EDITORIAL
The potential use of biological knowledge for nefarious purposes has attracted significant concern. The field of microbiology has come under particular scrutiny because some microbes and toxins are potential agents for bioterrorism and biological warfare. In 2005, the U.S. government established the National Science Advisory Board for Biosecurity (NSABB) to address issues related to biosecurity and dual-use research (http://osp.od.nih.gov/office-biotechnology-activities/biosecurity/nsabb). Over the past decade, the NSABB has considered several topics, including defining the boundary between research that requires no special oversight and research that could be misapplied, which is known as dual-use research of concern (DURC). One of the major accomplishments of the NSABB was to draft a definition for DURC as “life sciences research that, based on current understanding, can be reasonably anticipated to provide knowledge, information, products, or technologies that could be directly misapplied to pose a significant threat with broad potential consequences to public health and safety, agricultural crops and other plants, animals, the environment, materiel, or national security.” In addition to defining the type of research that should elicit heightened concern, the NSABB recommended that research be examined for DURC potential throughout its life span, from experimental conception to final dissemination of the results (http://osp.od.nih.gov/sites/default/files/resources/Framework%20for%20transmittal%20duplex%209-10-07.pdf) and developed tools for communicating findings that meet the definition (http://osp.od.nih.gov/sites/default/files/resources/Communication_Tools%20_Dual_Use_Potential.pdf). Furthermore, the NSABB sought to establish a culture of responsibility to mitigate risks associated with DURC that extended through the entire scientific enterprise and included journals and editors. In 2007, the American Society for Microbiology (ASM) responded to the NSABB directives by introducing a questionnaire in the manuscript referee review form used by its journals that asked reviewers to provide an assessment about whether the work involved experiments of concern.
Since the winter of 2012, the discipline of microbiology, and the field of virology in particular, has been convulsed by controversy about so-called “gain-of-function” (GOF) research, which involves an intense debate about the value of experimental work that imparts new properties such as increased host range, increased virulence, changes in transmissibility, and drug resistance to pathogens with pandemic potential, such as highly pathogenic avian influenza viruses (HPAIV) (1). This controversy began in 2011 with the submission of papers describing experiments involving the serial passage of H5N1 avian influenza virus in ferrets, which led to the selection of variants with capacity for ferret-to-ferret transmission. Four years later, a fierce debate continues with no end in sight about the value of information gained relative to the risks of conducting such work. Although the initial debate on GOF experiments was focused on biosecurity, the concerns have evolved to primarily encompass the area of biosafety. Experiments that increase host range, virulence, transmissibility, drug resistance, or some combination of these properties for dangerous microbes are thought by most to be DURC and have elicited considerable attention from journal editors, mainstream media, and the public.
The GOF debate has sensitized the microbiology community to the need for assessing manuscripts from the standpoint of biosecurity and biosafety in addition to the quality of the scientific work. A recent study of 127 editors in chief revealed that the majority agreed that it was important to consider biosecurity issues during manuscript review, but only a small minority had biosecurity experience (2). Furthermore, no editor had refused to publish a manuscript based on biosecurity grounds alone, and the study concluded that there was a need to develop standards for the review and publication of DURC (2). Indeed, we are aware of only one example of a journal allowing redaction of information based on biosecurity concerns (3, 4).
Some ASM journals have published papers that include potential DURC, and the ASM Journals Board has developed a process for evaluating such manuscripts prior to publication. We note that ASM publications, and in particular the Journal of Virology, were recently criticized for publishing a paper describing the adaptation of H7N1 HPAIV for transmission in ferrets without loss of virulence (5–7). Consequently, we provide here a description of the process used by ASM to evaluate manuscripts with potential DURC content. Our goal is to make the process transparent and illustrate the limitations of the current DURC framework. Given that very little has been published on how journals evaluate DURC-related manuscripts, we also hope that the description of the process provided here might be helpful to other journals.
DURC review at ASM journals.
After the 2011-2012 GOF controversy involving the H5N1 papers, the ASM instituted an ad hoc process of reviewing manuscripts with potential DURC content. Since that time, the process of reviewing manuscripts containing possible DURC has evolved into a formal process involving three distinct phases: screening, discussion, and decision (Fig. 1). The screening phase is designed to be rapid and unobtrusive while at the same time identifying manuscripts that require discussion. Screening begins with author-declared information in the submission cover letter, which can alert journal editors and reviewers to the need to consider biosafety and biosecurity issues in evaluating the work. After the manuscript arrives at ASM, the editorial staff screens the manuscript to determine whether the research involves one of the microbes or toxins listed in the Select Agents and Toxins List (SATL; http://www.selectagents.gov/SelectAgentsandToxinsList.html). If so, the manuscript receives special scrutiny. Submissions to the Journal of Virology are also screened for key phrases and wording that suggest DURC, such as “increased pathogenesis or virulence,” “increased transmission,” “escape from antibody,” “cross-species transmission,” and the type of work that has been associated with GOF experiments with HPAIV. Editors and reviewers enter the process when they evaluate the manuscript, with the latter being asked to specifically comment on DURC-related issues as part of the review process regardless of whether the content includes a microbe or toxin on the SATL or any of the targeted keywords. If anyone in the screening process identifies a DURC-related issue, the manuscript is referred to the editor in chief and chair of the Journals Board, for additional review. If no DURC-related issues are apparent, the manuscript proceeds to publication. However, if there is concern about DURC, the manuscript enters the discussion phase and is referred to the ASM Responsible Publication Committee (ARPC), which is composed of the five authors of this paper. When the paper under discussion comes from an ASM journal other than the Journal of Virology, mBio, and mSphere, which are represented on the ARPC by their editors in chief, that journal’s editor in chief serves as an ad hoc member of the committee. Members of the ARPC read the manuscript and generally confer initially by e-mail, which may progress to a teleconference if there are issues that require more in-depth discussion. At that point, the ARPC may solicit outside advice, including reaching out to the National Institutes of Health Office of Biotechnology Activities, the funding agency, the authors of the paper, or biosafety officers at the institution at which the research was conducted. Following evaluation by the ARPC, the manuscript moves to the decision phase with one of four possible outcomes: accept, reject, redact, or publish with an accompanying editorial explaining the decision to publish, describing the evaluation process, noting the biosafety and biosecurity risk mitigation in place, and highlighting the benefits of the research. Decisions of the ARPC do not require a unanimous vote. No paper has yet been redacted or rejected by the ARPC, although several have been published with accompanying editorials (6–10). Editorials explaining the decision to publish and the potential benefits of the research were advocated by the NSABB as a mechanism for enhanced communication with the public and also have been used by other journals publishing DURC-related papers (3, 11).
FIG 1 .
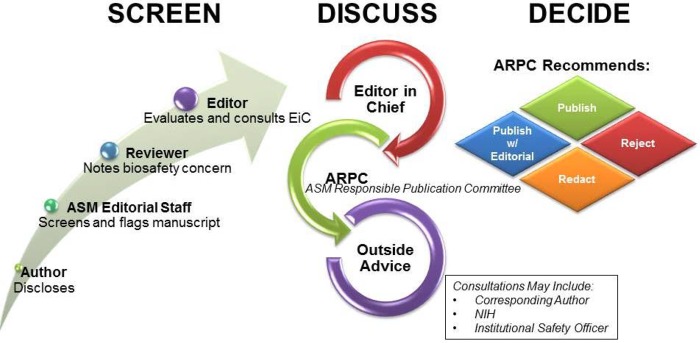
Scheme of the review process used by ASM journals for manuscripts containing DURC.
Limitations of DURC review.
Current manuscript review procedures are based on the NSABB DURC definition and on the SATL, both of which introduce significant limitations into the process. Although the NSABB DURC definition was a major step forward in establishing a mechanism for identifying research about which we ought to be most concerned, the DURC definition also requires a judgment call about whether such research can be “directly misapplied.” Making this judgment requires a risk assessment for which reviewers and editors may not be prepared unless they have had training in biosecurity and access to information about possible threats, which is unlikely, since such information is probably classified. Hence, a proper DURC assessment of the type envisioned by the NSABB is simply not possible at the journal level. This difficulty in making DURC assessments has led us to call for the establishment of a national board to help the scientific community assess DURC-related studies (12). Very few journals have editors who are experienced with DURC-related issues (2), and until such expertise is available, it will be difficult, if not impossible, to carry out the type of analysis envisioned by the NSABB. The ASM is fortunate to have some expertise in house, since three of the five members of the ARPC served on the NSABB and were intimately involved in drafting of DURC-related documents. However, other journals may not have access to this type of experience, and a national board to help with DURC review would meet that need (12). The reliance on the SATL employs a simple screening mechanism that allows journals to focus primarily on microbes and toxins deemed of greatest concern. However, it was noted previously that development of lists is a mechanism with the potential for increasing societal vulnerability by focusing attention on some microbes while ignoring others (13). In fact, the reliance on the SATL for screening manuscripts runs counter to the spirit of the DURC framework generated by the NSABB, which was not microbe specific. Therefore, screening on the basis of the SATL can miss papers that potentially meet the DURC criteria if these involve organisms that are not usually considered potential biological weapons. For example, a paper describing the enhancement of stress tolerance and virulence in an entomopathogenic fungus by metabolic engineering of dihydroxynaphthalene melanin biosynthesis genes, which was published in Applied and Environmental Microbiology (14), would not receive DURC review, since this organism is not on the SATL. Similarly, a study demonstrating that selection of entomopathogenic fungi capable of surviving at higher temperatures enhances virulence by defeating insect-induced fevers, published in BMC Biotechnology (15), would not attract attention unless someone was aware that the same approach would also defeat the enormous protection conferred by physiological temperatures in humans, which effectively restrict the growth of most potential fungal pathogens (16). To be clear, there is no evidence that the experiments reported in these papers pose any hazards. These examples are provided here solely to illustrate the limitations of list-based screens. Another consideration for editors who handle DURC-related papers is that rejection could simply result in submission to another journal that lacks DURC review protocols. Although the ARPC has not faced this situation, one can conceive of scenarios in which there would be reluctance by some journals to reject a paper containing worrisome DURC-related information. Clearly, there are important unresolved issues in the review of DURC-related papers, which is an area for continued attention by scientists, journals, and government agencies.
The horizon beyond DURC.
By the time a paper containing DURC-related content arrives on the desk of a journal editor, it comes at the very end of the oversight process envisioned by the NSABB. As such, the oversight process does not work well due to intrinsic limitations on the DURC formulation and the lack of appropriate expertise of journal editorial staff to evaluate this material. The NSABB cannot be faulted, however, for not anticipating the practical issues involved in evaluating DURC: it took a real-life example, the H5N1 ferret transmission controversy, to expose these issues. Two of us have argued for an approach to DURC-related research that focuses less on creating a filter for the evaluation of such research and more on prestudy development of consensus about the importance of the questions being asked and appropriate mitigation of any risks associated with the research (17). In fact, based on the experience and controversies involving the publication of GOF research in recent years, by the time such a paper gets to a journal, it is probably too late to prevent publication. In addition to the weaknesses described herein, in many cases the research has already been made public through conference presentations. Instead, a process that vets the work from inception to funding and envisions a publication plan based on answering important scientific questions with oversight provided by a national advisory board would be superior to the current approach to screening DURC-related manuscripts at the journal level. Furthermore, it is noteworthy that many manuscripts reporting DURC involving HPAIV and other microbes and toxins on the SATL originate from outside the United States, where NSABB recommendations do not apply. Since biosecurity and biosafety concerns with pathogens of pandemic potential could affect all of humanity, it is important to continue efforts to increase international awareness and understanding of these complex issues.
ACKNOWLEDGMENTS
We thank Erika Davies for designing Fig. 1 and Barbara Goldman for very helpful comments.
Footnotes
Citation Casadevall A, Dermody TS, Imperiale MJ, Sandri-Goldin RM, Shenk T. 2015. Dual-use research of concern (DURC) review at American Society for Microbiology journals. mBio 6(4):e01236-15. doi:10.1128/mBio.01236-15.
REFERENCES
- 1.Casadevall A, Imperiale MJ. 2014. Risks and benefits of gain-of-function experiments with pathogens of pandemic potential, such as influenza virus: a call for a science-based discussion. mBio 5:e01730-14. doi: 10.1128/mBio.01730-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patrone D, Resnik D, Chin L. 2012. Biosecurity and the review and publication of dual-use research of concern. Biosecur Bioterror 10:290–298. doi: 10.1089/bsp.2012.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hooper DC, Hirsch MS. 2014. Novel Clostridium botulinum toxin and dual use research of concern issues. J Infect Dis 209:167. doi: 10.1093/infdis/jit528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casadevall A, Enquist L, Imperiale MJ, Keim P, Osterholm MT, Relman DA. 2014. Redaction of sensitive data in the publication of dual use research of concern. mBio 5:e00991-13. doi: 10.1128/mBio.00991-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wain-Hobson S. 2014. An avian H7N1 gain-of-function experiment of great concern. mBio 5:e01882-14. doi: 10.1128/mBio.01882-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dermody TS, Sandri-Goldin RM, Shenk T. 2014. Sequence changes associated with respiratory transmission of H7N1 influenza virus in mammals. J Virol 88:6533–6534. doi: 10.1128/JVI.00886-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dermody TS, Casadevall A, Imperiale MJ, Sandri-Goldin RM, Shenk T. 2014. The decision to publish an avian H7N1 influenza virus gain-of-function experiment. mBio 5:e01985-14. doi: 10.1128/mBio.01985-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaffner DW, Drake HL. 2014. Botulinum neurotoxin subtype A4 originating from nontoxigenic Clostridium botulinum. Appl Environ Microbiol 80:7131–7132. doi: 10.1128/AEM.03001-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dermody TS, Sandri-Goldin RM, Shenk T. 2013. A new determinant of H5N1 influenza virus pathogenesis in mammals. J Virol 87:4795–4796. doi: 10.1128/JVI.00474-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drake HL. 2015. PilZ domain proteins of the plant pathogen Xanthomonas oryzae pv. oryzae function differentially in virulence. Appl Environ Microbiol 81:4233–4234. doi: 10.1128/AEM.01322-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirsch MS. 2013. Editorial comment regarding H5N1 influenza article in this issue. J Infect Dis 207:207. doi: 10.1093/infdis/jis528. [DOI] [PubMed] [Google Scholar]
- 12.Casadevall A, Dermody TS, Imperiale MJ, Sandri-Goldin RM, Shenk T. 2014. On the need for a national board to assess dual use research of concern. J Virol 88:6535–6537. doi: 10.1128/JVI.00875-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casadevall A, Relman DA. 2010. Microbial threat lists: obstacles in the quest for biosecurity? Nat Rev Microbiol 8:149–154. doi: 10.1038/nrmicro2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tseng MN, Chung PC, Tzean SS. 2011. Enhancing the stress tolerance and virulence of an entomopathogen by metabolic engineering of dihydroxynaphthalene melanin biosynthesis genes. Appl Environ Microbiol 77:4508–4519. doi: 10.1128/AEM.02033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Crecy E, Jaronski S, Lyons B, Lyons TJ, Keyhani NO. 2009. Directed evolution of a filamentous fungus for thermotolerance. BMC Biotechnol 9:74. doi: 10.1186/1472-6750-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robert VA, Casadevall A. 2009. Vertebrate endothermy restricts most fungi as potential pathogens. J Infect Dis 200:1623–1626. doi: 10.1086/644642. [DOI] [PubMed] [Google Scholar]
- 17.Imperiale MJ, Casadevall A. 2015. A new synthesis for dual use research of concern. PLoS Med 12:e1001813. doi: 10.1371/journal.pmed.1001813. [DOI] [PMC free article] [PubMed] [Google Scholar]


