ABSTRACT
The yeast Saccharomyces cerevisiae harbors several prions that constitute powerful models to investigate the mechanisms of epigenetic structural inheritance. [PSI+] is undoubtedly the best-known yeast prion and results from the conversion of the translation termination factor Sup35p into self-perpetuating protein aggregates. Structurally different conformers of Sup35p aggregates can lead to [PSI+] strains with weak or strong prion phenotypes. Yeast prions are faithfully transmitted from mother to daughter cells during cell division, upon cytoplasmic mixing during mating, or when Sup35p fibrils made in test tubes are introduced into spheroplasts. Virtually all living cells in the three domains of life, Bacteria, Archaea, and Eukarya, secrete small membrane vesicles in the extracellular space. These extracellular vesicles (EV) have gained increasing interest as vehicles for the intercellular transfer of signaling molecules, nucleic acids, and pathogenic factors, as well as prion-like protein aggregates associated with neurodegenerative diseases. To begin to explore the question of whether EV could represent a natural mean for yeast prion transmission from cell to cell, we purified these extracellular vesicles and assessed whether they contained Sup35p. Here, we show that Sup35p is secreted within EV released in the extracellular medium of yeast cultures. We demonstrate that Sup35p within EV isolated from strong and weak [PSI+] cells is in an infectious prion conformation. Among the possible implications of our work is the possibility of previously unsuspected EV-mediated horizontal cell-to-cell transfer of fungal prions.
IMPORTANCE
Most living cells in the three domains of life, Bacteria, Archaea, and Eukarya, secrete small membrane vesicles in the extracellular space. These extracellular vesicles (EV) were long viewed as “trash cans” by which cells disposed of unwanted macromolecules. EV gained renewed interest as their roles as vehicles for the cell-to-cell transfer of nucleic acids, signaling molecules, and pathogenic factors were recently uncovered. Of particular interest is their proposed role in the prion-like propagation of toxic protein aggregates in neurodegenerative diseases. Yeasts naturally harbor prion proteins that are excellent models to investigate the mechanisms of formation, propagation, and elimination of self-perpetuating protein aggregates. Here we show for the first time that a yeast prion is secreted within EV in its infectious aggregated state. A major implication of our work is the possibility of EV-mediated horizontal spread of fungal prions.
OBSERVATION
“Proteinaceous infectious particles,” or prions, are self-perpetuating alternate conformations of proteins that are responsible for heritable non-Mendelian traits in mammals, filamentous fungi, and yeast (1–5). The yeast Saccharomyces cerevisiae hosts many structurally and functionally unrelated proteins with prion properties (6). [PSI+] is undoubtedly the best-known yeast prion and results from the conversion of the translation termination factor Sup35p into fibrillar protein aggregates (6). Sup35p can populate structurally different heritable conformations that are at the origin of different [PSI+] strains, generally defined as weak or strong with respect to the severity of the nonsense suppression phenotypes they confer (6).
Yeast prions are faithfully transmitted from mother to daughter cell during cell division or upon cytoplasmic mixing during mating. It is also possible to induce the prion state efficiently by introducing cytosolic fractions from prion-containing strains or prions assembled in vitro within prion-free yeast cells (7, 8).
Cells from the three domains of life, Bacteria, Archaea, and Eukarya, secrete small membrane vesicles in the extracellular space (9–11). These extracellular vesicles (EV) comprise exosomes (~30 to 100 nm), which originate from the fusion of multivesicular bodies with the plasma membrane, and microvesicles (or ectosomes) (~100 to 1,000 nm), which form directly from the plasma membrane (9–11). EV have gained increased interest as their potential roles as vehicles for the intercellular transfer of nucleic acids, signaling molecules, and pathogenic factors have been uncovered over the past years (12, 13). EV also mediate clearance and possibly cell-to-cell transfer of protein aggregates associated with neurodegenerative diseases (14). In yeasts and fungi, EV mediate the export of a wide range of proteins, lipids, RNA, and polysaccharides (15–19).
Here we assess whether EV are vehicles for the export of [PSI+] prions in yeast. We purified EV from the extracellular medium of [psi−] and strong or weak [PSI+] yeast cultures and found that they all contained Sup35p. Importantly, we demonstrate that Sup35p within EV produced by strong and weak [PSI+] cells is in its aggregated infectious prion state. These findings are of importance, as they not only shed light on a new pathway for prion export in yeast but also provide a tool to investigate horizontal cell-to-cell transfer of genetic and epigenetic information in yeasts and possibly fungi.
Purification and characterization of yeast EV.
We first determined that maximal recovery of EV was achieved from the extracellular medium of yeast cultures grown to early stationary phase in rich yeast extract-peptone-dextrose (YPD) medium (see Text S1 in the supplemental material). We estimated that our purification procedure yielded ~2 × 1013 EV per liter of extracellular medium under these conditions, which corresponds to a final ratio of ~200 EV per yeast cell (calculation details can be found in Text S1).
Yeast EV were purified from a [psi−] strain and from [PSI+]S and [PSI+]W strains, bearing strong and weak prion variants, respectively, by using the optimized protocol described in Text S1 in the supplemental material (see also Fig. S1A in the supplemental material). EV preparations from all three strains had similar electrophoretic protein profiles when examined by SDS-PAGE (see Fig. S1B) and contained, as expected, membrane vesicles visible by electron microscopy (see Fig. S1C). The morphology of these vesicles was consistent from one preparation to another, most of them being round, ovoid, or cup-shaped (see Fig. S1C). The vesicle diameters spanned between ~20 and ~300 nm, with most EV in the ~50-to-100-nm range (see Fig. S1D), suggesting that they mainly correspond to exosomes. Thus, harboring or not strong and weak [PSI+] prion strains affected neither the yeast cells’ abilities to produce and secrete EV nor the size and morphology of EV.
Sup35p is localized inside EV originating from [psi−] and [PSI+] cells.
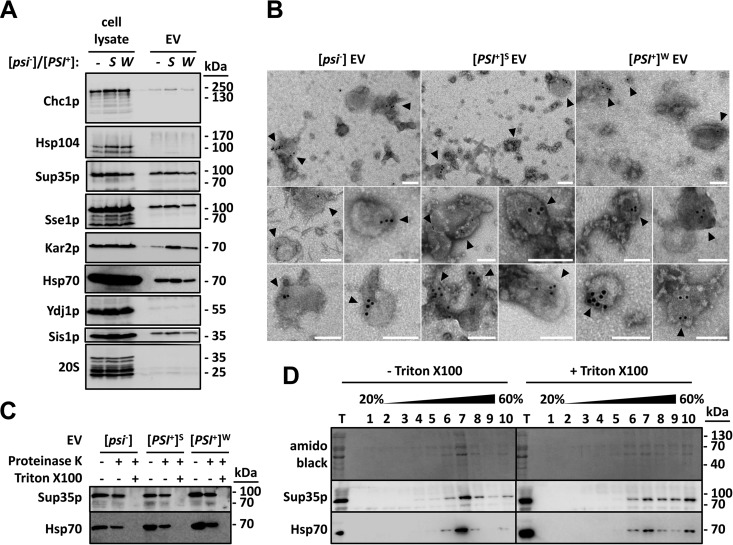
We found significant amounts of Sup35p in EV isolated from both [psi−] and strong or weak [PSI+] cells (Fig. 1A). Slight variations in the levels of Sup35p detected in EV were observed in biological replicates, e.g., independent preparations (data not shown). EV isolated from [psi−] cells contained in a consistent manner less Sup35p than those isolated from [PSI+] cells (see Fig. S2 in the supplemental material). Cytosolic molecular chaperones of the Hsp70 (Ssa1p), Hsp110 (Sse1p), and Hsp40 (Sis1p) families were detected within EV preparations (Fig. 1A). These also contained the clathrin heavy-chain protein Chc1p and the endoplasmic reticulum Hsp70 family member Kar2p, but they did not contain detectable amounts of proteasomes (20S) or the Ydj1p and Hsp104 molecular chaperones (Fig. 1A). With the notable exception of Sup35p, most of the proteins we found associated with EV were also identified in a proteomic study of yeast EV proteins (17). The reasons why Sup35p was not identified in that study are unclear, but this may have been due to the different yeast genetic backgrounds used and/or to the different growth conditions, particularly the use of Sabouraud dextrose broth instead of YPD agar (YPDA) medium and a growth temperature of 25°C instead of 30°C (17). All of these parameters may have consequences on protein expression levels and ultimately on the composition of the EV proteome. Furthermore, the yeast strains used by Oliveira et al. were likely in the [psi−] state and thus contained less Sup35p than our [PSI+] cells (for instance, see Fig. S2) (17). Alternatively, the identification of Sup35p may have not withstood the stringent filtering criteria that were applied at each step of the mass spectrometry analysis (17). Immunogold labeling and electron microscopy revealed the presence of Sup35p inside Triton X-100-permeabilized EV (Fig. 1B). EV were not stained by the secondary gold-conjugated antibody in the absence of primary anti-Sup35p antibodies, confirming that the immunogold labeling procedure was specific for Sup35p (see Fig. S3 in the supplemental material). Most (~75%) Sup35p-positive vesicles had a diameter of ~30 to 100 nm, suggesting that they correspond to exosomes (see Fig. S4 in the supplemental material). We demonstrated the EV intraluminal localization of Sup35p and Hsp70 by subjecting EV to proteinase K treatment. Sup35p and Hsp70 resisted cleavage in intact EV but not in the presence of Triton X-100 (Fig. 1C). Sup35p and Hsp70 colocalized with EV proteins in sucrose gradients, and their flotation in the gradients was altered upon solubilization of EV with Triton X-100 (Fig. 1D). Furthermore, Sup35p and Hsp70 remained associated with EV in sucrose gradient separations under high ionic strength (see Fig. S5 in the supplemental material). Taken together, these data show that Sup35p is packaged inside vesicles and secreted in the extracellular medium, both in [psi−] and weak or strong [PSI+] cells.
FIG 1 .
Sup35p is localized inside EV produced by [psi−] and [PSI+] cells. (A) The indicated strains were grown to early stationary phase in YPDA medium. Cell lysates and EV were then prepared and analyzed by SDS-PAGE and Western blotting using antibodies against the indicated proteins. (B) EV were fixed with 2% paraformaldehyde, adsorbed onto electron microscopy grids, and permeabilized with 0.02% Triton X-100 for 10 min. Immunogold labeling of Sup35p was then performed using primary polyclonal anti-Sup35p antibody and secondary anti-rabbit 10-nm-gold-conjugated antibodies. Electron microscopy grids were fixed with 1% glutaraldehyde for 5 min at room temperature and visualized by negative-stain electron microscopy. Bars, 100 nm. Arrowheads point to gold-labeled Sup35p-positive vesicles. (C) Purified EV were incubated with or without 2% Triton X-100 for 30 min on ice, before the addition of 0.01 mg ml−1 proteinase K. Reaction mixtures were further incubated for 15 min on ice and stopped with the addition of 2 mM phenylmethylsulfonyl fluoride. An untreated control reaction mixture (without Triton X-100 and without proteinase K) was run in parallel under identical conditions. Reaction products were then analyzed by SDS-PAGE and Western blotting using the indicated antibodies. (D) Purified EV produced by [PSI+]S cells were incubated with or without 1% Triton X-100 for 30 min at 4°C. The mixture was adjusted to 60% sucrose in 20 mM HEPES-OH (pH 7.5) and deposited in the bottom of an ultracentrifuge tube. Equal volumes of 40% and 20% (wt/wt) sucrose solutions in 20 mM HEPES-OH (pH 7.5) were successively layered on top of the EV suspensions. Following centrifugation for 17 h at 150,000 × g and at 4°C, 10 fractions were collected from the top of the gradients and analyzed by SDS-PAGE and Western blotting using the indicated antibodies (T stands for total and represents the input material).
Sup35p within [PSI+] EV retains an infectious state.
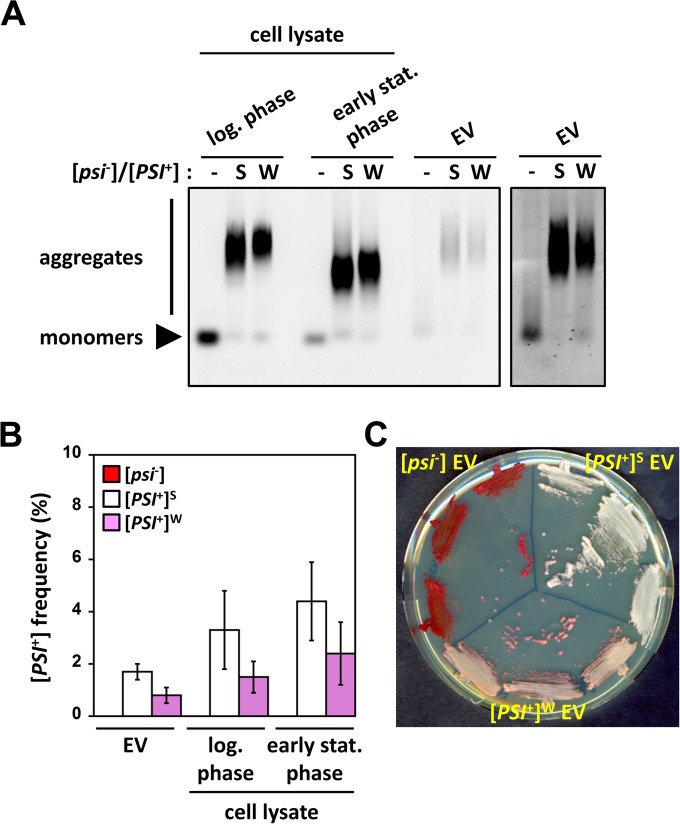
Remarkably, SDD-AGE analysis revealed that Sup35p in EV prepared from [PSI+] cells migrates as high-molecular-weight SDS-resistant polymers, whereas only monomeric Sup35p was detected in EV prepared from [psi−] cells (Fig. 2A, right panel). As reported previously (20), the size distribution of SDS-resistant Sup35p polymers visualized by SDD-AGE changed upon entry of cells into stationary phase (Fig. 2A; compare log-phase and early-stationary-phase [PSI+] samples). Sup35p SDS-resistant polymers appeared slightly larger in [PSI+]W than in [PSI+]S cell lysates, regardless of the growth phase, as reported previously (21, 22). The apparent molecular weight of Sup35p polymers within [PSI+] EV appeared much higher than that of Sup35p polymers in lysates of the early-stationary-phase cells from which they originated (Fig. 2A; compare early-stationary-phase and EV [PSI+] samples). The size distribution of Sup35p polymers within [PSI+] EV was, however, comparable to that of Sup35p polymers found within lysates prepared from exponentially growing cells (Fig. 2A; compare log-phase and EV [PSI+] samples). These observations suggest that most Sup35p prion particles are packaged during the exponential phase of growth before being progressively released in the extracellular medium, possibly in relation to glucose consumption (23). Alternatively, conformational rearrangements of Sup35p prion particles within EV could also account for the observed size differences between intracellular and intravesicular high-molecular-weight species. Regardless, our data suggest that both soluble and aggregated Sup35p molecular species are released from cells via export in EV. These results were confirmed by the finding that green fluorescent protein (GFP)-tagged Sup35p (see Text S1 in the supplemental material) displayed a punctate pattern (characteristic of aggregates) and a diffuse dim pattern (characteristic of soluble entities) in EV purified from [PSI+] and [psi−] cells, respectively, as visualized by fluorescence microscopy (see Fig. S6 in the supplemental material).
FIG 2 .
Sup35p within [PSI+] EV is in its aggregated strain-specific prion state. (A) Cell lysates (20 µg) from the indicated strains grown to log phase or early stationary phase and purified EV (20 µg) were analyzed by SDD-AGE followed by immunoblotting using anti-Sup35p antibodies. The right panel corresponds to a longer exposure of the same membrane (only EV lanes are shown). (B) EV and cell lysates were prepared as described for panel A. All samples (~1 to 10 µg total protein) were normalized by semiquantitative Western blotting to contain equivalent amounts of Sup35p and were then transformed into [psi−] spheroplasts. Random clones from each transformation reaction were then patched onto 1/4-YPD plates to assess their prion phenotype and to determine the [PSI+] conversion efficiency (mean ± standard error). Transformation with [psi−] EV or cell lysates did not yield any [PSI+] clones, and the [PSI+] conversion frequency was then equal to 0. (C) [PSI+] clones, obtained by transforming [psi−] spheroplasts with [PSI+]S or [PSI+]W EV, and [psi−] clones, obtained by transforming [psi−] spheroplasts with [psi−] EV, were streaked onto 1/4-YPD plates to assess their prion phenotype.
Remarkably, Sup35p prion particles within EV prepared from [PSI+] cells were able to confer the prion state when transformed into [psi−] cells (see Text S1 in the supplemental material for details) (Fig. 2B). [PSI+] formation frequencies obtained upon transformation of [psi−] spheroplasts with EV or cell lysates normalized to contain comparable amounts of aggregated Sup35p were within similar ranges (Fig. 2B). This indicates that Sup35p prion particles within EV fully retain their infectious potential. Importantly, Sup35p prion particles within EV also retained their strain-specific characteristics. Indeed, EV originating from strong [PSI+]S and weak [PSI+]W cells induced the formation of strong and weak [PSI+] cells, respectively, in the protein transformation experiments (Fig. 2C). As expected, [PSI+]S and [PSI+]W cell lysates induced the formation of strong and weak [PSI+] clones, respectively, in control experiments (see Fig. S7 in the supplemental material).
To our knowledge, our study provides the first evidence for EV-mediated export of a prion in yeast (Fig. 1). Of particular importance is the finding that prion particles within EV fully retain their infectious and strain-specific prion state (Fig. 2), meaning they could in principle mediate horizontal cell-to-cell propagation of yeast prions.
Packaging of soluble and aggregated Sup35p in EV.
We demonstrated using immunogold labeling and electron microscopy, proteolysis resistance assays, and flotation assays that both soluble and aggregated Sup35p are within EV, not at their surface (Fig. 1B, C, and D; see also Fig. S3 and S5 in the supplemental material). Sup35p was mostly found inside vesicles with a diameter of ~30 to 100 nm, e.g., exosomes (Fig. 1B; see also Fig. S3 in the supplemental material). A small proportion (25%) of Sup35p was detected in larger vesicles (~100 to 180 nm in diameter) that may, or may not, correspond to ectosomes directly originating from plasma membrane budding (Fig. 1B; see also Fig. S3) (9, 11). Given the limited differences in size and density, we were not able to obtain homogeneous preparations of each type of vesicle. In addition, specific differences in cargo content that may be helpful in distinguishing exosomes from ectosomes have yet to be established in yeast. Therefore, other strategies will be required to ascertain the exosomal or ectosomal origin of Sup35p-containing EV.
Important questions that also need to be addressed in future studies include how aggregated Sup35p prion particles are addressed to vesicles and which cellular factors (e.g., molecular chaperones) and/or cellular structures (e.g., cytoskeleton) assist in this process. Sup35p is a cytosolic protein that does not, to the extent of our knowledge, enter the secretory pathway. Surprisingly though, both soluble and aggregated Sup35p were found in EV (Fig. 2; see also Fig. S6 in the supplemental material). A plausible explanation for this localization would be either that Sup35p is secreted to the extracellular space or that it is directed toward autophagic destruction, or both (9, 24). The stochastic trapping of Sup35p into prevesicular structures is unlikely to account for Sup35p encapsulation within EV. Indeed, only a subset of cytosolic molecular chaperones was found within EV (Fig. 1A), suggesting the existence of cargo selectivity mechanisms.
The sizes of SDS-resistant Sup35p assemblies within [PSI+] EV and cell lysates from log-phase [PSI+] cells were alike (Fig. 2A). These Sup35p assemblies were, however, much larger than those found in lysates prepared from the early-stationary-phase [PSI+] cells harvested at the end of culturing (Fig. 2A). This observation suggests that the packaging of Sup35p aggregates within vesicles occurs in actively dividing cells. Entry into stationary phase upon glucose exhaustion may significantly slow vesicle formation and trafficking, but it may in turn trigger the release of preformed vesicles in the extracellular space (23). In line with this notion, we were not able to isolate significant amounts of EV from cell cultures in the log phase of growth (data not shown). Although less likely, we cannot rule out the alternate possibility that Sup35p aggregates are remodeled within EV or that bigger aggregates are preferentially targeted for EV-mediated release. Regardless, Sup35p prion particles within EV fully retained their infectious potential (Fig. 2B) and their ability to transmit their strain-specific conformations (Fig. 2C), indicating that they are unlikely to represent dead-end aggregation products.
In light of our data, EV-mediated export of prions does not seem to constitute a prion clearance mechanism, as [PSI+] is faithfully maintained during growth. However, packaging and export of prions (and possibly that of other aggregated or misfolded protein species) in EV may constitute a way to reduce the burden on the cellular protein quality-control machineries and improve cell fitness.
EV as vehicles for horizontal cell-to-cell transfer of yeast prions?
Our data show that EV constitute a natural source of infectious Sup35p particles, raising the intriguing possibility that EV could be new mediators of prion spreading among yeasts. Our attempts to induce prion formation by incubating [psi−] cells with purified EV or with EV-containing conditioned medium from [PSI+] cells were, as per now, inconclusive but did not yet rule out the existence of such a cell-to-cell transfer of yeast prions. Uptake of exogenous vesicles by yeasts requires overcoming the cell wall physical barrier, which appears to be an unsurmountable obstacle for such big particles, at least under standard laboratory growth conditions (11). The efficient internalization of EV trapped between the plasma membrane and the cell wall has been demonstrated to occur when the growth medium is replenished with glucose (23). In contrast, neither the mechanism by which EV are able to cross the yeast cell wall to be released in the medium nor that of EV capture and uptake from the medium are known (11). These processes may require particular and yet-to-be-identified growth conditions and/or environmental cues that may, for example, induce changes in the mechanical properties of the cell wall (25). Intercellular transfer of EV may also require direct cell-to-cell contacts, such as those occurring in colonies or biofilms. We can envision conditions in the wild where multiple individuals within a microbial community—yeast and/or other microbes—share a cell wall or analogous polysaccharide extracellular matrix that allows the exchange of information via the secretion and uptake of EV.
Conclusions.
EV are under intense scrutiny from the biomedical research community due to the key roles they may play in horizontal cell-to-cell communication and in the transfer of pathogenic and virulence factors, to their potential use as diagnostic markers in many cancers or neurodegenerative diseases, and to their use as vehicles for drug delivery. The findings we report here establish yeast prions as powerful tractable biological models to specifically investigate the molecular mechanisms of vesicle-mediated export of infectious protein assemblies and as a new tool to investigate the EV-mediated horizontal transfer of information in yeasts and fungi.
SUPPLEMENTAL MATERIAL
Materials and methods. Download
Characterization of EV purified from [psi−], [PSI+]S, and [PSI+]W yeast cultures. (A) Prion phenotypes of the strains used in this study were assessed on 1/4-YPD plates. (B) Purified EV from the indicated strains were analyzed by SDS-PAGE and silver staining. (C) Negative-stained electron micrographs of purified EV. Bars, 100 nm. (D) The size distribution of the indicated EV preparations was determined by analyzing electron micrographs, such as those shown in panel C, by using the ImageJ software. The number (n) of vesicles measured in each analysis is indicated (three independent EV preparations were analyzed and combined for each strain). Results from all EV preparations were combined to generate the “total” graph. Download
Slight variations in the levels of Sup35p were observed among different EV preparations. Purified EV (~3 µg) from [psi−] or [PSI+]S strains were analyzed by SDS-PAGE and Western blotting by using anti-Sup35p antibodies (upper panel). The membrane was stained with amido black (lower panel) to reveal total EV proteins. Download
EV are not stained by gold-conjugated secondary antibodies in the absence of primary anti-Sup35p antibody. EV were fixed with 2% paraformaldehyde, adsorbed onto electron microscopy grids, and permeabilized with 0.02% Triton X-100 for 10 min. Electron microscopy grids were incubated with anti-rabbit gold-conjugated antibodies, fixed with 1% glutaraldehyde, and then visualized by negative-stain electron microscopy. Bars, 100 nm. Download
Size distribution of immunogold-labeled Sup35p-positive vesicles. Immunogold labeling of Sup35p and negative-stain electron microscopy of EV isolated from the indicated strains were performed as described for Fig. 1B. The size distribution of immunogold-labeled Sup35p-positive vesicles was determined using the ImageJ software, and and results are presented as a box plot, showing the median (middle line within each box), 25th (lower boundaries of boxes) and 75th (upper boundaries of boxes) percentiles, the minimal and maximal values (whiskers), and outliers (dots). The number of immunogold-labeled Sup35p-positive vesicles analyzed for each EV type is indicated at the bottom of the plot. Download
Sup35p and Hsp70 colocalize with [PSI+]S EV in discontinuous sucrose gradients under low- or high-salt conditions. Purified EV from the [PSI+]S strain were adjusted to 1.75 M sucrose in phosphate-buffered saline (PBS; low salt condition) supplemented or not with 400 mM NaCl (high-salt condition) and deposited at the bottom of ultracentrifugation tubes. Equal volumes of 1.17 M and 0.58 M sucrose solutions in PBS (low-salt condition) supplemented or not with 400 mM NaCl (high-salt condition) were layered on top of the EV suspensions. Following centrifugation for 17 h at 150,000 × g at 4°C, eight fractions were collected from the tops of the gradients and analyzed by SDS-PAGE and Coomassie blue staining (upper panel) or Western blotting (middle and lower panels), using the indicated antibodies (T stands for total and represents the input material). (B) The fractions highlighted by colored dashed-line boxes from the sucrose gradients described for panel A were concentrated by centrifugation for 1 h at 100,000 × g at 4°C, washed in PBS, and then analyzed by immunogold labeling of Sup35p and by negative-stain electron microscopy. Bars, 100 nm. Arrowheads point to gold-labeled Sup35p-positive vesicles. Download
EV isolated from a [PSI+] sup35-GFP strain display a punctate fluorescence pattern. (A) Negative-stained electron micrographs of EV preparations from the indicated strains. Bars, 100 nm. (B) EV preparations from the indicated strains were directly observed under a fluorescence microscope. Bar, 50 µm. Download
Cell lysates from strong and weak [PSI+] strains induce strong and weak [PSI+] clones, respectively, in protein transformation assays. Representative [psi−] and [PSI+] clones obtained by transforming [psi−] spheroplasts with the indicated cell lysates (as described for Fig. 2B) were streaked on 1/4-YPD plates to assess their prion phenotype. Download
ACKNOWLEDGMENTS
We thank Tricia R. Serio, Jeffrey L. Brodsky, and Sandra K. Lemmon for the kind gift of strains and antibodies.
This work benefited from the facilities and expertise of the Imagif Cell Biology Unit, which is supported by the Conseil Général de l’Essonne, France bioimaging infrastructure and the Agence Nationale de la Recherche (ANR). M.K. and R.M. are supported by the Centre National de la Recherche Scientifique and the Agence Nationale de la Recherche (ANR-12-BS08-0013-02).
Footnotes
Citation Kabani M, Melki R. 2015. Sup35p in its soluble and prion states is packaged inside extracellular vesicles. mBio 6(4):e01017-15. doi:10.1128/mBio.01017-15.
Contributor Information
Susan Liebman, University of Minnesota, GCD.
Judith Berman, University of Nevada Reno.
REFERENCES
- 1.Cox BS. 1965. PSI, a cytoplasmic suppressor of super-suppressor in yeast. Heredity 20:505–521. doi: 10.1038/hdy.1965.65. [DOI] [Google Scholar]
- 2.Aigle M, Lacroute F. 1975. Genetical aspects of [URE3], a non-mitochondrial, cytoplasmically inherited mutation in yeast. Mol Gen Genet 136:327–335. doi: 10.1007/BF00341717. [DOI] [PubMed] [Google Scholar]
- 3.Prusiner SB. 1982. Novel proteinaceous infectious particles cause scrapie. Science 216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 4.Wickner RB. 1994. [URE3] as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae. Science 264:566–569. doi: 10.1126/science.7909170. [DOI] [PubMed] [Google Scholar]
- 5.Coustou V, Deleu C, Saupe S, Begueret J. 1997. The protein product of the het-s heterokaryon incompatibility gene of the fungus Podospora anserina behaves as a prion analog. Proc Natl Acad Sci U S A 94:9773–9778. doi: 10.1073/pnas.94.18.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liebman SW, Chernoff YO. 2012. Prions in yeast. Genetics 191:1041–1072. doi: 10.1534/genetics.111.137760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King CY, Diaz-Avalos R. 2004. Protein-only transmission of three yeast prion strains. Nature 428:319–323. doi: 10.1038/nature02391. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka M, Chien P, Naber N, Cooke R, Weissman JS. 2004. Conformational variations in an infectious protein determine prion strain differences. Nature 428:323–328. doi: 10.1038/nature02392. [DOI] [PubMed] [Google Scholar]
- 9.Colombo M, Raposo G, Théry C. 2014. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 10.Deatherage BL, Cookson BT. 2012. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect Immun 80:1948–1957. doi: 10.1128/IAI.06014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf JM, Casadevall A. 2014. Challenges posed by extracellular vesicles from eukaryotic microbes. Curr Opin Microbiol 22:73–78. doi: 10.1016/j.mib.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schorey JS, Cheng Y, Singh PP, Smith VL. 2015. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep 16:24–43. doi: 10.15252/embr.201439363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simons M, Raposo G. 2009. Exosomes—vesicular carriers for intercellular communication. Curr Opin Cell Biol 21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Rajendran L, Bali J, Barr MM, Court FA, Krämer-Albers EM, Picou F, Raposo G, van der Vos KE, van Niel G, Wang J, Breakefield XO. 2014. Emerging roles of extracellular vesicles in the nervous system. J Neurosci 34:15482–15489. doi: 10.1523/JNEUROSCI.3258-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albuquerque PC, Nakayasu ES, Rodrigues ML, Frases S, Casadevall A, Zancope-Oliveira RM, Almeida IC, Nosanchuk JD. 2008. Vesicular transport in Histoplasma capsulatum: an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell Microbiol 10:1695–1710. doi: 10.1111/j.1462-5822.2008.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peres da Silva R, Puccia R, Rodrigues ML, Oliveira DL, Joffe LS, César GV, Nimrichter L, Goldenberg S, Alves LR. 2015. Extracellular vesicle-mediated export of fungal RNA. Sci Rep 5:7763. doi: 10.1038/srep07763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliveira DL, Nakayasu ES, Joffe LS, Guimarães AJ, Sobreira TJ, Nosanchuk JD, Cordero RJ, Frases S, Casadevall A, Almeida IC, Nimrichter L, Rodrigues ML. 2010. Characterization of yeast extracellular vesicles: evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PLoS One 5:e11113. doi: 10.1371/journal.pone.0011113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, Nosanchuk JD, Almeida IC, Casadevall A. 2008. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell 7:58–67. doi: 10.1128/EC.00370-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodrigues ML, Nimrichter L, Oliveira DL, Frases S, Miranda K, Zaragoza O, Alvarez M, Nakouzi A, Feldmesser M, Casadevall A. 2007. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot Cell 6:48–59. doi: 10.1128/EC.00318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabani M, Redeker V, Melki R. 2014. A role for the proteasome in the turnover of Sup35p and in [PSI+] prion propagation. Mol Microbiol 92:507–528. doi: 10.1111/mmi.12572. [DOI] [PubMed] [Google Scholar]
- 21.Kryndushkin DS, Alexandrov IM, Ter-Avanesyan MD, Kushnirov VV. 2003. Yeast [PSI+] prion aggregates are formed by small Sup35 polymers fragmented by Hsp104. J Biol Chem 278:49636–49643. doi: 10.1074/jbc.M307996200. [DOI] [PubMed] [Google Scholar]
- 22.Kabani M, Cosnier B, Bousset L, Rousset JP, Melki R, Fabret C. 2011. A mutation within the C-terminal domain of Sup35p that affects [PSI+] prion propagation. Mol Microbiol 81:640–658. doi: 10.1111/j.1365-2958.2011.07719.x. [DOI] [PubMed] [Google Scholar]
- 23.Giardina BJ, Stanley BA, Chiang HL. 2014. Glucose induces rapid changes in the secretome of Saccharomyces cerevisiae. Proteome Sci 12:9. doi: 10.1186/1477-5956-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baixauli F, López-Otín C, Mittelbrunn M. 2014. Exosomes and autophagy: coordinated mechanisms for the maintenance of cellular fitness. Front Immunol 5:403. doi: 10.3389/fimmu.2014.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pelling AE, Sehati S, Gralla EB, Valentine JS, Gimzewski JK. 2004. Local nanomechanical motion of the cell wall of Saccharomyces cerevisiae. Science 305:1147–1150. doi: 10.1126/science.1097640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials and methods. Download
Characterization of EV purified from [psi−], [PSI+]S, and [PSI+]W yeast cultures. (A) Prion phenotypes of the strains used in this study were assessed on 1/4-YPD plates. (B) Purified EV from the indicated strains were analyzed by SDS-PAGE and silver staining. (C) Negative-stained electron micrographs of purified EV. Bars, 100 nm. (D) The size distribution of the indicated EV preparations was determined by analyzing electron micrographs, such as those shown in panel C, by using the ImageJ software. The number (n) of vesicles measured in each analysis is indicated (three independent EV preparations were analyzed and combined for each strain). Results from all EV preparations were combined to generate the “total” graph. Download
Slight variations in the levels of Sup35p were observed among different EV preparations. Purified EV (~3 µg) from [psi−] or [PSI+]S strains were analyzed by SDS-PAGE and Western blotting by using anti-Sup35p antibodies (upper panel). The membrane was stained with amido black (lower panel) to reveal total EV proteins. Download
EV are not stained by gold-conjugated secondary antibodies in the absence of primary anti-Sup35p antibody. EV were fixed with 2% paraformaldehyde, adsorbed onto electron microscopy grids, and permeabilized with 0.02% Triton X-100 for 10 min. Electron microscopy grids were incubated with anti-rabbit gold-conjugated antibodies, fixed with 1% glutaraldehyde, and then visualized by negative-stain electron microscopy. Bars, 100 nm. Download
Size distribution of immunogold-labeled Sup35p-positive vesicles. Immunogold labeling of Sup35p and negative-stain electron microscopy of EV isolated from the indicated strains were performed as described for Fig. 1B. The size distribution of immunogold-labeled Sup35p-positive vesicles was determined using the ImageJ software, and and results are presented as a box plot, showing the median (middle line within each box), 25th (lower boundaries of boxes) and 75th (upper boundaries of boxes) percentiles, the minimal and maximal values (whiskers), and outliers (dots). The number of immunogold-labeled Sup35p-positive vesicles analyzed for each EV type is indicated at the bottom of the plot. Download
Sup35p and Hsp70 colocalize with [PSI+]S EV in discontinuous sucrose gradients under low- or high-salt conditions. Purified EV from the [PSI+]S strain were adjusted to 1.75 M sucrose in phosphate-buffered saline (PBS; low salt condition) supplemented or not with 400 mM NaCl (high-salt condition) and deposited at the bottom of ultracentrifugation tubes. Equal volumes of 1.17 M and 0.58 M sucrose solutions in PBS (low-salt condition) supplemented or not with 400 mM NaCl (high-salt condition) were layered on top of the EV suspensions. Following centrifugation for 17 h at 150,000 × g at 4°C, eight fractions were collected from the tops of the gradients and analyzed by SDS-PAGE and Coomassie blue staining (upper panel) or Western blotting (middle and lower panels), using the indicated antibodies (T stands for total and represents the input material). (B) The fractions highlighted by colored dashed-line boxes from the sucrose gradients described for panel A were concentrated by centrifugation for 1 h at 100,000 × g at 4°C, washed in PBS, and then analyzed by immunogold labeling of Sup35p and by negative-stain electron microscopy. Bars, 100 nm. Arrowheads point to gold-labeled Sup35p-positive vesicles. Download
EV isolated from a [PSI+] sup35-GFP strain display a punctate fluorescence pattern. (A) Negative-stained electron micrographs of EV preparations from the indicated strains. Bars, 100 nm. (B) EV preparations from the indicated strains were directly observed under a fluorescence microscope. Bar, 50 µm. Download
Cell lysates from strong and weak [PSI+] strains induce strong and weak [PSI+] clones, respectively, in protein transformation assays. Representative [psi−] and [PSI+] clones obtained by transforming [psi−] spheroplasts with the indicated cell lysates (as described for Fig. 2B) were streaked on 1/4-YPD plates to assess their prion phenotype. Download