Abstract
Streptococcus thermophilus is a facultative anaerobic bacterium that has the ability to grow and survive in aerobic environments, but the mechanism for this remains unclear. In this study, the efeB gene, encoding a dye-decolorizing peroxidase, was identified in the genome of Streptococcus thermophilus CGMCC 7.179, and purified EfeB was able to decolorize reactive blue 5. Strikingly, genes encoding two components (TatA and TatC) of the twin-arginine translocation (TAT) system were also found in the same operon with the efeB gene. Knocking out efeB or tatC resulted in decreased growth of the strain under aerobic conditions, and complementation of the efeB-deficient strains with the efeB gene enhanced the biomass of the hosts only in the presence of the tatC gene. Moreover, it was proved for both S. thermophilus CGMCC 7.179 and Escherichia coli DE3 that EfeB could be translocated by the TAT system of S. thermophilus. In addition, the transcriptional levels of efeB and tatC increased when the strain was cultured under aerobic conditions. Overall, these results provide the first evidence that EfeB plays a role in protecting cells of S. thermophilus from oxidative stress, with the assistance of the TAT system.
INTRODUCTION
Streptococcus thermophilus is a Gram-positive bacterium of the genus Streptococcus, which comprises several harmful pathogenic species, such as Streptococcus pyogenes and Streptococcus pneumoniae. With long-term usage in fermented dairy products, S. thermophilus has lost its virulence-related genes and is given a “generally recognized as safe” (GRAS) status. This organism is commonly used with Lactobacillus delbrueckii subsp. bulgaricus or other lactobacilli for yogurt making as well as for the production of mozzarella, Swiss, and cheddar cheeses (1, 2).
However, S. thermophilus encounters various stress conditions during the fermentation and storage processes (3). Among these environmental stresses, the presence of toxic reactive oxygen species (ROS) is the most important survival challenge, as it affects the organism's growth, fermentative capabilities, and viability and, consequently, the texture and flavor of the final fermented products (4). Though S. thermophilus cannot eliminate oxygen by respiration and lacks catalase activity (5), it can grow in the presence of oxygen and has an inducible capacity to survive in the presence of low concentrations of superoxide and hydroxyl radicals (6, 7), suggesting that this bacterium has evolved a specific inducible defensive system against ROS damage.
In S. thermophilus, a single H2O-forming NADH oxidase is found, which could reduce the amount of intracellular O2 (8). A well-characterized antioxidant enzyme in S. thermophilus is the manganese-containing superoxide dismutase (SodA), which converts superoxide anions to molecular oxygen and hydrogen peroxide, and the activity of SodA is not regulated by O2 (9). Recently, a functional thioredoxin system composed of NADPH, a thioredoxin reductase, and thioredoxin was identified in S. thermophilus (10). This system provided protection against oxidative stress through its disulfide reductase activity regulating the protein dithiol/disulfide balance (11). Also, a bifunctional gamma-glutamate-cysteine ligase/glutathione synthetase (GshF) that deals with oxidative damage has been reported for S. thermophilus (12). However, how S. thermophilus metabolizes H2O2 remains unclear, and none of these proteins have been verified to be involved in the inducible defensive system against ROS damage.
To identify the genes involved in oxidative stress resistance, insertional mutagenesis was carried out experimentally with S. thermophilus CNRZ368, and the mutants were screened by menadione sensitivity and resistance. Among the mutant genes, the ossH gene showed 55% identity to the gene for a potential membrane-spanning permease of an Fe3+ ABC transporter (3), and the osrD gene showed significant identity to the genes for predicted iron-dependent peroxidases belonging to the family of dye-decolorizing peroxidases (13). Unfortunately, the genetic organization and physiological functions of these two genes were not further characterized.
Dye-decolorizing peroxidases were classified as a novel peroxidase family because of their broad substrate specificity, low pH optima, lack of a conserved active site distal histidine, and structural divergence from classical plant and animal peroxidases (14). They can decolorize a broad spectrum of dyes by utilizing H2O2 as an electron acceptor. Large amounts of putative dye-decolorizing peroxidases have been registered in the PeroxiBase database (http://peroxibase.toulouse.inra.fr/), but few of them have been characterized (15). Interestingly, the reported bacterial dye-decolorizing peroxidases from Escherichia coli O157:H7 (YcdB/EfeB), Bacillus subtilis 168 (YwbN/EfeB), and Staphylococcus aureus subsp. aureus N315 (FepB) were verified to be substrates of the twin-arginine translocation (TAT) system (16–18).
The TAT system is present in the cytoplasmic membranes of most bacteria and archaea and has the highly unusual property of transporting fully folded proteins across the cytoplasmic membrane. The TAT system in E. coli includes five components (TatA, TatB, TatC, TatD, and TatE), while it comprises two components (TatA and TatC) in most Gram-positive bacteria. The TAT system has been proved to be essential for viability in a few bacteria and archaea (19). However, the function of the TAT pathway still remains unknown for S. thermophilus, since this organism exhibits a striking level of gene decay (10% pseudogenes) (20).
In this study, S. thermophilus CGMCC 7.179, isolated from the traditional fermented dairy products of Inner Mongolia, was partially sequenced. A dye-decolorizing peroxidase gene (efeB) and two TAT component genes (tatC and tatA) were identified in the S. thermophilus CGMCC 7.179 genome, and they were located in the same operon. The protective role of EfeB against oxidative stress was investigated, and the functionality of the TAT system was analyzed. This is the first demonstration that a dye-decolorizing peroxidase is translocated by the TAT pathway in S. thermophilus.
MATERIALS AND METHODS
Plasmids, bacterial strains, and growth conditions.
The strains and plasmids used in this study are listed in Table 1. S. thermophilus CGMCC 7.179 was isolated from a traditional yogurt of Inner Mongolia, China. S. thermophilus strains were propagated in M17 broth (Oxoid, Basingstoke, United Kingdom) supplemented with 1% (wt/vol) lactose (LM17 broth) anaerobically at 42°C. Escherichia coli DH5α was used as the host for standard cloning procedures. All E. coli strains were grown aerobically in Luria-Bertani (LB) broth at 37°C. Chloramphenicol or erythromycin was added at 5 μg/ml or 2.5 μg/ml, respectively, for S. thermophilus. Antibiotics for E. coli were used at the following final concentrations: ampicillin, 100 μg/ml; chloramphenicol, 25 μg/ml; and kanamycin, 50 μg/ml.
TABLE 1.
Strains and plasmids used in this study
| Bacterial strain or plasmid | Genotype or characteristics | Reference or source |
|---|---|---|
| Strains | ||
| S. thermophilus strains | ||
| CGMCC 7.179 | Wild-type strain | |
| ST16814 | CGMCC 7.179 ΔtatC | This study |
| ST1314 | CGMCC 7.179 ΔefeB | This study |
| ST16503 | CGMCC 7.179 ΔtatC ΔefeB | This study |
| CGMCC 7.179/SP-CAT | CGMCC 7.179 with pSec-SP-CAT | This study |
| ST16814/SP-CAT | ST16814 with pSec-SP-CAT | This study |
| ST1314/Sec | ST1314 with pSec:Leiss:Nuc | This study |
| ST1314/efeB | ST1314 with pSec-efeB | This study |
| ST16503/Sec | ST16503 with pSec:Leiss:Nuc | This study |
| ST16503/efeB | ST16503 with pSec-efeB | This study |
| E. coli strains | ||
| DH5α | F− ϕ80lacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK+) phoA supE44 λ− thi-1 gyrA96 relA1 | |
| DE3 | F− ompT hsdSB(rB− mB−) gal dcm (DE3) | |
| DE3/pET | DE3 with pET22b | This study |
| dE | DE3 ΔtatE | This study |
| dEAD | DE3 ΔtatE ΔtatABCD | This study |
| dEAD/efeB | dEAD with pET-efeB | This study |
| dEAD/efeB-TatCA | dEAD with pET-efeB and pSec-tatCA | This study |
| Plasmids | ||
| pG+ host4 | Ermr; temperature-sensitive vector | 27 |
| pG+ efeUp-Down | pG+ host4 derivative with upstream and downstream sequences of efeB gene | This study |
| pG+ tatUp-Down | pG+ host4 derivative with upstream and downstream sequences of tatC gene | This study |
| pET22b | Apr; carries T7 promoter and lac operator | Novagen |
| pET-efeB | Apr; expresses EfeB under T7 promoter control | This study |
| pSec:Leiss:Nuc | pWV01 replicon; expresses Nuc under PnisA control; Cmr | 28 |
| pSec-efeB | pSec:Leiss:Nuc derivative; expresses EfeB | This study |
| pSec-SP-CAT | pSec:Leiss:Nuc derivative; expresses SP-CAT | This study |
| pSec-tatCA | pSec:Leiss:Nuc derivative; expresses TatCA | This study |
| pKD46 | Apr; λ Red recombinase expression | 29 |
| pKD4 | Apr Kanr; kan cassette template | 29 |
| pCP20 | Apr Cmr; FLP recombinase expression | 30 |
Multiple-sequence alignment.
Multiple-sequence alignment was performed using Clustal X, version 2.0 (21), and ESPript 3.0 (22). The amino acid sequence of EfeB was aligned with those of YcdB, YwbN, FepB, DyP, MsP1, MsP2, and PoDyP (16–18, 23–25).
Construction of plasmids.
The primers used in this study are listed in Table 2. All the molecular manipulations in this study were performed as described previously (26). Taq polymerase, restriction enzymes, T4 DNA ligase (TaKaRa, Tokyo, Japan), and Pfu polymerase (TransGen, Beijing, China) were used according to standard procedures.
TABLE 2.
Oligonucleotide primers used in this study
| Primer | Sequence (5′-3′)a | Restriction site |
|---|---|---|
| efUpF | AAACTCGAGTCATTATTAATGATAAAACGACGAT | XhoI |
| efUpR | TTTTCTTATTTCCCATCTAGGATAA | |
| efDownF | TTATCCTAGATGGGAAATAAGAAAAACTCCTCGTTTTGCTTGGG | |
| efDownR | AAAAGATCTTAATTTCACGGTTAGCCCCTG | BglII |
| tUpF | AGGCTCGAGAAAAACGGCTGCGGCATCTATG | XhoI |
| tUpR | TCTGTCGACGCTGCTTCACCGTCACAAAGGC | SalI |
| tDownF | GATGTCGACAAAGGAAATCTCATGGGAATTC | SalI |
| tDownR | CCTCTGCAGTTAACCAGTGTATGAACCGTAG | PstI |
| KanF | GTGTAGGCTGGAGCTGCTTC | |
| KanR | ATGGGAATTAGCCATGGTCC | |
| EupF | CTGGATACCACCTGTTATATGGTG | |
| EupR | GAAGCAGCTCCAGCCTACACAGATACCTTCTTGACATATAACCGG | |
| EdownF | GGACCATGGCTAATTCCCATCGTGGCGAGCAGGACGC | |
| EdownR | GAAACCAATGAAGAGATTATTGAAG | |
| AupF | GCGCCAGGGCAAGTATTTAC | |
| AupR | GAAGCAGCTCCAGCCTACACACATGTTCCTCTGTGGTAGATGATG | |
| DdownF | GGACCATGGCTAATTCCCATAGTTTGCGGAACTCGGTGTT | |
| DdownR | GATTCATACCACGACTATCAACGC | |
| efF | AACATATGACTGATAAAAAATTTTTAGACCAA | NdeI |
| efR | ATAGTCGACTTAGTGGTGGTGGTGGTGGTGTTCAAAGAGTGCTTGACCAAGG | SalI |
| SPF | ATGACTGATAAAAAATTTTTAGACC | |
| SPR | AGCAGATGCACCAGAAAGAC | |
| PtatF | AAGGATCCGTGAAAAGACAAATCGTGGACTC | BamHI |
| PtatR | GGTCTAAAAATTTTTTATCAGTCATGAATGTCTCCTATTTTCAAGATAGT | |
| PcmF | AAAAGATCTCTGCAGTCGACGGCAATAGTTAC | BglII |
| PcmR | GGTCTAAAAATTTTTTATCAGTCATTTGATATGCCTCCTAAATTTTTATC | |
| cmF | GTCTTTCTGGTGCATCTGCTATGAACTTTAATAAAATTGATTTAG | |
| cmR | AAAAGATCTCTGTAATATAAAAACCTTC | BglII |
| RepF | AAAAGATCTTCAGCAAATAACTCTTTTTCTTTG | BglII |
| RepR | AAAAGATCTATTGTAAAAGTGTCACTGCTGCTAG | BglII |
| P1F | AAAAGATCTAGCGCGCCAAAGAAGTAG | BglII |
| P1R | AAGAATTCATATGTCGAGCCTCCTGAAGTACTG | NdeI |
| TatCF | AACATATGGCAAGAAGTAGAGATGAGATG | NdeI |
| TatAR | AAGTCGACCTAGTCTTTAGTTTTGTCTTCTTCG | SalI |
| RT-16sF | CTAACTACGTGCCAGCAGC | |
| RT-16sR | GGTTGAGCCACAGCCTTTA | |
| RT-efF | ATGATGAACAAGTAGCCTTCC | |
| RT-efR | CCTTACGGTCACCAATAGC | |
| RT-tatF | GCTGTTCTCTTTGAATTTCCTGTAG | |
| RT-tatR | GTGTCAGAATCACCGCCAAA | |
| ST tufF | GGTGCGATCCTTGTAGTTGCA | |
| ST tufR | ACACCAACCTGACGTGAAAGAA | |
| RPOST-F | ACTGTCATTGTTGCTTGGAATG | |
| RPOST-R | AGCTGAGGTTACTGCTGGAGAT |
The restriction sites in the primer sequences are underlined.
To knock out the efeB gene in S. thermophilus CGMCC 7.179, pG+ efeUp-Down was constructed as follows. Sequences upstream (amplified with primers efUpF and efUpR) and downstream (amplified with primers efDownF and efDownR) of the efeB gene were PCR amplified from the genomic DNA of S. thermophilus CGMCC 7.179 and spliced by an overlap extension PCR using primers efUpR and efDownF; subsequently, the fused fragment was inserted into the temperature-sensitive vector pG+ host4 (27). Plasmid pG+ tatUp-Down was constructed by the same procedure, using primers tUpF, tUpR, tDownF, and tDownR. To express EfeB, the efeB gene from S. thermophilus CGMCC 7.179 was amplified by a PCR with primers efF and efR. The PCR product was digested with NdeI and SalI and inserted into the corresponding sites of pET22b, resulting in plasmid pET-efeB. To perform efeB complementation, plasmid pSec-efeB was constructed as follows. The Ptat promoter was amplified from the S. thermophilus CGMCC 7.179 genome by a PCR using primers PtatF and PtatR, and efeB was amplified from the S. thermophilus CGMCC 7.179 genome by a PCR using primers SPF and efR. Ptat and efeB were fused, and the fusion fragment was digested with BamHI and SalI and inserted into the Lactococcus lactis/E. coli shuttle vector pSec:Leiss:Nuc (28) digested with BglII and SalI. To test the interaction of the TAT system and the EfeB signal peptide (SP), the sequence of the putative EfeB SP (amplified with primers SPF and SPR) was fused between the promoter (Pcm; amplified with primers PcmF and PcmR) and the chloramphenicol acetyltransferase gene (CAT; amplified with primers cmF and cmR), generating Pcm-SP-CAT. The fusion fragment was then digested with BglII and ligated with the digested replicon RepAC, amplified by PCR from the vector pSec:Leiss:Nuc, generating plasmid pSec-SP-CAT. To constitutively express TatCA, P1x (amplified with primers P1F and P1R), which is the promoter of the xyl operon from Lactobacillus fermentum 1001, and the tatCA sequence were amplified (with TatCF and TatAR) and digested with NdeI. The P1x-tatCA fragment was amplified from ligated P1x and tatCA by a PCR using primers P1F and TatAR, and the PCR product was digested with BglII and SalI and inserted into the corresponding sites of pSec:Leiss:Nuc, resulting in pSec-tatCA.
Gene deletion.
To knock out the efeB gene in S. thermophilus CGMCC 7.179, pG+ efeUp-Down was used to perform homologous double-crossover recombination in the S. thermophilus CGMCC 7.179 genome as described previously (27), generating the efeB deletion mutant ST1314, and the efeB gene deletion was verified by PCR amplification with primers efUpF and efDownR. The same procedures were carried out with sequences homologous to the up- and downstream regions of tatC (with plasmid pG+ tatUp-Down) in S. thermophilus CGMCC 7.179 and ST1314, generating ST16814 and ST16503, respectively.
For deletion of tatE in E. coli DE3, the kanamycin resistance cassette of plasmid pKD4 was amplified by a PCR using primers KanF and KanR. Subsequently, it was fused with Eup (amplified with primers EupF and EupR) and Edown (amplified with primers EdownF and EdownR), which had about 300 bp of sequence (each) homologous to the up- and downstream regions of tatE. Homologous recombination of the PCR product with the chromosomal tatE allele was carried out in E. coli DE3 as described previously, using the λ Red recombinase expression plasmid pKD46 (29). The kanamycin resistance cassette was eliminated by using the temperature-sensitive plasmid pCP20 (30), by which the new strain dE was obtained. The same procedures were carried out with sequences homologous to the up- and downstream regions of tatABCD in dE, resulting in the dEAD strain, in which tatE and tatABCD were deleted.
Determination of numbers of CFU.
The number of CFU was calculated by a previously described method (31). Cultures were 10-fold serially diluted, and the dilution time (Tdilution) of the original sample was 0. For objective dilution, 5-μl samples were pipetted onto a plate containing 1.5% agar medium. The plates were air dried and then incubated until colonies were visible, with an average size of 200 to 500 μm. The colony number of every drop (Ncolony) was then counted. The CFU concentration (number of CFU per milliliter) was calculated by using the following equation:
Detection of tolerance of S. thermophilus to oxidative stress.
After overnight static incubation at 42°C, S. thermophilus and its derivatives were diluted 50-fold in 5 ml fresh LM17 broth and incubated at 42°C with shaking (200 rpm) at a ratio of liquid to airspace of approximately 1 to 3, and the optical density at 600 nm (OD600) and number of CFU per milliliter were measured at 2-h intervals. Meanwhile, static cultures were used as controls.
Expression and purification of EfeB.
Plasmid pET-efeB was transformed into E. coli dEAD, generating dEAD/efeB. EfeB with six histidine residues at the C terminus was overproduced by induction with isopropyl-β-d-thiogalactopyranoside (IPTG; Sangon, China) at a final concentration of 0.5 mM when the OD600 of cultures reached 0.8. After a further 14 h of growth at 16°C, the cells were harvested by centrifugation at 8,000 × g for 5 min and resuspended in 10 ml phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 2 mM KH2PO4, pH 7.4). Cells were broken by use of an ultrasonic cell crusher, and cell debris was removed by centrifugation. EfeB was purified by nickel affinity chromatography following the procedure described in the pET system manual (Novagen, Inc.). The purified protein was quantified using the Bradford protein assay. The cell extracts and purified protein were separated by SDS-PAGE (12% acrylamide) (32).
EfeB enzyme assay.
Dye-decolorizing peroxidase activity was detected as described previously (33), with modifications. Briefly, reactive blue 5 (RB5; Yongxing Dye, Jinan, China), a representative anthraquinone dye, was selected as the substrate and dissolved in 25 mM citrate buffer (pH 5.5) to a final concentration of 360 μM. Two hundred microliters of enzyme solution (0.4 mg/ml) was mixed with 100 μl substrate solution and 0.2 mM H2O2. The reaction mixture was then incubated at 42°C for 30 min. The enzyme activity was calculated by measuring the decrease in absorbance at 600 nm of RB5 (ε600 = 8,000 M−1 cm−1). One unit of enzyme activity was defined as the amount of enzyme required for the decolorization of 1 mmol of RB5 (34). In control experiments, a boiled enzyme solution was used instead of the enzyme solution, or H2O was used instead of H2O2.
Test of genetic complementation of efeB.
pSec:Leiss:Nuc and pSec-efeB were transformed into strains ST1314 and ST16503. After overnight static cultivation at 42°C, strains ST1314/efeB, ST1314/Sec, ST16503/efeB, and ST16503/Sec were diluted 50-fold in 5 ml fresh LM17 broth and incubated at 42°C with shaking (200 rpm) at a ratio of liquid to airspace of approximately 1 to 3. The OD600 was measured at 2-h intervals.
Translocation analysis of the EfeB signal peptide.
The function of tatC in S. thermophilus CGMCC 7.17 was investigated by a previously reported method for positive selection for a loss of TAT function (35). Briefly, the plasmid pSec-SP-CAT was transformed into S. thermophilus CGMCC 7.179 and ST16814, generating strains CGMCC 7.179/SP-CAT and ST16814/SP-CAT, respectively. Strains CGMCC 7.179/SP-CAT and ST16814/SP-CAT were grown to stationary phase and 10-fold serially diluted. Five-microliter aliquots of a 10-fold dilution were pipetted onto plates containing 5, 8, 9, 10, 11, and 15 μg/ml chloramphenicol. Five-microliter aliquots of a 105-fold dilution were pipetted onto plates without chloramphenicol as a control experiment.
Coexpression of efeB and tatCA in E. coli.
Plasmid pSec-tatCA was transformed into dEAD/efeB. After overnight incubation at 37°C, dEAD/efeB-TatCA, dEAD/efeB, and DE3/pET were diluted 50-fold in 5 ml fresh LB broth and further incubated until the OD600 reached 0.8. IPTG was added to a final concentration of 0.5 mM. After a further 14 h of growth at 16°C, the cells were removed by centrifugation at 10,000 × g for 5 min. The cell extracts and supernatant were separated by SDS-PAGE (12% acrylamide). For EfeB immunoblotting, the supernatant was separated by SDS-PAGE (12% acrylamide) and transferred to a polyvinylidene difluoride (PVDF) membrane (36). Immunoreactive bands were visualized using an anti-His antibody (Novagen).
Transcriptional analysis by real-time qPCR.
RNA isolation and real-time quantitative PCR (qPCR) were performed as described previously (37). Briefly, exponential-phase cells from 1-ml cultures were collected by centrifugation at 7,000 × g for 5 min, and the total RNA was extracted by use of an RNA Simple total RNA kit (Tiangen, Beijing, China) according to the manufacturer's protocols. The quantity and purity of RNA were determined spectrophotometrically at 260 nm and 280 nm. Reverse transcription was performed with random 6-mer primers and an oligo(dT) primer, using a PrimeScript RT reagent kit (TaKaRa, Tokyo, Japan) according to the manufacturer's instructions. Real-time qPCR was performed with SYBR Premix Ex TaqII (TaKaRa, Tokyo, Japan) by applying the protocol for a real-time PCR detection system (Bio-Rad, Hercules, CA). The efeB transcript was PCR amplified with the primers RT-efF and RT-efR, and the tatC transcript was PCR amplified with the primers RT-tatF and RT-tatR. The 16S rRNA gene, the elongation factor Tu (tuf) gene, and the RNA polymerase subunit alpha (rpoA) gene, which were used as internal standards, were amplified with the primers RT-16sF and RT-16sR, ST tuff and ST tufR (38), and RPOST-F and RPOST-R (39), respectively.
Nucleotide sequence accession number.
The GenBank accession number for the efeOBU-tatCA operon is KR106994.
RESULTS
Genetic organization of the efeB gene.
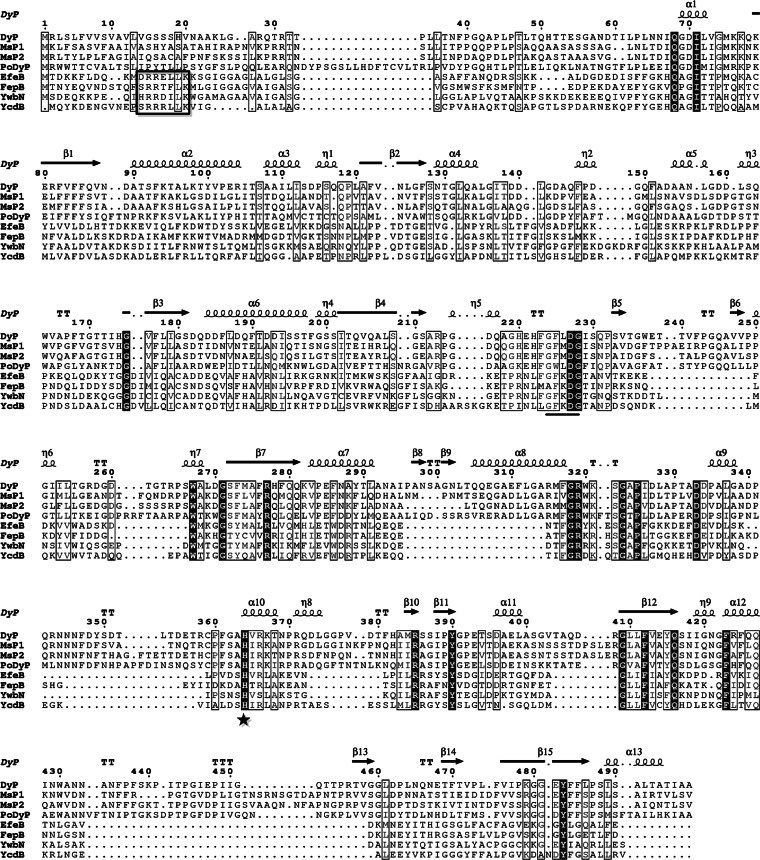
To investigate why S. thermophilus CGMCC 7.179 can grow under aerobic conditions, a genome-wide search of its genome for peroxidase genes revealed an ortholog of dye-decolorizing peroxidase genes which was an allele of the osrD gene. This gene was named efeB in accordance with the names of the dye-decolorizing peroxidase genes from E. coli and B. subtilis. The complete DNA sequence of efeB is 1,206 bp long. The deduced amino acid sequence of EfeB was aligned with those of three bacterial dye-decolorizing peroxidases (FepB, YwbN, and YcdB) and four fungal dye-decolorizing peroxidases (DyP, MsP1, MsP2, and PoDyP). The heme-binding His313 residue and the highly conserved G-X-X-D-G box of dye-decolorizing peroxidases were found in EfeB (Fig. 1). In particular, EfeB showed 48.13% similarity to FepB of Staphylococcus aureus N315.
FIG 1.
Multiple-sequence alignment of EfeB and dye-decolorizing peroxidases. All sequences are displayed as full-length sequences before processing. The secondary structure of DyP is shown at the top of each set of sequences. Abbreviations: α, α-helix; β, β-sheet; η, 310-helix; TT, β-turn. Perfectly matched residues are displayed in white on black. Similar amino acid residues are boxed with a thin line. The His313 residue of EfeB is indicated with a star. The G-X-X-D-G motif is underlined. The S/T-R-R-X-F-L-K motif is boxed with a bold line.
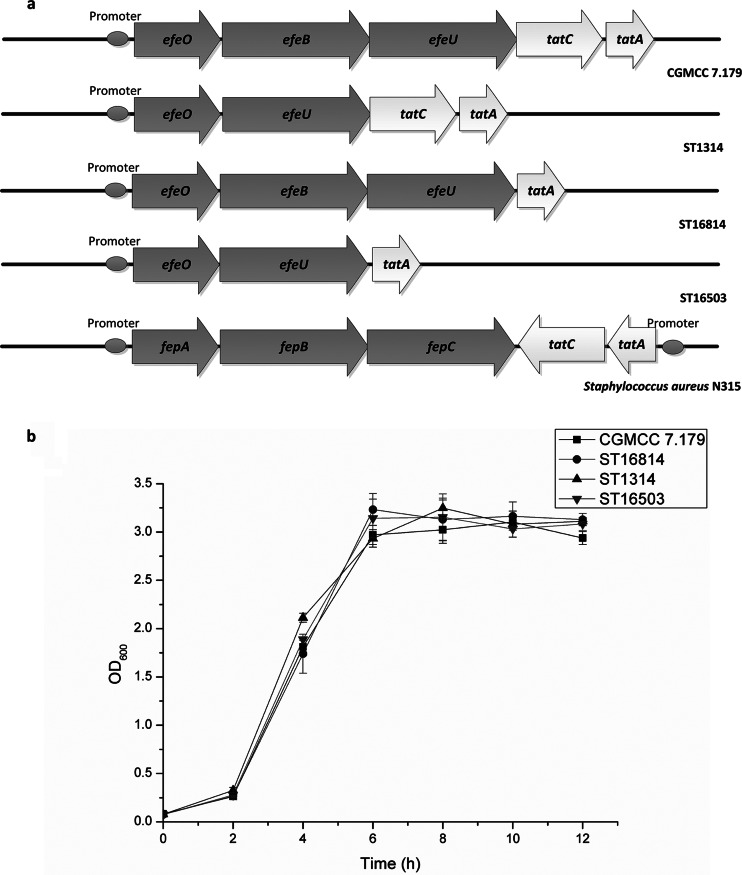
When the sequence alignment was focused on the bacterial dye-decolorizing peroxidases, a typical twin-arginine signal peptide containing the consensus motif S/T-R-R-X-F-L-K was revealed at the N terminus of EfeB (Fig. 1). Genetic organization analysis showed that the efeB gene was located in a 5-gene cluster containing genes encoding a putative periplasmic iron transport lipoprotein (efeO), a putative dye-decolorizing peroxidase (efeB), a putative iron permease (efeU), and two putative components of a TAT system (tatC and tatA) (Fig. 2a). In contrast to the genetic organization of the tatCA genes in Staphylococcus aureus N315, the tatCA genes in S. thermophilus CGMCC 7.179 had the same orientation as the efeOBU genes (Fig. 2a). It was surmised that these 5 genes were probably located in the same operon.
FIG 2.
(a) Gene organization of the efeOBU-tatCA operon in S. thermophilus CGMCC 7.197 (wild type), ST1314 (efeB deleted), ST16814 (tatC deleted), and ST16503 (efeB and tatC deleted) and Staphylococcus aureus N315. The operon encodes five proteins. Arrows indicate the orientations of the genes. (b) Growth curves for S. thermophilus CGMCC 7.197 (■), ST1314 (▲), ST16814 (●), and ST16503 (▼) under anaerobic conditions.
Function of EfeB in defense against oxidative stress.
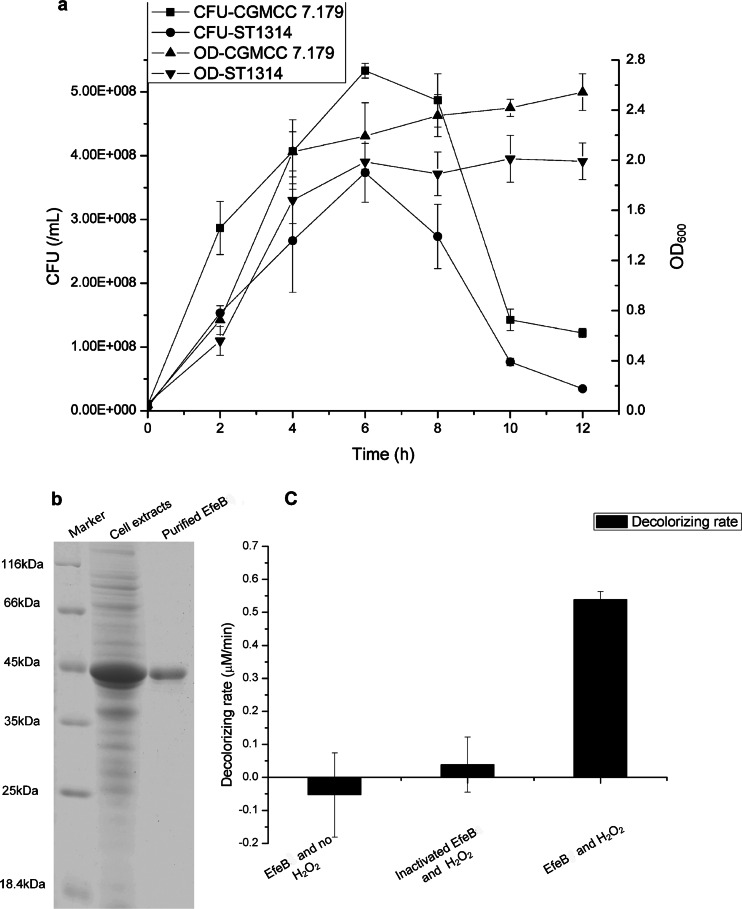
To examine the protective role of EfeB against oxidative stress, the efeB-deficient mutant ST1314 was generated in S. thermophilus CGMCC 7.179 by homologous double-crossover recombination (Fig. 2a). The deletion of the efeB gene was verified by PCR amplification with primers efUpF and efDownR. Strain CGMCC 7.179 and its derivative ST1314 displayed the same growth rate under anaerobic conditions (Fig. 2b). To detect the tolerance of strains CGMCC 7.179 and ST1314 to oxygen, their optical densities and viable counts were measured when they were grown aerobically in LM17 medium. As shown in Fig. 3a, both the OD600 and the number of CFU of the efeB-deficient strain ST1314 were lower than those of the wild-type strain CGMCC 7.179 throughout the whole growth period. After incubation for 6 h, the viable cell counts of both strains CGMCC 7.179 and ST1314 reached the maximum, with a gap of about 160 million/ml (30.02%), indicating that the deletion of the efeB gene resulted in an increase of the sensitivity of S. thermophilus cells to oxygen. Therefore, EfeB was able to protect cells against oxidative stress.
FIG 3.
Function, purification, and activity of EfeB. (a) Numbers of CFU and OD600 values for S. thermophilus CGMCC 7.197 and ST1314 under aerobic conditions. Symbols: ■, number of CFU of CGMCC 7.197; ●, number of CFU of ST1314; ▲, OD600 of CGMCC 7.197; ▼, OD600 of ST1314. (b) SDS-PAGE analysis of EfeB in cell extract sample or purified sample. (c) RB5 decolorization by EfeB, with controls of inactivated EfeB and supplying no H2O2.
EfeB enzyme activity assay.
According to sequence alignment, EfeB is distinctly related to the dye-decolorizing peroxidase family. To investigate the possible peroxidase activity of EfeB, this protein was first expressed in tatABCDE-deficient E. coli dEAD and purified by nickel affinity chromatography. As shown in Fig. 3b, SDS-PAGE analysis of the purified EfeB protein revealed a visible protein band with a molecular mass of 45 kDa, which was consistent with the theoretical molecular mass of EfeB plus the His tag. The enzyme activity of EfeB was biochemically detected with the substrate RB5, which is a staple substrate of dye-decolorizing peroxidases. The results showed that the purified EfeB protein had the capability to decolorize RB5 with the aid of H2O2, displaying a specific activity of 6.74 U/mg. When EfeB was inactivated by boiling or H2O instead of H2O2 was added to the reaction system, no decolorization was observed, suggesting that EfeB is a member of the dye-decolorizing peroxidase family (Fig. 3c).
Dependence of EfeB on the TAT system.
A TAT substrate search was performed in the whole genome of a completely sequenced S. thermophilus strain, and the dye-decolorizing peroxidase was the only candidate TAT-exported protein (8). Taken together, the genetic construction of the efeB gene and the translocation pathway of other bacterial dye-decolorizing peroxidases suggested that EfeB might be translocated by the TAT system. To clarify the TAT dependence of EfeB, tatC-deficient mutants ST16814 and ST16503 were generated in S. thermophilus CGMCC 7.179 and ST1314, respectively. Strains CGMCC 7.179, ST16814, ST1314, and ST16503 displayed the same growth rate under anaerobic conditions (Fig. 2b). After the strains were cultured aerobically, the growth curves for strains ST16503, ST16814, and ST1314 displayed the same pattern, and all of them showed obviously slower growth than that of the wild-type strain CGMCC 7.179 (Fig. 4a). Therefore, both EfeB and TatC are necessary for the antioxidant response. In other words, EfeB and TatC are interdependent in defending against oxidative stress.
FIG 4.
Function of the tatC gene. (a) Growth curves for S. thermophilus CGMCC 7.197 (■), ST16814 (▲), ST1314 (●), and ST16503 (⭑) under shaking conditions. (b) Growth curves for S. thermophilus ST1314/Sec (▼), ST1314/efeB (◆), ST16503/Sec (◀), and ST16503/efeB (▶) under shaking conditions.
To further test whether the function of EfeB depended on the presence of TatC, the efeB gene was genetically complemented in strains ST1314 and ST16503. Strains ST1314/efeB, ST1314/Sec, ST16503/efeB, and ST16503/Sec were grown aerobically. As shown in Fig. 4b, the OD600 values for strain ST1314/efeB and strain ST1314/Sec were 1.89 and 1.68, respectively. The significant growth improvement of the complemented ST1314/efeB strain revealed the positive effect of EfeB on the defense against oxidative stress in the presence of the TAT system. However, the growth of the ST16503/efeB strain was not restored compared to that of ST16503/Sec, indicating that EfeB indeed depends on the TAT system to defend against oxidative stress.
Translocation analysis of the EfeB signal peptide, with CAT as a reporter protein.
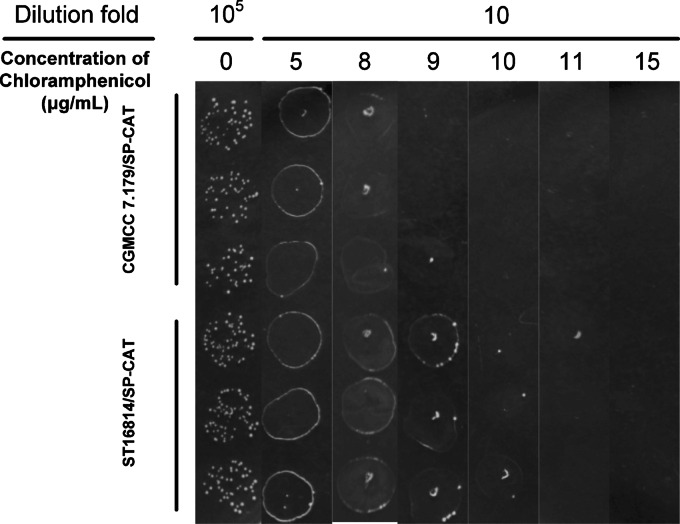
In E. coli, chloramphenicol acetyltransferase (CAT) is equipped with a typical TAT signal peptide to positively select for a loss of Tat function (35). In the present study, the SP sequence from the efeB gene was fused with the CAT gene. The difference in resistance to chloramphenicol between S. thermophilus CGMCC 7.179/SP-CAT and ST16814/SP-CAT was used to reflect the interaction between the TAT system and the signal peptide of EfeB. Strains CGMCC 7.179/SP-CAT and ST16814/SP-CAT were cultured to stationary phase in LM17 broth. To confirm that these two strains had no growth difference in the absence of chloramphenicol, 5-μl samples of 105-fold dilutions of their cultures were first pipetted onto LM17 plates. Subsequently, their sensitivity to chloramphenicol was measured by pipetting 5-μl samples of 10-fold dilutions on plates containing chloramphenicol at various concentrations (5, 8, 9, 10, 11, and 15 μg/ml). As shown in Fig. 5, the recombinant strains CGMCC 7.179/SP-CAT and ST16814/SP-CAT showed almost the same survival in the absence of chloramphenicol. With an increase of the chloramphenicol concentration, strain CGMCC 7.179/SP-CAT became more sensitive to chloramphenicol than strain ST16814/SP-CAT. When the concentration of chloramphenicol reached 9 μg/ml, the growth of CGMCC 7.179/SP-CAT was completely inhibited, while the lawn of ST16814/SP-CAT could easily be observed, suggesting that ST16814/SP-CAT displayed a stronger capability to tolerate chloramphenicol than that of CGMCC 7.179/SP-CAT. Therefore, the TAT system could translocate EfeB SP-fused CAT in CGMCC 7.179.
FIG 5.
Chloramphenicol resistance of S. thermophilus CGMCC 7.197/SP-CAT and ST16814/SP-CAT. Samples of 105-fold dilutions were pipetted onto plates without chloramphenicol, and 10-fold dilutions were pipetted onto plates containing chloramphenicol at various concentrations (5, 8, 9, 10, 11, and 15 μg/ml). Each sample was used 3 times.
Translocation of EfeB by the TAT system in vitro.
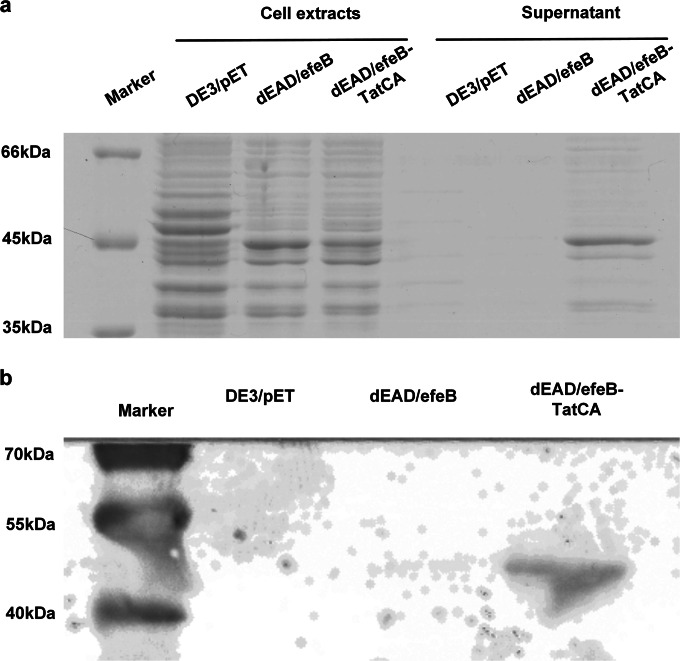
To further verify that EfeB was translocated by the Tat system, the subcellular location of EfeB was analyzed in the tatABCDE-deficient mutant E. coli dEAD/efeB and its tatCA complementation strain, dEAD/efeB-TatCA. After induction by IPTG and culture for 14 h at 16°C, cell extracts and supernatants of E. coli dEAD/efeB and dEAD/efeB-TatCA cultures were separated by SDS-PAGE. The results showed a visible protein band with a molecular mass of 45 kDa for the cell extracts of dEAD/efeB and dEAD/efeB-TatCA as well as the supernatant of the dEAD/efeB-TatCA cultures, but not for the supernatant of the strain dEAD/efeB culture (Fig. 6a). Therefore, EfeB was successfully expressed in E. coli dEAD/efeB, but it was not translocated across the cytoplasmic membrane into the medium. When TatCA was expressed simultaneously in E. coli dEAD/efeB-TatCA, TatCA served as the translocon to drive EfeB across the cytoplasmic membrane. This result was also confirmed by Western blot analysis (Fig. 6b), in which a single corresponding band was visible only for the supernatants of E. coli dEAD/efeB-TatCA cultures. Together, the data showed that EfeB could be translocated by the S. thermophilus CGMCC 7.179 TAT system in vitro.
FIG 6.
Coexpression of EfeB and TatCA in E. coli. (a) SDS-PAGE analysis of EfeB in cell extracts or supernatants of DE3/pET, dEAD/efeB, and dEAD/efeB-TatCA. (b) Western blot of EfeB in supernatants of DE3/pET, dEAD/efeB, and dEAD/efeB-TatCA.
Transcriptional analysis of the efeOBU-tatCA operon.
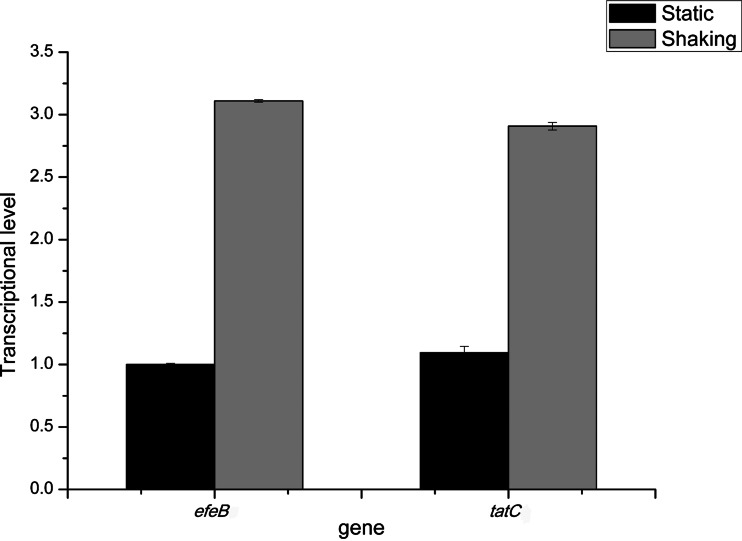
To investigate whether the efeOBU-tatCA operon was induced by O2, the transcriptional levels of the efeB and tatC genes under anaerobic and aerobic conditions were quantified by real-time qPCR. Total RNA was extracted from cells whose OD600 had reached 1.0. As shown in Fig. 7, the transcriptional level of the efeB gene under anaerobic conditions was defined as 1, and it reached 3.11 ± 0.01 under aerobic conditions. Interestingly, corresponding with efeB, the same transcriptional pattern was observed for tatC, with levels of 1.06 ± 0.01 and 2.91 ± 0.03 under anaerobic and aerobic conditions, respectively. Therefore, it was concluded that transcription of the efeB and tatC genes was induced by aerobic conditions. These data also further strengthened our hypothesis that the efeB and tatC genes are organized in the same operon in S. thermophilus. Excitingly, this efeOBU-tatCA operon might be the first component found to be involved in the inducible defensive system against ROS damage of S. thermophilus.
FIG 7.
Transcriptional levels of efeB and tatC under static (black columns) and shaking (gray columns) conditions. The transcriptional level of the efeB gene under static conditions was defined as 1.
DISCUSSION
S. thermophilus is one of the most widely used bacteria in the dairy industry. Owing to its extensive industrial application and survival challenges, many studies have been performed on the stress resistance (especially ROS resistance) of S. thermophilus. Although several ROS resistance enzymes are well characterized, very little information regarding the response to peroxide stress was revealed. In the present study, the efeB gene in the S. thermophilus CGMCC 7.179 genome was identified as a gene encoding a dye-decolorizing peroxidase. Functional analysis showed that EfeB can defend against oxidative toxicity. Strikingly, the protective role of EfeB depended on the genomically adjacent TAT system, as EfeB was a substrate of the TAT system.
In the genome of S. thermophilus CGMCC 7.179, the efeB gene was found to be located in a putative operon with four other genes: efeU, efeO, tatC, and tatA. Interestingly, the efeO gene is an allele of the ossH gene, which was found by insertional mutagenesis (3). Therefore, there was ample reason to believe that this operon was related to the capability of S. thermophilus CGMCC 7.179 to defend against oxidative stress. The amino acid sequence of EfeB was aligned with those of seven dye-decolorizing peroxidases. The heme-binding His313 residue (40) and the highly conserved G-X-X-D-G box (15) of dye-decolorizing peroxidases were found in EfeB. Specifically, EfeB showed a high level of similarity to the dye-decolorizing peroxidase FepB. Therefore, EfeB might be a member of the dye-decolorizing peroxidase family, by which S. thermophilus CGMCC 7.179 may eliminate H2O2.
To investigate the protective role of EfeB against oxidative stress, the efeB-deficient mutant ST1314 was generated by homologous double-crossover recombination. Strains CGMCC 7.179 and ST1314 displayed the same growth rate and OD600 under static conditions. However, the growth and maximum number of CFU of strain ST1314 were obviously lower than those of the wild-type strain CGMCC 7.179 when the strains were exposed to air. The distinct decrease in the number of living bacteria indicated that the efeB gene actually worked to defend against oxidative stress. Sequence alignment showed that EfeB contains a conserved His313 residue to link heme molecules, but S. thermophilus cannot synthesize heme; therefore, whether the peroxidase activation of EfeB depends on heme from the environment needs to be studied further.
Although in silico analysis of the efeB gene showed that it is a putative dye-decolorizing peroxidase, its function of eliminating H2O2 needed to be confirmed by biochemical testing. Reactive blue 5 is the most commonly used substrate to test the activity of dye-decolorizing peroxidases (33, 34, 41, 42). The EfeB protein purified from dEAD/efeB displayed a specific activity toward RB5 of 6.74 U/mg, which is 1/3 that of the DyP protein from Geotrichum candidum Dec 1 (34). Although the specific activity of EfeB was somewhat low, it still significantly indicated that EfeB is a member of the dye-decolorizing peroxidase family. However, the natural substrate of EfeB is unknown, and its specific activity may be higher with other substrates in vivo.
It has been reported that the bacterial dye-decolorizing peroxidases FepB, YcdB, and YwbN are secreted by the Tat pathway (16–18). In S. thermophilus CGMCC 7.179, genes encoding two components of the TAT system were located in the same operon as the efeB gene. Furthermore, a typical twin-arginine signal peptide exists at the N terminus of EfeB. Since TatC is essential in all TAT systems (19), the tatC gene was deleted to investigate the relationship between EfeB and the TAT system. The experimental data on the deletion of the tatC gene and the complementation of the efeB gene revealed the interdependence of EfeB and the TAT system in defending against oxidative stress.
However, direct evidence to verify the translocation of EfeB by the TAT system remained poor. We found that overexpression of EfeB in S. thermophilus CGMCC 7.179 is lethal (data not shown), so a method used for positive selection of Tat function in E. coli was performed to test the translocation of the EfeB signal peptide. CAT inactivates the antibiotic chloramphenicol by acetylation using acetyl-coenzyme A, which is present only in the cytoplasm (35). Thus, when CAT is fused to the SP of EfeB, cells that export the SP-CAT fusion protein should be more sensitive to chloramphenicol than cells that fail to export the fusion protein. The experimental results showed that ST16814/SP-CAT possessed a stronger capability to tolerate chloramphenicol than that of CGMCC 7.179/SP-CAT, suggesting that the Tat system of S. thermophilus CGMCC 7.179 can export small quantities of SP-CAT across the cytoplasmic membrane. However, the resistance difference was quite slight, which may have resulted from the expression of the TAT system being too low to produce enough transport capability.
The difference in chloramphenicol resistance between strains CGMCC 7.179/SP-CAT and ST16814/SP-CAT was so small that the deduction appeared not to be very persuasive. Considering that components from other bacterial TAT systems can work efficiently in E. coli (43, 44), EfeB and TatCA were coexpressed in E. coli dEAD. EfeB could be observed in the supernatant of dEAD/efeB-TatCA by SDS-PAGE, which suggested that a large amount of EfeB was exported. While EfeB was expressed in dEAD/efeB, no EfeB existed in the supernatant. Obviously, TatCA was able to export EfeB efficiently. Thus, it was credible that TatC protected the cells by translocating EfeB across the cytoplasmic membrane.
To our knowledge, none of the characterized antioxidant enzymes are involved in the inducible defensive system against ROS damage in S. thermophilus. To test whether the efeOBU-tatCA operon was inducible, the transcriptional levels of the efeB and tatC genes were quantified under anaerobic and aerobic conditions. Compared with the levels in cells cultured anaerobically, the efeB and tatC transcriptional levels were tripled after aerobic culture, suggesting that the efeOBU-tatCA operon could be involved in the inducible defensive system against ROS damage. The transcriptional levels of the efeB and tatC genes were almost the same under the same conditions, which further indicated that efeB and tatC are located in the same operon.
In conclusion, EfeB is the first peroxidase identified in S. thermophilus, and it provides a novel defense against oxidative stress. This oxidative stress-inducible peroxidase could use an anthraquinone dye as an electron donor, which protected cells from the toxicity of both H2O2 and anthraquinone. Moreover, experimental evidence was provided to indicate that EfeB is translocated by the TAT system in S. thermophilus. The S. thermophilus TAT system identified in this study is unique among lactic acid bacteria (LAB), so it is a promising translocon for the construction of an LAB extracellular protein expression system for the translocation of proteins that are difficult to secrete.
ACKNOWLEDGMENTS
We thank N. Galleron for kindly providing the plasmid pSec:leiss:Nuc. We also thank I. Biswas and E. Maguin for their generous gift of the plasmid pG+ host4.
This work was supported by the National Natural Science Foundation of China (grants 31471715 and 31271905) and the Hi-Tech Research and Development Program of China (grant 2011AA100902).
REFERENCES
- 1.Tamime A, Deeth H. 1980. Yogurt technology and biochemistry. J Food Prot 43:939–977. [DOI] [PubMed] [Google Scholar]
- 2.Fox PF, McSweeney PL, Cogan TM, Guinee TP. 2004. Cheese: chemistry, physics and microbiology: general aspects, vol 1 Elsevier Academic Press, London, United Kingdom. [Google Scholar]
- 3.Thibessard A, Borges F, Fernandez A, Gintz B, Decaris B, Leblond-Bourget N. 2004. Identification of Streptococcus thermophilus CNRZ368 genes involved in defense against superoxide stress. Appl Environ Microbiol 70:2220–2229. doi: 10.1128/AEM.70.4.2220-2229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van de Guchte M, Serror P, Chervaux C, Smokvina T, Ehrlich SD, Maguin E. 2002. Stress responses in lactic acid bacteria. Antonie Van Leeuwenhoek 82:187–216. doi: 10.1023/A:1020631532202. [DOI] [PubMed] [Google Scholar]
- 5.Condon S. 1987. Responses of lactic acid bacteria to oxygen. FEMS Microbiol Lett 46:269–280. doi: 10.1111/j.1574-6968.1987.tb02465.x. [DOI] [Google Scholar]
- 6.Thibessard A, Leblond-Bourget N, Fernandez A, Gintz B, Decaris B. 2001. Response of Streptococcus thermophilus CNRZ368 and its colonial variants to oxidative stress: evidence for an inducible defence system. Lait 81:311–316. doi: 10.1051/lait:2001134. [DOI] [Google Scholar]
- 7.Thibessard A, Fernandez A, Gintz B, Leblond-Bourget N, Decaris B. 2001. Hydrogen peroxide effects on Streptococcus thermophilus CNRZ368 cell viability. Res Microbiol 152:593–596. doi: 10.1016/S0923-2508(01)01234-7. [DOI] [PubMed] [Google Scholar]
- 8.Hols P, Hancy F, Fontaine L, Grossiord B, Prozzi D, Leblond-Bourget N, Decaris B, Bolotin A, Delorme C, Ehrlich SD. 2005. New insights in the molecular biology and physiology of Streptococcus thermophilus revealed by comparative genomics. FEMS Microbiol Rev 29:435–463. [DOI] [PubMed] [Google Scholar]
- 9.Chang SK, Hassan HM. 1997. Characterization of superoxide dismutase in Streptococcus thermophilus. Appl Environ Microbiol 63:3732–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marco S, Rullo R, Albino A, Masullo M, De Vendittis E, Amato M. 2013. The thioredoxin system in the dental caries pathogen Streptococcus mutans and the food-industry bacterium Streptococcus thermophilus. Biochimie 95:2145–2156. doi: 10.1016/j.biochi.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Lu J, Holmgren A. 2014. The thioredoxin antioxidant system. Free Radic Biol Med 66:75–87. doi: 10.1016/j.freeradbiomed.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 12.Wang T, Lu W, Lu S, Kong J. 2015. Protective role of glutathione against oxidative stress in Streptococcus thermophilus. Int Dairy J 45:41–47. doi: 10.1016/j.idairyj.2015.01.015. [DOI] [Google Scholar]
- 13.Fernandez A, Thibessard A, Borges F, Gintz B, Decaris B, Leblond-Bourget N. 2004. Characterization of oxidative stress-resistant mutants of Streptococcus thermophilus CNRZ368. Arch Microbiol 182:364–372. doi: 10.1007/s00203-004-0712-2. [DOI] [PubMed] [Google Scholar]
- 14.Ogola HJO, Kamiike T, Hashimoto N, Ashida H, Ishikawa T, Shibata H, Sawa Y. 2009. Molecular characterization of a novel peroxidase from the cyanobacterium Anabaena sp. strain PCC 7120. Appl Environ Microbiol 75:7509–7518. doi: 10.1128/AEM.01121-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugano Y. 2009. DyP-type peroxidases comprise a novel heme peroxidase family. Cell Mol Life Sci 66:1387–1403. doi: 10.1007/s00018-008-8651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jongbloed JD, Grieger U, Antelmann H, Hecker M, Nijland R, Bron S, Van Dijl JM. 2004. Two minimal Tat translocases in Bacillus. Mol Microbiol 54:1319–1325. doi: 10.1111/j.1365-2958.2004.04341.x. [DOI] [PubMed] [Google Scholar]
- 17.Sturm A, Schierhorn A, Lindenstrauss U, Lilie H, Brüser T. 2006. YcdB from Escherichia coli reveals a novel class of Tat-dependently translocated hemoproteins. J Biol Chem 281:13972–13978. doi: 10.1074/jbc.M511891200. [DOI] [PubMed] [Google Scholar]
- 18.Biswas L, Biswas R, Nerz C, Ohlsen K, Schlag M, Schäfer T, Lamkemeyer T, Ziebandt A-K, Hantke K, Rosenstein R, Götz F. 2009. Role of the twin-arginine translocation pathway in Staphylococcus. J Bacteriol 191:5921–5929. doi: 10.1128/JB.00642-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer T, Berks BC. 2012. The twin-arginine translocation (Tat) protein export pathway. Nat Rev Microbiol 10:483–496. doi: 10.1038/nrmicro2814. [DOI] [PubMed] [Google Scholar]
- 20.Bolotin A, Quinquis B, Renault P, Sorokin A, Ehrlich SD, Kulakauskas S, Lapidus A, Goltsman E, Mazur M, Pusch GD, Fonstein M, Overbeek R, Kyprides N, Purnelle B, Prozzi D, Ngui K, Masuy D, Hancy F, Burteau S, Boutry M, Delcour J, Goffeau A, Hols P. 2004. Complete sequence and comparative genome analysis of the dairy bacterium Streptococcus thermophilus. Nat Biotechnol 22:1554–1558. doi: 10.1038/nbt1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larkin MA, Blackshields G, Brown N, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 22.Robert X, Gouet P. 2014. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res 42:W320–W324. doi: 10.1093/nar/gku316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheibner M, Hülsdau B, Zelena K, Nimtz M, De Boer L, Berger RG, Zorn H. 2008. Novel peroxidases of Marasmius scorodonius degrade β-carotene. Appl Microbiol Biotechnol 77:1241–1250. doi: 10.1007/s00253-007-1261-9. [DOI] [PubMed] [Google Scholar]
- 24.Faraco V, Piscitelli A, Sannia G, Giardina P. 2007. Identification of a new member of the dye-decolorizing peroxidase family from Pleurotus ostreatus. World J Microbiol Biotechnol 23:889–893. doi: 10.1007/s11274-006-9303-5. [DOI] [Google Scholar]
- 25.Sugano Y, Sasaki K, Shoda M. 1999. cDNA cloning and genetic analysis of a novel decolorizing enzyme, peroxidase gene dyp from Geotrichum candidum Dec 1. J Biosci Bioeng 87:411–417. doi: 10.1016/S1389-1723(99)80087-5. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 27.Maguin E, Duwat P, Hege T, Ehrlich D, Gruss A. 1992. New thermosensitive plasmid for gram-positive bacteria. J Bacteriol 174:5633–5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Loir Y, Gruss A, Ehrlich S, Langella P. 1998. A nine-residue synthetic propeptide enhances secretion efficiency of heterologous proteins in Lactococcus lactis. J Bacteriol 180:1895–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cherepanov PP, Wackernagel W. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9–14. doi: 10.1016/0378-1119(95)00193-A. [DOI] [PubMed] [Google Scholar]
- 31.Sieuwerts S, de Bok FA, Mols E, de Vos WM, Vlieg JE. 2008. A simple and fast method for determining colony forming units. Lett Appl Microbiol 47:275–278. doi: 10.1111/j.1472-765X.2008.02417.x. [DOI] [PubMed] [Google Scholar]
- 32.Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Liu C, Li B, Yuan H, Yang J, Zheng B. 2012. Identification and molecular characterization of a novel DyP-type peroxidase from Pseudomonas aeruginosa PKE117. Appl Biochem Biotechnol 166:774–785. doi: 10.1007/s12010-011-9466-x. [DOI] [PubMed] [Google Scholar]
- 34.Sugano Y, Nakano R, Sasaki K, Shoda M. 2000. Efficient heterologous expression in Aspergillus oryzae of a unique dye-decolorizing peroxidase, DyP, of Geotrichum candidum Dec 1. Appl Environ Microbiol 66:1754–1758. doi: 10.1128/AEM.66.4.1754-1758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hicks MG, Lee PA, Georgiou G, Berks BC, Palmer T. 2005. Positive selection for loss-of-function tat mutations identifies critical residues required for TatA activity. J Bacteriol 187:2920–2925. doi: 10.1128/JB.187.8.2920-2925.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Towbin H, Staehelin T, Gordon J. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A 76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo T, Kong J, Zhang L, Zhang C, Hu S. 2012. Fine tuning of the lactate and diacetyl production through promoter engineering in Lactococcus lactis. PLoS One 7:e36296. doi: 10.1371/journal.pone.0036296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calles-Enríquez M, Eriksen BH, Andersen PS, Rattray FP, Johansen AH, Fernandez M, Ladero V, Alvarez MA. 2010. Sequencing and transcriptional analysis of the Streptococcus thermophilus histamine biosynthesis gene cluster: factors that affect differential hdcA expression. Appl Environ Microbiol 76:6231–6238. doi: 10.1128/AEM.00827-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.La Gioia F, Rizzotti L, Rossi F, Gardini F, Tabanelli G, Torriani S. 2011. Identification of a tyrosine decarboxylase gene (tdcA) in Streptococcus thermophilus 1TT45 and analysis of its expression and tyramine production in milk. Appl Environ Microbiol 77:1140–1144. doi: 10.1128/AEM.01928-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X, Du Q, Wang Z, Zhu D, Huang Y, Li N, Wei T, Xu S, Gu L. 2011. Crystal structure and biochemical features of EfeB/YcdB from Escherichia coli O157: ASP235 plays divergent roles in different enzyme-catalyzed processes. J Biol Chem 286:14922–14931. doi: 10.1074/jbc.M110.197780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim SJ, Shoda M. 1999. Purification and characterization of a novel peroxidase from Geotrichum candidum Dec 1 involved in decolorization of dyes. Appl Environ Microbiol 65:1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugano Y, Matsushima Y, Shoda M. 2006. Complete decolorization of the anthraquinone dye reactive blue 5 by the concerted action of two peroxidases from Thanatephorus cucumeris Dec 1. Appl Environ Microbiol 73:862–871. [DOI] [PubMed] [Google Scholar]
- 43.Barnett JP, Eijlander RT, Kuipers OP, Robinson C. 2008. A minimal Tat system from a Gram-positive organism: a bifunctional TatA subunit participates in discrete TatAC and TatA complexes. J Biol Chem 283:2534–2542. doi: 10.1074/jbc.M708134200. [DOI] [PubMed] [Google Scholar]
- 44.Fritsch MJ, Krehenbrink M, Tarry MJ, Berks BC, Palmer T. 2012. Processing by rhomboid protease is required for Providencia stuartii TatA to interact with TatC and to form functional homo-oligomeric complexes. Mol Microbiol 84:1108–1123. doi: 10.1111/j.1365-2958.2012.08080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]