Abstract
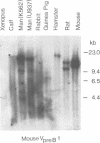
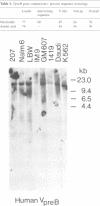
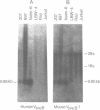
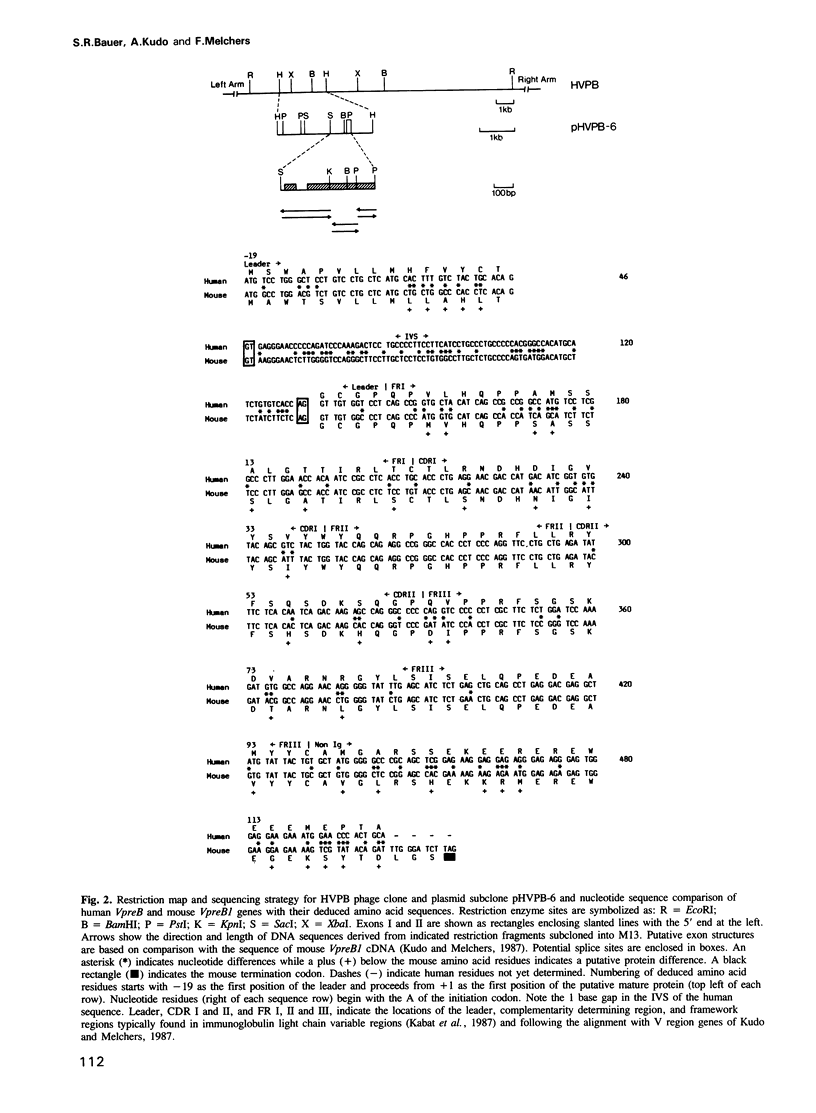
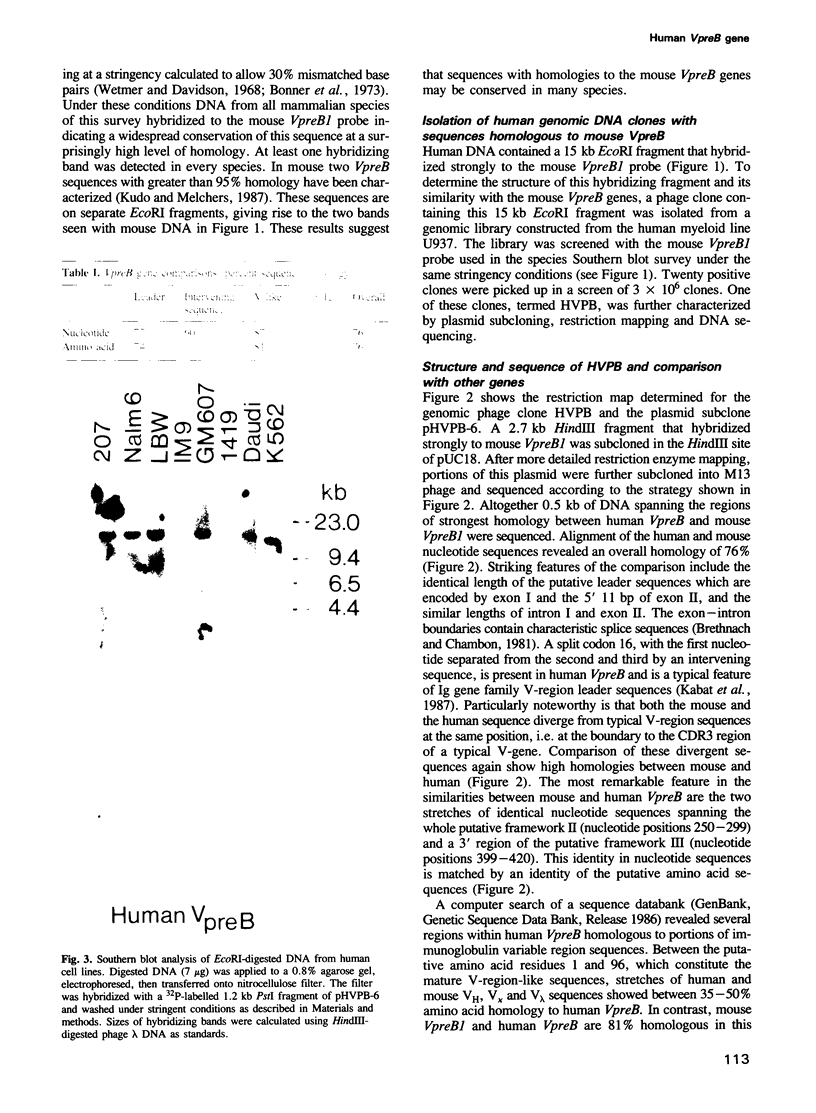
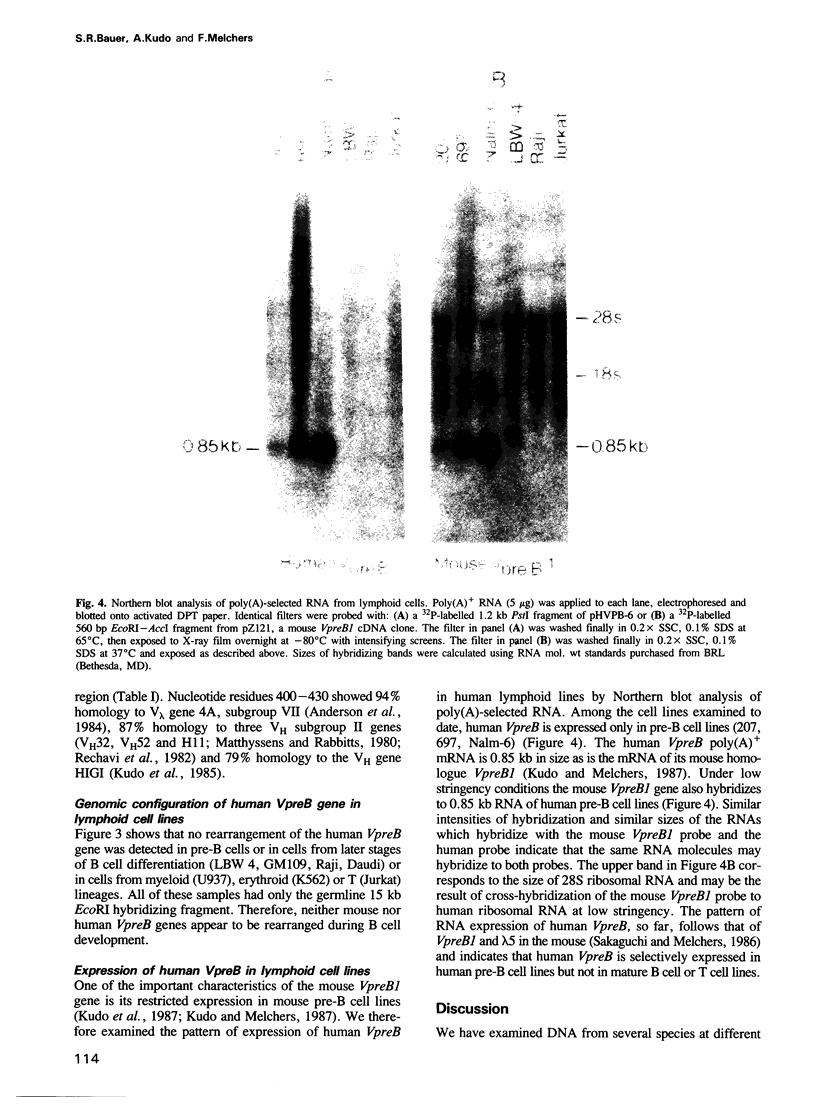
DNA from several mammals, including humans, was found to contain one or more restriction enzyme digested DNA fragments which hybridized to the mouse VpreB gene under stringencies demonstrating at least 70% nucleotide sequence homologies, indicating that the VpreB locus may be widespread and highly conserved among mammals. A human VpreB genomic clone was isolated and sequenced. Two exons and the intervening intron are spaced almost identically as in the mouse VpreB1 gene, and show 76% sequence homology to the mouse gene. As in the mouse VpreB1 gene, the 5' end of the human VpreB gene contains characteristic features of Ig domains, while the 3' end is Ig non-related. This 3' Ig non-related structure of the VpreB gene(s) may, therefore, have existed before the speciation of humans and mice over 65 million years ago. Sequences encoding the entire putative second framework region and a stretch in the third framework region are identical in human and mouse VpreB. the human VpreB gene appears to be selectively expressed in human pre-B cell lines as an 0.85 kb poly(A)+ RNA. Its expression promises to be a useful marker for the detection of normal and malignant human pre-B lymphocytes.
Full text
PDF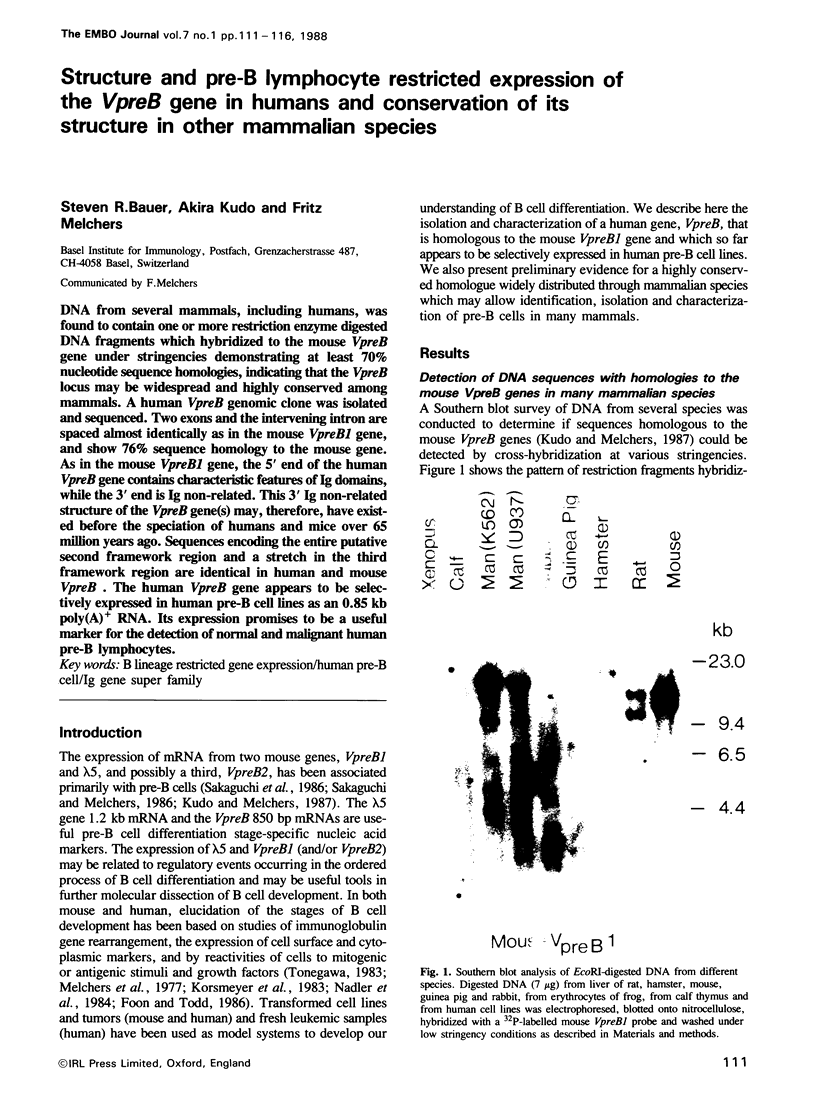


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amzel L. M., Poljak R. J. Three-dimensional structure of immunoglobulins. Annu Rev Biochem. 1979;48:961–997. doi: 10.1146/annurev.bi.48.070179.004525. [DOI] [PubMed] [Google Scholar]
- Anderson M. L., Szajnert M. F., Kaplan J. C., McColl L., Young B. D. The isolation of a human Ig V lambda gene from a recombinant library of chromosome 22 and estimation of its copy number. Nucleic Acids Res. 1984 Sep 11;12(17):6647–6661. doi: 10.1093/nar/12.17.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson L. C., Nilsson K., Gahmberg C. G. K562--a human erythroleukemic cell line. Int J Cancer. 1979 Feb;23(2):143–147. doi: 10.1002/ijc.2910230202. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- EPSTEIN M. A., BARR Y. M. CHARACTERISTICS AND MODE OF GROWTH OF TISSUE CULTURE STRAIN (EB1) OF HUMAN LYMPHOBLASTS FROM BURKITT'S LYMPHOMA. J Natl Cancer Inst. 1965 Feb;34:231–240. doi: 10.1093/jnci/34.2.231. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Findley H. W., Jr, Cooper M. D., Kim T. H., Alvarado C., Ragab A. H. Two new acute lymphoblastic leukemia cell lines with early B-cell phenotypes. Blood. 1982 Dec;60(6):1305–1309. [PubMed] [Google Scholar]
- Foon K. A., Todd R. F., 3rd Immunologic classification of leukemia and lymphoma. Blood. 1986 Jul;68(1):1–31. [PubMed] [Google Scholar]
- Hendershot L., Levitt D. Differential regulation of membrane and secretory mu chain synthesis in human beta cell lines. Regulation of membrane mu or secreted mu. J Exp Med. 1982 Dec 1;156(6):1622–1634. doi: 10.1084/jem.156.6.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz R., Hozier J., LeBien T., Minowada J., Gajl-Peczalska K., Kubonishi I., Kersey J. Characterization of a leukemic cell line of the pre-B phenotype. Int J Cancer. 1979 Feb;23(2):174–180. doi: 10.1002/ijc.2910230206. [DOI] [PubMed] [Google Scholar]
- Klein E., Klein G., Nadkarni J. S., Nadkarni J. J., Wigzell H., Clifford P. Surface IgM-kappa specificity on a Burkitt lymphoma cell in vivo and in derived culture lines. Cancer Res. 1968 Jul;28(7):1300–1310. [PubMed] [Google Scholar]
- Klobeck H. G., Solomon A., Zachau H. G. Contribution of human V kappa II germ-line genes to light-chain diversity. Nature. 1984 May 3;309(5963):73–76. doi: 10.1038/309073a0. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J., Arnold A., Bakhshi A., Ravetch J. V., Siebenlist U., Hieter P. A., Sharrow S. O., LeBien T. W., Kersey J. H., Poplack D. G. Immunoglobulin gene rearrangement and cell surface antigen expression in acute lymphocytic leukemias of T cell and B cell precursor origins. J Clin Invest. 1983 Feb;71(2):301–313. doi: 10.1172/JCI110770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo A., Ishihara T., Nishimura Y., Watanabe T. A cloned human immunoglobulin heavy chain gene with a novel direct-repeat sequence in 5' flanking region. Gene. 1985;33(2):181–189. doi: 10.1016/0378-1119(85)90092-7. [DOI] [PubMed] [Google Scholar]
- Kudo A., Melchers F. A second gene, VpreB in the lambda 5 locus of the mouse, which appears to be selectively expressed in pre-B lymphocytes. EMBO J. 1987 Aug;6(8):2267–2272. doi: 10.1002/j.1460-2075.1987.tb02500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo A., Sakaguchi N., Melchers F. Organization of the murine Ig-related lambda 5 gene transcribed selectively in pre-B lymphocytes. EMBO J. 1987 Jan;6(1):103–107. doi: 10.1002/j.1460-2075.1987.tb04725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Tiemeier D., Enquist L. EK2 derivatives of bacteriophage lambda useful in the cloning of DNA from higher organisms: the lambdagtWES system. Science. 1977 Apr 8;196(4286):175–177. doi: 10.1126/science.322278. [DOI] [PubMed] [Google Scholar]
- Matthyssens G., Rabbitts T. H. Structure and multiplicity of genes for the human immunoglobulin heavy chain variable region. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6561–6565. doi: 10.1073/pnas.77.11.6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers F., Andersson J., Phillips R. A. Ontogeny of murine B lymphocytes: development of Ig synthesis and of reactivities to mitogens and to anti-Ig-antibodies. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):147–158. doi: 10.1101/sqb.1977.041.01.019. [DOI] [PubMed] [Google Scholar]
- Nadler L. M., Korsmeyer S. J., Anderson K. C., Boyd A. W., Slaughenhoupt B., Park E., Jensen J., Coral F., Mayer R. J., Sallan S. E. B cell origin of non-T cell acute lymphoblastic leukemia. A model for discrete stages of neoplastic and normal pre-B cell differentiation. J Clin Invest. 1984 Aug;74(2):332–340. doi: 10.1172/JCI111428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechavi G., Bienz B., Ram D., Ben-Neriah Y., Cohen J. B., Zakut R., Givol D. Organization and evolution of immunoglobulin VH gene subgroups. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4405–4409. doi: 10.1073/pnas.79.14.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi N., Berger C. N., Melchers F. Isolation of a cDNA copy of an RNA species expressed in murine pre-B cells. EMBO J. 1986 Sep;5(9):2139–2147. doi: 10.1002/j.1460-2075.1986.tb04477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi N., Melchers F. Lambda 5, a new light-chain-related locus selectively expressed in pre-B lymphocytes. Nature. 1986 Dec 11;324(6097):579–582. doi: 10.1038/324579a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U., Tosi M., Pittet A. C., Fabiani L., Wellauer P. K. Tissue-specific expression of mouse alpha-amylase genes. J Mol Biol. 1980 Sep 5;142(1):93–116. doi: 10.1016/0022-2836(80)90208-9. [DOI] [PubMed] [Google Scholar]
- Selsing E., Miller J., Wilson R., Storb U. Evolution of mouse immunoglobulin lambda genes. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4681–4685. doi: 10.1073/pnas.79.15.4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]