ABSTRACT
Plant-derived compounds and other natural substances are a rich potential source of compounds that kill or attenuate pathogens that are resistant to current antibiotics. Medieval societies used a range of these natural substances to treat conditions clearly recognizable to the modern eye as microbial infections, and there has been much debate over the likely efficacy of these treatments. Our interdisciplinary team, comprising researchers from both sciences and humanities, identified and reconstructed a potential remedy for Staphylococcus aureus infection from a 10th century Anglo-Saxon leechbook. The remedy repeatedly killed established S. aureus biofilms in an in vitro model of soft tissue infection and killed methicillin-resistant S. aureus (MRSA) in a mouse chronic wound model. While the remedy contained several ingredients that are individually known to have some antibacterial activity, full efficacy required the combined action of several ingredients, highlighting the scholarship of premodern doctors and the potential of ancient texts as a source of new antimicrobial agents.
IMPORTANCE
While the antibiotic potential of some materials used in historical medicine has been demonstrated, empirical tests of entire remedies are scarce. This is an important omission, because the efficacy of “ancientbiotics” could rely on the combined activity of their various ingredients. This would lead us to underestimate their efficacy and, by extension, the scholarship of premodern doctors. It could also help us to understand why some natural compounds that show antibacterial promise in the laboratory fail to yield positive results in clinical trials. We have reconstructed a 1,000-year-old remedy which kills the bacteria it was designed to treat and have shown that this activity relies on the combined activity of several antimicrobial ingredients. Our results highlight (i) the scholarship and rational methodology of premodern medical professionals and (ii) the untapped potential of premodern remedies for yielding novel therapeutics at a time when new antibiotics are desperately needed.
INTRODUCTION
Antibiotic resistance is a clear and present danger to human health, and there are worryingly few new antibiotics in the developmental pipeline (1). A rich source of new antimicrobials potentially resides in medieval and early modern medical texts: microbial infection has been a constant presence throughout human history, and manuscript evidence shows that early modern and premodern societies used a range of natural compounds to treat conditions that are clearly recognizable as microbial infections. Some substances used in premodern treatments for infection (e.g., extracts from garlic [2–5]) show antimicrobial or virulence-reducing effects under certain conditions, but experiments that test the antibiotic activities of entire historical remedies are few and far between. This is an important omission, because the efficacy of these “ancientbiotics” could rely on the combined activity of their various ingredients. This would lead us to underestimate their efficacy and, by extension, the scholarship of premodern doctors. It could also help to explain why the in vitro antibacterial activity of individual compounds (e.g., garlic [6]) does not always reliably translate into in vivo antimicrobial potential.
Bald’s Leechbook (7) (Materials and Methods) is an English medical text from the Anglo-Saxon period. The manuscript was written in the 10th century and contains remedies for various ailments, including clearly recognizable microbial infections. Medieval medicine has generally been dismissed as backwards or superstitious, but recent scholars have suggested that among the remedies there may be methods and recipes that indicate a more factual application (8–10). One of Bald’s remedies, a salve for a “wen” or lump in the eye (Fig. 1), is particularly interesting to the modern microbiologist. It aims to treat a condition caused by bacterial infection and contains ingredients with the potential for antimicrobial activity. The recipe instructs the reader to crush garlic and a second Allium species (whose translation into modern English is ambiguous), combine these with wine and oxgall (bovine bile), and leave the mixture to stand in a brass or bronze vessel for 9 days and nights.
FIG 1 .

Bald’s eyesalve. A facsimile of the recipe, taken from the manuscript known as Bald’s Leechbook (London, British Library, Royal 12, D xvii). The original text reads as follows. “Ƿyrc eaȝsealf ƿiþ ƿænne: ȝenim cropleac ⁊ ȝarleac beȝea emfela, ȝecnuƿe ƿel tosomne, ȝenim ƿin ⁊ fearres ȝeallen beȝean emfela ȝemenȝ ƿiþ þy leaces, do þonne on arfæt læt standan niȝon niht on þæm arfæt aƿrinȝ þurh claþ ⁊ hlyttre ƿel, do on horn ⁊ ymb niht do mid feþre on eaȝe; se betsta læcedom.” This is translated into modern English as follows. “Make an eyesalve against a wen: take equal amounts of cropleac [an Allium species] and garlic, pound well together, take equal amounts of wine and oxgall, mix with the alliums, put this in a brass vessel, let [the mixture] stand for nine nights in the brass vessel, wring through a cloth and clarify well, put in a horn and at night apply to the eye with a feather; the best medicine.” © The British Library Board, Royal 12 D Xll xvii. Reproduced with permission.
The “wen” is most likely a sty: an infection of an eyelash follicle. Today, most styes are caused by the Gram-positive bacterium Staphylococcus aureus (11), which also causes other severe and persistent infections of various tissues. The spread of methicillin-resistant S. aureus (MRSA) strains has exacerbated this problem (11, 12). MRSA is particularly associated with chronic wound infections, which affect increasing numbers of people and are often highly resistant to treatment (11, 13).
The ingredients combined to treat this infection appear promising to the modern microbiologist. Allium species produce a range of antimicrobial compounds (3). Ajoene, which is found in garlic (A. sativum), and allicin, found in garlic, ramsons (A. ursinum), and spring onions (A. fistulosum), may prevent biofilm formation by S. aureus and other bacterial species (4) and interfere with quorum sensing and virulence in Pseudomonas aeruginosa (5). Other antimicrobial compounds produced by Allium species include flavonoids, such as quercetin, kaempferol, and their derivatives, found in leek (A. ampeloprasum/A. porrum) and onion (A. cepa) (3), and the antimicrobial peptide Ace-AMP1, so far only characterized in onion (14). Bile may also be antibacterial: it is generally thought to limit bacterial overgrowth in the host small intestine (15). Wine may act as a source of plant-derived antimicrobial small molecules or simply as a solvent for the extraction of compounds from the plant matter in the recipe. Finally, it is possible that copper salts may leach from the vessel in which the eyesalve is prepared. Copper surfaces prevent bacterial growth (16), and host-derived copper plays a role in immune defense (17). Interestingly, there is evidence that Allium-derived compounds may act synergistically with other antimicrobial agents, including copper (18) and tobramycin (2, 5).
We therefore sought to test the effect of Bald’s eyesalve on S. aureus and to determine whether any antibacterial activity found could be attributed to a single ingredient or was reliant on combining the ingredients according to the instructions laid down by “Bald.”
(Our work was put forward as a poster/oral presentation for the Society for General Microbiology Annual Conference 2015, Birmingham, United Kingdom, 30 March to 2 April 2015.)
RESULTS
Reconstruction of Bald’s eyesalve.
We reconstructed four independent batches of the recipe (batches A to D; see Materials and Methods). For each batch, we made two variants of the recipe, one using onion (ES-O) and one using leek (ES-L) for the ambiguous Allium species. We chose these because they both produce antimicrobial flavonoids that are not found in garlic, and onion also produces an antimicrobial peptide (3, 19). We also made preparations of the individual ingredients at approximately the same concentrations as they are found in the recipe (batches A and B), versions of the recipe with single ingredients omitted (batch C), and a version of the recipe which was tested before and after the 9-day waiting period (batch D). All of these were prepared in glass bottles, to which we added squares of brass sheet to simulate the brass/bronze vessel. The complete recipes resulted in clear, slightly acidic (pH 4.6 to 4.7) brownish liquids. The full recipes were apparently self-sterilizing: we cultured bacteria from aliquots of the leek and oxgall preparations but not from aliquots of ES-O or ES-L (see Data Set S1 in the supplemental material).
Bald’s eyesalve kills planktonic and biofilm cultures of S. aureus.
As shown by the results in Fig. 2A, both ES-O and ES-L prevented growth of planktonic cultures of S. aureus growing aerobically at 37°C in synthetic wound fluid (SWF) (20). Aliquots of cultures were plated out at the end of the incubation period, and no colonies were observed (detection limit, 30 CFU). Furthermore, 5 µl of each culture was transferred to 1 ml of fresh SWF and incubated overnight at 37°C, but no growth was observed, showing that Bald’s eyesalve was bactericidal, rather than simply bacteriostatic, under these conditions.
FIG 2 .
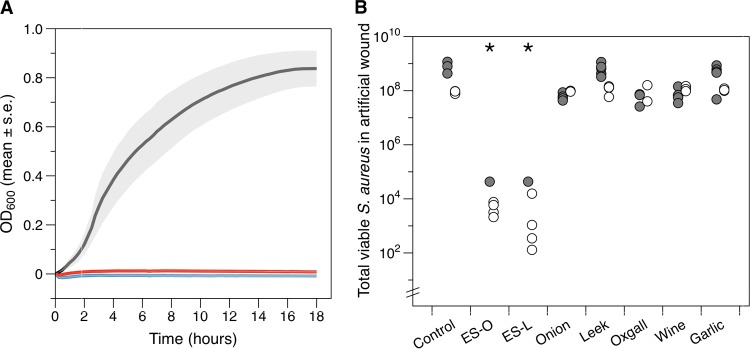
Bald’s eyesalve kills S. aureus in planktonic culture and in a synthetic wound biofilm model. (A) One hundred microliters of sterile distilled water (black line), the onion variant of the eyesalve (ES-O, red line), or the leek variant of the eyesalve (ES-L, blue line) (both from batch A) was added to a 200-µl mid-log-phase culture (104 to 105 cells) of S. aureus in synthetic wound fluid (SWF), and the optical density of the culture was measured during 18 h of incubation at 37°C. The mean results from four replica populations and the associated standard errors (shaded intervals; too small to see for ES-O and ES-L) are shown. (B) Two hundred microliters of ES-O or ES-L (batch A, filled circles, and batch B, open circles) or of each individual ingredient preparation was added to five 1-day-old cultures of S. aureus growing at 37°C in a synthetic wound (400-µl synthetic wound fluid rendered semisolid by adding 2 mg·ml−1 collagen). After 24 h of further incubation, the collagen was dissolved to recover cells for agar plate counts. The control treatment was sterile distilled water left to stand for 9 days in the presence of brass, which was also present in all other preparations, to simulate the presence of a copper alloy vessel (see Materials and Methods). Asterisks denote treatments whose results were significantly different from those of the control.
However, during infection, bacteria often exist as dense, antibiotic-resistant biofilms in a matrix of self-excreted polysaccharides and soft tissue from the host (11, 13, 21). We therefore grew S. aureus for 24 h at 37°C in a synthetic model of soft tissue infection comprised of SWF solidified with collagen (20). We exposed the resulting mature biofilms to batches A and B of ES-O or ES-L or preparations of the individual ingredients for a further 24 h (Fig. 2B). A generalized linear model revealed a significant effect of treatment (χ27 1.5e+10, P < 0.001); post hoc Tukey tests showed that only the results for ES-O and ES-L were significantly different from the results for the control treatment (P < 0.001), causing marked reductions in the numbers of viable cells recovered from synthetic wounds. None of the individual ingredients alone had a significant effect on viable cell counts. There was also a significant effect of batch (χ21 4.2e+9, P < 0.001) and a significant interaction between batch and treatment (χ27 2.0e+9, P < 0.001); this was because the full recipes of batch B were slightly more bactericidal than those of batch A, and the effects of the individual ingredient preparations differed between batches. The full recipes retained bactericidal activity after 30 days of storage at 4°C (see Fig. S1 in the supplemental material).
Bald’s eyesalve is only effective when the recipe is followed carefully.
We then made a third batch (batch C) of ES-O and ES-L, along with variants in which individual ingredients were systematically omitted. These were tested against S. aureus in the synthetic wound biofilm model. A generalized linear model revealed a significant effect of treatment (χ211 3.1e+9, P < 0.001). We used Dunnett’s test to test comparisons that were of interest. First, we contrasted the results of each recipe with those of the control. Both ES-O and ES-L caused a significant reduction in viable cell numbers (t21 = 6.38, P < 0.001, and t21 = 6.25, P < 0.001, respectively); this was comparable to the results for batches A and B (Fig. 3). When onion/leek was dropped from the common recipe base, partial bactericidal activity was still observed (t21 = 3.38, P = 0.015). Bactericidal activity was also retained when brass or oxgall was dropped from either ES-O (t21 = 6.00, P < 0.001, and t21 = 3.80, P = 0.005, respectively) or ES-L (t21 = 7.08, P < 0.001, and t21 = 5.38, P < 0.001, respectively). Dropping either garlic or wine from ES-O or ES-L completely removed the bactericidal activity: the viable cell counts were not significantly different from the results for the control (P ≥ 0.166). We then compared the results for the modified recipes that retained bactericidal activity with the results for their parent recipes. The bactericidal power of the common recipe base when onion/leek was omitted was significantly impaired compared with that of either ES-O or ES-L (t6 = 9.307, P = 0.005, and t6 = 18.2, P < 0.001, respectively). Omitting brass from either ES-O or ES-L had no effect on their bactericidal activities (t10 = 0.684, P = 0.897, and t10 = 0.685, P = 0.896, respectively). Omitting oxgall decreased the bactericidal activity of ES-O (t10 = 4.66, P = 0.001) but not that of ES-L (t10 = 0.710, P = 0.885).
FIG 3 .
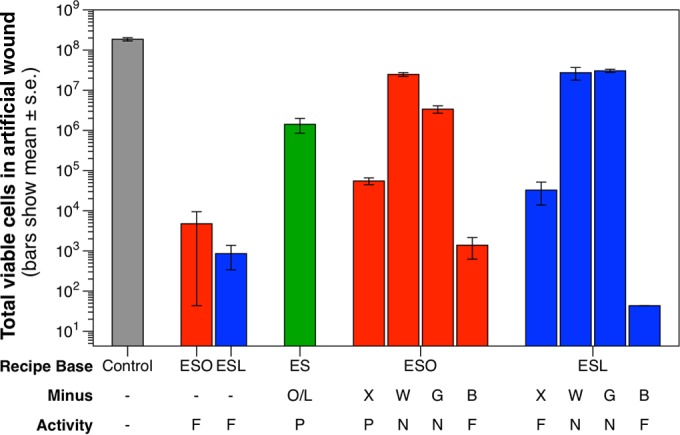
The activity of Bald’s eyesalve against S. aureus biofilms requires several ingredients. Two hundred microliters of the onion variant of the eyesalve (ES-O), the leek variant of the eyesalve (ES-L) (batch C), or preparations missing a single ingredient were added to three 1-day-old cultures of S. aureus in synthetic wounds, and bactericidal activity quantified as described in Fig. 2. Red bars show the results for the full or reduced versions of ES-O, blue bars show the results for the full or reduced versions of ES-L, and the green bar shows the result for a variant of the eyesalve with neither onion nor leek (ES). The control treatment (grey bar) was sterile distilled water. Individual ingredients dropped out of the recipe (Minus) are coded as follows: O, onion; L, leek; X, oxgall; W, wine; G, garlic; B, brass. Reduced recipes were assessed as having full (F), partial (P), or no (N) activity in comparison with the results for the control treatment and the appropriate complete recipe.
The combination of garlic, wine, and the other Allium species thus appears crucial for the full efficacy of Bald’s eyesalve, and when onion is used in place of leek, oxgall is also necessary for full activity. Brass may be dispensable, but it is possible that its presence may facilitate activity against other bacterial species; additionally, using a brass pot may have allowed the Anglo-Saxon physician to start work with an uncontaminated vessel, as the copper content could prevent bacteria colonizing its surface.
Batch D of Bald’s eyesalve was used to test whether the 9-day waiting period was important. ES-O and ES-L, with a water control, were tested in the synthetic wound biofilm model (i) immediately after preparation and (ii) after 9 days (Fig. 4). Analysis of variance (ANOVA) revealed significant effects of treatment (F2,24 = 110.9, P < 0.001), time (immediate/after 9 days) (F1,24 = 410.2, P < 0.001), and their interaction (F2,24 = 44.3, P < 0.001). Post hoc Tukey tests showed that while both fresh and 9-day-old ES-O and ES-L killed cells (comparisons versus the respective controls, P ≤ 0.037), the number of viable cells left after treatment with either version of the eyesalve was lower when the eyesalve had been left to stand for 9 days prior to use (both P < 0.001). ES-O and ES-L caused a 2-log drop in viable S. aureus cells if used immediately after preparation but a 4- to 5-log drop if used after 9 days (Fig. 4; consistent with data in Fig. 2B and 3).
FIG 4 .
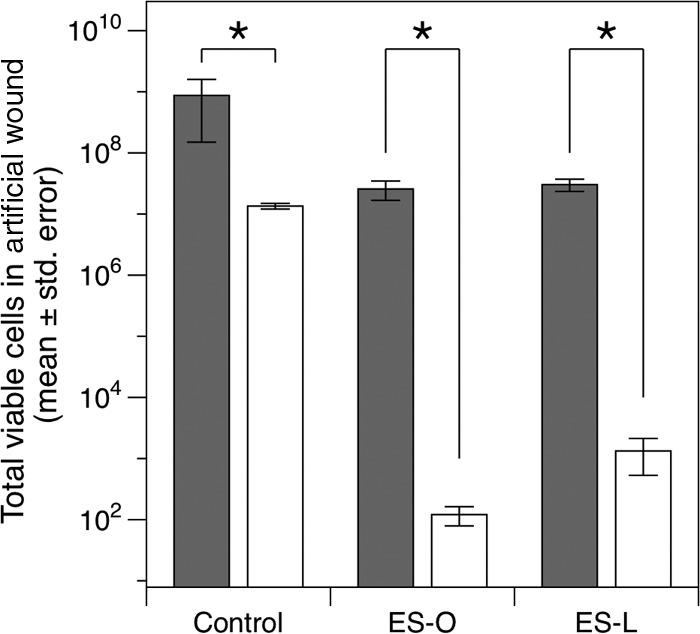
The activity of Bald’s eyesalve against S. aureus biofilms requires the 9-day waiting period specified by the recipe. Immediately after preparation, 200 µl of the onion variant of the eyesalve (ES-O), the leek variant of the eyesalve (ES-L) (batch D), or sterile distilled water was added to five 1-day-old cultures of S. aureus growing at 37°C in synthetic wounds, and the bactericidal activity quantified as previously described (filled bars); the experiment was repeated using ES-O and ES-L (batch D) after the 9-day waiting period specified by Bald (open bars). Asterisks denote treatments whose results were significantly different from those of the control. While the control cultures for the 9-day experiment grew to slightly lower densities than the control for the fresh eyesalve (P = 0.005), this difference was small compared with the differences observed for ES-O and ES-L (1 to 2 log versus 4 to 5 log difference).
Bald’s eyesalve kills MRSA in chronically infected animal tissue.
All the ingredients in Bald’s eyesalve are edible, and the likelihood of copper accumulating to levels toxic to mammalian cells seems low. Therefore, we tested its efficacy against S. aureus in a mouse model of chronic wound infection (22) using a thoroughly modern pathogen: methicillin-resistant S. aureus (MRSA) (12). Mice were administered wounds as previously described (22) and infected with MRSA. Four days postinfection, wounds were excised and submerged in ES-O, ES-L (Batch B), sterile saline, or the clinical last-line antibiotic vancomycin (100 µg·ml−1) for 4 h. As shown by the results in Fig. 5, vancomycin did not cause significant reductions in viable bacteria during this relatively very short exposure time (comparison of least-squares means adjusted for the experimental block) (t16 = 1.45, P = 0.491). However, ES-O and ES-L caused statistically significant drops in the numbers of viable cells recovered from wounds (approximately 10-fold reductions) (t16 = 3.94, P = 0.006, and t16 = 3.66, P = 0.010, respectively). Batches A and B of ES-O and ES-L were also tested in the mouse wound model after 3 months of storage at 4°C, but without the vancomycin comparison (see Fig. S2 in the supplemental material): in this experiment, ES-L but not ES-O caused a statistically significant drop in the numbers of viable cells recovered from wounds (an approximately 10-fold reduction). Thus, while there is some interbatch variability in activity, Bald’s eyesalve shows potential as an antistaphylococcal agent in vivo.
FIG 5 .
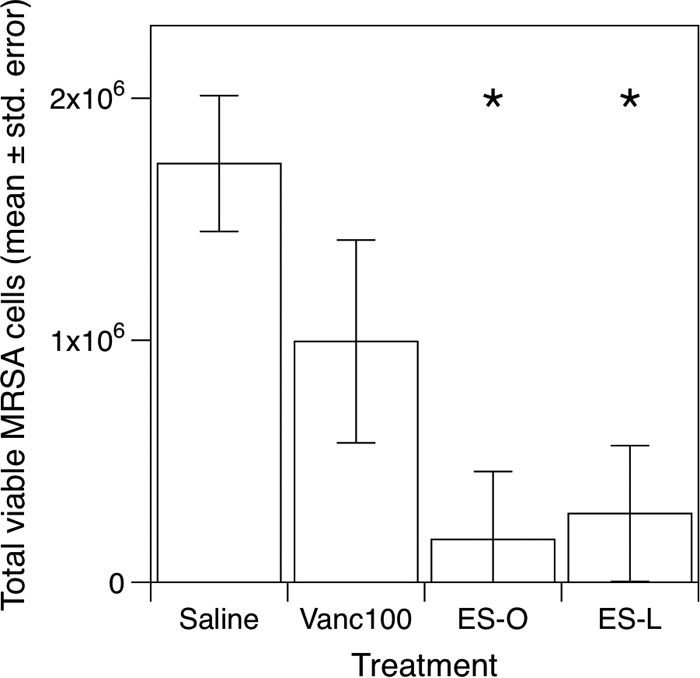
Bactericidal activity of Bald’s eyesalve in a mouse chronic wound model of MRSA infection. Six adult female Swiss-Webster mice were administered wounds and infected with ca. 105 CFU S. aureus Mu50. Four days postinfection, mice were euthanized. Wound tissue was excised and cut into either three (n = 3 mice) or four (n = 3 mice) equal pieces, which were weighed, submerged in 300 µl sterile saline (one replicate from n = 6 mice), 100 µg·ml−1 vancomycin (one replicate from n = 3 mice), the onion variant of the eyesalve (ES-O; one replicate from n = 6 mice), or the leek variant of the eyesalve (ES-L; one replicate from n = 6 mice) (both ES-O and ES-L were from batch B) for 4 h, and then rinsed in sterile saline and homogenized. Viable bacteria were enumerated, and the counts standardized per gram of tissue. Asterisks denote treatments whose results were significantly different from those of the control.
DISCUSSION
In summary, we have reconstituted a 1,000-year-old remedy for bacterial infection and shown that it kills the most common cause of the infection it was designed to treat. Bald’s eyesalve eliminates S. aureus in planktonic culture and reduces viable cell numbers by several orders of magnitude in a synthetic model of established biofilm infection. This effect depends upon the combination of several ingredients and upon the 9-day storage period specified in the recipe. Furthermore, a highly antibiotic-resistant strain of S. aureus is susceptible to Bald’s eyesalve when infected tissue from a mouse chronic wound model is exposed to the recipe. In this model, significant bactericidal activity was observed after a brief period of exposure during which the current last-line clinical antibiotic (vancomycin) did not kill significant numbers of bacteria. Our findings contrast with those of a previous attempt to test Bald’s eyesalve by Brennessel et al. (23), who found it ineffective against S. aureus in disk diffusion assays; however, these authors do not specify the methods of preparation and do not give quantitative results or details of replication, so we do not know exactly how their tests were conducted.
Future work will explore the mechanism(s) by which Bald’s eyesalve kills S. aureus. We note that several of the ingredients may damage biological membranes. Bile salts act as surfactants (15), the Ace-AMP1 antimicrobial peptide present in onion is thought to have lipid-binding activity (19), and saponins, present in a wide variety of Allium spp., can bind the sterols present in biological membranes (3). Other antimicrobial compounds present in Allium spp. have been shown to act as enzyme inhibitors (allicin and flavonoids) (3). Wine may be a source of further plant-derived antimicrobial small molecules, or it may simply act as a solvent for the extraction of compounds from the other ingredients in the recipe, with the 9-day waiting period providing time for molecule extraction.
It is interesting that copper was entirely dispensable for antistaphylococcal activity in our synthetic wound model. Copper has been shown to have broad-spectrum antibacterial activity (16, 17), and we would expect copper salts to accumulate in our preparations: we observed copper compounds forming on the surface of the brass squares used to simulate the preparation vessel, and Brennessel et al. (who used an actual brass pot), found that copper salts accumulated to high levels in their version of Bald’s eyesalve (M. Drout, personal communication). It is possible that we simply did not have a large enough copper surface to allow copper to accumulate to biologically active levels in our recipe. Alternatively, the copper vessel itself may have allowed the Anglo-Saxon physician to begin work with an uncontaminated pot due to the resistance of copper surfaces to bacterial colonization. A third possibility is that copper is entirely dispensable: copper alloy bowls of the Anglo-Saxon period can be highly decorated (even inlaid with silver) and appear as grave goods in high status burials as a marker of feasting power (24), and perhaps the more humble vessels may have been a marker of the physician’s professional status.
The combined activity of molecules derived from the different ingredients might explain why the activity of Bald’s eyesalve is greater than the sum of its parts: the mixture may attack the bacteria on several fronts. This is an important consideration for future work on natural antimicrobial compounds, as strong in vitro activity of individual compounds has not always translated into positive in vivo results (6). Alternatively, novel compounds may be formed as molecules from different ingredients react over the 9-day storage period specified by the recipe. Future research into antibiotics derived from natural materials would benefit from considering potential combinatorial activity of different ingredients, and premodern medical texts provide an excellent starting point for this strategy. Such work will require extensive collaborative research involving historians, linguists, microbiologists, and analytical/medicinal chemists.
When we describe Bald’s eyesalve as being “designed” to treat eye infection, we do not use the term lightly. There has been considerable debate about the levels of scholarship and scientific method among early medieval medical practitioners (8, 9, 25). This may be partly because of an almost complete absence of theoretical material, and the fact that the surviving corpus of Anglo-Saxon medicine seems to be compilations of excerpts and treatises (26). Furthermore, many recipes—such as the one that requires having a virgin get water from an eastward-flowing spring for a cyst “which pains the heart,” from the mid-11th century Lacnunga—look rather dubious as remedies. Medicine, as some scholars have claimed, was a craft and not a science in early medieval Europe (26), and there was a lack of explanatory frameworks. Texts like Bald’s Leechbook were most likely composed in a monastic environment, and in many text sources, there is a conflation of spiritual and practical healing. More credence has been given to later medieval texts, associated with the School of Salerno and the growth of university-based training (27).
However, our finding that the combination of ingredients used is crucial for bactericidal activity supports the hypothesis that this “ancientbiotic” was systematically constructed based on empirical knowledge. The fact that Anglo-Saxon recipes do not state detailed amounts of each component requires the practitioners to have had some knowledge about how much of each ingredient to use. It is also notable that numerous “alternative” recipes are often given for a condition—indicating that a trained physician could adapt treatments when necessary. If medieval physicians really did use observation and experience to design effective antimicrobial medicines, then this predates the generally accepted date for the adoption of a rational scientific method (the formation of the Royal Society in the mid-17th century) and the modern age of antibacterial medicine (Lister’s use of carbolic acid in the late 19th century) by several hundred years.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
We used a standard laboratory wild-type strain of S. aureus (Newman) for the initial in vitro tests of antibacterial activity. For the mouse chronic wound model, we used S. aureus Mu50 (ATCC 700699). Synthetic wound fluid (50% [wt/vol] fetal bovine serum, 50% peptone water) (20) was used as the growth medium for all experiments. All cultures were grown aerobically at 37°C.
Bald’s eyesalve.
The recipe (Fig. 1) calls for equal amounts (“begea emfela”) of the Allium species and equal amounts of wine and oxgall, but it is not clear whether these two pairs of quantities are equal to each other, nor whether ingredients were measured by weight or volume. We decided to combine equal amounts by volume of all of the recipe ingredients. For the complete eyesalve recipes, 25 ml peeled, finely chopped garlic bulb was mixed with either 25 ml peeled, finely chopped yellow onion or 25 ml finely chopped leek (leaves), and these ingredients were crushed with a mortar and pestle for 2 min (the recipe asks for it to be pounded well: “gecnuwe wel tosomne”). Vegetables were purchased fresh from a greengrocer. Twenty-five milliliters of wine (Pennard Organic Wines) was added to the crushed alliums; we used an organic English white wine produced by a vineyard situated 7 miles from the village of Panborough in Somerset: a charter of ad 956 tells us that the Anglo-Saxon monastery of St. Mary’s Abbey in Glastonbury held land rights to a vineyard at Panborough (28). We then added 25 ml bovine bile salts (Fluka/Sigma Aldrich) dissolved in water at a concentration of 87 mg·ml−1; this is the natural concentration of bile salts in the bovine gallbladder (source, Sigma Aldrich technical support). The mixture of alliums, wine, and oxgall was placed in a sterile 250-ml Duran bottle and, following the reasoning of Brennessel et al. (23), nine clean and sterile 15-mm squares of 0.51-mm 260 brass sheet (Hypertriton, Inc., Canada) were added to the bottle to simulate the copper alloy vessel (“arfæt”). The lid was closed and the bottle wrapped in foil (as the original vessel would not have been transparent and we did not know if any ingredients were light sensitive). The recipes, denoted ES-O for the version containing onion and ES-L for the version containing leek, were then left refrigerated at 4°C for 9 days. While the Anglo-Saxons did not have refrigerators, neither did they have centrally heated buildings: we reasoned that the daily fluctuations in ambient temperature in the laboratory caused by heating during working hours were probably less realistic than a constant cool temperature.
To test the antibacterial effects of the individual ingredients of the recipe, when we made batches A and B, we also combined 6 ml of each ingredient (the vegetables were finely chopped and crushed as described above) with 17 ml of distilled water in a foil-wrapped glass bottle containing two 15-mm squares of the brass sheet and left at 4°C for 9 days. This is an approximate scaling down of the recipe given the constraints imposed by measuring chopped vegetables accurately and by requiring a whole number of brass squares. To provide a control treatment, 23 ml distilled water and two 15-mm squares of the brass sheet were left at 4°C for 9 days.
We repeated the preparation of full recipes and individual ingredients twice, using fresh ingredients each time, to produce two independent batches of the eyesalve or individual ingredient preparations (batches A and B). We later repeated the preparation a third time (batch C), making complete ES-O and ES-L recipes plus versions of the recipes that were each missing one ingredient, using the scaled-down, 24-ml total volume that was used for the individual ingredient preparations for batches A and B.
The recipe calls for the mixture to be strained and “purified well” (“hlyttre wel”) before use or further storage. We debated how to simulate this and whether we should sterilize the mixtures. We decided that any microbes present in the ingredients are part of the “natural” state of the medicine and would not have been removed by filtering through cloth (probably linen). Therefore, we briefly centrifuged each recipe/ingredient to pellet the solid material and filtered the remaining liquid through a filter small enough to remove solid particles of vegetable matter but large enough to let bacterial cells through (5 µm). Because the oxgall does not fully dissolve in the mixture, the finished eyesalve contains a fine white precipitate (especially noticeable when taken directly from cold storage). All versions of the eyesalve or its ingredients were thus briefly vortexed before use to ensure a homogenous suspension.
Batch D of the complete recipes was made as described above but in two subbatches from the same ingredients. One subbatch was made without brass, shaken for 10 s, and then filtered and used in experiments immediately. Brass was added to the other subbatch, and this was stored for 9 days at 4°C, following the original recipe.
Growth and testing of planktonic cultures in synthetic wound fluid.
Cultures of S. aureus were grown for 6 h (mid-log phase) in SWF at 37°C on an orbital shaker, and 200-µl aliquots of this culture (containing 104 to 105 cells) were transferred to 12 wells of a 96-well microtiter plate. One hundred microliters of sterile distilled water, ES-O, or ES-L (Batch A only) was added to four wells each. Cultures were incubated at 37°C in a microplate spectrophotometer (Tecan Infinite) for 18 h with periodic shaking; every 15 min, the cultures were shaken for a few seconds and the optical density at 600 nm (OD600) of each well was measured. Because the eyesalve recipes were colored, we standardized the OD readings by subtracting the OD for each well at time zero.
Culturing and treatment of synthetic wound infections.
S. aureus was streaked onto LB agar and incubated aerobically at 37°C for 24 h. Multiple colonies were pooled to inoculate starter cultures in SWF. These were incubated aerobically at 37°C on an orbital shaker for 6 h. The cultures were then diluted to an OD600 of 0.05 in SWF, and 100-µl amounts added to wells of 24-well cell culture plates containing synthetic wound medium. Synthetic wounds comprised 400 µl polymerized collagen (2 mg·ml−1 collagen, 0.01% acetic acid, 60% [vol/vol] SWF, 0.01 M sodium hydroxide). For experiments on batches A and B of the eyesalve, additional synthetic wounds were mock inoculated with 100 µl sterile distilled water instead of with S. aureus to reveal any growth of bacteria resident in the eyesalve or its component ingredients. Synthetic wounds were incubated aerobically at 37°C for 24 h to allow the establishment of bacterial biofilms within the collagen matrix.
Batches A and B of the full recipes and individual ingredient preparations were tested as follows. For S. aureus-infected wounds and the no-infection control, five replica synthetic wounds were overlaid with 200 µl of each complete recipe or individual ingredients. A further five infected and noninfected synthetic wounds were overlaid with 200 µl sterile distilled water to provide mock-treated controls. Treated synthetic wounds were then incubated aerobically at 37°C for 24 h. The collagen was then depolymerized by treating each wound with 500 µl 0.5 mg·ml−1 collagenase. An aliquot of each wound was serially diluted and plated on LB agar to enumerate the number of viable cells present.
For batch C (full recipes and recipes missing a single ingredient), this experiment was repeated but each preparation was tested in triplicate. For batch D (full recipes before and after 9-day storage), each preparation was tested in five replica wounds. For batches C and D, only a single no-infection control wound was used for each experiment; this was visually inspected to confirm the absence of contaminating bacterial growth and not analyzed further.
Mouse chronic wound infections.
Adult female Swiss-Webster mice were administered wounds as previously described (22) and infected with approximately 105 CFU S. aureus Mu50. At 4 days postinfection, mice were euthanized and their wound tissue was extracted and cut into 3 equal pieces, which were weighed. Wound sections were then submerged in 300 µl sterile saline, ES-O, or ES-L for 4 h, after which they were rinsed in sterile saline and homogenized. Wound homogenates were then serially diluted and plated on Mueller-Hinton agar with 4% NaCl and 6 µg/ml oxacillin (MRSA screen agar) to determine CFU/g tissue. This experiment was carried out twice on separate days, and data from the two blocks combined in one analysis. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee of Texas Tech University Health Sciences Center (protocol number 07044), and an NC3Rs ARRIVE checklist (29; see also www.nc3rs.org.uk) is available for this work.
Statistical analyses.
All data were analyzed with R (version 2.14.0; R Foundation for Statistical Computing, http://www.R-project.org). Different models and their residuals were explored to find the most appropriate analysis for each data set. The data sets for S. aureus growth in synthetic wounds treated with batches A to C of the recipes/ingredients were highly skewed; therefore, these data sets were analyzed using generalized linear models with Poisson error. ANOVAs of log-transformed data were used to analyze (i) the 30-day retest of batches A and B in synthetic wound infections and (ii) the test for an effect of the 9-day waiting period using batch D. In all analyses where both batches A and B were used, we tested for effects of treatment, batch, and their interaction. Post hoc comparisons were conducted using the multcomp package (30). For the S. aureus synthetic wound data for batch C, one replica wound treated with eyesalve minus onion/leek had to be dropped from the data set, as it completely dried out during incubation. Data on MRSA viable cell counts in mouse chronic wounds were not suitable for analysis using general linear models and so were analyzed by fitting least-squares means for each treatment adjusted for the effect of the experimental block; Tukey tests were then used to test pairwise differences between treatments, with P values adjusted for multiple comparisons. The growth curves of the S. aureus reporter strains were compared using the compare GrowthCurves function of the statmod package (version 1.4.21; http://CRAN.R-project.org/package=statmod). For each strain, growth curves from different treatments were compared using 1,000 permutations of the data and the Holm-Bonferroni correction for multiple comparisons.
Experimental data and R code have been deposited with Dryad.org (doi:10.5061/dryad.mn17p).
SUPPLEMENTAL MATERIAL
Bacterial species cultured from recipes and ingredient preparations. Download
Bald’s eyesalve retains antistaphylococcal activity for 30 days of storage. The onion (ES-O) and leek (ES-L) variants of the recipe (batches A and B) retained bactericidal activity against S. aureus biofilms in synthetic wounds after 30 days of storage (ANOVA, treatment, F2,24 = 217, P < 0.01; batch, F1,24 = 0.16, P = 0.693; and interaction, F2,24 = 0.196, P = 0.823). Download
Bald’s eyesalve kills MRSA in a mouse chronic wound infection. Adult female Swiss-Webster mice were administered wounds and infected with ca. 105 CFU S. aureus Mu50. Four days postinfection, mice were euthanized. Wound tissue was extracted and cut into three equal pieces, which were weighed, submerged in 300 µl sterile saline, ES-O, or ES-L (batches A and B, after 3 months of storage at 4°C) for 4 h, and then rinsed in sterile saline and homogenized. Viable bacteria were enumerated by plating. ES-L but not ES-O killed MRSA in wounds (ANOVA, F2,12 = 6.17, P = 0.014; post hoc Tukey test versus the control, ES-O, P = 0.069, and ES-L, P = 0.014). There was no effect of eyesalve batch (ANOVA, batch, F1,12 = 0.443, P = 0.518; interaction, F2,12 = 0.583, P = 0.573). The graph shows the means of data from six mice per treatment (three for each batch of the eyesalve). Download
ACKNOWLEDGMENTS
S.P.D., F.H., C.L., and A.E.L.R. conceived the study; C.L. and F.H. identified the recipe; A.E.L.R., F.H., K.P.R., and R.G. conducted the experimental work; F.H. analyzed the data; and all authors contributed to manuscript preparation.
This work was funded by the University of Nottingham Interdisciplinary Centre for Analytical Science (sandpit grant to F.H., A.E.L.R., C.L., and S.P.D.), the Natural Environment Research Council (NE/J007064/1 to S.P.D.), the Human Science Frontier Programme (RGY0081/2012 to S.P.D.), and the University of Nottingham Institute for Medieval Research and School of English Research Committee (C.L.).
We thank the two anonymous reviewers. The development of the work benefitted from discussions with Conor Kostick, Steve Atkinson, Erin Connelly, Laura Piddock, James Gurney, Jason Millington, Mike Drout, and Marvin Whiteley. We also thank Emma Rayner for sourcing the manuscript facsimile used in Fig. 1, and the British Library for permission to reproduce it.
The authors declare no conflicts of interest.
Footnotes
Citation Harrison F, Roberts AEL, Gabrilska R, Rumbaugh KP, Lee C, Diggle SP. 2015. A 1,000-year-old antimicrobial remedy with antistaphylococcal activity. mBio 6(4):e01129-15. doi:10.1128/mBio.01129-15.
REFERENCES
- 1.Review on Antimicrobial Resistance 2014. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. http://amr-review.org.
- 2.Bjarnsholt T, Jensen PO, Rasmussen TB, Christophersen L, Calum H, Hentzer M, Hougen HP, Rygaard J, Moser C, Eberl L, Høiby N, Givskov M. 2005. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiology 151:3873–3880. doi: 10.1099/mic.0.27955-0. [DOI] [PubMed] [Google Scholar]
- 3.Lanzotti V, Bonanomi G, Scala F. 2013. What makes Allium species effective against pathogenic microbes? Phytochem Rev 12:751–772. doi: 10.1007/s11101-013-9295-3. [DOI] [Google Scholar]
- 4.Nidadavolu P, Amor W, Tran PL, Dertien J, Colmer-Hamood JA, Hamood AN. 2012. Garlic ointment inhibits biofilm formation by bacterial pathogens from burn wounds. J Med Microbiol 61:662–671. doi: 10.1099/jmm.0.038638-0. [DOI] [PubMed] [Google Scholar]
- 5.Jakobsen TH, Van Gennip M, Phipps RK, Shanmugham MS, Christensen LD, Alhede M, Skindersoe ME, Rasmussen TB, Friedrich K, Uthe F, Jensen PØ, Moser C, Nielsen KF, Eberl L, Larsen TO, Tanner D, Høiby N, Bjarnsholt T, Givskov M. 2012. Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob Agents Chemother 56:2314–2325. doi: 10.1128/AAC.05919-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smyth AR, Cifelli PM, Ortori CA, Righetti K, Lewis S, Erskine P, Holland ED, Givskov M, Williams P, Cámara M, Barrett DA, Knox A. 2010. Garlic as an inhibitor of Pseudomonas aeruginosa quorum sensing in cystic fibrosis—a pilot randomized controlled trial. Pediatr Pulmonol 45:356–362. doi: 10.1002/ppul.21193. [DOI] [PubMed] [Google Scholar]
- 7.Cockayne O. 1864–1866. Leechdoms, wortcunning and starcraft: being a collection of documents, for the most part never before printed, illustrating the history of science before the Norman conquest. Rolls series 35th, 3 Vols. Longman, Green, Longman, Roberts, and Green, London, United Kingdom. [Google Scholar]
- 8.Cameron ML. 1993. Anglo-Saxon medicine. Cambridge University; Press, Cambridge, United Kingdom. [Google Scholar]
- 9.Horden P. 2000. What’s wrong with early medieval medicine? Soc Hist Med 24:2–25. [Google Scholar]
- 10.Meaney A. 2000. The practice of medicine in England about the year 1000. Soc Hist Med 13:221–237. doi: 10.1093/shm/13.2.221. [DOI] [PubMed] [Google Scholar]
- 11.Archer NK, Mazaitis MJ, Costerton JW, Leid JG, Powers ME, Shirtliff ME. 2011. Staphylococcus aureus biofilms: properties, regulation, and roles in human disease. Virulence 2:445–459. doi: 10.4161/viru.2.5.17724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Köck R, Becker K, Cookson B, van Gemert-Pijnen JE, Harbarth S, Kluytmans J, Mielke M, Peters G, Skov RL, Struelens MJ, Tacconelli E, Navarro Torné A, Witte W, Friedrich AW. 2010. Methicillin-resistant Staphylococcus aureus (MRSA): burden of disease and control challenges in Europe. Euro Surveill 15:19688. [DOI] [PubMed] [Google Scholar]
- 13.Kirketerp-Møller K, Zulkowski K, James G. 2011. Chronic wound colonization, infection, and biofilms, p 11-24. In Bjarnsholt T, Jensen PØ, Moser C, Høiby N (ed), Biofilm infections. Springer, New York, NY. [Google Scholar]
- 14.Soininen TH, Jukarainen N, Soininen P, Auriola SO, Julkunen-Tiitto R, Oleszek W, Stochmal A, Karjalainen RO, Vepsäläinen JJ. 2014. Metabolite profiling of leek (Allium porrum L) cultivars by 1H NMR and HPLC-MS. Phytochem Anal 25:220–228. doi: 10.1002/pca.2495. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann AF, Eckmann L. 2006. How bile acids confer gut mucosal protection against bacteria. Proc Natl Acad Sci U S A 103:4333–4334. doi: 10.1073/pnas.0600780103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grass G, Rensing C, Solioz M. 2011. Metallic copper as an antimicrobial surface. Appl Environ Microbiol 77:1541–1547. doi: 10.1128/AEM.02766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaturvedi KS, Henderson JP. 2014. Pathogenic adaptations to host-derived antibacterial copper. Front Cell Infect Microbiol 4:3. doi: 10.3389/fcimb.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogita A, Fujita K, Taniguchi M, Tanaka T. 2006. Dependence of synergistic fungicidal activity of Cu2+ and allicin, an allyl sulfur compound from garlic, on selective accumulation of the ion in the plasma membrane fraction via allicin-mediated phospholipid peroxidation. Planta Med 72:875–880. doi: 10.1055/s-2006-947167. [DOI] [PubMed] [Google Scholar]
- 19.Tassin S, Broekaert WF, Marion D, Acland DP, Ptak M, Vovelle F, Sodano P. 1998. Solution structure of ace-AMP, a potent antimicrobial protein extracted from onion seeds. Structural analogies with plant nonspecific lipid transfer proteins. Biochemistry 37:3623–3637. doi: 10.1021/bi9723515. [DOI] [PubMed] [Google Scholar]
- 20.Werthén M, Henriksson L, Jensen PØ, Sternberg C, Givskov M, Bjarnsholt T. 2010. An in vitro model of bacterial infections in wounds and other soft tissues. APMIS 118:156–164. doi: 10.1111/j.1600-0463.2009.02580.x. [DOI] [PubMed] [Google Scholar]
- 21.Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 22.Dalton T, Dowd SE, Wolcott RD, Sun Y, Watters C, Griswold JA, Rumbaugh KP. 2011. An in vivo polymicrobial biofilm wound infection model to study interspecies interactions. PLoS One 6:e27317. doi: 10.1371/journal.pone.0027317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brennessel B, Drout MDC, Gravel R. 2005. A reassessment of the efficacy of Anglo-Saxon medicine. Anglo Saxon Engl 34:183–195. doi: 10.1017/S0263675105000086. [DOI] [Google Scholar]
- 24.Lee C. 2007. Feasting the dead: food and drink in Anglo-Saxon burial rituals. Boydell & Brewer, Woodbridge, United Kingdom. [Google Scholar]
- 25.Grattan JHG, Singer C. 1952. Anglo-Saxon magic and medicine: illustrated specially from the semi-pagan text “Lacnunga.” Oxford University Press, London, United Kingdom. [Google Scholar]
- 26.Liuzza RM. 2011. Anglo-Saxon prognostics: an edition and translation of texts from London, British Library, MS Cotton Tiberius A.iii. D.S. Brewer, Cambridge, United Kingdom. [Google Scholar]
- 27.Banham D. 2011. Dun, Oxa and Pliny the great physician: attribution and authority in Old English medical texts. Soc Hist Med 24:57–73. doi: 10.1093/shm/hkq104. [DOI] [Google Scholar]
- 28.Hooke D. 1990. A note on the evidence for vineyards and orchards in Anglo-Saxon England. J Wine Res 1:77–80. doi: 10.1080/09571269008717858. [DOI] [Google Scholar]
- 29.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. 2010. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biom J 50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bacterial species cultured from recipes and ingredient preparations. Download
Bald’s eyesalve retains antistaphylococcal activity for 30 days of storage. The onion (ES-O) and leek (ES-L) variants of the recipe (batches A and B) retained bactericidal activity against S. aureus biofilms in synthetic wounds after 30 days of storage (ANOVA, treatment, F2,24 = 217, P < 0.01; batch, F1,24 = 0.16, P = 0.693; and interaction, F2,24 = 0.196, P = 0.823). Download
Bald’s eyesalve kills MRSA in a mouse chronic wound infection. Adult female Swiss-Webster mice were administered wounds and infected with ca. 105 CFU S. aureus Mu50. Four days postinfection, mice were euthanized. Wound tissue was extracted and cut into three equal pieces, which were weighed, submerged in 300 µl sterile saline, ES-O, or ES-L (batches A and B, after 3 months of storage at 4°C) for 4 h, and then rinsed in sterile saline and homogenized. Viable bacteria were enumerated by plating. ES-L but not ES-O killed MRSA in wounds (ANOVA, F2,12 = 6.17, P = 0.014; post hoc Tukey test versus the control, ES-O, P = 0.069, and ES-L, P = 0.014). There was no effect of eyesalve batch (ANOVA, batch, F1,12 = 0.443, P = 0.518; interaction, F2,12 = 0.583, P = 0.573). The graph shows the means of data from six mice per treatment (three for each batch of the eyesalve). Download


