Abstract
Genes encoding the alpha and beta subunits of the T-cell receptor (TCR) for antigen require rearrangement events for functional expression. In the case of the immunoglobin genes, rearrangement events have been shown to be necessary, but they are not sufficient for full gene expression. The regulation of TCR genes, apart from the requirement for rearrangement, remains to be elucidated. The T-lymphoma cell clone SL12.4 actively transcribes both TCR-alpha and -beta genes and the cells contain nuclear TCR precursor transcripts. However, the cells fail to accumulate appreciable quantities of mature TCR-alpha and -beta mRNAs in either the nucleus or the cytoplasm. The protein synthesis inhibitor cycloheximide (CHX) induces a 20-fold increase in mature TCR-alpha transcript accumulation without a concomitant increase in TCR-alpha gene transcription suggesting that CHX reverses the nuclear post-transcriptional events which prevent mature TCR-alpha mRNA accumulation. CHX also induces full length TCR-beta transcripts greater than 90-fold while TCR-beta gene transcription increases only 2- to 4-fold. The calcium ionophore A23187 induces the accumulation of TCR-alpha but not -beta transcripts; and in contrast to CHX, it increases the rate of TCR-alpha gene transcription and the expression of large nuclear TCR-alpha precursor transcripts. Since CHX and A23187 mediated induction of TCR mRNA is both rapid and reversible, it is unlikely that new DNA rearrangements are responsible for the induction. Collectively, the data show that the accumulation of mature TCR-alpha and -beta transcripts in SL12.4 cells can be coordinately or independently induced by nuclear events involving both transcriptional and posttranscriptional mechanisms.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert F., Hua C., Truneh A., Pierres M., Schmitt-Verhulst A. M. Distinction between antigen receptor and IL 2 receptor triggering events in the activation of alloreactive T cell clones with calcium ionophore and phorbol ester. J Immunol. 1985 Jun;134(6):3649–3655. [PubMed] [Google Scholar]
- Alterman R. B., Ganguly S., Schulze D. H., Marzluff W. F., Schildkraut C. L., Skoultchi A. I. Cell cycle regulation of mouse H3 histone mRNA metabolism. Mol Cell Biol. 1984 Jan;4(1):123–132. doi: 10.1128/mcb.4.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawerman G. Determinants of messenger RNA stability. Cell. 1987 Jan 16;48(1):5–6. doi: 10.1016/0092-8674(87)90346-1. [DOI] [PubMed] [Google Scholar]
- Calame K. L. Mechanisms that regulate immunoglobulin gene expression. Annu Rev Immunol. 1985;3:159–195. doi: 10.1146/annurev.iy.03.040185.001111. [DOI] [PubMed] [Google Scholar]
- Carneiro M., Schibler U. Accumulation of rare and moderately abundant mRNAs in mouse L-cells is mainly post-transcriptionally regulated. J Mol Biol. 1984 Oct 5;178(4):869–880. doi: 10.1016/0022-2836(84)90316-4. [DOI] [PubMed] [Google Scholar]
- Clark S. P., Yoshikai Y., Taylor S., Siu G., Hood L., Mak T. W. Identification of a diversity segment of human T-cell receptor beta-chain, and comparison with the analogous murine element. 1984 Sep 27-Oct 3Nature. 311(5984):387–389. doi: 10.1038/311387a0. [DOI] [PubMed] [Google Scholar]
- Davey M. P., Bongiovanni K. F., Kaulfersch W., Quertermous T., Seidman J. G., Hershfield M. S., Kurtzberg J., Haynes B. F., Davis M. M., Waldmann T. A. Immunoglobulin and T-cell receptor gene rearrangement and expression in human lymphoid leukemia cells at different stages of maturation. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8759–8763. doi: 10.1073/pnas.83.22.8759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton R., Birnie G. D., Knowler J. T. Post-transcriptional regulation of rat liver gene expression by glucocorticoids. Nucleic Acids Res. 1985 Sep 25;13(18):6467–6482. doi: 10.1093/nar/13.18.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furley A. J., Mizutani S., Weilbaecher K., Dhaliwal H. S., Ford A. M., Chan L. C., Molgaard H. V., Toyonaga B., Mak T., van den Elsen P. Developmentally regulated rearrangement and expression of genes encoding the T cell receptor-T3 complex. Cell. 1986 Jul 4;46(1):75–87. doi: 10.1016/0092-8674(86)90861-5. [DOI] [PubMed] [Google Scholar]
- Gascoigne N. R., Chien Y., Becker D. M., Kavaler J., Davis M. M. Genomic organization and sequence of T-cell receptor beta-chain constant- and joining-region genes. Nature. 1984 Aug 2;310(5976):387–391. doi: 10.1038/310387a0. [DOI] [PubMed] [Google Scholar]
- Gerster T., Picard D., Schaffner W. During B-cell differentiation enhancer activity and transcription rate of immunoglobulin heavy chain genes are high before mRNA accumulation. Cell. 1986 Apr 11;45(1):45–52. doi: 10.1016/0092-8674(86)90536-2. [DOI] [PubMed] [Google Scholar]
- Graves R. A., Marzluff W. F. Rapid reversible changes in the rate of histone gene transcription and histone mRNA levels in mouse myeloma cells. Mol Cell Biol. 1984 Feb;4(2):351–357. doi: 10.1128/mcb.4.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M. F. Differentiation-linked leukemogenesis in lymphocytes. Science. 1986 Nov 7;234(4777):697–704. doi: 10.1126/science.3535067. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Hermanowski A. L., Ziff E. B. Effect of protein synthesis inhibitors on growth factor activation of c-fos, c-myc, and actin gene transcription. Mol Cell Biol. 1986 Apr;6(4):1050–1057. doi: 10.1128/mcb.6.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney T., Jr Characterization of mouse 45S ribosomal RNA subspecies suggests that the first processing cleavage occurs 600 +/- 100 nucleotides from the 5' end and the second 500 +/- 100 nucleotides from the 3' end of a 13.9 kb precursor. Nucleic Acids Res. 1985 Jul 11;13(13):4905–4919. doi: 10.1093/nar/13.13.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley-Hyde J. M., Lynch R. G. The physiology of B cells as studied with tumor models. Annu Rev Immunol. 1986;4:621–649. doi: 10.1146/annurev.iy.04.040186.003201. [DOI] [PubMed] [Google Scholar]
- Harpold M. M., Evans R. M., Salditt-Georgieff M., Darnell J. E. Production of mRNA in Chinese hamster cells: relationship of the rate of synthesis to the cytoplasmic concentration of nine specific mRNA sequences. Cell. 1979 Aug;17(4):1025–1035. doi: 10.1016/0092-8674(79)90341-6. [DOI] [PubMed] [Google Scholar]
- Hays E. F., Weinroth S. E., MacLeod C. L., Kitada S. Tumorigenicity of T lymphoma/T lymphoma hybrids and T lymphoma/normal cell hybrids. Int J Cancer. 1986 Oct 15;38(4):597–601. doi: 10.1002/ijc.2910380421. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Cohen D. I., Nielsen E. A., Davis M. M. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984 Mar 8;308(5955):149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- Hyman R., Cunningham K., Stallings V. Effect of gene dosage on cell-surface expression of Thy-1 antigen in somatic cell hybrids between Thy-1- Abelson-leukemia-virus induced lymphomas and Thy-1+ mouse lymphomas. Immunogenetics. 1981;12(3-4):381–395. doi: 10.1007/BF01561678. [DOI] [PubMed] [Google Scholar]
- Ishihara T., Kudo A., Watanabe T. Induction of immunoglobulin gene expression in mouse fibroblasts by cycloheximide treatment. J Exp Med. 1984 Dec 1;160(6):1937–1942. doi: 10.1084/jem.160.6.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao H. T., Nevins J. R. Alteration of cellular gene expression in adenovirus transformed cells by post-transcriptional mechanisms. Nucleic Acids Res. 1986 Sep 25;14(18):7253–7263. doi: 10.1093/nar/14.18.7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M., Siu G., Hood L. E., Shastri N. The molecular genetics of the T-cell antigen receptor and T-cell antigen recognition. Annu Rev Immunol. 1986;4:529–591. doi: 10.1146/annurev.iy.04.040186.002525. [DOI] [PubMed] [Google Scholar]
- Leys E. J., Crouse G. F., Kellems R. E. Dihydrofolate reductase gene expression in cultured mouse cells is regulated by transcript stabilization in the nucleus. J Cell Biol. 1984 Jul;99(1 Pt 1):180–187. doi: 10.1083/jcb.99.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod C. L., Hays E. F., Hyman R., Bourgeois S. A new murine model system for the in vitro development of thymoma cell heterogeneity. Cancer Res. 1984 May;44(5):1784–1790. [PubMed] [Google Scholar]
- MacLeod C. L., Minning L., Gold D. P., Terhorst C., Wilkinson M. Negative trans-regulation of T-cell antigen receptor/T3 complex mRNA expression in murine T-lymphoma somatic cell hybrids. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6989–6993. doi: 10.1073/pnas.83.18.6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod C. L., Weinroth S. E., Streifinger C., Glaser S. M., Hays E. F. SL12 murine T-lymphoma: a new model for tumor cell heterogeneity. J Natl Cancer Inst. 1985 Apr;74(4):875–882. [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Ohashi P. S., Mak T. W., Van den Elsen P., Yanagi Y., Yoshikai Y., Calman A. F., Terhorst C., Stobo J. D., Weiss A. Reconstitution of an active surface T3/T-cell antigen receptor by DNA transfer. Nature. 1985 Aug 15;316(6029):606–609. doi: 10.1038/316606a0. [DOI] [PubMed] [Google Scholar]
- Owen F. L., Strauss W. M., Murre C., Duby A. D., Hiai H., Seidman J. G. AKR murine thymic leukemias are from a distinct thymic cell lineage and do not express the beta chain of the T-cell antigen receptor. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7434–7437. doi: 10.1073/pnas.83.19.7434. [DOI] [PMC free article] [PubMed] [Google Scholar]
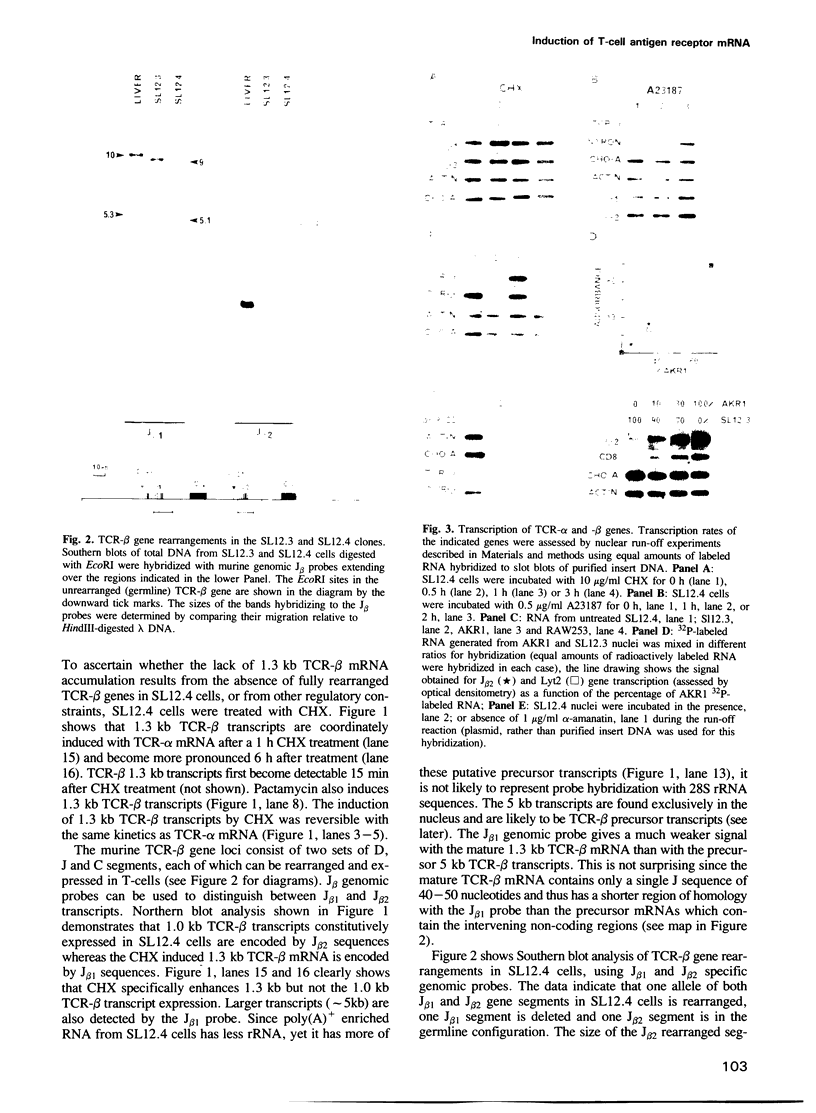
- Raulet D. H., Garman R. D., Saito H., Tonegawa S. Developmental regulation of T-cell receptor gene expression. Nature. 1985 Mar 7;314(6006):103–107. doi: 10.1038/314103a0. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Dieckmann B., Vannice J. L., Trahey M., McCormick F. Inhibition of protein synthesis stimulates the transcription of human beta-interferon genes in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3964–3968. doi: 10.1073/pnas.81.13.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Lugo J. P. Differentiation and cell division in the mammalian thymus. Dev Biol. 1985 Nov;112(1):1–17. doi: 10.1016/0012-1606(85)90114-9. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Lindsten T., Fowlkes B. J., van den Elsen P., Terhorst C., Davis M. M., Germain R. N., Schwartz R. H. Expression of genes of the T-cell antigen receptor complex in precursor thymocytes. 1985 Jun 27-Jul 3Nature. 315(6022):765–768. doi: 10.1038/315765a0. [DOI] [PubMed] [Google Scholar]
- Sangster R. N., Minowada J., Suciu-Foca N., Minden M., Mak T. W. Rearrangement and expression of the alpha, beta, and gamma chain T cell receptor genes in human thymic leukemia cells and functional T cells. J Exp Med. 1986 Jun 1;163(6):1491–1508. doi: 10.1084/jem.163.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schümperli D. Cell-cycle regulation of histone gene expression. Cell. 1986 May 23;45(4):471–472. doi: 10.1016/0092-8674(86)90277-1. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986 Dec 26;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Siu G., Kronenberg M., Strauss E., Haars R., Mak T. W., Hood L. The structure, rearrangement and expression of D beta gene segments of the murine T-cell antigen receptor. 1984 Sep 27-Oct 3Nature. 311(5984):344–350. doi: 10.1038/311344a0. [DOI] [PubMed] [Google Scholar]
- Sive H. L., Heintz N., Roeder R. G. Regulation of human histone gene expression during the HeLa cell cycle requires protein synthesis. Mol Cell Biol. 1984 Dec;4(12):2723–2734. doi: 10.1128/mcb.4.12.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass H. R., Dembić Z., Steinmetz M., von Boehmer H. Expression of T-cell antigen receptor genes during fetal development in the thymus. Nature. 1985 May 16;315(6016):232–233. doi: 10.1038/315232a0. [DOI] [PubMed] [Google Scholar]
- Snodgrass H. R., Kisielow P., Kiefer M., Steinmetz M., von Boehmer H. Ontogeny of the T-cell antigen receptor within the thymus. Nature. 1985 Feb 14;313(6003):592–595. doi: 10.1038/313592a0. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Lesley J., Trotter J., Hyman R. Thymocyte subpopulation enriched for progenitors with an unrearranged T-cell receptor beta-chain gene. Nature. 1985 Jun 20;315(6021):666–669. doi: 10.1038/315666a0. [DOI] [PubMed] [Google Scholar]
- Truneh A., Albert F., Golstein P., Schmitt-Verhulst A. M. Calcium ionophore plus phorbol ester can substitute for antigen in the induction of cytolytic T lymphocytes from specifically primed precursors. J Immunol. 1985 Oct;135(4):2262–2267. [PubMed] [Google Scholar]
- Vaessen R. T., Houweling A., van der Eb A. J. Post-transcriptional control of class I MHC mRNA expression in adenovirus 12-transformed cells. Science. 1987 Mar 20;235(4795):1486–1488. doi: 10.1126/science.3823900. [DOI] [PubMed] [Google Scholar]
- Vannice J. L., Taylor J. M., Ringold G. M. Glucocorticoid-mediated induction of alpha 1-acid glycoprotein: evidence for hormone-regulated RNA processing. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4241–4245. doi: 10.1073/pnas.81.14.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R., Briskin M., Carter C., Govan H., Taylor A., Kincade P. A labile inhibitor blocks immunoglobulin kappa-light-chain-gene transcription in a pre-B leukemic cell line. Proc Natl Acad Sci U S A. 1986 Jan;83(2):295–298. doi: 10.1073/pnas.83.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. F., Calame K. The endogenous immunoglobulin heavy chain enhancer can activate tandem VH promoters separated by a large distance. Cell. 1985 Dec;43(3 Pt 2):659–665. doi: 10.1016/0092-8674(85)90238-7. [DOI] [PubMed] [Google Scholar]
- Wilkinson M. F., Morris A. G. Gamma-interferon induction in human lymphoblasts compared with fresh mononuclear leucocytes: earlier synthesis, rapid shut-off and enhancement of yields by metabolic inhibitors. Immunology. 1984 May;52(1):175–179. [PMC free article] [PubMed] [Google Scholar]
- Winoto A., Mjolsness S., Hood L. Genomic organization of the genes encoding mouse T-cell receptor alpha-chain. 1985 Aug 29-Sep 4Nature. 316(6031):832–836. doi: 10.1038/316832a0. [DOI] [PubMed] [Google Scholar]
- Wiskocil R., Weiss A., Imboden J., Kamin-Lewis R., Stobo J. Activation of a human T cell line: a two-stimulus requirement in the pretranslational events involved in the coordinate expression of interleukin 2 and gamma-interferon genes. J Immunol. 1985 Mar;134(3):1599–1603. [PubMed] [Google Scholar]
- Yagüe J., White J., Coleclough C., Kappler J., Palmer E., Marrack P. The T cell receptor: the alpha and beta chains define idiotype, and antigen and MHC specificity. Cell. 1985 Aug;42(1):81–87. doi: 10.1016/s0092-8674(85)80103-3. [DOI] [PubMed] [Google Scholar]
- Young H. A., Varesio L., Hwu P. Posttranscriptional control of human gamma interferon gene expression in transfected mouse fibroblasts. Mol Cell Biol. 1986 Jun;6(6):2253–2256. doi: 10.1128/mcb.6.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]