Abstract
The objective of this position statement from the Saudi Gastroenterology Association is to guide gastroenterologists on the use of tumor necrosis factor-alfa (TNF-α) antagonists for the treatment of the idiopathic inflammatory bowel diseases, Crohn's disease, and ulcerative colitis. In this article, we summarize the relevant literature regarding the safety and efficacy of TNF-α antagonists, highlight relevant safety concerns specific to the environment in Saudi Arabia, and provide specific recommendations for the use of these agents.
Keywords: Crohn's disease, inflammatory bowel disease, position statement, Saudi Gastroenterology Association, TNF antagonists, ulcerative colitis
The idiopathic inflammatory bowel diseases (IBD), ulcerative colitis (UC), and Crohn's disease (CD) are characterized by dysregulated immune responses to gut microbiota in genetically predisposed individuals.[1,2,3] UC, which is limited to the large bowel, typically presents with bloody diarrhea and abdominal cramps.[2] CD can affect any part of the gastrointestinal tract from the mouth to the anus.[2,4] UC and CD patients usually experience abdominal pain, diarrhea, and fatigue.[1] Uncontrolled inflammation from IBD can result in either local or systemic complications that are associated with reduced quality of life and an increased risk of mortality.[5] The risk of colorectal cancer (CRC) is increased in UC depending on extent, age of onset, duration of disease, presence of primary sclerosing cholangitis, and family history of colorectal cancer.[6,7,8] Colectomy is frequently required to treat dysplasia, cancer, or medically refractory disease.[9] In distinction, CD cannot be “cured” with surgery. However, bowel resection is frequently required to manage disease-related complications such as abscess/fistula formation or strictures that result in bowel obstruction.[10]
The incidence of IBD is increasing in North America, Europe, and Asia.[11,12,13,14,15] The Middle East in general and the Kingdom of Saudi Arabia (KSA) in particular16,17,18] are also affected by this phenomenon. Although multiple environmental factors have been implicated as potential causes for the increased incidence of IBD, urbanization, cigarette smoking, and changes in the gut microbiome[19,20,21] are the most consistently identified associations. The societal burden due to both the costs of therapy and loss of work productivity has increased considerably as a consequence of the rising incidence of IBD.[22]
Management of IBD
Traditional management of IBD has been based on controlling symptoms. Medical management has featured an incremental stepwise approach—the “therapeutic pyramid”—that specifies matching treatment selection to severity of symptoms with intensification of therapy based on response.
However, several emerging concepts are causing modification of this approach. First, risk profiling is becoming an important part of IBD management, which potentially allows selective use of highly effective therapies in patients at the greatest risk of disease-related complications. High-risk features for CD include diagnosis at an early age; ileal, foregut, or perianal disease; cigarette smoking; need for bowel resection; endoscopically detectable deep ulcerations; and presentation with a disease-related complication.[23] However, existing clinical risk prediction models have not been prospectively validated.[24,25] In UC, the presence of extensive disease, deep colonic ulcerations, frequent bowel movements, an elevated C-reactive protein (CRP) serum concentration, corticosteroid dependence or resistance, hospitalization at the time of diagnosis, and fulminant colitis increases the risk of colectomy.[26] In clinical practice, disease activity and intensity of treatment is usually based on a global assessment of symptoms and endoscopy. Although the Mayo Clinic Score (MCS)[27] and the Crohn's disease activity index (CDAI)[28] are standard instruments for assessment of disease activity, in clinical trials they are not widely used in clinical practice.
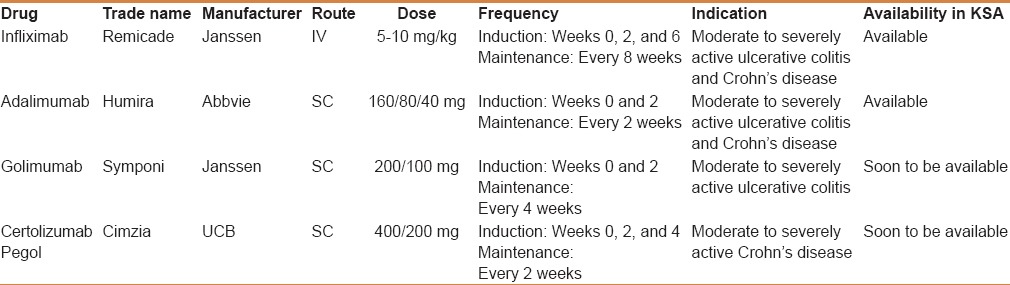
Conventional therapies for IBD include agents that target the inflammatory cascade either locally in the gut (eg, 5-aminosalicylic acid agents (5ASA) and budesonide) or systematically (corticosteroids and immunosuppressants such as thiopurines [azathioprine (AZA) and 6-mercaptopurine (6 MP)] and methotrexate (MTX)).[29,30,31] Tumor necrosis factor-alfa (TNF-α) plays a central role in the perpetuation of chronic inflammation. Over the past two decades monoclonal antibodies directed against TNF-α[3] have been used to treat numerous inflammatory disorders including rheumatoid arthritis, psoriasis, and IBD.[32] TNF-α antagonists used for the treatment of IBD include infliximab “Remicade” (IFX), adalimumab “Humira”(ADA), certolizumab pegol “Cimzia” (CZP), and golimumab “Symponi” (GOL). IFX and ADA are approved by the US Food and Drug administration (FDA) for the treatment of UC and CD. CZP is only approved for the treatment of CD in the United States in addition to a few other countries such as Switzerland. GOL has been recently approved for the treatment of UC in the United States and Canada. Currently, in KSA only IFX and ADA are approved by the Saudi Food and Drug administration (SFDA) for management of CD and UC. However, GOL and CZP are likely to become available in the future [Table 1].
Table 1.
TNF-α antagonists used for the treatment of inflammatory bowel disease
The efficacy of TNF antagonists in the treatment of CD
IFX, ADA, and CZP have been extensively studied for the management of CD. The key studies are summarized in the following sections.
Infliximab
Infliximab (IFX) was first studied in a small 12-week dose finding randomized controlled trial that evaluated induction therapy. Four weeks after a single infusion of either 5 mg/kg, 10 mg/kg, or 20 mg/kg of infliximab, higher rates of clinical response (81% vs 50% vs 64% vs17%, for drug and placebo, respectively, P < 0.001) and clinical remission (33% for pooled drug doses vs 4% placebo, P = 0.005) were observed in patients receiving IFX.[33] In the ACCENT1 Phase III trial, 576 patients with moderate to severely active CD received induction therapy with 5 mg/kg of IFX at week 0 and were then assessed for clinical response at week 2. Responders were subsequently randomized to one of three groups: 5 mg/kg of IFX at weeks 2 and 6 and then every 8 weeks thereafter until week 46 (Group I), repeat infusions of 5 mg/kg IFX at the same timepoints (Group II), or 5 mg/kg IFX at weeks 2 and 6 followed by 10 mg/kg (Group III). The co-primary endpoints comprised the proportion of patients who responded to induction at week 2 who demonstrated remission (CDAI < 150) at week 30 and the time to loss of response up to week 54 in patients who initially responded to induction therapy. Patients who received IFX were more likely to sustain clinical remission at weeks 23, 30, and 110 compared with patients assigned to placebo (odds ratio (OR) 2.7, 95% CI 1.6–4.6).[34]
Adalimumab
ADA was initially studied in a small phase IIa induction trial that recruited CD patients who had lost response or became intolerant to IFX.[35] Subsequently, the CLASSICI study evaluated 299 patients with moderate to severely active biologic-naïve CD, who were randomized patients to one of three ADA dose regimens (40/20, 80/40, or 160/80 mg) or placebo at weeks 0 and 2. The primary endpoint was clinical remission at week 4 defined as a CDAI score <150 points. Significantly higher rates of remission were observed in the 160/80 ADA group[36,37] than in the placebo group (36% vs 12%, respectively, P = 0.001). ADA was then studied as a maintenance agent in the CHARM trial in which all participants received an induction regimen consisting of 80 mg of ADA at week 0 followed by 40 mg at week 2. At the end of the induction phase (week 4), patients were stratified according to their response (decrease in CDAI ≥ 70 points from baseline) and randomized to receive placebo, ADL 40 mg every other week (eow), or ADA 40 mg weekly for up for 56 weeks. The co-primary end points were the proportion of randomized responders with clinical remission (CDAI < 150) at weeks 26 and week 56. More patients assigned to either ADL regimen were in clinical remission at both week 26 and week 56 (36%, 41%, and 12%, respectively; P < 0.001) than those who received placebo (40%, 47%, and 17%, respectively; P < 0.001). No important efficacy or safety differences were observed between the weekly and every other week ADA maintenance regimens.
Certolizumab pegol
CZP was studied in several large randomized controlled trials. Schrieber et al. initially evaluated CZP induction therapy in a phase II placebo-controlled trial in which 292 patients with moderate-to-severe CD participated. Patients were assigned to subcutaneous CZP 100, 200, or 400 mg or placebo at weeks 0, 4, and 8. The primary endpoint was the proportion of patients with a clinical response (CDAI decrease from baseline of >70 points) at week 12. Although higher rates of clinical response were observed for CZP 400 mg throughout the study, especially at week 10 (CZP 400 mg vs placebo: 52.8% vs 30.1%; P = 0.006), a statistically significant difference was not observed for the primary endpoint at week 12 (CZP 400 mg: 44.4%; placebo: 35.6%; P = 0.278). However, a post hoc subgroup analysis of patients with an elevated baseline C-reactive protein (CRP) concentration (≥10 mg/L, n = 119) demonstrated a more pronounced treatment effect at week 12 (CZP 400 mg: 53.1%; placebo: 17.9%; P = 0.005).[38] The efficacy of CZP was subsequently evaluated as an induction and maintenance agent for CD in two large multicenter randomized controlled trials (PRECISE1 and 2). In PRECISE1, 662 adult patients with moderate-to-severe CD were stratified according to their baseline CRP concentrations and then randomized to receive 400 mg CZP or placebo at weeks 0, 2, 4, and then every 4 weeks for a total of 26 weeks. The co-primary endpoints were clinical response at week 6 alone and at weeks 6 and 26 combined. In patients with an elevated concentration of CRP at baseline, 37% of patients who received CZP had a clinical response at week 6 compared with 26% of those assigned to placebo (P = 0.04). Corresponding values of 22% and 12% were seen for the combined outcome of response at both weeks 6 and 26, respectively (P = 0.05). Rates of clinical remission did not significantly differ between the two groups (week 6: 17 vs 22%, P = 0.17 and week 6 and 22: 10% vs 14%, P = 0.07).[39] In PRECISE2, 425 adult patients with moderate-to-severe CD who had initially responded to open-label induction therapy with CZP at weeks 0, 2, and 4 were randomized to either receive 400 mg of CZP or placebo every 4 weeks for a total of 26 weeks. At week 26, 48% of week 6 responders who continued CZP therapy were in clinical remission (CDAI < 150) compared with 29% of those treated with placebo (P < 0.001).[40]
Summary and recommended approach
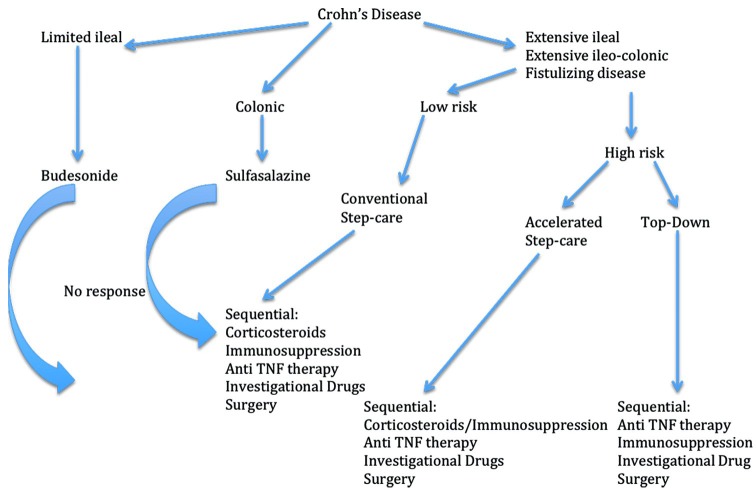
Patients with objective evidence of disease activity who do not respond to or who are dependent on corticosteroids and those at high risk for disease-related complications should be treated with a TNF-α antagonist in combination with an immunosuppressant. Alternatively, patients who demonstrate high-risk features may be considered for accelerated step-care or top–down therapy[41] [Figure 1].
Figure 1.
A therapeutic algorithm for Crohn's disease treatment
The efficacy of TNF antagonists in the treatment of UC
IFX, ADA, and golimumab (GOL) have been extensively studied as induction and maintenance agents for UC. Studies that evaluated these drugs are described in the following sections.
Infliximab
Two large multicenter placebo-controlled trials, the Active Ulcerative Colitis Trial 1 and 2 (ACT1 and ACT2) studies, evaluated 364 patients each with moderate to severely active UC. Patients were randomly assigned to receive 5 or 10 mg/kg of IFX or placebo at weeks 0, 2, 6, and every 8 weeks thereafter for 46 weeks. The primary endpoint was clinical response, defined as a decrease in the Mayo Clinic Score (MCS) of at least 3 points and a minimum total score reduction of at least 30%, with an accompanying decrease in the subscore for rectal bleeding of at least 1 point or an absolute rectal bleeding subscore of 0 or 1 measured at weeks 8 or 52. In ACT1, 69% of patients who received 5 mg/kg of IFX and 61% of those who received 10 mg/kg of IFX demonstrated a clinical response at week 8, compared with 37% of those who received placebo (P < 0.001 for both comparisons with placebo). Similarly, in the ACT2 trial, 64% of patients who received 5 mg/kg of IFX and 69% of those who received 10 mg/kg of IFX demonstrated a clinical response at week 8, as compared with 29% of those who received placebo (P < 0.001 for both comparisons with placebo). In both studies, patients who were treated with IFX were more likely to have a clinical response at week 30 (P ≤ 0.002 for all comparisons). In ACT1, more patients who received either dose of IFX had a clinical response at week 54 (45% and 44%, respectively) compared with those who received placebo (20%, P < 0.001 for both comparisons).[27] IFX was subsequently approved in multiple jurisdictions for the treatment of moderate to severely active UC in ambulatory patients.
Adalimumab
In 2012, the FDA-approved ADA for the induction and maintenance of remission in UC based on results from the Ulcerative Colitis Long-Term Remission and Maintenance with Adalimumab trials 1 and 2 (ULTRA1 and ULTRA2). These studies were similar in design to the ACT trials; however, in ULTRA2 approximately 40% of the participants had failed IFX therapy. In ULTRA1, which was exclusively an evaluation of induction therapy, 18.5% of the patients who received ADA 160/80 mg (P = 0.031 vs placebo) and 10.0% of those who received ADA 80/40 mg (P = 0.833 vs placebo) were in remission, compared with 9.2% of patients treated with placebo.[42] In the ULTRA2 study, 16.5% and 9.3% (P = 0.019) at week 8, and 17.3% and 8.5% (P = 0.004) at week 52, of patients treated with ADA and placebo, respectively, were in clinical remission.[43] ADA was subsequently approved in many jurisdictions for the treatment of moderate to severely active UC. No head-to-head trial has compared ADA with IFX; therefore the choice of therapy is dependent on patient, physician, and payer preferences. Patients with mild-to-moderate disease who fail to respond to firstline agents such as oral and rectal aminosalicylates, corticosteroids, or thiopurines, or those with corticosteroid dependence should also be considered for treatment with TNF-α antagonist. It is noteworthy that prolonged treatment with corticosteroids has been consistently linked with significant morbidity and early mortality [hazard ratio (HR) = 2.14, 95% CI = 1.55–2.95; P < 0.001].[44]
Golimumab
Data are available from two large multicenter randomized controlled trials (PURSUIT) that evaluated the effectiveness of GOL induction and maintenance therapy for moderate to severely active UC.[45,46] The PURSUIT SC trial, integrated a phase IIb dose-finding studies and a phase III maintenance study. The primary endpoint for the dose-finding trial was clinical response at week 6. GOL was superior to placebo for inducing clinical response (51.0% and 54.9% for patients receiving 200 mg/100 mg and 400 mg/200 mg of GOL, respectively, compared with 30.3% of patients treated with placebo (both comparisons, P ≤ 0.0001)). GOL-treated patients also had higher rates of clinical remission (17.8% for 200/100 mg GOL and 17.9% for 200/400 mg GOL vs 6.4% for placebo), endoscopic remission (42.3% for 200/100 mg GOL and 45.1% for 200/400 mg GOL vs 28.7% for placebo) and improvement in quality of life at week 6 compared with those that received placebo (P ≤ 0.0014, all comparisons). Additionally, in the maintenance component of PURSUIT, GOL-treated patients had higher rates of clinical response (47.0% of patients treated with 50 mg of GOL, 49.7% of patients treated with 100 mg GOL, and 31.2% of patients treated with placebo (P = 0.010 and P < 0.001, respectively) at week 54. Consistently higher rates of clinical remission and endoscopic remission were also seen at weeks 34 and 52 in patients who received GOL.
In summary, GOL is an effective alternative to either IFX or ADA depending on availability and patient preference. It is however noteworthy that no studies have evaluated GOL in patients with a previous history of failure to respond to other TNF-α antagonists.
Summary and recommended approach
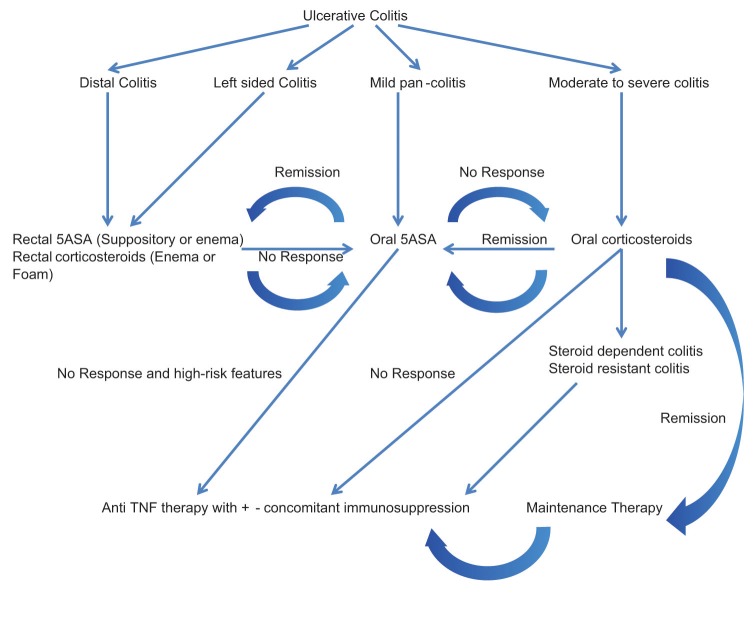
For UC patients presenting with high-risk features or those who are resistant/dependent on corticosteroids or who fail first line therapy with 5-ASA agents, a TNF-α antagonist in combination with an immunosuppressant is recommended.
A treatment algorithm for moderate to severely active UC, based on these principles is shown in Figure 2.
Figure 2.
A therapeutic algorithm for ulcerative colitis treatment
Challenges associated with TNF antagonist therapy for inflammatory bowel disease
Primary and secondary treatment failure
TNF-α antagonists are highly effective and safe for the induction and maintenance of remission for both UC and CD, and have primarily used in step-care; as “apex” agents.[47] However, approximately one third of patients fail to respond to induction therapy “primary failure.”[48] Although the relative prevalence of the specific mechanism responsible for primary treatment failure are currently unknown, multiple factors contribute to the problem including high rates of drug clearance that result in inadequate drug concentrations, immunogenicity with development of antidrug antibodies (ADAs), the dominance of inflammatory pathways driven by cytokines other than TNF-α, and the presence of alternate disease processes such as superimposed infection, bile salts diarrhea, and irritable bowel syndrome (IBS) are dominant causes.[49]
Secondary loss of response in patients who initially benefit from treatment is also an important clinical problem. Approximately, 40% of responders require dose intensification or lose response to drug over time.[50] The factors previously identified as causes of primary failure are also responsible for this problem. Based on these considerations, increased emphasis has been placed on the need to optimize TNF antagonist therapy and prevent treatment failure.
Strategies to prevent sensitization
Therapeutic monoclonal antibodies are foreign proteins that can elicit a host humoral immune response. Immunogenicity can result in treatment failure through multiple mechanisms. First antiidiotypic ADAs can block drugs from binding to TNF-α, which directly neutralizes biological activity. Second, drugs binding to other epitopes can result in the formation of immune complexes and enhanced clearance of drug through the reticuloendothelial system in the liver and spleen. Consequently, the pharmacokinetics of the antibody is affected leading to loss of efficacy. Finally, development of ADAs is associated with a higher incidence of adverse drug reactions and withdrawal from therapy.[4,51,52] Sensitization can be prevented by avoiding intermittent therapy, co-administration of an immunosuppressant such as AZA or MTX.[47,53] and theoretically, by maintenance of an adequate serum trough level.[54] Generally speaking, co-administration of an immunosuppressant reduces the risk of sensitization at one year, from low single digit rates to less than 5%.
As noted previously, a considerable heterogeneity among patients exits in the clearance of monoclonal antibodies. Although some of the factors responsible for this variability have been identified, the majority of determinants remain unknown.[55] Thus therapeutic drug monitoring through measurement of serum drug concentrations and ADAs has emerged as a potential opportunity for optimizing treatment success with TNF antagonists. Suboptimal drug concentrations have been consistently associated with low rates of treatment success in induction studies[54,56,57,58] and are commonly observed in patients with secondary loss of response. Although the traditional approach to manage secondary loss of response has been to either empirically decrease the dosing interval, increase the dose, or to switch agents,[50] this approach is not cost-effective and may not result in optimal efficacy.[59] Based on these studies, an algorithm has been developed for the use of therapeutic drug monitoring in patients with a secondary loss of response [Table 2]. In patients with low serum IFX trough levels (<3 μg/mL) and no ADAs, IFX dose escalation or interval reduction can be considered. Patients with an undetectable or low drug concentration and high concentration ADAs should be switched to another TNF antagonist because they have previously responded to a member of the class and generally speaking ADAs are not cross-reactive. Conversely, if adequate serum trough concentrations are detected, switching to an out of class medication such as vedolizumab is preferred.[24,60]
Table 2.
A guide to managing secondary loss of response to TNF-α antagonists

At present, TDM is recommended only for the management of secondary loss of response; however, several other potential applications exist that may prove useful when further data becomes available.
The role of combination therapy
Multiple theoretical reasons exist that support the concept that combinations of drugs might be more effective than monotherapy in IBD. These include the observation that concomitant immunosuppression increases serum concentrations of TNF antagonists, decreases the risk of sensitization, and overall potentiates their activity.[4]
One of the first studies to investigate the role of concomitant immunosuppression in IBD was conducted by the Groupe d’Etude Thérapeutique des Affections Inflammatoires du tube Digestif (GETAID), a French group that evaluated 113 patients with active CD, based on a CDAI score > 150, despite 6 months of treatment with corticosteroids. Patients were first categorized according to previous exposure to thiopurines (55 were actively receiving a thiopurine and 55 were thiopurine naïve) then randomized to IF × 5 mg/kg (n = 57) or placebo (n = 78) at weeks 0, 2, and 6. The total follow up was 52 weeks and the primary endpoint was corticosteroid-free remission at week 24 (CDAI < 150).[61] More patients treated with combination therapy (IFX plus a thiopurine) demonstrated corticosteroid-free remission at week 24 (57% vs 29%, P = 0.003), week 12 (75% vs 38%, P < 0.001), and week 52 (40% vs 22%, P = 0.04) compared with patients solely treated with thiopurine monotherapy. In another multicenter open-label trial conducted by D’Haens et al., 133 CD patients who were corticosteroid-, antimetabolite-, and biologic-naïve were randomized to early combined immunosuppression, that is, “top–down” therapy (n = 67, 5 mg/kg IFX at weeks 0, 2, and 6 plus AZA) or conventional treatment, that is, “step up” therapy (n = 66, corticosteroids sequentially followed by AZA and IFX if needed).[41] The co-primary study endpoints were corticosteroid-free and bowel resection-free remission at weeks 26 and 52. At week 26, 60% of patients receiving early combined immunosuppression were in clinical remission compared with 35.9% of patients in the conventional therapy group (P = 0.0062). After 52 weeks, 61.5% of patients in the early combined immunosuppression group were in clinical remission compared with 42.2% of patients in the conventional treatment group (P = 0.028). Adverse events were not substantially different in the two groups. Data from the Study of Biologic and Immunomodulator Naive Patients in Crohn's Disease (SONIC) trial showed that combination therapy is superior to monotherapy, with IFX alone or AZA alone. In this multicenter trial, 508 patients with biologic and immunosuppressant-naïve moderate-to-severe CD were randomized to receive AZA plus placebo, IFX plus placebo, or IFX and AZA in combination. IFX was given as 5 mg/kg IV at weeks 0, 2, 6, and then every 8 weeks and AZA was given as 2.5 mg/kg/day orally. More patients treated with AZA/IFX (56.8%) were in corticosteroid-free remission at week 26 than those treated with either agent alone (44% for IFX, P = 0.02, and 30% for AZA, P < 0.001).[62]
Given that combination therapy with MTX has consistently been shown to increase the efficacy of TNF antagonists in RA, Feagan et al. conducted a multicenter Canadian double blind placebo controlled trial that investigated the effectiveness of IFX in combination with MTX for treating corticosteroid-dependent CD.[63] In this study, patients with active disease were treated with infliximab induction and maintenance therapy in conjunction with prednisone. They were then randomized to either 25 mg of sc MTX or placebo. The primary endpoint of the trial, which was the time to treatment failure, defined as failure to achieve corticosteroid-free remission (CDAI less than 150) at week 14 or failure to subsequently maintain remission for an additional 50 weeks, did not differ between the MTX and placebo groups. At the end of 50 weeks of treatment, only 30% of patients in both the groups had failed therapy. However, MTX was protective against development of ADAs (20% vs 4%, P = 0.01) and the median serum IFX concentration was higher in patients receiving combination therapy (6.35 μg/mL compared with 3.75 μg/mL; P = 0.08). These observations are difficult to explain in that MTX therapy was associated with the former beneficial effects yet no difference in clinical efficacy was observed between the experimental groups. One possibility is that the results of the trial were confounded by the uniform application of corticosteroid therapy, which resulted in very high rates of treatment success. Future studies should further evaluate the role of combining corticosteroid and TNF antagonist induction therapy.
A recently published Canadian cluster randomized controlled trial compared conventional step-care (CSC) to an accelerated step-care (ASC) approach that featured the early use of combined immunosuppression. The primary endpoint was the proportion of patients in corticosteroid-free remission at 12 months. Although this outcome was not met, remission rates were 66% for ASC vs 61.9% for CSC, (P = 0.52). The time to occurrence of major adverse outcomes (HR = 0.73, 95% CI 0.62–0.86, P < 0.001), surgery (HR = 0.69, 95% CI 0.50–0.97, P = 0.03, ARR 2.9%, NNT 34.1), and disease-related complications (HR = 0.73, 95% CI 0.61–0.87, P < 0.001, ARR 6·5%, NNT 15.3) was reduced by this approach (REACT). No differences were observed in the rates of serious infection and mortality.[64]
The efficacy of combination therapy has also been evaluated in UC. In the UC-SUCCES trial, 239 TNF-αntagonist-naïve patients with moderate to severely active UC were randomly assigned to AZA, IFX, or combination therapy. At the end of 16 weeks of treatment, the proportions of patients in corticosteroid-free remission in the three groups were 23.7% (P = 0.032, compared with IFX/AZA), 22.1% (0.017, compared with IFX/AZA) and 39.7%.[65] Based on these data combined therapy is likely a more effective strategy for the treatment of UC than TNF antagonist monotherapy.
Special patient populations requiring anti TNF-α therapy
Fistulizing Crohn's disease
Fistulizing disease is high-risk phenotype of CD. No randomized studies have specifically evaluated the efficacy of combination therapy for the treatment of fistulizing disease. The best data regarding the efficacy of TNF antagonists comes from two RCTs that exclusively evaluated patients with fistulizing disease. Present et al. assigned 94 adult patients with fistulizing CD to receive 5 mg/kg of IFX, 10 mg/kg of IFX, or placebo at weeks 0, 2, and 6. The primary endpoint was a reduction of 50% or more from baseline in the number of draining fistulas observed at two or more consecutive study visits. At the end of 6 weeks of treatment more patients assigned to IFX experienced treatment success than those who received placebo (68% for the 5 mg/kg group, 56% for the 10 mg/kg group, and 26% for placebo, P = 0.002 and P = 0.02, respectively).[66] Subsequently, IFX maintenance therapy was evaluated in the ACCENT2 trial. Three hundred and six patients with at least one draining abdominal or perineal fistula were included. Reduced fistula drainage was seen in 68% of patients treated with IFX 5 mg/kg, in 56% of patients treated with IFX 10 mg/kg and 26% of those who received placebo (P = 0.002 and 0.02, respectively). Complete closure of fistula openings was observed in 55% of the IFX 5 mg/kg group and 38% of the IFX 10 mg/kg group, versus13% of the placebo group (P = 0.001 and 0.04, respectively).[67]
Data regarding the efficacy of ADA for the treatment of fistulizing disease are limited to a post-hoc analysis of the CHARM trial, in which following induction with an 80 mg\40 mg regimen, patients who responded were randomized to ADA 40 mg eow or ADA 40 mg weekly for 56 weeks. In the post hoc analysis, the percentage of patients with fistula closure at week 26 who continued to have fistula closure at week 56 was higher in patients treated with ADA than in those treated with placebo (30% and 13% for combined ADA groups and placebo group, respectively, at week 26, P = 0.043; and 33% and 13% for combined ADA groups and placebo group, respectively, at week 56, P = 0.016).[68]
Patients with fistulizing disease are at high risk for disease-related complications. Therefore, management of these patients requires close collaboration between gastroenterologists and surgeons. Combination therapy with TNF antagonist and an immunosuppressant is the mainstay of medical treatment. Surgical management is based on provision of adequate drainage. Placement of setons is frequently used for this purpose. Diversion of the fecal stream may be necessary in refractory cases.
Postoperative therapy for CD
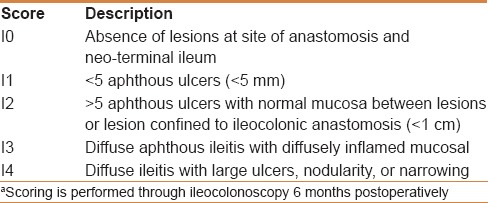
Recurrence of CD after ileal resection is a common and important clinical problem. The Rutgeert's score [Table 3] has been used to assess the risk of symptomatic recurrence based on the endoscopic appearance of the neo-terminal ileum approximately 6 months after a resection. This score classifies patients into grades 0–i4 depending on the number and severity of ulcers in the neoterminal ileum, the state of mucosa between ulcers and the presence or absence of stenosis.
Table 3.
The Rutgeert's scoring systema for postoperative risk stratification of Crohn's disease
In a small trial by Regueiro et al., 24 CD patients were randomized to receive 5 mg/kg of IFX or placebo within 4 weeks of an ileocolonic resection with the primary outcome of endoscopic recurrence after one year of follow up. A significantly lower proportion of patients treated with IFX developed endoscopic recurrence compared with placebo-treated patients (9.1% vs 84.6%, P = 0.0006).[69] More recently, a multicenter Australian trial, the Post-Operative Crohn's Endoscopic Recurrence (POCER) study, evaluated different postsurgical medical regimens in a “treat to target” approach targeting mucosal healing as the primary endpoint after 18 months of follow-up based on a Rutgeert's score of 0 or i1. Patients were classified into high risk (active smoker, perforating disease, ≥2 previous bowel resections) or low-risk patients for endoscopic recurrence. All patients received metronidazole for 3 months and high-risk patients received a thiopurine, if tolerated, or ADA if not. Patients were then randomized to undergo an endoscopic evaluation of the anastomosis line, 6 months postoperatively or no endoscopic evaluation. Escalation of therapy was then based on the 6 months evaluation such that low-risk patients received AZT and high-risk patients received more frequent doses of ADA if previously on ADA or ADA every 2 weeks if previously of AZT, if endoscopic recurrence was found based on a Rutgeert's score >i1. Escalation of therapy occurred in 39% of patients surveyed 6 months postoperatively and 18-months endoscopic recurrence occurred in 49% of surveyed versus 67% of unsurveyed patients (P = 0.028).[70]
In our opinion, patients at a high risk of early recurrence of CD postoperatively (cigarette smoking, penetrating disease, or Rutgeert's score of i2 or higher 6 months following surgery) should be treated with a combination of a TNF-α antagonist and an immunosuppressant to prevent clinical recurrence.
Treatment of severe ulcerative colitis
Approximately 40% of patients with severe UC fail to respond to intravenous corticosteroid therapy.[26,71,72] The most widely acceptable definition of severe UC is based on the Truelove and Witts criteria[73] of an attack characterized by more than six bloody stools per day and one or more of the following findings: Temperature >37.8°C; pulse rate >90/min; hemoglobin <10.5 g/dL; or erythrocyte sedimentation rate >30 mm/h. Treatment of these patients prior to the availability of IFX consisted of intravenous cyclosporine or colectomy. Based on the success of IFX for the management of ambulatory patients with UC, Jarnerot et al. evaluated the efficacy and safety of IFX therapy in patients with severe disease who had failed treatment with parenteral corticosteroids. Forty-five patients were randomly assigned to receive IFX or placebo on day 4 or on day 6–8. The primary endpoint was colectomy or death at 3 months post randomization. A 7/24 patients in the IFX group and 14/21 patients in the placebo group required a colectomy (P = 0.017; OR = 4.9; 95% CI 1.4–17).[74] A more recent study by Laharie et al., compared IFX with cyclosporin in an open-label randomized controlled trial. Clinical response at day 7 was observed in 60% of patients treated with cyclosporin versus 54% of those treated with IFX (absolute risk difference 6%; 95% CI − 7 to 19; P = 0.52) indicating that cyclosporine is not markedly superior to IFX.[75]
Given the favorable safety profile most clinicians now opt for IFX therapy in their practice. Use of IFX in this patient population is associated with very high rates of drug clearance. Aggressive dose intensification may be required to manage this problem.[55]
Safety of TNF antagonists
The occurrence of serious infection is the primary safety concern for this class of drugs.[76] Although it is widely perceived that opportunistic pathogens such as tuberculosis (TB), histoplasmosis and pneumocystis are the most common pathogens encountered, this impression is incorrect. Bacterial pneumonia and intra-abdominal sepsis in patients with fistulizing CD are the most common serious infectious complications. The former usually occurs in older patients with multiple medical problems. The risk of infectious complications can be reduced by screening for latent TB and chronic viral hepatitis, blood testing for antimetabolite-related neutropenia, and immunization against pneumonia causing organisms (influenza and pneumococcal vaccine). The presence of pelvic sepsis should be ruled out by MRI or examination under anesthesia before TNF-α antagonists are initiated in patients with fistulizing CD.[77]
Although it is widely believed by patients and many physicians that TNF antagonist therapy is associated with an increased risk of cancer, epidemiologic data do not support this viewpoint. Strong evidence shows no increased risk of the common solid organ cancers. Furthermore, the risk of non-melanoma skin cancer (NMSC), a “sentinel” malignancy for immunosuppression-related cancer risk, is not elevated. A modest increase in the risk of lymphoma has not been definitively excluded but no compelling evidence supports such an association. Data from a recent study suggesting an increased risk of melanoma has not been observed in other studies. In contrast, epidemiologic data indicate an increased risk of NMSC and lymphoma in patients treated with AZA. Young males are at higher risk for hepatosplenic T-cell lymphoma (HSTCL), a rare condition that has been mainly reported in patients treated with thiopurines-based combination therapy.[78] These associations have not been seen with MTX therapy.
Other uncommon serious complications associated with TNF antagonist therapy include demyelinating diseases, drug-induced lupus, and worsening of congestive heart failure. A common problem is development of psoriaform lesions, which occurs in up to 15% of patients receiving TNF antagonists and typically regresses with discontinuation of therapy.[79]
Pregnancy
TNF-α antagonists have been used in pregnancy with favorable results and are classified by the US FDA as category B drugs.[80,81] However, dosing adjustment should be attempted to minimize drug exposure in the third trimester because transplacental transfer of antibodies from the maternal to the fetal circulation occurs at that time. Fetal catabolism of monoclonal antibodies is less efficient in neonates and a TNF antagonist can be expected to persist in the fetal serum to approximately 6 months of age.[80,81,82,83] Cases of disseminated disease have occurred following administration of BCG vaccine.[84,85] No live viral or bacterial vaccines should be administered to these children during the first 6 months of life. This policy precludes Rotavirus immunization, which is a live attenuated vaccine that is usually given at 3 months of age.[84,85,86,87] CTZ pegol is a Fab and therefore lacks the Fc component of the antibody, which is responsible for transplacental transfer by active transit. Accordingly, fetal exposure to CZP only results from passive transfer, and drug concentrations in neonates are markedly lower than in infants exposed in utero to either IFX or ADA.[88]
Special considerations in KSA
The risk of reactivation of latent tuberculosis
The increased risk of tuberculosis with TNF antagonist therapy as a result of re-activation of latent disease is an important concern in KSA.[89] In 2013, the incidence rate was 5447 cases/year with an annual death rate of 894.[90] Clinicians prescribing TNF antagonists should be aware of several key points about this problem. First, although careful evaluation for the presence of latent TB infection should always be undertaken by taking an exposure history, performing a chest radiography examination, and ordering a gamma-interferon release assay, clinicians should be aware that false-negative tests can occur.[91,92] Thus a high index of suspicion is needed. Second, extrapulmonic disease is common and difficult to diagnose. Third, re-activation often occurs within the first 6 months of treatment notwithstanding that patients residing in a hyperendemic area may acquire a primary infection at any time. Accordingly, annual testing is recommended for patients receiving long-term therapy. Finally, INH treatment will be required in many circumstances and gastroenterologists need to be aware of the risk factors for drug-induced hepatitis and monitor patients for this complication.[93] Although it is desirable to delay initiation of TNF antagonist therapy for as long as possible in a patient who requires treatment with INH, in many cases the patient's disease severity will make this impossible. Consultation with an experienced infectious disease consultant is valuable in managing these patients.[94]
Sun exposure and the risk of skin cancer
Patients with IBD who are treated with thiopurines are at an increased risk of developing non-melanoma skin cancer (basal cell and squamous cell carcinoma).[95,96,97,98,99] Prescribing thiopurines to patients with a history of recurrent non-melanoma skin cancer should be avoided. Additionally, avoidance of prolonged sun exposure, proper sun screening, and frequent skin examinations for surveillance in patients treated with thiopurines is required. Discontinuation of thiopurines should be considered in patients who develop NMSC.
CONCLUSIONS
TNF antagonists are effective and relatively safe agents for the treatment of moderate to severely active CD and UC. Combination therapy with either AZA or MTX is a preferred strategy for most patients. Patients at high risk for disease-related complications should receive early treatment. The risk of reactivation of latent TB with TNF-α antagonists is an important concern to the Saudi population of IBD patients given the high prevalence of the disease in KSA. Vigilant screening for latent TB is a critical component of patient management. Therapeutic drug monitoring is an effective strategy that delivers both economic and practical benefit to clinical practice.
RECOMMENDATIONS
TNF-α antagonists IFX, ADA, and CZP can be used to treat CD as first line therapy in patients with severe disease, high-risk features, fistulizing disease, and following failure of conventional therapies. Step-care, accelerated step-care, and top–down care are all acceptable approaches
IFX, ADA, and GOL are treatment options for UC patients with moderate to severely active disease, steroid-dependent disease, or history of failure to conventional therapy
IFX can be used as a rescue therapy for severe UC requiring hospitalization and postoperatively for the prevention of CD recurrence
TNF-α antagonists can be used as monotherapy or more preferably in combination with an immunosuppressant to reduce the risk of drug sensitization and secondary failure of treatment
Patients being initiated on anti-TNF-α therapy should be counseled on the potential side effects associated with this class of drugs and adequately screened for chronic hepatitis and latent pulmonary tuberculosis prior to receiving therapy
Careful attention should be directed toward receiving vaccinations against seasonal viral influenza and bacterial pneumonia prior to and annually and every five years after initiation of therapy, respectively
TNF antagonist should be administered according to scheduled dosing and not intermittently. Dose escalation should be guided by serum drug concentrations where possible
Gastroenterologists and colorectal surgeons should collaborate in the management of fistulizing disease. Imaging and EUA should be used to guide treatment of their patients. Antibiotics, seton placement, fistulotomy, and surgical drainage are used for active abscesses. Anti-TNF-α therapy should be started only after abscesses are eradicated
Anti TNF-α agents can be given during pregnancy but infants born to mothers treated with anti-TNF-α agents should not receive live attenuated vaccines during the first six months of life
Careful screening for latent pulmonary and extra pulmonary TB is required prior to initiation of anti-TNF-α therapy. In the presence of a positive PPD skin test, interferon-gamma release assay or changes consistent with possible pulmonary TB on chest radiography, consultation with infectious disease specialist is recommended
Sun exposure should be limited in patients treated with combination therapy due to the increased risk of NMSC. Sunscreen should be used judiciously in this patient population especially in patients above age 60 years. Frequent skin examination for surveillance of NMSC is also recommended.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Baumgart DC, Sandborn WJ. Crohn's disease. Lancet. 2012;380:1590–605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 2.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713–25. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 3.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 4.Baert F, Noman M, Vermeire S, Van Assche G, D’ Haens G, Carbonez A, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med. 2003;348:601–8. doi: 10.1056/NEJMoa020888. [DOI] [PubMed] [Google Scholar]
- 5.Bewtra M, Kaiser LM, TenHave T, Lewis JD. Crohn's disease and ulcerative colitis are associated with elevated standardized mortality ratios: A meta-analysis. Inflamm Bowel Dis. 2013;19:599–613. doi: 10.1097/MIB.0b013e31827f27ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ananthakrishnan AN, Cagan A, Gainer VS, Cheng SC, Cai T, Szolovits P, et al. Mortality and extraintestinal cancers in patients with primary sclerosing cholangitis and inflammatory bowel disease. J Crohns Colitis. 2014;8:956–63. doi: 10.1016/j.crohns.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baars JE, Kuipers EJ, van Haastert M, Nicolaï JJ, Poen AC, van der Woude CJ. Age at diagnosis of inflammatory bowel disease influences early development of colorectal cancer in inflammatory bowel disease patients: A nationwide, long-term survey. J Gastroenterol. 2012;47:1308–22. doi: 10.1007/s00535-012-0603-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN. Ulcerative colitis and Crohn's disease: A comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35:1590–2. doi: 10.1136/gut.35.11.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: A meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10:639–45. doi: 10.1016/j.cgh.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Debruyn JC, Soon IS, Hubbard J, Wrobel I, Panaccione R, Kaplan GG. Nationwide temporal trends in incidence of hospitalization and surgical intestinal resection in pediatric inflammatory bowel diseases in the United States from 1997 to 2009. Inflamm Bowel Dis. 2013;19:2423–32. doi: 10.1097/MIB.0b013e3182a56148. [DOI] [PubMed] [Google Scholar]
- 11.Hope B, Shahdadpuri R, Dunne C, Broderick AM, Grant T, Hamzawi M, et al. Rapid rise in incidence of Irish paediatric inflammatory bowel disease. Arch Dis Child. 2012;97:590–4. doi: 10.1136/archdischild-2011-300651. [DOI] [PubMed] [Google Scholar]
- 12.Martín-de-Carpi J, Rodríguez A, Ramos E, Jiménez S, Martínez-Gómez MJ, Medina E. SPIRIT-IBD Working Group of Sociedad Española de Gastroenterología, Hepatología y Nutricion Pediátrica. Increasing incidence of pediatric inflammatory bowel disease in Spain (1996-2009): The SPIRIT Registry. Inflamm Bowel Dis. 2013;19:73–80. doi: 10.1002/ibd.22980. [DOI] [PubMed] [Google Scholar]
- 13.Vind I, Riis L, Jess T, Knudsen E, Pedersen N, Elkjaer M, et al. DCCD study group. Increasing incidences of inflammatory bowel disease and decreasing surgery rates in Copenhagen City and County, 2003-2005: A population-based study from the Danish Crohn colitis database. Am J Gastroenterol. 2006;101:1274–82. doi: 10.1111/j.1572-0241.2006.00552.x. [DOI] [PubMed] [Google Scholar]
- 14.Zheng JJ, Zhu XS, Huangfu Z, Shi XH, Guo ZR. Prevalence and incidence rates of Crohn's disease in mainland China: A meta-analysis of 55 years of research. J Dig Dis. 2010;11:161–6. doi: 10.1111/j.1751-2980.2010.00431.x. [DOI] [PubMed] [Google Scholar]
- 15.Benchimol EI, Manuel DG, Guttmann A, Nguyen GC, Mojaverian N, Quach P, et al. Changing age demographics of inflammatory bowel disease in Ontario, Canada: A population-based cohort study of epidemiology trends. Inflamm Bowel Dis. 2014;20:1761–9. doi: 10.1097/MIB.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 16.Al-Mofarreh MA, Al-Mofleh IA. Emerging inflammatory bowel disease in saudi outpatients: A report of 693 cases. Saudi J Gastroenterol. 2013;19:16–22. doi: 10.4103/1319-3767.105915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aljebreen AM, Alharbi OR, Azzam NA, Almalki AS, Alswat KA, Almadi MA. Clinical epidemiology and phenotypic characteristics of Crohn's disease in the central region of Saudi Arabia. Saudi J Gastroenterol. 2014;20:162–9. doi: 10.4103/1319-3767.132993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alharbi OR, Azzam NA, Almalki AS, Almadi MA, Alswat KA, Sadaf N, et al. Clinical epidemiology of ulcerative colitis in Arabs based on the Montreal classification. World J Gastroenterol. 2014;20:17525–31. doi: 10.3748/wjg.v20.i46.17525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castiglione F, Diaferia M, Morace F, Labianca O, Meucci C, Cuomo A, et al. Risk factors for inflammatory bowel diseases according to the “hygiene hypothesis”: A case-control, multi-centre, prospective study in Southern Italy. J Crohns Colitis. 2012;6:324–9. doi: 10.1016/j.crohns.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Soon IS, Molodecky NA, Rabi DM, Ghali WA, Barkema HW, Kaplan GG. The relationship between urban environment and the inflammatory bowel diseases: A systematic review and meta-analysis. BMC Gastroenterol. 2012;12:51. doi: 10.1186/1471-230X-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michail S, Durbin M, Turner D, Griffiths AM, Mack DR, Hyams J, et al. Alterations in the gut microbiome of children with severe ulcerative colitis. Inflamm Bowel Dis. 2012;18:1799–808. doi: 10.1002/ibd.22860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rocchi A, Benchimol EI, Bernstein CN, Bitton A, Feagan B, Panaccione R, et al. Inflammatory bowel disease: A Canadian burden of illness review. Can J Gastroenterol. 2012;26:811–7. doi: 10.1155/2012/984575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vermeire S, van Assche G, Rutgeerts P. Review article: Altering the natural history of Crohn's disease--evidence for and against current therapies. Aliment Pharmacol Ther. 2007;25:3–12. doi: 10.1111/j.1365-2036.2006.03134.x. [DOI] [PubMed] [Google Scholar]
- 24.Mosli MH, Sandborn WJ, Kim RB, Khanna R, Al-Judaibi B, Feagan BG. Toward a personalized medicine approach to the management of inflammatory bowel disease. Am J Gastroenterol. 2014;109:994–1004. doi: 10.1038/ajg.2014.110. [DOI] [PubMed] [Google Scholar]
- 25.Levesque BG, Sandborn WJ, Ruel J, Feagan BG, Sands BE, Colombel JF. Converging goals of treatment of inflammatory bowel disease from clinical trials and practice. Gastroenterology. 2015;148:37–51.e1. doi: 10.1053/j.gastro.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Travis SP, Farrant JM, Ricketts C, Nolan DJ, Mortensen NM, Kettlewell MG, et al. Predicting outcome in severe ulcerative colitis. Gut. 1996;38:905–10. doi: 10.1136/gut.38.6.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–76. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 28.Best WR, Becktel JM, Singleton JW, Kern F., Jr Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439–44. [PubMed] [Google Scholar]
- 29.Talley NJ, Abreu MT, Achkar JP, Bernstein CN, Dubinsky MC, Hanauer SB, et al. American College of Gastroenterology IBD Task Force. An evidence-based systematic review on medical therapies for inflammatory bowel disease. Am J Gastroenterol. 2011;106(Suppl 1):S2–26. doi: 10.1038/ajg.2011.58. [DOI] [PubMed] [Google Scholar]
- 30.Chande N, Wang Y, MacDonald JK, McDonald JW. Methotrexate for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2014;8:CD006618. doi: 10.1002/14651858.CD006618.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel V, Wang Y, MacDonald JK, McDonald JW, Chande N. Methotrexate for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2014;8:CD006884. doi: 10.1002/14651858.CD006884.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ford AC, Sandborn WJ, Khan KJ, Hanauer SB, Talley NJ, Moayyedi P. Efficacy of biological therapies in inflammatory bowel disease: Systematic review and meta-analysis. Am J Gastroenterol. 2011;106:644–59. doi: 10.1038/ajg.2011.73. quiz 660. [DOI] [PubMed] [Google Scholar]
- 33.Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's Disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997;337:1029–35. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 34.Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. ACCENT I Study Group. Maintenance infliximab for Crohn's disease: The ACCENT I randomised trial. Lancet. 2002;359:1541–9. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 35.Sandborn WJ, Hanauer S, Loftus EV, Jr, Tremaine WJ, Kane S, Cohen R, et al. An open-label study of the human anti-TNF monoclonal antibody adalimumab in subjects with prior loss of response or intolerance to infliximab for Crohn's disease. Am J Gastroenterol. 2004;99:1984–9. doi: 10.1111/j.1572-0241.2004.40462.x. [DOI] [PubMed] [Google Scholar]
- 36.Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: The CLASSIC-I trial. Gastroenterology. 2006;130:323–33. doi: 10.1053/j.gastro.2005.11.030. quiz 591. [DOI] [PubMed] [Google Scholar]
- 37.Schreiber S, Sandborn WJ. CLASSIC-I study the efficacy of adalimumab. Gastroenterology. 2006;130:1929–30. doi: 10.1053/j.gastro.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 38.Schreiber S, Rutgeerts P, Fedorak RN, Khaliq-Kareemi M, Kamm MA, Boivin M, et al. A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn's disease. Gastroenterology. 2005;129:807–18. doi: 10.1053/j.gastro.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 39.Sandborn WJ, Feagan BG, Stoinov S, Honiball PJ, Rutgeerts P, Mason D, et al. PRECISE 1 Study Investigators. Certolizumab pegol for the treatment of Crohn's disease. N Engl J Med. 2007;357:228–38. doi: 10.1056/NEJMoa067594. [DOI] [PubMed] [Google Scholar]
- 40.Schreiber S, Khaliq-Kareemi M, Lawrance IC, Thomsen OØ, Hanauer SB, McColm J, et al. PRECISE 12 Study Investigators. Maintenance therapy with certolizumab pegol for Crohn's disease. N Engl J Med. 2007;357:239–250. doi: 10.1056/NEJMoa062897. [DOI] [PubMed] [Google Scholar]
- 41.D’Haens G, Baert F, van Assche G, Caenepeel P, Vergauwe P, Tuynman H, et al. Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: An open randomised trial. Lancet. 2008;371:660–7. doi: 10.1016/S0140-6736(08)60304-9. [DOI] [PubMed] [Google Scholar]
- 42.Reinisch W, Sandborn WJ, Hommes DW, D’Haens G, Hanauer S, Schreiber S, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: Results of a randomised controlled trial. Gut. 2011;60:780–7. doi: 10.1136/gut.2010.221127. [DOI] [PubMed] [Google Scholar]
- 43.Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D’Haens G, Wolf DC, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257–65.e1-3. doi: 10.1053/j.gastro.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 44.Lichtenstein GR, Feagan BG, Cohen RD, Salzberg BA, Diamond RH, Price S, et al. Serious infection and mortality in patients with Crohn's disease: More than 5 years of follow-up in the TREATTM registry. Am J Gastroenterol. 2012;107:1409–22. doi: 10.1038/ajg.2012.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. PURSUIT-SC Study Group. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:85. doi: 10.1053/j.gastro.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 46.Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. PURSUIT-Maintenance Study Group. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:96–109.e1. doi: 10.1053/j.gastro.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Akobeng AA, Sandborn WJ, Bickston SJ, Chande N, Shackelton LM, Nelson S, et al. Tumor necrosis factor-alpha antagonists twenty years later: What do Cochrane reviews tell us? Inflamm Bowel Dis. 2014;20:2132–41. doi: 10.1097/MIB.0000000000000218. [DOI] [PubMed] [Google Scholar]
- 48.Papamichael K, Gils A, Rutgeerts P, Levesque BG, Vermeire S, Sandborn WJ, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: Evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis. 2015;21:182–97. doi: 10.1097/MIB.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 49.D’Haens GR, Panaccione R, Higgins PD, Vermeire S, Gassull M, Chowers Y, et al. The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn's and Colitis Organization: When to start, when to stop, which drug to choose, and how to predict response? Am J Gastroenterol. 2011;106:199–213. doi: 10.1038/ajg.2010.392. [DOI] [PubMed] [Google Scholar]
- 50.Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn's disease: A review. Am J Gastroenterol. 2009;104:760–7. doi: 10.1038/ajg.2008.88. [DOI] [PubMed] [Google Scholar]
- 51.Lee LY, Sanderson JD, Irving PM. Anti-infliximab antibodies in inflammatory bowel disease: Prevalence, infusion reactions, immunosuppression and response, a meta-analysis. Eur J Gastroenterol Hepatol. 2012;24:1078–85. doi: 10.1097/MEG.0b013e32835558cf. [DOI] [PubMed] [Google Scholar]
- 52.Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): A meta-analysis. Am J Gastroenterol. 2013;108:40–8. doi: 10.1038/ajg.2012.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rutgeerts P, Diamond RH, Bala M, Olson A, Lichtenstein GR, Bao W, et al. Scheduled maintenance treatment with infliximab is superior to episodic treatment for the healing of mucosal ulceration associated with Crohn's disease. Gastrointest Endosc. 2006;63:433–42. doi: 10.1016/j.gie.2005.08.011. quiz 464. [DOI] [PubMed] [Google Scholar]
- 54.Afif W, Loftus EV, Jr, Faubion WA, Kane SV, Bruining DH, Hanson KA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol. 2010;105:1133–9. doi: 10.1038/ajg.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ordás I, Feagan BG, Sandborn WJ. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2012;10:1079. doi: 10.1016/j.cgh.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 56.Karmiris K, Paintaud G, Noman M, Magdelaine-Beuzelin C, Ferrante M, Degenne D, et al. Influence of trough serum levels and immunogenicity on long-term outcome of adalimumab therapy in Crohn's disease. Gastroenterology. 2009;137:1628–40. doi: 10.1053/j.gastro.2009.07.062. [DOI] [PubMed] [Google Scholar]
- 57.Maser EA, Villela R, Silverberg MS, Greenberg GR. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn's disease. Clin Gastroenterol Hepatol. 2006;4:1248–54. doi: 10.1016/j.cgh.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 58.Seow CH, Newman A, Irwin SP, Steinhart AH, Silverberg MS, Greenberg GR. Trough serum infliximab: A predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2010;59:49–54. doi: 10.1136/gut.2009.183095. [DOI] [PubMed] [Google Scholar]
- 59.Velayos FS, Kahn JG, Sandborn WJ, Feagan BG. A test-based strategy is more cost effective than empiric dose escalation for patients with Crohn's disease who lose responsiveness to infliximab. Clin Gastroenterol Hepatol. 2013;11:654–66. doi: 10.1016/j.cgh.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 60.Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, et al. GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. doi: 10.1056/NEJMoa1215734. [DOI] [PubMed] [Google Scholar]
- 61.Lémann M, Mary JY, Duclos B, Veyrac M, Dupas JL, Delchier JC, et al. Groupe d’Etude Therapeutique des Affections Inflammatoires du Tube Digestif (GETAID). Infliximab plus azathioprine for steroid-dependent Crohn's disease patients: A randomized placebo-controlled trial. Gastroenterology. 2006;130:1054–61. doi: 10.1053/j.gastro.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 62.Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, et al. SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383–95. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 63.Feagan BG, McDonald JW, Panaccione R, Enns RA, Bernstein CN, Ponich TP, et al. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn's disease. Gastroenterology. 2014;146:681–8.e1. doi: 10.1053/j.gastro.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 64.Khanna R, Levesque BG, Bressler B, Zou G, Stitt L, Greenberg GR, et al. Early combined immunosuppression for the management of Crohn's disease: A community-based cluster randomized trial. Lancet. 2015 doi: 10.1016/S0140-6736(15)00068-9. In press. [DOI] [PubMed] [Google Scholar]
- 65.Panaccione R, Ghosh S, Middleton S, Márquez JR, Scott BB, Flint L, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146:392–400.e3. doi: 10.1053/j.gastro.2013.10.052. [DOI] [PubMed] [Google Scholar]
- 66.Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398–405. doi: 10.1056/NEJM199905063401804. [DOI] [PubMed] [Google Scholar]
- 67.Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350:876–85. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- 68.Colombel JF. The CHARM trial of adalimumab in Crohn's disease. Gastroenterol Hepatol. 2006;2:486–8. [PMC free article] [PubMed] [Google Scholar]
- 69.Regueiro M, Schraut W, Baidoo L, Kip KE, Sepulveda AR, Pesci M, et al. Infliximab prevents Crohn's disease recurrence after ileal resection. Gastroenterology. 2009;136:441–50.e1. doi: 10.1053/j.gastro.2008.10.051. quiz 716. [DOI] [PubMed] [Google Scholar]
- 70.Kamm MA, De Cruz PP, Wright EK, Hamilton AL, Ritchie KJ, Krejany EO, et al. Optimising post-operative Crohn's disease management: Best drug therapy alone versus endoscopic monitoring, disease evolution, and faecal calprotectin monitoring. The POCER study. J Crohns Colitis. 2014;8(Suppl 1):S13. [Google Scholar]
- 71.Baron JH, Connell AM, Kanaghinis TG, Lennard-Jones JE, Jones AF. Out-patient treatment of ulcerative colitis. Comparison between three doses of oral prednisone. Br Med J. 1962;2:441–3. doi: 10.1136/bmj.2.5302.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2:1041–8. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Truelove SC, Witts LJ. Cortisone in ulcerative colitis; preliminary report on a therapeutic trial. Br Med J. 1954;2:375–8. doi: 10.1136/bmj.2.4884.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Järnerot G, Hertervig E, Friis-Liby I, Blomquist L, Karlén P, Grännö C, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: A randomized, placebo-controlled study. Gastroenterology. 2005;128:1805–11. doi: 10.1053/j.gastro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 75.Laharie D, Bourreille A, Branche J, Allez M, Bouhnik Y, Filippi J, et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: A parallel, open-label randomised controlled trial. Lancet. 2012;380:1909–15. doi: 10.1016/S0140-6736(12)61084-8. [DOI] [PubMed] [Google Scholar]
- 76.Zhang D, Xiong B, Li X, Xu T, Yu M. Meta-analysis: Serious adverse events in Crohn's disease patients treated with TNF-αlpha inhibitors. Hepatogastroenterology. 2013;60:1333–42. doi: 10.5754/hge121057. [DOI] [PubMed] [Google Scholar]
- 77.Chebli JM, Gaburri PD, Chebli LA, da Rocha Ribeiro TC, Pinto AL, Ambrogini Júnior O, et al. A guide to prepare patients with inflammatory bowel diseases for anti-TNF-α therapy. Med Sci Monit. 2014;20:487–98. doi: 10.12659/MSM.890331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Deepak P, Sifuentes H, Sherid M, Stobaugh D, Sadozai Y, Ehrenpreis ED. T-cell non-Hodgkin's lymphomas reported to the FDA AERS with tumor necrosis factor-alpha (TNF-α) inhibitors: Results of the REFURBISH study. Am J Gastroenterol. 2013;108:99–105. doi: 10.1038/ajg.2012.334. [DOI] [PubMed] [Google Scholar]
- 79.Kip KE, Swoger JM, Grandinetti LM, Barrie AM, 3rd, Greer JB, Regueiro MD. Tumor necrosis factor α antagonist-associated psoriasis in inflammatory diseases: An analysis of the FDA adverse event reporting system. Inflamm Bowel Dis. 2013;19:1164–72. doi: 10.1097/MIB.0b013e31828075bd. [DOI] [PubMed] [Google Scholar]
- 80.Bortlik M, Machkova N, Duricova D, Malickova K, Hrdlicka L, Lukas M, et al. Pregnancy and newborn outcome of mothers with inflammatory bowel diseases exposed to anti-TNF-α therapy during pregnancy: Three-center study. Scand J Gastroenterol. 2013;48:951–8. doi: 10.3109/00365521.2013.812141. [DOI] [PubMed] [Google Scholar]
- 81.Mahadevan U, Kane S, Sandborn WJ, Cohen RD, Hanson K, Terdiman JP, et al. Intentional infliximab use during pregnancy for induction or maintenance of remission in Crohn's disease. Aliment Pharmacol Ther. 2005;21:733–8. doi: 10.1111/j.1365-2036.2005.02405.x. [DOI] [PubMed] [Google Scholar]
- 82.Molnár T, Farkas K, Nagy F, Lakatos PL, Miheller P, Nyári T, et al. Pregnancy outcome in patients with inflammatory bowel disease according to the activity of the disease and the medical treatment: A case-control study. Scand J Gastroenterol. 2010;45:1302–6. doi: 10.3109/00365521.2010.503967. [DOI] [PubMed] [Google Scholar]
- 83.Zelinkova Z, de Haar C, de Ridder L, Pierik MJ, Kuipers EJ, Peppelenbosch MP, et al. High intra-uterine exposure to infliximab following maternal anti-TNF treatment during pregnancy. Aliment Pharmacol Ther. 2011;33:1053–8. doi: 10.1111/j.1365-2036.2011.04617.x. [DOI] [PubMed] [Google Scholar]
- 84.Cheent K, Nolan J, Shariq S, Kiho L, Pal A, Arnold J. Case Report: Fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn's disease. J Crohns Colitis. 2010;4:603–5. doi: 10.1016/j.crohns.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 85.Heller MM, Wu JJ, Murase JE. Fatal case of disseminated BCG infection after vaccination of an infant with in utero exposure to infliximab. J Am Acad Dermatol. 2011;65:870. doi: 10.1016/j.jaad.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 86.Khan N, Asim H, Lichtenstein GR. Safety of anti-TNF therapy in inflammatory bowel disease during pregnancy. Expert Opin Drug Saf. 2014;13:1699–708. doi: 10.1517/14740338.2014.973399. [DOI] [PubMed] [Google Scholar]
- 87.Mahadevan U, Cucchiara S, Hyams JS, Steinwurz F, Nuti F, Travis SP, et al. The London Position Statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn's and Colitis Organisation: Pregnancy and pediatrics. Am J Gastroenterol. 2011;106:214–24. doi: 10.1038/ajg.2010.464. [DOI] [PubMed] [Google Scholar]
- 88.Marchioni RM, Lichtenstein GR. Tumor necrosis factor-α inhibitor therapy and fetal risk: A systematic literature review. World J Gastroenterol. 2013;19:2591–602. doi: 10.3748/wjg.v19.i17.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Solovic I, Sester M, Gomez-Reino JJ, Rieder HL, Ehlers S, Milburn HJ, et al. The risk of tuberculosis related to tumour necrosis factor antagonist therapies: A TBNET consensus statement. Eur Respir J. 2010;36:1185–206. doi: 10.1183/09031936.00028510. [DOI] [PubMed] [Google Scholar]
- 90.Murray CJ, Ortblad KF, Guinovart C, Lim SS, Wolock TM, Roberts DA, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:1005–70. doi: 10.1016/S0140-6736(14)60844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carmona L, Gómez-Reino JJ, Rodríguez-Valverde V, Montero D, Pascual-Gómez E, Mola EM, et al. Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. Arthritis Rheum. 2005;52:1766–72. doi: 10.1002/art.21043. [DOI] [PubMed] [Google Scholar]
- 92.Kavanagh PM, Gilmartin JJ, O’Donnell J, O’Flanagan D. Tumour necrosis factor-alpha and tuberculosis: Guidance from the National TB Advisory Committee. Ir Med J. 2008;101:6–7. [PubMed] [Google Scholar]
- 93.Thongraung W, Sittidach M, Khwansuwan P, Sariyasuntorn K, Wongsampan S. Evaluation of the physicians’ approach to the diagnosis and treatment of patients with antituberculosis drug-induced hepatotoxicity. J Eval Clin Pract. 2012;18:1119–25. doi: 10.1111/j.1365-2753.2011.01706.x. [DOI] [PubMed] [Google Scholar]
- 94.Andrisani G, Armuzzi A, Papa A, Marzo M, Felice C, Pugliese D, et al. Comparison of Quantiferon-TB Gold versus tuberculin skin test for tuberculosis screening in inflammatory bowel disease patients. J Gastrointestin Liver Dis. 2013;22:21–5. [PubMed] [Google Scholar]
- 95.Abbas AM, Almukhtar RM, Loftus EV, Jr, Lichtenstein GR, Khan N. Risk of melanoma and non-melanoma skin cancer in ulcerative colitis patients treated with thiopurines: A nationwide retrospective cohort. Am J Gastroenterol. 2014;109:1781–93. doi: 10.1038/ajg.2014.298. [DOI] [PubMed] [Google Scholar]
- 96.Long MD, Martin CF, Pipkin CA, Herfarth HH, Sandler RS, Kappelman MD. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143:390–399.e1. doi: 10.1053/j.gastro.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peyrin-Biroulet L, Khosrotehrani K, Carrat F, Bouvier AM, Chevaux JB, Simon T, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011;141:1621–28. doi: 10.1053/j.gastro.2011.06.050. e1-5. [DOI] [PubMed] [Google Scholar]
- 98.Setshedi M, Epstein D, Winter TA, Myer L, Watermeyer G, Hift R. Use of thiopurines in the treatment of inflammatory bowel disease is associated with an increased risk of non-melanoma skin cancer in an at-risk population: A cohort study. J Gastroenterol Hepatol. 2012;27:385–9. doi: 10.1111/j.1440-1746.2011.06865.x. [DOI] [PubMed] [Google Scholar]
- 99.Singh H, Nugent Z, Demers AA, Bernstein CN. Increased risk of nonmelanoma skin cancers among individuals with inflammatory bowel disease. Gastroenterology. 2011;141:1612–20. doi: 10.1053/j.gastro.2011.07.039. [DOI] [PubMed] [Google Scholar]