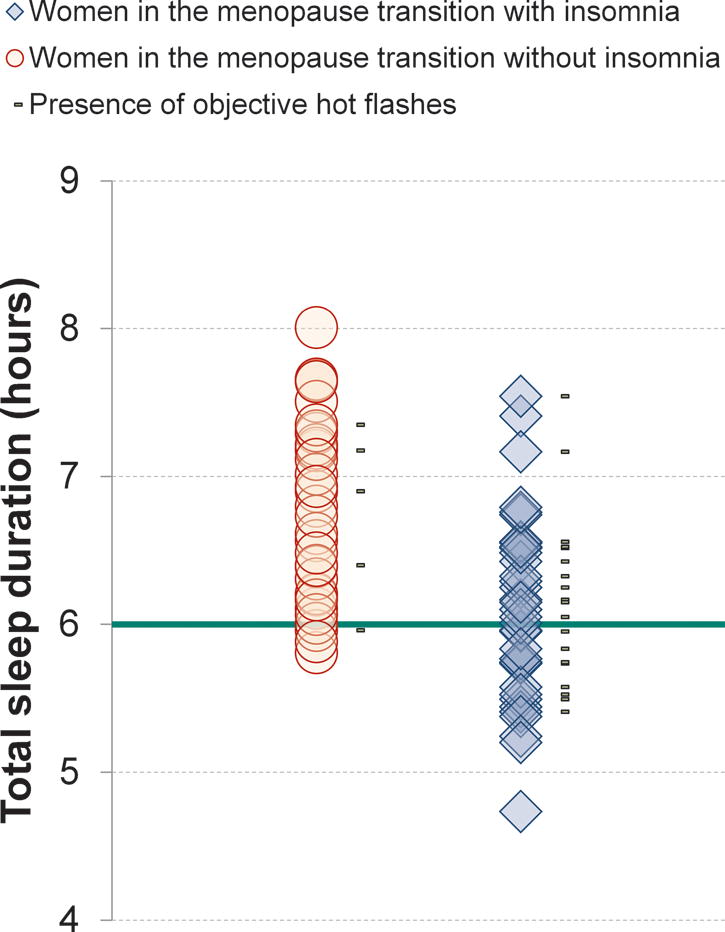
Polysomnographic-defined sleep duration in women with (n = 38) and without (n = 34) insomnia on a single overnight recording in the laboratory. Eighteen insomniacs and three controls had less than six hours total sleep duration (green line on the graph). Gray marks are placed next to the symbols of women in whom hot flashes were objectively identified based on skin conductance measurements.
The multiple regression model was significant for awakening index (number of awakenings per hour of sleep, R2=0.16, F5,66=2,58, p=0.034), with Presence of objective hot flashes as the only significant factor. Women having hot flashes had more awakenings per hour of sleep than women without hot flashes (t=2.17, p=0.033). The regression model was also significant for TIB (R2=0.16, F5,66=2,47, p=0.041) with Presence of objective hot flashes as a significant factor. Women having hot flashes had more time in bed compared to those without hot flashes (t=2.00, p=0.049). None of the models predicting percentage time in any sleep stage was significant.
The multiple regression model for self-reported total sleep time was significant (R2=0.18, F5,63=2.81, p=0.024) with Group as the only significant factor: women with insomnia reported a shorter sleep duration compared with controls (t=−2.37, p=0.021) (Table 2).
Insomniacs were more likely than controls to have at least one objective hot flash during the PSG recording (20 of 36 insomniacs versus 5 of 31 controls, χ2=11.07, p<0.001). 59 hot flashes were recorded in the insomniac group whereas 13 hot flashes were recorded in controls.
EEG microstructure
Absolute EEG power for delta, theta, alpha, sigma and beta bands within N2, N3 and REM sleep, are provided in Supplementary Table 3. The only regression model with a significant effect of Group was for beta EEG power in REM sleep (R2=0.18, F5,66=2.81, p=0.023) with insomniacs showing elevated beta EEG power compared to non-insomniacs (t=2.11, p=0.038). Phase of the menstrual cycle was also a significant factor in the model (t=3.41, p=0.001): women recorded in the luteal phase had higher beta EEG power in REM sleep. The model for sigma EEG power in REM sleep was significant (R2=0.16, F5,66=2.51, p=0.038), with Phase of the menstrual cycle as the only significant factor (t=3.25, p=0.002): women recorded in the luteal phase had higher sigma EEG power in REM sleep than women recorded at other times. Finally, the model for alpha EEG power in N3 sleep was significant (R2=0.19, F5,66=3.14), with Phase of the menstrual cycle as the only significant factor (t=3.21, p=0.002): women recorded in the luteal phase had higher alpha power than women recorded at other times.
Relationships with hormone levels
There were no significant relationships between FSH or estradiol levels and beta EEG power in any sleep stage in insomniacs or non-insomniacs. Higher FSH levels (N=64, R=0.44, t=3.87, p<0.001) and lower estradiol levels (N=64, R=−0.47, t=−4.16, p<0.001) correlated with more objective nocturnal hot flashes.
Sleep diary assessments
Variables derived from sleep diaries, showing the women’s usual sleep patterns, are shown in Table 4. Women with insomnia reported a shorter sleep duration (p=0.044), more wake after sleep onset (WASO, p=0.002), more nocturnal awakenings (p=0.050), and more hot flashes (p=0.012) than controls. Insomniacs also showed higher night-to-night variability in WASO than controls (p<0.001). Ten insomniacs and one control had self-reported average sleep durations of less than 6 hours.
Table 4.
Sleep patterns and night-to-night variability in sleep as assessed by 2 weeks of sleep diaries in women in the menopause transition with (n = 32) and without (n = 29) insomnia.
| Women with insomnia (Mean ± SD, [95%CI]) |
Women without insomnia (Mean ± SD, [95%CI]) |
t | p | ||
|---|---|---|---|---|---|
| Sample (No.) | 32 | 29 | – | – | |
| Nights analyzed | 12.8 ± 1.7 [12.2–13.4] | 12.9 ± 1.9 [12.2–13.6] | 0.27 | 0.79 | |
| Lights-out time (hh:mm) | 23:08 ± 00:37 [22:55–23.21] | 23:05 ± 00:40 [22:50–23:20] | 0.33 | 0.75 | |
| Night-to-night variability | 00:47 ± 00:19 [00:40–00:53] | 00:45 ± 00:21 [00:37–00:53] | −0.35 | 0.73 | |
| Wake-up time (hh:mm) | 06:32 ± 00:54 [06:12–06:51] | 06:34 ± 00:48 [06:16–06:52] | 0.16 | 0.87 | |
| Night-to-night variability | 00:50 ± 00:18 [00:43–00:56] | 00:45 ± 00:19 [00:38–00:52] | −0.97 | 0.34 | |
| TIB (min) | 443.9 ± 44.3 [428.0–459.9] | 449.4 ± 37.8 [435.0–463.7] | 0.51 | 0.61 | |
| Night-to-night variability | 59.5 ± 19.2 [52.5–66.4] | 57.9 ± 23.8 [48.9–66.9] | −0.28 | 0.78 | |
| TST (min) | 396.4 ± 53.9 [376.9–415.8] | 421.6 ± 40.0 [406.3–436.8] | 2.06 | 0.044 | ◄ |
| Night-to-night variability | 67.7 ± 21.2 [60.1–75.4] | 60.6 ± 24.5 [51.3–69.9] | −1.22 | 0.23 | |
| SOL (min) | 13.8 ± 11.2 [9.7–17.8] | 11.4 ± 8.8 [8.1–14.8] | −1.00 | 0.37 | |
| Night-to-night variability | 9.8 ± 8.9 [6.6–13.0] | 10.5 ± 13.3 [5.4–15.5] | 0.23 | 0.82 | |
| WASO (min) | 33.8 ± 22.4 [25.7–41.8] | 17.0 ± 17.1 [10.5–23.5] | −3.27 | 0.002 | ◄ |
| Night-to-night variability | 30.0 ± 18.1 [23.5–36.5] | 15.1 ± 14.6 [9.6–20.7] | −3.52 | <0.001 | ◄ |
| N awakenings | 2.1 ± 1.3 [1.6–2.6] | 1.5 ± 1.0 [1.1–1.9] | −2.00 | 0.050 | |
| N hot flashes | 1.6 ± 2.3 [0.8–2.5] | 0.5 ± 1.0 [0.1–0.8] | −2.58 | 0.012 | ◄ |
p<0.05; TIB = Time in Bed; TST = Total Sleep Time; SOL = Sleep Onset Latency; WASO = Wake After Sleep Onset
4. Discussion
Sleep difficulties are common and cause clinical distress in a significant proportion of women approaching menopause. We show that women with no past-history of sleep difficulties, who developed DSM-IV insomnia disorder in the approach to menopause, have a severe sleep deficit, with shorter sleep duration, more WASO, and poorer sleep efficiency compared to women in the menopause transition without insomnia. Nocturnal physiological hot flashes are more common in insomniacs and contribute to nocturnal awakenings.
Several studies have evaluated PSG-defined sleep in peri- compared with post- and pre-menopausal women, with most showing no difference in PSG-defined sleep patterns (Joffe et al., 2010). The present study is the first, to our knowledge, to investigate PSG and EEG sleep measures within a group of women all in the menopause transition, comparing those who had or had not developed insomnia in association with their transition. The sleep deficit in the insomniacs is substantial; PSG data indicated that they slept, on average, 6 hours and 4 minutes, with 47.4% of them sleeping less than 6 hours. The shorter sleep duration in insomniacs compared with controls (whose average sleep was 6 hours and 46 minutes) was evident in subjective sleep ratings and was not specific to the laboratory environment, being apparent across several days of monitoring with sleep diaries. The National Sleep Foundation recommends 7–9 hours of sleep in healthy adults; less than 6 hours is not recommended (Hirshkowitz et al., 2015) and is associated with increased risk for poor health outcomes including stroke and diabetes (Chen et al., 2008; Holliday et al., 2013; Irwin, 2015).
Insomnia also is an independent risk factor for poor health (Irwin, 2015) and the most dramatic consequences arise when insomnia and short sleep duration coexist. Individuals with combined insomnia and PSG-defined short sleep duration (<6 hours) have an increased risk of hypertension (Vgontzas et al., 2009a; Fernandez-Mendoza et al., 2012) and Type-2 Diabetes (Vgontzas et al., 2009b), and deficits in executive attention (Fernandez-Mendoza et al., 2010). The presence of PSG-defined short sleep duration (<6 hours) in insomnia is a strong predictor of persistent insomnia years later (Vgontzas et al., 2012).
While many women approaching menopause report that their sleep becomes poorer (Joffe et al., 2010; Kravitz and Joffe, 2011), not all women have persistent sleep complaints that interfere with their daily functioning. We have previously shown that predisposing factors like personality traits of higher neuroticism, lower agreeableness, and lower conscientiousness, and a history of depression and/or premenstrual dysphoric disorder are evident in women who developed insomnia in the approach to menopause (Sassoon et al., 2014). A major precipitating factor in the context of menopause-associated insomnia is menopausal symptoms-most prominently hot flashes-which can also act as perpetuating factors. Our findings that self-reported and physiological hot flashes are more common in women with insomnia than controls and that the presence of objective hot flashes predicts the number of PSG-defined awakenings argue for a role of hot flashes in contributing to sleep disturbance. These findings support early work showing an association between waking episodes and menopausal hot flashes (Erlik et al., 1981; Gonen et al., 1986) and that of Joffe et al. (2013), who used a Gonadotropin-releasing hormone agonist model in young women to show that hot flashes interrupt PSG-defined sleep. As shown in our previous work (de Zambotti et al., 2014), hot flash-associated wake time can vary in its contribution to overall WASO. Reasons why hot flashes contribute to variable amounts of wakefulness between women could relate to severity and duration of hot flashes as well as individual traits such as elevated anxiety, leading to greater difficulty in returning to sleep following a hot flash. Higher levels of anxiety and psychological symptoms are common in insomnia (Hiller et al., in press), and the insomniacs in our study scored higher on the psychological domain of the Greene Climacteric Scale and had more severe depression symptoms, although none of the women met DSM-IV criteria for a depressive disorder. However, too few controls had physiological hot flashes for us to investigate whether hot flash-associated wake time differed between groups.
A higher number of nocturnal hot flashes was associated with lower estradiol and higher FSH levels, supporting a large body of evidence showing an association between changes in these hormones across the menopause transition and self-reported hot flashes (see Randolph Jr et al., 2005). Hormone therapy effectively treats hot flashes and consequently alleviates subjective sleep problems in many women (Polo-Kantola, 2011). Treatment of hot flashes with either selective serotonin or serotonin-norepinephrine reuptake inhibitors also reduces insomnia symptoms in peri- and post-menopausal women with hot flashes (Ensrud et al., 2012; Ensrud et al., 2015; Pinkerton et al., 2015). Further trials are needed to determine the effectiveness of these and other treatments in improving PSG-defined sleep in women with insomnia that develops in the context of the menopause transition.
Factors other than hot flashes may contribute to the development of insomnia. Most notably, the approach to menopause is characterized by erratic and overall declining estradiol levels, and increasing FSH levels (Santoro, 2005). Animal studies provide evidence for an impact of reproductive hormones on sleep-wake regulatory mechanisms (Mong et al., 2011) and there is evidence for an association between reproductive hormone levels and objective and subjective sleep quality in perimenopausal women (Hollander et al., 2001; Dennerstein et al., 2007; Kravitz et al., 2008; Sowers et al., 2008;de Zambotti et al., in press). However, we recently showed that the relationships between FSH and PSG-defined wake measures we found in pre- and peri- menopausal women without insomnia were not evident in perimenopausal insomniacs (de Zambotti et al., in press). Menstrual cycle related hormonal changes may also impact sleep in women who are still cycling in the menopause transition. We found that being in the luteal phase predicted WASO and alpha power in NREM sleep and high frequency activity (sigma and beta bands) in REM sleep, suggesting increased arousal. These findings support data from the SWAN study showing that actigraphy-derived sleep measures varied with menstrual phase in midlife women, with total sleep time and sleep efficiency being lowest in the premenstrual (late-luteal) phase (Zheng et al., 2015). However, since there were only 11 women in the luteal phase and the analysis was between groups, our findings should be interpreted cautiously.
While we found substantial group differences in PSG-defined sleep measures, there was little evidence of differences in sleep EEG measures apart from higher beta EEG power (but only during REM sleep) in insomniacs. Higher beta EEG power during sleep is taken as an indicator of cortical arousal and is a common finding in studies of insomniacs (Bonnet and Arand, 2010). A previous study that investigated quantitative sleep EEG measures in perimenopausal women found higher beta EEG activity during NREM and REM sleep, partly explained by the greater frequency of self-reported hot flashes, in late peri- and postmenopausal women compared to early peri- and premenopausal women (Campbell et al., 2011); however, they did not compare EEG measures between women with and without insomnia. Menopausal stage (early versus late transition) was not a significant factor in any of the multiple regression models for EEG frequency bands in our analysis and we did not find any significant relationships between FSH or estradiol levels and beta EEG activity in any sleep stage in an exploratory analysis. Future studies should consider a longitudinal analysis of changes in hormones in association with changes in the sleep EEG spectrum.
Our study has limitations. The analysis was cross-sectional. A longitudinal analysis is needed to track changes in sleep in relation to hormonal changes and the development of hot flashes in women with predisposing risk factors for insomnia compared to controls as well as to track whether sleep disruption resolves when hot flashes resolve. We did not evaluate impact of poor objective sleep quality on cognitive function or daytime performance in the insomniacs. However, as a requirement for a diagnosis of insomnia, all women reported daytime impact and/or distress associated with their sleep complaint. Our findings relate to non-treatment seeking women from the community who developed insomnia during the menopause transition. Possibly, women with pre-existing insomnia may be impacted differently by the menopause transition. Our study is also limited by having only one night of PSG data in the analysis. However, we incorporated several days of sleep diaries into the analysis, which supported the major findings from the laboratory-based PSG study, providing greater ecological validity to our study.
A notable strength of our study is that we compared two groups of well-characterized women, matched according to age and menopausal status. Women were clinically interviewed to determine the presence of insomnia that had developed in the context of the menopausal transition. We used PSG and quantitative EEG measures to assess objective sleep quality and we also evaluated physiological hot flashes. Finally, we used clinical PSG to confirm the absence of breathing-related or periodic limb movement sleep disorders, which are important predictors of objective sleep quality in midlife women (Freedman and Roehrs, 2007).
5. Conclusions
Difficulty sleeping in women who developed insomnia in the context of the menopause transition is not limited to their perception, being supported by a measurable PSG-defined sleep deficit. Hot flashes, measured in more than half of the insomniacs, contribute to a poorer PSG sleep. Insomnia in midlife women should be routinely assessed and appropriately treated early on to reduce the risk for psychological and medical adverse consequences and to maintain a better quality of life.
Supplementary Material
Hgihlights.
Insomnia that develops in the approach to menopause is poorly characterized
Perimenopausal women with insomnia have a measurable sleep deficit
Hot flashes, frequent in insomniacs, contribute to their poorer sleep quality
Insomnia in perimenopausal women should be treated to avoid negative consequences
Acknowledgments
This study was performed at the SRI International (Menlo Park, CA, USA) and was supported by National Institutes of Health, Grant HL103688 to Dr Fiona C Baker. Hormone analysis was conducted by The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core, which is supported by the Eunice Kennedy Shriver NICHD/NIH (NCTRI) Grant P50-HD28934.
We thank our research assistants Justin Greco, David Sugarbaker, David Dresser, Stephanie Claudatos, Sarah Inkelis, Lena Kardos, and Rebecca Carr for their excellent effort in collecting data for this project. We also thank Dr. Harold Javitz for his statistical expertise in guiding the analysis of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: none.
References
- Beck A, Steer R, Brown G. Beck Depression Inventory-II (BDI-II) San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Berecki-Gisolf J, Begum N, Dobson AJ. Symptoms reported by women in midlife: menopausal transition or aging? Menopause. 2009;16:1021–1029. doi: 10.1097/gme.0b013e3181a8c49f. [DOI] [PubMed] [Google Scholar]
- Bonnet M, Arand D. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Buysse DJ. Insomnia. JAMA. 2013;309:706–716. doi: 10.1001/jama.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Cheng Y, Germain A, Moul DE, Franzen PL, Fletcher M, Monk TH. Night-to-Night Sleep Variability in Older Adults with and Without Chronic Insomnia. Sleep Med. 2010;11:56–64. doi: 10.1016/j.sleep.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I, Bromberger J, Buysse D, Hall M, Hardin K, Kravitz H, Matthews K, Rasor M, Utts J, Gold E. Evaluation of the association of menopausal status with delta and beta EEG activity during sleep. Sleep. 2011;34:1561–1568. doi: 10.5665/sleep.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J-C, Brunner RL, Ren H, Wassertheil-Smoller S, Larson JC, Levine DW, Allison M, Naughton MJ, Stefanick ML. Sleep Duration and Risk of Ischemic Stroke in Postmenopausal Women. Stroke; a journal of cerebral circulation. 2008;39:3185–3192. doi: 10.1161/STROKEAHA.108.521773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zambotti M, Colrain IM, Baker FC. Interaction between reproductive hormones and physiological sleep in women. J Clin Endocrinol Metab. doi: 10.1210/jc.2014-3892. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zambotti M, Colrain IM, Javitz HS, Baker FC. Magnitude of the impact of hot flashes on sleep in perimenopausal women. Fertil Steril. 2014;102:1708–1715. e1701. doi: 10.1016/j.fertnstert.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of neuroscience methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dennerstein L, Lehert P, Burger H, Guthrie J. New findings from non-linear longitudinal modelling of menopausal hormone changes. Hum Reprod Update. 2007;13:551–557. doi: 10.1093/humupd/dmm022. [DOI] [PubMed] [Google Scholar]
- Ensrud K, Guthrie K, Hohensee C, Caan B, Carpenter J, Freeman E, LaCroix A, Landis C, Manson J, Newton K. Effects of Estradiol and Venlafaxine on Insomnia Symptoms and Sleep Quality in Women with Hot Flashes. Sleep. 2015;38:97–108. doi: 10.5665/sleep.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensrud KE, Joffe H, Guthrie KA, Larson JC, Reed SD, Newton KM, Sternfeld B, LaCroix AZ, Landis CA, Woods NF. Effect of escitalopram on insomnia symptoms and subjective sleep quality in healthy menopausal women with hot flashes: a randomized controlled trial. Menopause. 2012;19:848–855. doi: 10.1097/gme.0b013e3182476099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlik Y, Tataryn I, Meldrum D, Lomax P, Bajorek J, Judd H. Association of waking episodes with menopausal hot flushes. JAMA. 1981;245:1741–1744. [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Calhoun S, Bixler EO, Pejovic S, Karataraki M, Liao D, Vela-Bueno A, Ramos-Platon MJ, Sauder KA, Vgontzas AN. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep. 2010;33:459–465. doi: 10.1093/sleep/33.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Vgontzas A, Liao D, Shaffer M, Vela-Bueno A, Basta M, Bixler E. Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort. Hypertension. 2012;60:929–935. doi: 10.1161/HYPERTENSIONAHA.112.193268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Version 2.0. Biometrics Research Department, New York State Psychiatric Institute; New York, NY: 1998. [Google Scholar]
- Freedman R. Laboratory and ambulatory monitoring of menopausal hot flashes. Psychophysiology. 1989;26:573–579. doi: 10.1111/j.1469-8986.1989.tb00712.x. [DOI] [PubMed] [Google Scholar]
- Freedman R, Roehrs T. Sleep disturbance in menopause. Menopause. 2007;14:826–829. doi: 10.1097/GME.0b013e3180321a22. [DOI] [PubMed] [Google Scholar]
- Gonen R, Sharf M, Lavie P. The association between mid-sleep waking episodes and hot flushes in post-menopausal women. Journal of Psychosomatic Obstetrics & Gynecology. 1986;5:113–117. [Google Scholar]
- Greene JG. Constructing a standard climacteric scale. Maturitas. 1998;29:25–31. doi: 10.1016/s0378-5122(98)00025-5. [DOI] [PubMed] [Google Scholar]
- Hiller RM, Johnston A, Dohnt H, Lovato N, Gradisar M. Assessing cognitive processes related to insomnia: A review and measurement guide for Harvey’s cognitive model for the maintenance of insomnia. Sleep Med Rev. doi: 10.1016/j.smrv.2014.11.006. in press. [DOI] [PubMed] [Google Scholar]
- Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, Hazen N, Herman J, Katz ES, Kheirandish-Gozal L. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health: Journal of the National Sleep Foundation. 2015 doi: 10.1016/j.sleh.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Hollander L, Freeman E, Sammel M, Berlin J, Grisso J, Battistini M. Sleep quality, estradiol levels, and behavioral factors in late reproductive age women. Obstet Gynecol. 2001;98:391397. doi: 10.1016/s0029-7844(01)01485-5. [DOI] [PubMed] [Google Scholar]
- Holliday E, Magee C, Kritharides L, Banks E, Attia J. Short sleep duration is associated with risk of future diabetes but not cardiovascular disease: a prospective study and meta-analysis. PloS one. 2013;8:e82305. doi: 10.1371/journal.pone.0082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber C. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. American Academy of Sleep Medicine 2007 [Google Scholar]
- Irwin M. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol. 2015;66:143–172. doi: 10.1146/annurev-psych-010213-115205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe H, Massler A, Sharkey K. Evaluation and management of sleep disturbance during the menopause transition. Semin Reprod Med. 2010;28:404–421. doi: 10.1055/s-0030-1262900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe H, White D, Crawford S, McCurnin K, Economou N, Connors S, Hall J. Adverse effects of induced hot flashes on objectively recorded and subjectively reported sleep: results of a gonadotropin-releasing hormone agonist experimental protocol. Menopause. 2013;20:905–914. doi: 10.1097/GME.0b013e31828292d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalleinen N, Polo-Kantola P, Himanen S, Alhola P, Joutsen A, Urrila A, Polo O. Sleep and the menopause-do postmenopausal women experience worse sleep than premenopausal women? Menopause Int. 2008;14:97–104. doi: 10.1258/mi.2008.008013. [DOI] [PubMed] [Google Scholar]
- Kravitz H, Joffe H. Sleep during the perimenopause: a SWAN story. Obstet Gynecol Clin North Am. 2011;38:567–586. doi: 10.1016/j.ogc.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz HM, Zhao X, Bromberger JT, Gold EB, Hall MH, Matthews KA, Sowers MR. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31:979–990. [PMC free article] [PubMed] [Google Scholar]
- Mong J, Baker F, Mahoney M, Paul K, Schwartz M, Semba K, Silver R. Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J Neurosci. 2011;31:16107–16116. doi: 10.1523/JNEUROSCI.4175-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk T, Reynolds C, Kupfer D, Buysse D, Coble P, Hayes A, MacHen M, Petrie S, Ritenour A. The Pittsburgh Sleep Diary. J Sleep Res. 1994;3:111–120. [PubMed] [Google Scholar]
- Morin CM, Espie CA. Insomnia: A Clinical Guide to Assessment and Treatment. Springer; 2003. [Google Scholar]
- Ohayon M. Severe hot flashes are associated with chronic insomnia. Arch Intern Med. 2006;166:1262–1268. doi: 10.1001/archinte.166.12.1262. [DOI] [PubMed] [Google Scholar]
- Pinkerton JV, Joffe H, Kazempour K, Mekonnen H, Bhaskar S, Lippman J. Low-dose paroxetine (7.5 mg) improves sleep in women with vasomotor symptoms associated with menopause. Menopause. 2015;22:50–58. doi: 10.1097/GME.0000000000000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo-Kantola P. Sleep problems in midlife and beyond. Maturitas. 2011;68:224–232. doi: 10.1016/j.maturitas.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Randolph J, Jr, Sowers M, Bondarenko I, Gold E, Greendale G, Bromberger J, Brockwell S, Matthews K. The relationship of longitudinal change in reproductive hormones and vasomotor symptoms during the menopausal transition. J Clin Endocrinol Metab. 2005;90:6106–6112. doi: 10.1210/jc.2005-1374. [DOI] [PubMed] [Google Scholar]
- Santoro N. The menopausal transition. Am J Med. 2005;118(Suppl 12B):18–13. doi: 10.1016/j.amjmed.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Sassoon S, de Zambotti M, Colrain I, Baker F. Association between personality traits and DSM-IV diagnosis of insomnia in peri-and postmenopausal women. Menopause. 2014;21:602–611. doi: 10.1097/GME.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaver J, Giblin E, Lentz M, Lee K. Sleep patterns and stability in perimenopausal women. Sleep. 1988;11:556–561. doi: 10.1093/sleep/11.6.556. [DOI] [PubMed] [Google Scholar]
- Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N. Executive summary: Stages of Reproductive Aging Workshop (STRAW) Fertil Steril. 2001;76:874–878. doi: 10.1016/s0015-0282(01)02909-0. [DOI] [PubMed] [Google Scholar]
- Sowers M, Zheng H, Kravitz H, Matthews K, Bromberger J, Gold E, Owens J, Consens F, Hall M. Sex steroid hormone profiles are related to sleep measures from polysomnography and the Pittsburgh Sleep Quality Index. Sleep. 2008;31:1339–1349. [PMC free article] [PubMed] [Google Scholar]
- The North American Menopause Society. Chapter 1: Overview of Menopause, Menopause Practice: A Clinician’s Guide. North American Menopause Society (4th) 2010 [Google Scholar]
- van Dijk G, Kavousi M, Troup J, Franco O. Health issues for menopausal women: The top 11 conditions have common solutions. Maturitas. 2015;80:24–30. doi: 10.1016/j.maturitas.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Vgontzas A, Fernandez-Mendoza J, Bixler E, Singareddy R, Shaffer M, Calhoun S, Liao D, Basta M, Chrousos G. Persistent insomnia: the role of objective short sleep duration and mental health. Sleep. 2012;35:61–68. doi: 10.5665/sleep.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009a;32:491–497. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia With Objective Short Sleep Duration Is Associated With Type 2 Diabetes: A population-based study. Diabetes Care. 2009b;32:1980–1985. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R, Kalilani L, DiBenedetti D, Zhou X, Fehnel S, Clark R. Healthcare seeking and treatment for menopausal symptoms in the United States. Maturitas. 2007;58:348–358. doi: 10.1016/j.maturitas.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Woods NF, Mitchell ES. Sleep symptoms during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Sleep. 2010;33:539–549. doi: 10.1093/sleep/33.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young T, Rabago D, Zgierska A, Austin D, Laurel F. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep. 2003;26:667–672. doi: 10.1093/sleep/26.6.667. [DOI] [PubMed] [Google Scholar]
- Zheng H, Harlow S, Kravitz H, Bromberger J, Buysse D, Matthews K, Gold E, Owens J, Hall M. Actigraphy-defined measures of sleep and movement across the menstrual cycle in midlife menstruating women: Study of Women’s Health Across the Nation Sleep Study. Menopause. 2015;22:66–74. doi: 10.1097/GME.0000000000000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


