Abstract
OBJECTIVE
To assess the safety, tolerability, and feasibility of adult allogeneic bone marrow–derived mesenchymal precursor cells (MPCs) in type 2 diabetes inadequately controlled with metformin either alone or with one additional oral antidiabetic agent.
RESEARCH DESIGN AND METHODS
The study was a dose-escalating randomized placebo-controlled trial assessing one intravenous (IV) infusion of MPCs (rexlemestrocel-L; Mesoblast Inc.) 0.3 × 106/kg (n = 15), 1.0 × 106/kg (n = 15), or 2.0 × 106/kg (n = 15) or placebo (n = 16). Study duration was 12 weeks.
RESULTS
Subjects (21 women, 40 men) with a mean ± SD baseline HbA1c 8.3 ± 1.0% (67 ± 10.9 mmol/mol), BMI 33.5 ± 5.5 kg/m2, and diabetes duration 10.1 ± 6.0 years were enrolled at 18 U.S. sites. No acute adverse events (AEs) were associated with infusion. No serious AEs, serious hypoglycemia AEs, or discontinuations due to AEs over 12 weeks were found. No subjects developed donor-specific anti-HLA antibodies or became sensitized. The safety profile was comparable among treatment groups. Compared with placebo, a single IV infusion of rexlemestrocel-L reduced HbA1c at all time points after week 1. The adjusted least squares mean ± SE dose-related differences in HbA1c from placebo in the rexlemestrocel-L groups ranged from −0.1 ± 0.2% (−1.1 ± 2.2 mmol/mol) to −0.4 ± 0.2% (4.4 ± 2.2 mmol/mol) at 8 weeks and from 0.0 ± 0.25% to −0.3 ± 0.25% (−3.3 ± −2.7 mmol/mol) at 12 weeks (P < 0.05 for 2.0 × 106/kg dose at 8 weeks). The clinical target HbA1c <7% (53 mmol/mol) was achieved by 33% (5 of 15) of the subjects who received the 2.0 × 106/kg dose vs. 0% of those who received placebo (P < 0.05).
CONCLUSIONS
This short-term study demonstrates the safety and feasibility of up to 246 million MPCs in subjects with type 2 diabetes.
Introduction
The natural history of type 2 diabetes is characterized by progressively deteriorating glycemic control due to worsening insulin resistance and diminished insulin secretion (1–3). Metformin is the most widely recommended first-line pharmacotherapy for the management of type 2 diabetes and is now generally initiated along with diet and exercise at the time of diagnosis (1,2). However, metformin monotherapy frequently becomes insufficient to maintain glycemic goals in the face of progressive insulin resistance and β-cell failure, and many patients require multiple oral and/or injectable antihyperglycemic agents (3,4). These therapies control glycemia but do not reverse the underlying pathophysiology. Therapeutic agents that modify the progression of the disease beyond reducing hyperglycemia alone may provide additional long-term health benefits. Disease-modifying therapies introduced early in the course of diabetes may offset the microvascular and macrovascular damage of diabetes evident in 10–25% of newly diagnosed patients (5) and contribute to the vast individual, societal, and economic burden of the disease (6).
Multiple lines of research have implicated inflammation in the pathophysiology of type 2 diabetes (7–9), leading to the clinical investigation of new therapeutic agents with anti-inflammatory properties (10). The anti-inflammatory properties of adult bone marrow–derived mesenchymal lineage cells (11) may potentially address a novel pathway contributing to the pathogenesis of type 2 diabetes, as suggested by the observed effects of these cells on hyperglycemia in preclinical models of diabetes (12,13) and pilot clinical studies (14,15). The current study establishes the overall safety of rexlemestrocel-L (Mesoblast Inc.) and explores its glucose-lowering efficacy in subjects with type 2 diabetes with insufficient glycemic control on a stable regimen of antidiabetic therapy. This cell product is allogeneic mesenchymal lineage cells that are a STRO-3 immunoselected, culture-expanded, immature subfraction of adult bone marrow–derived mononuclear cells.
Research Design and Methods
Subjects
The study population included ∼60 adults ≤80 years of age with type 2 diabetes (HbA1c ≥7.0% to <10.5% [≥53 to <91 mmol/mol] at screening) who were receiving a stable therapeutic dose of metformin either alone or in combination with one other oral antidiabetic medication (except a thiazolidinedione) for at least 3 months before screening. Women of childbearing potential who were surgically sterile or agreed to use contraception during the entire study were eligible to participate. Exclusion criteria were C-peptide level ≤0.8 ng/mL, systolic blood pressure ≥170 mmHg, or diastolic blood pressure ≥110 mmHg; type 1 diabetes, diabetes resulting from pancreatic injury, or secondary forms of diabetes; acute metabolic diabetes complications (e.g., ketoacidosis, hyperosmolar coma) within 6 months of screening; severe hypoglycemia (defined as requiring third-party assistance) or repeated or frequent hypoglycemic episodes within 1 month before screening; insulin therapy within 6 months of screening except if used transiently for <7 days for intercurrent illness; New York Heart Association class III or IV heart failure; myocardial infarction or stroke within 6 months of screening; diagnosed and/or treated malignancy (except for treated basal cell or squamous small cell carcinoma of the skin with no evidence of recurrence); and presence of ≥20% anti-HLA antibody flow panel reactive antibody (PRA) class I or II and/or antibody specificities to donor HLA antigens. Complete eligibility criteria are provided in Supplementary Table 1.
Study Design
This multicenter, randomized, single-blind, placebo-controlled, sequential, dose-escalation study assessed the safety and tolerability of a single intravenous (IV) infusion of rexlemestrocel-L in subjects with type 2 diabetes suboptimally controlled on metformin alone or metformin plus one other oral antidiabetic medication. The study was conducted at 18 centers in the U.S. and consisted of an initial screening period not to exceed 8 weeks, a 12-week single-blind treatment period, and a 2-year poststudy safety follow-up period, including immune system responses, clinical laboratory parameters, and annual chest X-rays. The primary study, comprising the screening period and 12-week treatment period, was conducted between June 2012 and October 2013. The study was approved by the institutional review boards of the participating centers and was conducted in accordance with the principles of the Declaration of Helsinki and International Conference on Harmonisation Good Clinical Practice guidelines. All participants provided written informed consent.
Method of Assigning Subjects to Treatment Groups
At the baseline visit, an interactive voice response system/interactive web response system was used to randomize eligible subjects into the study and to assign treatment group allocations. Subjects were randomized to receive one of the following three rexlemestrocel-L doses or placebo in a 3:1 ratio using a sequential, escalating dose cohort paradigm: cohort 1, 0.3 × 106/kg (n = 15) or placebo (n = 5); cohort 2, 1.0 × 106/kg (n = 15) or placebo (n = 5); and cohort 3, 2.0 × 106/kg (n = 15) or placebo (n = 5). The computer-generated randomization to study treatment within each cohort was balanced by stratification, using permuted blocks, based on screening HbA1c <8.0% or ≥8.0% (64 mmol/mol). Each treatment was administered by IV infusion on day 0 after baseline assessments. Subjects were blinded to treatment allocation, but the investigators, pharmacist, and sponsor were not.
Study Treatment and Follow-up
The investigational product rexlemestrocel-L comprises STRO-3 immunoselected allogeneic MPCs derived from adult bone marrow mononucleated cells from healthy paid donors aged 18–45 years, culture-expanded in media supplemented with FBS, and formulated and cryopreserved in 4.0% dimethyl sulfoxide, 50% Alpha modified Eagle’s medium, and 42.5% ProFreeze. Donor and process testing were conducted for a prespecified panel of transmissible infectious diseases, karyotype, tumorigenicity, sterility, endotoxins, and mycoplasma. Cell procurement, processing, cryopreservation, and storage procedures were performed by a contract manufacturing facility under Current Good Manufacturing Practice conditions. The product is characterized by surface antigen expression of STRO-1, CC-9 (CD146), and HLA class I and II and cell count and viability. Cryopreserved products were shipped to study sites in temperature-monitored shipping dewars in the vapor phase of liquid nitrogen and thawed immediately before use. The MPC product or saline placebo was suspended in 100 mL normal saline and infused with filtration over 45 min. Complete details are provided in the Supplementary Data “Description of Investigational Product.”
Vital signs and oxygen saturation were monitored continuously during and for 6 h postinfusion. During the 12-week study period, all subjects remained on their background diabetes treatment and received standard-of-care management of their diabetes (16). Subjects completed glycemic diaries and received periodic reminders for lifestyle management. Rescue therapy was administered in case of unacceptable hyperglycemia (fasting plasma glucose [FPG] 270 mg/dL [15 mmol/L] weeks 4–8 and 240 mg/dL [13.3 mmol/L] weeks 8–12) at the discretion of the investigators. Any approved oral antidiabetic agent except thiazolidinediones could be used as deemed appropriate and consistent with clinical practice guidelines (16).
Study Oversight
This study was sponsored by Mesoblast Inc. and designed by the sponsor with input from the authors and the contract research organization (CRO) Medpace, Inc. (Cincinnati, OH). The CRO held the main database, and its employees performed the statistical analyses. All authors participated in manuscript preparation, made the decision to submit the manuscript for publication, and vouch for the completeness and accuracy of the data.
An independent Data Monitoring Committee comprising independent physicians with expertise in diabetes, other relevant internal medicine specialties, and the conduct of clinical trials along with an independent biostatistician ensured subjects’ safety. The Data Monitoring Committee reviewed all safety data for each dose cohort when all subjects in that cohort completed the week 1 visit, and if there were no safety concerns, a certificate of nonobjection to study continuation and escalation to the next dose cohort was issued to the sponsor. Stopping rules were prespecified in the study protocol.
Statistical Analyses
This study was designed to assess safety and tolerability of MPC therapy. Accordingly, there was no formal statement of null and alternative hypotheses and no accompanying power analysis to ensure a high probability of rejecting the null hypothesis of no difference between rexlemestrocel-L and placebo in case it was in fact false. Analyses of efficacy end points were primarily descriptive and hypothesis generating. P values for selected efficacy analyses are shown for exploratory purposes.
All efficacy analyses were carried out on the intention-to-treat (ITT) population, which was defined as all randomized subjects who received study treatment and had at least one evaluable postbaseline HbA1c or FPG measurement before receiving antihyperglycemic rescue medication. For subjects who required rescue therapy or in the event of missing data at week 12, the exploratory efficacy end point was defined as the last assessment before administration of rescue medication carried forward to the week 12 end point. The primary efficacy variable was the change in HbA1c from baseline to week 12 with the last observation carried forward (LOCF).
Demographic and baseline characteristics were summarized for all randomized subjects by treatment group. Efficacy end points were analyzed using summary statistics and frequency tables. The treatment difference was obtained through an ANCOVA model, with treatment and HbA1c strata (<8% or ≥8% [64 mmol/mol]) as factors and the baseline value as covariate. The difference in least squares means (LSMs) and corresponding SEs are presented. The same statistical model was applied to changes in HbA1c analyzed by prespecified subgroups based on baseline HbA1c, background antidiabetic medication, age, sex, and other demographic variables.
The prespecified exploratory analyses of efficacy were performed for hypothesis generation and were not adjusted for multiplicity. The number and percentage of subjects at an HbA1c target of <7% (53 mmol/mol) at week 12 with LOCF was assessed by two-sided 95% exact binomial CI within treatment, and for the between-treatment group comparison, the Fisher exact test was used for the comparison of each MPC group versus placebo. The number and percentage of subjects who required glycemic rescue therapy and time to glycemic rescue are summarized descriptively.
Safety was assessed by adverse events, cardiovascular events, clinical laboratory measurements (hematology, blood chemistry, and urinalysis), vital signs, 12-lead electrocardiograms, physical examination findings, fundus examination, chest X-rays, pulmonary function tests, and review of antibody specificity testing for anti-HLA class I and II antibodies as well as antimurine and antibovine antibodies. All safety analyses were applied to the safety population and defined as all subjects who received study treatment and had at least one follow-up safety evaluation.
Assay Procedures
Insulin, osteocalcin, and C-peptide levels were determined by electrochemiluminescence immunoassay. Plasma glucose was measured by photometry. IL-6, IL-1β, tumor necrosis factor-α (TNF-α), and adiponectin were measured by ELISA, and hs-CRP was determined by nephelometry. Immune profiling consisted of determining flow PRAs by flow cytometry and the presence of donor-specific antibodies. Assays were performed using One Lambda, Inc., reagents as well as a Luminex platform according to the manufacturers’ instructions. A positive donor-specific antibody was identified when the antibody specificities were directed to the MPC donor HLA antigens.
Changes in the Conduct of the Study
There was one protocol amendment (version 3, dated 7 September 2012) to broaden eligibility for inclusion of subjects receiving metformin monotherapy to inclusion of subjects receiving metformin alone or metformin plus one other oral nonthiazolidinedione antidiabetic agent for at least 3 months before treatment.
Results
Subjects
Sixty-one subjects were enrolled at 18 centers in the U.S. between 28 June 2012 and 3 July 2013. The disposition of subjects is shown in Supplementary Fig. 1. All subjects completed the 12-week study. Briefly, of 189 subjects screened, 128 were not randomized with the most common reason (120 subjects) of not meeting inclusion or exclusion criteria; 61 were randomized, all of whom completed the 12-week study period. One subject was excluded from the ITT population analysis because rescue medication was administered before any postbaseline efficacy assessments. Demographic characteristics and key metabolic parameters at baseline are shown in Table 1.
Table 1.
Baseline and demographic characteristics
| Rexlemestrocel-L | ||||
|---|---|---|---|---|
| Parameter | Placebo | 0.3 × 106/kg | 1.0 × 106/kg | 2.0 × 106/kg |
| (N = 16) | (N = 15) | (N = 15) | (N = 15) | |
| Sex, n (%) | ||||
| Male | 12 (75.0) | 10 (66.7) | 9 (60.0) | 9 (60.0) |
| Female | 4 (25.0) | 5 (33.3) | 6 (40.0) | 6 (40.0) |
| Age, years | 58.7 ± 7.3 | 57.7 ± 8.2 | 55.3 ± 11.4 | 57.2 ± 6.6 |
| Race, n (%) | ||||
| Caucasian | 15 (93.8) | 14 (93.3) | 14 (93.3) | 12 (80.0) |
| African American | 1 ( 6.3) | 0 ( 0.0) | 1 (6.7) | 1 (6.7) |
| Asian | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (6.7) |
| Other | 0 (0.0) | 1 (6.7) | 0 (0.0) | 2 (13.4) |
| Height, cm | 171.5 ± 9.3 | 168.1 ± 6.5 | 171.2 ± 10.8 | 168.7 ± 9.8 |
| Baseline weight, kg | 95.9 ± 20.2 | 98.6 ± 21.3 | 101.7 ± 21.4 | 92.6 ± 16.9 |
| BMI, kg/m2 | 32.6 ± 6.2 | 34.8 ± 6.5 | 34.4 ± 4.7 | 32.4 ± 4.5 |
| Duration of diabetes, years | 9.8 ± 6.7 | 10.8 ± 7.3 | 10.2 ± 5.7 | 9.6 ± 4.5 |
| Baseline HbA1c (%) | 8.2 ± 0.8 | 8.3 ± 0.8 | 8.6 ± 1.1 | 7.9 ± 1.1 |
| mmol/mol | 66 ± 8.7 | 67 ± 8.7 | 70 ± 12 | 63 ± 12 |
| <8% | 7 (43.8) | 7 (46.7) | 5 (33.3) | 8 (53.3) |
| ≥8% | 9 (56.3) | 8 (53.3) | 10 (66.7) | 7 (46.7) |
| Antidiabetic regimen, n (%) | ||||
| Metformin only | 11 (68.8) | 11 (73.3) | 6 (40.0) | 7 (46.7) |
| Metformin + one oral agent | 5 (31.3) | 4 (26.7) | 9 (60.0) | 8 (53.3) |
| Fasting glucose (mg/dL) | 183 ± 55.0 | 194.6 ± 67.4 | 197.5 ± 30.5 | 166.1 ± 38.8 |
| mmol/L | 10.2 ± 3.2 | 10.8 ± 3.7 | 11.0 ± 1.7 | 9.2 ± 2.2 |
Data are mean ± SD or n (%).
Safety
All subjects received the full study treatment infusion. No adverse events were reported during infusion or the 6-h postinfusion monitoring period. In total, 27 (44.3%) subjects had any treatment-emergent adverse event (TEAE) during the 12-week study period (6 [40.0%], 10 [66.7%], and 5 [33.3%] in the 0.3 × 106/kg, 1.0 × 106/kg, and 2.0 × 106/kg rexlemestrocel-L groups, respectively, and 6 [37.5%] in the placebo group). One subject in the 0.3 × 106/kg group had a severe TEAE of abdominal pain; all other TEAEs were mild or moderate in severity. The most common TEAE in MPC-treated subjects was upper respiratory infection, which two (13.3%) in the 1.0 × 106/kg group experienced. TEAEs of sinusitis and posttraumatic pain were each experienced by two subjects in the placebo group (Table 2).
Table 2.
Summary of TEAEs during the 12-week study period
| Rexlemestrocel-L |
||||
|---|---|---|---|---|
| Placebo (n = 16) | 0.3 × 106/kg (n = 15) | 1.0 × 106/kg (n = 15) | 2.0 × 106/kg (n = 15) | |
| Subjects with any TEAEs | 6 (37.5) | 6 (40.0) | 10 (66.7) | 5 (33.3) |
| Subjects with any treatment-related TEAEs† | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (6.7) |
| Subjects with any SAEs | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Adverse events leading to discontinuation | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Subjects with TEAEs (≥2 subjects in any group) by system organ class and preferred term | ||||
| Infections and infestations | 3 (18.8) | 0 (0.0) | 3 (20.0) | 3 (20.0) |
| Nasopharyngitis‡ | 1 (6.3) | 0 (0.0) | 1 (6.7) | 1 (6.7) |
| Sinusitis | 2 (12.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Upper respiratory tract infection | 0 (0.0) | 0 (0.0) | 2 (13.3) | 0 (0.0) |
| Folliculitis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (6.7) |
| Fungal skin infection | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (6.7) |
| Gastroenteritis viral | 1 (6.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Urinary tract infection | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (6.7) |
| Injury, poisoning, and procedural complications | 2 (12.5) | 0 (0.0) | 2 (13.3) | 0 (0.0) |
| Posttraumatic pain | 2 (12.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Contusion | 0 (0.0) | 0 (0.0) | 1 (6.7) | 0 (0.0) |
| Fall | 1 (6.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Muscle strain | 0 (0.0) | 0 (0.0) | 1 (6.7) | 0 (0.0) |
Data are n (%). Percentage was calculated using the number of subjects in the column heading as the denominator. TEAEs are adverse events that started on or after the dose date of study treatment.
†As assessed by the investigator.
‡Although a subject may have had two or more TEAEs, the subject is counted only once within a system organ class category. The same subject may contribute to two or more preferred terms in the same system organ class category.
No subjects had a serious adverse event (SAE) during the 12-week primary study period, and no subjects discontinued as a result of a TEAE. Three subjects reported SAEs after the 12-week study period: One subject in the 0.30 × 106/kg group with a history of myocardial infarction and three-vessel coronary artery bypass graft surgery showed evidence of cardiac ischemia during a routine exercise stress test on day 87, one subject in the 1.0 × 106/kg group was hospitalized after a motor vehicle accident, and one subject in the placebo group was hospitalized for pneumonia and acute exacerbation of chronic obstructive pulmonary disease. None of these SAEs were treatment related.
In total, three (4.9%) subjects had a hypoglycemia event. One subject in the 0.3 × 106/kg group had two episodes of documented symptomatic hypoglycemia that were resolved with a snack. One subject in the 1.0 × 106/kg group had a minor episode of asymptomatic hypoglycemia with no action taken, and one subject in the placebo group had five episodes of asymptomatic hypoglycemia with no action taken and one episode of documented symptomatic hypoglycemia that resolved with a snack. No subjects in the 2.0 × 106/kg group had a hypoglycemia event.
Pulmonary and upper respiratory adverse events and acute allergic and immunologic adverse events were of special interest because systemically infused cells are known to pass through the lungs and the cells are from unrelated donors, respectively. In addition, because the investigational product is living cells from an unrelated donor, unwanted tissue formation is a theoretical potential risk. During the 12-week study period, no allergic or immunologic adverse events were reported. Five subjects experienced a respiratory, thoracic, or mediastinal adverse event (Table 2). One subject in the 0.3 × 106/kg group experienced mild allergic rhinitis, and one subject experienced a moderate cough. In the 1.0 × 106/kg group, one subject experienced a mild dry cough, one experienced moderate sinus congestion, and one experienced mild shortness of breath (Table 2). None of these adverse events occurred acutely with study infusion or were considered related to study treatment, and all resolved without sequelae.
No clinically meaningful differences in mean changes or individual subject changes in laboratory parameters or physical examination findings from baseline to the end of the 12-week study period were noted. Overall, there were no meaningful changes in pulmonary function; however, one subject in the 1.0 × 106/kg group had a significant decrease in expired volumes due to generalized edema at week 12. There were no clinically meaningful changes in the fundus examination. No subjects developed antibodies specific to the donor HLA, and no trends in changes in anti-HLA antibody response were noted across dose groups or as the study progressed to the week 12 end point (see Supplementary Data “Information on Immune System Responses Over Time”).
Exploratory Efficacy
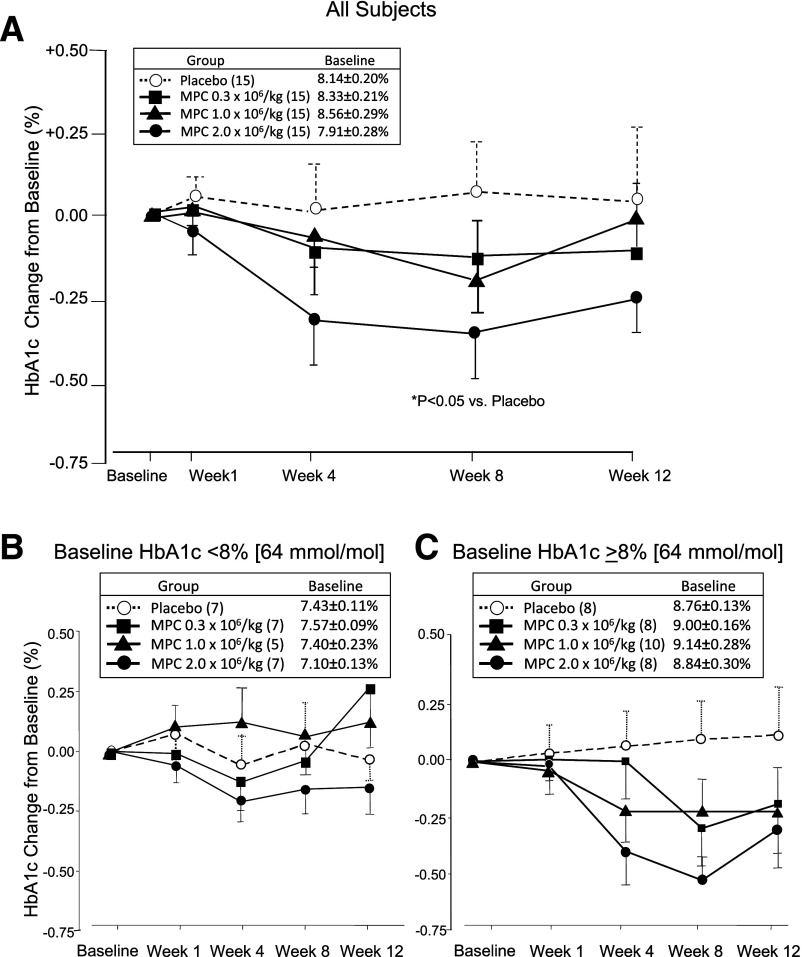
The primary exploratory efficacy parameter was the effect of a single IV administration of rexlemestrocel-L on HbA1c over 12 weeks. For all time points after week 1, there was a numerical decrease in HbA1c levels in the rexlemestrocel-L groups and a small increase in HbA1c in the placebo group (Fig. 1). Relative to placebo, the LSM change in HbA1c was 0.0 ± 0.25% for the 0.3 × 106/kg group, −0.1 ± 0.25% for the 1.0 × 106/kg group, and −0.3 ± 0.25% for the 2.0 × 106/kg group at week 12. The nadir in HbA1c for the highest-dose group appeared to occur at week 8 (P < 0.05 for 2.0 × 106/kg vs. placebo).
Figure 1.
HbA1c change from baseline over time by group in ITT population with LOCF in all subjects (A) and by subgroup of baseline HbA1c <8% (B) or ≥8% (C). Data are mean ± SEM with week 12 end point or last observation before administration of rescue medication carried forward. P value based on LSM difference from placebo derived from ANCOVA model using treatment and screening HbA1c strata as factors and baseline HbA1c value as covariate.
At week 12, the HbA1c target of <7% was achieved by two (13.3%) subjects in the 0.3 × 106/kg group, one (6.7%) in the 1.0 × 106/kg group, five (33.3%) in the 2.0 × 106/kg group, and none in the placebo group (P < 0.05 for the highest rexlemestrocel-L dose vs. placebo) (Supplementary Fig. 2). Relative to placebo, the LSM difference in the change in FPG at week 12 showed no trends across treatment groups (Table 3).
Table 3.
Baseline and week 12 change from baseline metabolic parameters and biomarkers
| Rexlemestrocel-L | |||||
|---|---|---|---|---|---|
| Placebo | 0.3 × 106/kg | 1.0 × 106/kg | 2.0 × 106/kg | Treatment | |
| N = 15 | N = 15 | N = 15 | N = 15 | P value† | |
| Body weight (kg) | 96.53 ± 20.69 | 98.59 ± 21.30 | 101.71 ± 21.38 | 92.57 ± 16.9 | |
| Change from baseline (kg) | −0.52 ± 2.38 | −0.70 ± 2.28 | −0.46 ± 2.77 | −0.19 ± 2.99 | 0.942 |
| n = 15 | n = 15 | n = 15 | n = 15 | ||
| FPG (mg/dL) | 179.9 ± 55.5 | 194.6 ± 67.4 | 197.5 ± 30.5 | 166.1 ± 38.8 | |
| mmol/L | 10.0 ± 3.1 | 10.8 ± 3.7 | 11.0 ± 1.7 | 9.2 ± 2.2 | |
| Change from baseline (mg/dL) | −18.0 ± 58.9 | −2.6 ± 50.6 | −11.8 ± 53.5 | −5.9 ± 48.5 | 0.554 |
| mmol/L | −1.0 ± 3.3 | −0.1 ± 2.8 | −0.7 ± 3.0 | −0.3 ± 2.7 | |
| n = 15 | n = 15 | n = 15 | n = 15 | ||
| Fasting plasma insulin (μIU/mL) | 17.05 ± 10.37 | 12.21 ± 8.15 | 17.78 ± 8.58 | 12.89 ± 9.61 | |
| Change from baseline (μIU/mL) | 2.81 ± 12.16 | 2.37 ± 3.24 | −3.95 ± 11.08 | −1.29 ± 5.6 | 0.158 |
| n = 15 | n = 15 | n = 13 | n = 15 | ||
| C-peptide (ng/mL) | 3.965 ± 1.586 | 3.590 ± 1.118 | 4.201 ± 1.583 | 3.599 ± 1.507 | |
| Change from baseline (ng/mL) | 0.631 ± 1.354 | 0.302 ± 0.822 | −0.213 ± 1.226 | 0.122 ± 0.906 | 0.226 |
| n = 15 | n = 15 | n = 15 | n = 14 | ||
| LDL (mmol/L) | 2.8 ± 1.0 | 2.6 ± 0.8 | 2.8 ± 1.0 | 3.0 ± 0.8 | |
| Change from baseline (%) | 5.1 ± 43.42 | 7.7 ± 14.77 | 8.3 ± 35.69 | −5.2 ± 22.68 | 0.945 |
| n = 15 | n = 12 | n = 15 | n = 14 | ||
| HDL (mmol/L) | 1.1 ± 0.31 | 1.0 ± 0.28 | 1.1 ± 0.27 | 1.21 ± 0.38 | |
| Change from baseline (%) | 2.2 ± 15.30 | 11.3 ± 19.18 | −2.2 ± 13.81 | −4.1 ± 19.39 | 0.168 |
| n = 15 | n = 12 | n = 15 | n = 14 | ||
| Triglycerides (mmol/L) | 1.89 ± 0.94 | 2.52 ± 1.77 | 2.23 ± 1.19 | 2.50 ± 1.91 | |
| Change from baseline (%) | −0.9 ± 23.55 | 1.6 ± 30.26 | −0.7 ± 21.51 | −2.5 ± 33.54 | 0.947‡ |
| n = 15 | n = 13 | n = 15 | n = 14 | ||
| hs-CRP (mg/L) | 3.71 ± 3.11 | 4.15 ± 5.10 | 4.03 ± 3.34 | 3.49 ± 2.71 | |
| Change from baseline (mg/L) | −0.30 ± 2.07 | 1.62 ± 5.99 | −0.11 ± 2.62 | 0.41 ± 2.17 | 0.571‡ |
| n = 15 | n = 15 | n = 15 | n = 15 | ||
| Adiponectin (μg/mL) | 5.448 ± 4.787 | 5.281 ± 1.862 | 6.191 ± 4.261 | 6.120 ± 2.878 | |
| Change from baseline (μg/mL) | −0.789 ± 2.00 | 0.311 ± 0.790§ | 0.271 ± 1.165§ | −0.259 ± 1.12 | 0.054 |
| n = 15 | n = 13 | n = 15 | n = 14 | ||
| TNF-α (pg/mL) | 2.025 ± 0.739 | 1.859 ± 0.445 | 1.776 ± 0.416 | 2.893 ± 4.126 | |
| Change from baseline (pg/mL) | 0.226 ± 0.496 | 0.027 ± 0.346§ | 0.158 ± 0.291 | −0.826 ± 3.391 | 0.201 |
| n = 15 | n = 13 | n = 14 | n = 14 | ||
| IL-6 (pg/mL) | 2.486 ± 1.347 | 1.997 ± 0.908 | 2.231 ± 1.081 | 3.449 ± 4.196 | |
| Change from baseline (pg/mL) | −0.144 ± 0.937 | 0.022 ± 0.491 | 0.444 ± 1.697 | −1.388 ± 3.73 | 0.396 |
| n = 15 | n = 13 | n = 14 | n = 14 | ||
Data are mean ± SD.
Treatment P values obtained from an ANCOVA model with treatment and screening HbA1c strata (<8% or ≥8%) as factors and baseline value as covariate.
Treatment P value obtained from Cochran-Mantel-Haenszel test in a nonparametric ANCOVA model with treatment as a factor adjusting for screening HbA1c strata and baseline value.
P < 0.05 for LSM difference from placebo.
Glycemic rescue therapy was required by two (13.3%) subjects in the 0.3 × 106/kg group, one (6.7%) in the 2.0 × 106/kg group, and one (6.7%) in the placebo group. No subjects in the 1.0 × 106/kg group required rescue therapy. Selected efficacy parameters are shown in Table 3. Reductions in insulin and TNF-α relative to placebo and increases in adiponectin relative to placebo were observed (Table 3) but did not reach statistical significance on the overall ANCOVAs. The reduction in fasting insulin was greater in the 1.0 × 106/kg group versus placebo (P < 0.05), the decrease in TNF-α was greater in the 0.3 × 106/kg group versus placebo (P < 0.05), and the increase in adiponectin was greater in both the 0.3 × 106/kg and the 1.0 × 106/kg groups versus placebo (P < 0.05). No trends were observed for treatment effects on body weight, C-peptide level, lipid profile, meal tolerance test (data not shown), renal function (urinary albumin-to-creatinine ratio [data not shown]), and hs-CRP.
Changes in HbA1c over time by subgroup of baseline HbA1c are shown in Fig. 1. In subjects with a baseline HbA1c <8% (64 mmol/mol), the LSM difference from placebo in the HbA1c change from baseline to week 12 was 0.4 ± 0.3%, 0.2% ± 0.3%, and −0.2 ± 0.3% for the 0.3 × 106/kg, 1.0 × 106/kg, and 2.0 × 106/kg groups, respectively. In subjects with a baseline HbA1c ≥8% (64 mmol/mol), the LSM difference from placebo in the HbA1c change from baseline to week 12 was −0.2 ± 0.40%, −0.2 ± 0.38%, and −0.4 ± 0.41% for the 0.3 × 106/kg, 1.0 × 106/kg, and 2.0 × 106/kg groups, respectively. No meaningful differences in mean changes in HbA1c and FPG were observed between other prespecified subgroups, including those based on age, sex, race, ethnicity, or cardiometabolic risk factors.
Conclusions
To our knowledge, this controlled trial is the first to investigate cell therapy using adult allogeneic mesenchymal lineage cells in subjects with type 2 diabetes. The primary aim of the study was to assess safety and tolerability of cell therapy rather than to provide a definitive assessment of glycemic efficacy. Theoretical potential risks of a living cell therapy of special interest in this study were acute immunogenic responses to the donor HLA, pulmonary/upper respiratory reactions, and unwanted tissue formation. The infusion of rexlemestrocel-L was well tolerated by all subjects. Overall, the safety profile was comparable among treatment groups. The HbA1c target of <7% at week 12 was achieved in 8 of 45 subjects treated with rexlemestrocel-L versus 0 of 15 subjects treated with placebo.
A number of characteristics of bone marrow–derived adult mesenchymal lineage cells contribute to the salutary effects of these cells observed in animal models of diabetes and related complications (13,17). In particular, secretion of a broad range of bioactive molecules, such as growth factors, cytokines, and chemokines, and the ability to respond to the microenvironment of injury and inflammation with the secretion of anti-inflammatory cytokines contribute to the cells’ biologically significant role under injury conditions (18,19). The secretory activity and subsequent paracrine effects of these cells in vivo is central to their clinical potential (18) because little evidence suggests that they engraft when administered systemically (20). The immunomodulatory role of these cells through altering the cytokine secretion profiles of many immune cell types is also established (17).
Mesoblast Inc. has developed a process to immunoselect MPCs (rexlemestrocel-L), a population of multipotent stromal stem cells present in perivascular niches from adult bone marrow mononuclear cells, and to expand these cells in culture for investigational allogeneic use (21). The immunoselection process uses antibodies against characteristic cell surface markers expressed by MPCs, such as STRO-1 and STRO-3 (22,23). In contrast, traditional mesenchymal stem cell preparation involves plastic adherence isolation (23). Immunoselected MPCs are enriched for colony-forming unit fibroblasts and demonstrate proliferative capacity, production of various cytokines and growth factors, and in vitro developmental capacities along the bone, fat, and cartilage lineages under defined culture conditions (24,25). In head-to-head comparisons of STRO-3 immunoselected MPCs and density-gradient selected MSCs from the same human donor, Psaltis et al. (23) showed that MPCs had greater residual population doubling capacity and secreted higher levels of paracrine factors necessary for imparting antiapoptotic effects on target cells, proangiogenic effects, and possibly immunomodulatory effects.
Human mesenchymal lineage cells have an immune-privileged phenotype and represent a potentially advantageous cell type for transplantation into an allogeneic host without HLA matching or recipient immunosuppression (25). The current findings that no subjects treated with rexlemestrocel-L treatment developed antibodies specific to the donor HLA or showed clinically relevant increases in either class I or II PRA are consistent with the immunotolerant profile of this cell type. However, immune tolerance has not been universally confirmed in preclinical and clinical studies and warrants further surveillance (25). These cells demonstrate low-level immunogenicity associated with negativity for HLA class II and CD80 and CD86 coimmunostimulatory molecules and exert potential immunomodulatory effects; namely, inhibition of T-cell proliferation (25) and release of soluble factors (24) that may have anti-inflammatory effects. An anti-inflammatory effect of rexlemestrocel-L resulting in improved glycemic control is suggested by similar mild effects reported with canakinumab and anakinra (26,27).
There are several limitations to this study. The sample size was too small to show a significant effect on glycemic control. It was not possible to balance the HbA1c values among the active dose cohorts because of the sequential, dose-escalating study design. At baseline, mean HbA1c and FPG levels were somewhat lower in the highest dose rexlemestrocel-L group compared with the other groups, although these differences were not statistically significant (Table 1). Imbalances among the treatment arms in baseline values were handled statistically by application of ANCOVA procedures using baseline values as covariates. The protocol amendment allowing inclusion of subjects on a background regimen of metformin plus one other oral antidiabetic medication was made midway through the enrollment of the first dose cohort. Accordingly, more subjects in this dose group received metformin monotherapy compared with the later cohorts. Finally, this study evaluated the effect of a single infusion of rexlemestrocel-L on HbA1c over a period of 12 weeks. Although there was evidence of biological activity within this brief period, longer study is needed for long-term safety surveillance and to assess the durability of effect and the possible need for an optimal frequency of repeat administration. The ongoing 2-year extension phase will provide additional safety information, including immune system responses and cancer surveillance. Despite these limitations, the safety and feasibility results of this study support further investigations to evaluate the use of MPCs in type 2 diabetes and its complications in appropriately sized and powered studies of longer duration, possibly including multiple or periodic cell administrations.
Supplementary Material
Article Information
Acknowledgments. The authors thank Mei Chen, Medpace, Inc., for developing the statistical analysis plan and performing all statistical analyses.
Funding. V.A.F. and clinical research at Tulane are supported in part by 1U54-GM-104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Duality of Interest. This study was funded by Mesoblast Inc. J.S.S., V.A.F., and J.R. have served as consultants to Mesoblast Inc. (considered by their institutional conflict of interest committee to be not significant). K.R.S. is an employee of Mesoblast Inc.
The sponsor of the study, Mesoblast Inc., funded the study, participated in the study design, and provided oversight to the CRO for data collection, data review, and the clinical study report.
Author Contributions. J.S.S., V.A.F., and J.R. enrolled study subjects, had full access to the study data, interpreted the study results, wrote and revised the manuscript, and approved the final manuscript. K.R.S. served as study director for the trial, drafted the protocol and manuscript, and approved the final manuscript. J.S.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this article were presented in abstract form at the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 13–17 June 2014.
Footnotes
Clinical trial reg. no. NCT01576328, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc14-2830/-/DC1.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al.; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD) . Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raz I, Riddle MC, Rosenstock J, et al. Personalized management of hyperglycemia in type 2 diabetes: reflections from a Diabetes Care Editors’ Expert Forum. Diabetes Care 2013;36:1779–1788 [DOI] [PMC free article] [PubMed]
- 3.Turner RC, Cull CA, Frighi V, Holman RR; UK Prospective Diabetes Study (UKPDS) Group . Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA 1999;281:2005–2012 [DOI] [PubMed] [Google Scholar]
- 4.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benhalima K, Wilmot E, Khunti K, Gray LJ, Lawrence I, Davies M. Type 2 diabetes in younger adults: clinical characteristics, diabetes-related complications and management of risk factors. Prim Care Diabetes 2011;5:57–62 [DOI] [PubMed] [Google Scholar]
- 6.van Dieren S, Beulens JW, van der Schouw YT, Grobbee DE, Neal B. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil 2010;17(Suppl. 1):S3–S8 [DOI] [PubMed] [Google Scholar]
- 7.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011;11:98–107 [DOI] [PubMed] [Google Scholar]
- 8.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006;116:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov 2014;13:465–476 [DOI] [PubMed] [Google Scholar]
- 10.Goldfine AB, Fonseca V, Shoelson SE. Therapeutic approaches to target inflammation in type 2 diabetes. Clin Chem 2011;57:162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther 2012;20:14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee RH, Seo MJ, Reger RL, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A 2006;103:17438–17443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yañez AJ, Conget PA. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant 2008;14:631–640 [DOI] [PubMed] [Google Scholar]
- 14.Estrada EJ, Valacchi F, Nicora E, et al. Combined treatment of intrapancreatic autologous bone marrow stem cells and hyperbaric oxygen in type 2 diabetes mellitus. Cell Transplant 2008;17:1295–1304 [DOI] [PubMed] [Google Scholar]
- 15.Bhansali A, Upreti V, Khandelwal N, et al. Efficacy of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cells Dev 2009;18:1407–1416 [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association . Standards of medical care in diabetes—2011. Diabetes Care 2011;34(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volarevic V, Arsenijevic N, Lukic ML, Stojkovic M. Concise review: mesenchymal stem cell treatment of the complications of diabetes mellitus. Stem Cells 2011;29:5–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meirelles LdaS, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev 2009;20:419–427 [DOI] [PubMed] [Google Scholar]
- 19.Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol 2011;6:457–478 [DOI] [PubMed] [Google Scholar]
- 20.von Bahr L, Batsis I, Moll G, et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells 2012;30:1575–1578 [DOI] [PubMed] [Google Scholar]
- 21.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood 1991;78:55–62 [PubMed] [Google Scholar]
- 22.Gronthos S, Fitter S, Diamond P, Simmons PJ, Itescu S, Zannettino ACW. A novel monoclonal antibody (STRO-3) identifies an isoform of tissue nonspecific alkaline phosphatase expressed by multipotent bone marrow stromal stem cells. Stem Cells Dev 2007;16:953–963 [DOI] [PubMed] [Google Scholar]
- 23.Psaltis PJ, Paton S, See F, et al. Enrichment for STRO-1 expression enhances the cardiovascular paracrine activity of human bone marrow-derived mesenchymal cell populations. J Cell Physiol 2010;223:530–540 [DOI] [PubMed] [Google Scholar]
- 24.See F, Seki T, Psaltis PJ, et al. Therapeutic effects of human STRO-3-selected mesenchymal precursor cells and their soluble factors in experimental myocardial ischemia. J Cell Mol Med 2011;15:2117–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atoui R, Chiu RC. Concise review: immunomodulatory properties of mesenchymal stem cells in cellular transplantation: update, controversies, and unknowns. Stem Cells Transl Med 2012;1:200–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ridker PM, Howard CP, Walter V, et al.; CANTOS Pilot Investigative Group . Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation 2012;126:2739–2748 [DOI] [PubMed] [Google Scholar]
- 27.Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 2007;356:1517–1526 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.