Abstract
GAD autoantibodies (GADAs) are sensitive markers of islet autoimmunity and type 1 diabetes. They form the basis of robust prediction models and are widely used for the recruitment of subjects at high risk of type 1 diabetes to prevention trials. However, GADAs are also found in many individuals at low risk of diabetes progression. To identify the sources of diabetes-irrelevant GADA reactivity, we analyzed data from the 2009 and 2010 Diabetes Autoantibody Standardization Program GADA workshop and found that binding of healthy control sera varied according to assay type. The characterization of control sera found positive by radiobinding assay (RBA), but negative by ELISA, showed that many of these sera reacted to epitopes in the N-terminal region of the molecule. This finding prompted development of an N-terminally truncated GAD65 radiolabel, 35S-GAD65(96–585), which improved the performance of most GADA RBAs participating in an Islet Autoantibody Standardization Program GADA substudy. These detailed workshop comparisons have identified a source of disease-irrelevant signals in GADA RBAs and suggest that N-terminally truncated GAD labels will enable more specific measurement of GADAs in type 1 diabetes.
Introduction
Accurate prediction of type 1 diabetes depends on islet autoantibody measurement. The presence of autoantibodies directed against multiple islet antigens confers a high risk of disease (1,2), and improved performance of individual islet autoantibody assays would enable more efficient recruitment of high-risk subjects to therapeutic prevention trials. GAD autoantibodies (GADAs) are the most widely used marker for type 1 diabetes, but to achieve optimum disease sensitivity the threshold for GADA positivity is often set at the 99th percentile, a level that exceeds the lifetime risk of disease development (3). Many individuals found to be GADA positive with current assays are therefore unlikely to progress to type 1 diabetes, making the development of more specific GADA assays a high priority (4).
The Diabetes Antibody Standardization Program (DASP) was established in 2001 with the aim of improving islet autoantibody assay performance and concordance among laboratories (5). DASP has facilitated the rapid evaluation and adoption of novel autoantibody assays (6–8), and this work continues under the mantle of the Islet Autoantibody Standardization Program (IASP). During the lifetime of the DASP/IASP, there have been major improvements in assay performance and comparability, but the specificity of GADA assays can still vary by as much as 10% between laboratories that achieve similar sensitivity (9).
Closer analysis of recent DASP/IASP workshops has revealed systematic differences in the reactivity of individual healthy control sera between ELISAs and radiobinding assays (RBAs). Several control sera showed increased binding of GAD65 in the majority of RBAs, despite being found negative in most ELISAs, while the converse was true for other control sera. We therefore investigated the binding characteristics of those control sera found positive more commonly by RBA to identify sources of disease-irrelevant signals and, using this information, set out to develop more specific GADA assays.
Research Design and Methods
DASP/IASP Workshops
Analysis was performed on samples included in the 2009 and 2010 DASP workshops as well as a GADA substudy in the 2012 IASP workshop (Supplementary Fig. 1). In each workshop, laboratories received uniquely coded sets of blinded sera from 50 patients with newly diagnosed type 1 diabetes that were contributed by several centers around the world, together with up to 100 U.S. blood donors without a family history of diabetes, who were used as healthy control subjects (Supplementary Table 1). Type 1 diabetes was diagnosed by local centers on the basis of clinical characteristics. All samples were collected within 14 days of starting insulin treatment. The 90 control sera included in DASP 2010 were also among the 100 control sera used in DASP 2009. Sera were prepared and frozen in 100-μL aliquots and distributed by the Centers for Disease Control and Prevention or the University of Florida, as previously described (10). Laboratories were asked to test samples for GADAs using the assay formats of their choice, to provide details of their assay protocols, and to report assay results, including raw data, to the DASP/IASP for analysis. Assay parameters varied between and within different formats. Major differences included the volume of serum used, buffer constituents, primary incubation time, separation method, washing technique, and standardization method. To reduce the variation between RBAs, a standard method protocol was developed that fixed these aspects of the technique, thereby allowing for greater comparability between laboratories (11). In the DASP 2009 workshop, 42 laboratories from 19 countries reported results for 56 GADA assays. In the DASP 2010 workshop, 39 laboratories from 19 countries reported results for 53 GADA assays. In the IASP 2012 workshop, 10 laboratories from seven countries participated in a GADA substudy using noncommercial RBAs (Supplementary Data).
Assessment of Epitope Specificities
The epitope specificities of selected GADA workshop control sera were assessed using plasmids encoding full-length GAD65, GAD67, and truncated GAD65, as well as GAD65–GAD67 chimeras (12). GAD67, GAD67(1–101)/GAD65(96–440)/GAD67(453–594), and GAD67(1–243)/GAD65(235–444)/GAD67(453–594) were cloned into pGEM-T Easy (Promega, Madison, WI), while GAD65(1–95)/GAD67(101–594) and GAD67(1–452)/GAD65(445–585) were cloned into pGEM-3 (Promega). GAD65(46–585) and GAD65(96–585) were cloned into pTNT (Promega). All plasmids were provided by author V.L. apart from the pTNT plasmid pThGAD65 encoding full-length GAD65 (courtesy of Åke Lernmark, Lund University, Malmö, Sweden). Samples were assayed for GADAs using the standard assay protocol (11) with 35S-methionine–labeled antigens made by in vitro transcription and translation of GAD65–GAD67 chimeras, truncated GAD65, and full-length GAD65. To further characterize GADA binding, selected DASP 2010 workshop sera were also assayed for GADA(1–585) and GADA(96–585) using the standard assay protocol with and without the addition of 5 or 0.05 pmol/well recombinant full-length GAD65 (Diamyd Medical AB, Stockholm, Sweden). Using this approach, the median percentage displacement of GADA binding was calculated as 100 × (cpm label alone − cpm label with unlabeled GAD)/cpm label alone) with a minimum set at 0%. A lack of displacement at 5 pmol/well would indicate a lack of specificity for GAD65, while a lack of displacement at 0.05 pmol/well would suggest that the antibodies were of low affinity, especially when the levels of binding were low (13,14).
For the IASP 2012 GADA substudy, participating laboratories generated 35S-labeled GAD using the pTNT plasmid (Promega) vector encoding GAD65(96–585) distributed by author V.L., as well as their usual plasmid encoding full-length GAD65 or 125I-labeled human recombinant full-length GAD65. Prior to the GADA substudy, coded DASP 2010 sets were assayed by three selected laboratories using both 35S-labeled GAD65(96–585) and GAD65(46–585) encoded in the same vector (Supplementary Fig. 1).
Data Analysis
Categorical variables were compared using the χ2 test. Areas under the curve (AUCs) for the different assays from receiver operator characteristic analysis were compared using the Wilcoxon signed rank test. When laboratory-assigned positive-negative calls were analyzed according to assay format, only those assays with a specificity >90% were included. For all analyses, a two-tailed P value of 0.05 was considered significant. Statistical analyses were performed using the Statistics Package for Social Sciences Version 19 (IBM, New York, NY).
Results
The Pattern of GADA Reactivity in Healthy Individuals Is Associated With Assay Format
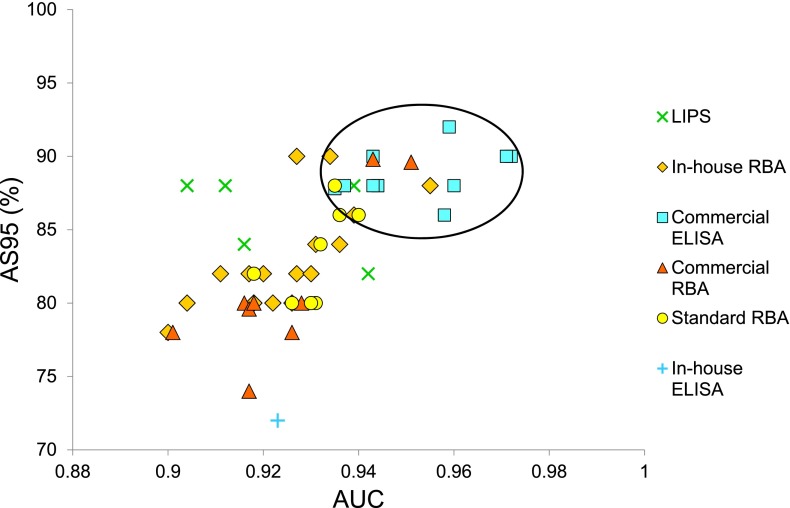
The median laboratory-assigned sensitivities and specificities of GADA assays in the DASP 2010 workshop were 86% (range 34–92%) and 94% (range 68–100%), respectively. According to threshold-independent measures (10), the adjusted sensitivity at 95% specificity (AS95), and the AUC, the commercial ELISA showed the best overall performance (Fig. 1). When assay results were analyzed according to assay format, the positive-negative calls for control sera often clustered according to assay type (Fig. 2A). For example, control serum LQ21235 was scored positive by 8 of 10 laboratories using the commercial ELISA, but by none of the other assays. In contrast, control sera N51532, N56575, N59932, S8531, and N53371 were found positive by none of the laboratories using the commercial ELISA compared with 19 (54%), 18 (51%), 13 (37%), 10 (29%), and 10 (29%) of the other 35 assays, respectively. Another difference between assays was that serum N54357 was scored positive by 6 of 8 RBAs using the commercial kit with iodinated GAD65 antigen, but not by any of the other 37 assays. In contrast to the pattern observed in control subjects, no clear assay-specific differences in the reactivity of patient sera were seen (data not shown).
Figure 1.
The AS95 plotted against the AUC from receiver operator characteristic analysis of GADA assays participating in the DASP 2010 workshop. Using these threshold-independent measures, better performance is demonstrated by those assays located toward the top right-hand corner (circled), a cluster that includes all the commercial ELISAs.
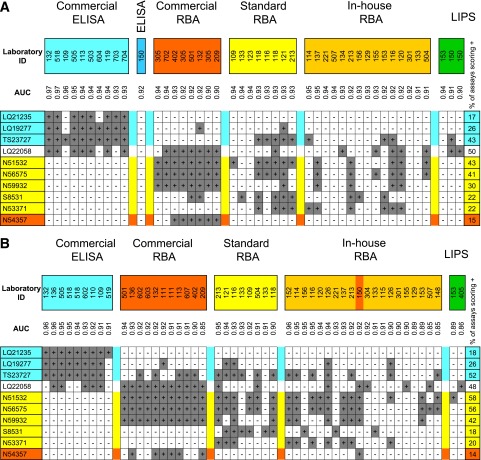
Figure 2.
Heatmaps of laboratory-defined positive-negative calls for the 10 healthy control sera found positive most often in the DASP 2010 (A) and the same sera in the DASP 2009 (B) for those assays with a laboratory-defined specificity of >90%, sorted according to assay type. Positive-negative calls were found to cluster according to assay type; sera shaded in blue were found positive most commonly by commercial ELISAs, those in yellow by RBAs, and those in orange by RBAs using 125I-labeled GAD65. ID, identification.
Assays Show a Consistent Pattern of Reactivity Over Time
The pattern of positive-negative calls for control sera in the DASP 2009 workshop was very similar to that in the DASP 2010 (Fig. 2B). The serum found positive exclusively by ELISA in DASP 2010, LQ21235, was positive in all nine ELISAs, but in none of the other assays. The five sera found positive by none of the laboratories using the commercial ELISA, but at least 30% of other assays in DASP 2010, were again consistently negative by ELISA and positive in 20–71% of other assays. Serum N54357 was positive in 6 of 11 commercial RBAs as well as the only other RBA using iodinated antigen, but in none of the other 38 assays.
Characterization of Control Samples Called Positive in DASP 2010
To determine whether the pattern of positivity in control subjects could be explained by differences in assay-specific reactivity to particular GADA epitopes, selected control samples were assayed by RBA using 35S-labeled GAD65, GAD67, and GAD65/67 chimeras (12) (Fig. 3). Of the three samples found positive more often by ELISA, LQ19277 showed dominant binding to the N-terminal of GAD67 and weak binding to full-length GAD65, while sera LQ21235 and TS23727 recognized epitopes restricted to the N-terminal of GAD65 that were dependent on amino acids 46–95. However, as expected for a serum found positive exclusively by ELISA, LQ21235 showed very low levels of binding in these RBA experiments. Of the five control samples found positive more often by RBAs, three (N51532, N56575, and N59932) showed weak reactivity with the middle region of GAD65. A fourth serum (S8531) showed reactivity restricted to the N-terminal of GAD65. The fifth serum, N53371, bound predominantly to epitopes in the N-terminal region of GAD65 with weaker responses to GAD67.
Figure 3.
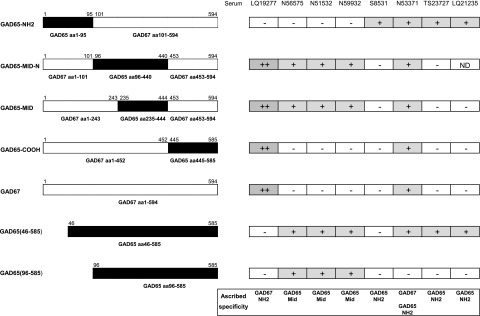
Epitope specificity of eight healthy control sera from the DASP 2010 workshop that showed assay-related differences in reactivity. The left panel shows the different GAD constructs used to assess epitope specificity with regions derived from GAD65 shown in black, and those from GAD67 shown in white. The right panel shows the reactivity of the control sera with these GAD constructs and the epitope reactivity ascribed to those sera based on the pattern of binding with the different constructs. Four sera (S8531, N53371, TS23727, and LQ21235) showed reactivity with GAD65 N-terminal epitopes that was abolished by deletion of the first 95 amino acids (aa). Three control sera (N56575, N51532, and N59932) showed weak reactivity with the middle (MID) region of GAD65 (amino acids 235–444), and this binding was not reduced by use of the N-terminally truncated labels.
Specificity of binding to 35S-labeled GAD65(1–585) and GAD65(96–585) in these sera was confirmed by competitive displacement with excess (5 pmol/well) unlabeled GAD65. The median displacements of GADA binding in all eight sera were 60% (range 39–78%) with 35S-GAD65(1–585) and 72% (range 70–76%) in the three sera found positive with 35S-GAD65(96–585) (Supplementary Fig. 2A). This compares with median displacements of binding in six GADA-positive patients (Immunology of Diabetes Society samples 004, 005, 006, 009, 097, and 195) of 87% (range 68–98%) and 91% (range 71–98%) for 35S-labeled GAD65(1–585) and GAD65(96–585), respectively (Supplementary Fig. 2B).
To identify sera with low-affinity antibodies, GADA binding was competed at a low concentration of unlabeled GAD65. Competition with 0.05 pmol/well unlabeled GAD65 caused median displacements of binding by the six patient sera of 65% (range 40–86%) and 76% (range 61–87%) with 35S-labeled GAD65(1–585) and GAD65(96–585), respectively (Supplementary Fig. 2D). In contrast, the median displacements of binding in the three sera showing weak reactivity with the middle region of GAD65 (N51532, N56575, and N59932) were 0% (range 0–15%) with 35S-labeled GAD65(1–585) and 14% (range 9–24%) with 35S-labeled GAD65(96–585), indicating that these samples had low-affinity antibodies (Supplementary Fig. 2C).
Evaluation of GADA Assays Using GAD65(46–585) and GAD65(96–585) Plasmids
To investigate whether replacing GAD65(1–585) with N-terminally truncated GAD65 constructs could improve GADA assay performance, three laboratories assayed a new coded set of samples from DASP 2010 with 35S- labels generated using plasmids encoding GAD65(1–585), GAD65(46–585), and GAD65(96–585). In each laboratory, the highest AS95 (88%) was achieved using the GAD65(96–585) construct (Supplementary Fig. 3).
Evaluation of GAD65(96–585) in the IASP 2012 GADA Substudy
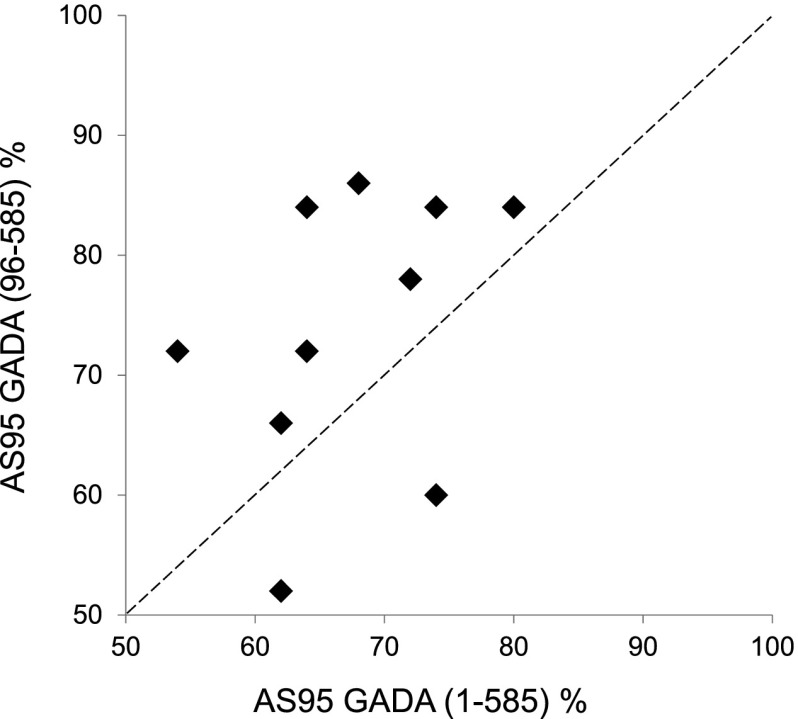
The potential of the GAD65(96–585) radiolabel to improve the performance of GADA RBAs was assessed by 10 laboratories in the IASP 2012 GADA substudy. Participating laboratories assayed coded IASP sets using both 35S- or 125I-labeled GAD65(1–585) and 35S-labeled GAD65(96–585). Of the 10 laboratories, 8 showed higher AS95 values with GAD65(96–585) (Fig. 4). Changes in the AS95 with the GAD65(96–585) label ranged widely from −14% to 20%, showing that, even within RBAs, the reactivity of different sera is strongly influenced by local assay conditions.
Figure 4.
AS95 for 10 RBAs using 35S or 125I-GAD65(1–585) and 35S-GAD65(96–585) to measure workshop samples in the IASP 2012 GADA substudy. The improved performance of assays using the N-terminally truncated GAD is shown by the eight laboratories that lie above the line of equivalence (hatched line).
Discussion
Using data from DASP workshops, we have shown that the binding of GAD65 by healthy control sera segregated according to assay format. Some control sera reacted preferentially in the commercial ELISA but not in the RBA, while others found positive in many RBAs showed no binding in ELISAs. A high proportion of the control sera found positive by RBAs targeted epitopes in the N-terminal region of GAD65, which are less commonly recognized by diabetes-relevant antibodies (15–18). These findings prompted the construction of a plasmid encoding GAD65(96–585) that was suitable for generating a N-terminally truncated radiolabel. In the IASP 2012 GADA substudy, 8 of 10 RBAs using this plasmid achieved a higher adjusted sensitivity than those using full-length GAD65, indicating that the performance of many RBAs could be improved by the use of N-terminally truncated GAD radiolabels.
Earlier islet autoantibody workshops have shown differences in assay performance that could be ascribed to particular characteristics. The higher performance of IA-2 autoantibody assays using plasmids expressing the intracellular region rather than the IA-2(256–556/630–979) or full-length constructs led to more widespread adoption of intracellular IA-2 autoantibody assays (9,10). The clear superiority of RBAs over ELISAs for measuring insulin autoantibodies has meant that RBAs have been used almost exclusively for insulin autoantibody measurement (19). The differences we observed with the GADA assay format were more subtle, but, by focusing on signals generated by healthy control sera, we were able to identify an important source of disease-irrelevant signals in the N-terminus affecting RBAs. Despite the superior overall performance of the GADA ELISA, analogous modification of the capture antigen in the ELISA format may improve the specificity of the assay. Even in the most robust GADA ELISAs, false-positive signals from sera acquired from healthy individuals contribute significantly to the higher background levels of binding that define assay thresholds, limiting our ability to assign true β-cell autoimmunity.
Birth cohort prospective studies of relatives of patients with type 1 diabetes have shown that diabetes-relevant autoantibody epitope reactivity typically spreads from the COOH-terminal and middle (PLP) regions to the N-terminal domains of the molecule (12,16). Autoantibodies to the N-terminal region typically constitute a relatively minor component of GAD65 autoreactivity and alone confer little association with type 1 diabetes (17). However, we cannot exclude the possibility that a very small proportion of sera from patients with type 1 diabetes may bind exclusively to the N-terminus and will be missed by the truncated antigen. Furthermore, in patients with other forms of diabetes such as latent autoimmune diabetes in adults or slowly progressive type 1 diabetes, epitopes in the N-terminal region may constitute a larger proportion of the anti-GAD response (20,21). The potential benefit of using truncated antigen to test for these conditions therefore needs to be evaluated. Cross-reactive N-terminal restricted GADA may mark an early phase of autoimmunity in neurological conditions such as Stiff Person Syndrome (SPS) (22–24), although as those disorders are rare and SPS itself is associated with very high GADA titers (25), the low level N-terminal restricted antibodies found in healthy control subjects are more likely attributable to cross-reactivity of irrelevant antibodies.
The commercial ELISA showed good sensitivity and specificity in the DASP and IASP workshops (9). This assay relies on the autoantibody forming a bridge between immobilized GAD65 on the plate and biotinylated GAD65 in solution with detection by streptavidin peroxidase (26). Access to N-terminal epitopes may be hindered in this configuration, preventing the binding of the N-terminally restricted antibodies detected by many RBAs. This could also partly explain why luminescence immunoprecipitation (LIPS) and electrochemiluminescence assays (27,28) showed good specificity in recent workshops. However, we cannot exclude that other mechanisms may be responsible for the lack of binding of these N-terminally restricted control sera in the ELISA. Some of the assay-dependent differences in recognition of DASP/IASP control sera are likely to be related to antibody affinity. The three control sera found reactive with the middle region of GAD in RBAs, but found negative by ELISA, showed minimal displacement at low levels of competing GAD65, which suggests that these sera contain low-affinity antibodies. This may be why these antibodies were not detected by the commercial ELISA or the electrochemiluminescence assay, as the bridging format they use favors the recognition of high-affinity antibodies, which are more closely associated with diabetes progression (17,29). The performance of RBAs using N-terminally truncated GAD65 labels may therefore be further improved by including affinity measurements.
A major strength of this study was the availability of data from a number of DASP and IASP workshops, which allowed us to identify consistent patterns in the reactivity of control sera according to assay format. The original design of the DASP workshops (10), with the inclusion of a relatively large number of control sera, has again been vindicated, as it allowed us to identify systematic differences in reactivity, which would have been impossible with a smaller number of samples. These sample sets distributed to laboratories are, however, still limited with regard to sample number, as well as ethnicity, age, and their cross-sectional nature. Other important systematic variations in GADA binding by healthy control sera may be identified in different cohorts. Furthermore, only 10 laboratories participated fully in the IASP 2012 GADA substudy, which restricted our ability to determine whether use of the GAD65(96–585) label could enhance assay performance.
Measurement of GADAs is fundamental to most strategies aimed at the prediction and characterization of type 1 diabetes, but there has been concern that, despite their high sensitivity, GADAs are often less closely associated with diabetes progression than are other islet autoantibodies such as IA-2A and ZnT8A (30). The DASP and IASP workshops have revealed assay-related differences in the binding of GAD65 by control sera that should aid the development of more specific GADA assays. If the promise shown by the N-terminally truncated GAD65(96–585) antigen probe to improve the specificity of GADA RBAs without loss of sensitivity is confirmed in large prospective studies, we would advocate its adoption for population screening in combination with other islet autoantibodies to identify individuals who are at high risk of progression to type 1 diabetes.
Supplementary Material
Article Information
Acknowledgments. The authors thank the patients and healthy donors who have contributed blood to the Diabetes Antibody Standardization Program and the Islet Autoantibody Standardization Program, as well as their physicians. The authors also thank Kyla Chandler at the University of Bristol for assistance with the competitive displacement assays.
Funding. The Diabetes Antibody Standardization Program was funded at the Centers for Disease Control and Prevention by grants PL105-33, 106-310, 106-554, and 107-360, which were administered by the National Institutes of Health. The Islet Autoantibody Standardization Program was funded at the University of Florida by Catalog of Federal Domestic Assistance grant #93.847: Diabetes, Digestive, and Kidney Diseases Extramural Research of the National Institute of Diabetes and Digestive and Kidney Diseases. V.L. and C.B worked within the framework of the Italian Ministry of Research “Ivascomar project, Cluster Tecnologico Nazionale Scienze della Vita ALISEI” and were supported by the Associazione Italiana per la Ricerca sul Cancro “AIRC bando 5 × 1000 N_12182” grant. P.A. was supported by grants from the German Federal Ministry of Education and Research (BMBF) to the Competence Network for Diabetes Mellitus (FKZ 01GI0805) and to the German Center for Diabetes Research (DZD e.V.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.J.K.W., V.L., and P.A. researched the data, contributed to the discussion, and wrote the manuscript. M.S., P.W.M., D.L.P., W.E.W., R.W., C.B., and S.K. researched the data and reviewed and edited the manuscript. B.A. reviewed and edited the manuscript. A.J.K.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 49th Annual Meeting of the European Association for the Study of Diabetes, Barcelona, Spain, 23–27 September 2013, and at the 13th International Immunology of Diabetes Society Congress, Mantra Lorne, Victoria, Australia, 7–11 December 2013.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db14-1693/-/DC1.
A complete list of the participating laboratories can be found in the Supplementary Data online.
References
- 1.Bingley PJ, Gale EA; European Nicotinamide Diabetes Intervention Trial (ENDIT) Group . Progression to type 1 diabetes in islet cell antibody-positive relatives in the European Nicotinamide Diabetes Intervention Trial: the role of additional immune, genetic and metabolic markers of risk. Diabetologia 2006;49:881–890 [DOI] [PubMed] [Google Scholar]
- 2.Orban T, Sosenko JM, Cuthbertson D, et al.; Diabetes Prevention Trial-Type 1 Study Group . Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 2009;32:2269–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haller MJ, Atkinson MA, Schatz D. Type 1 diabetes mellitus: etiology, presentation, and management. Pediatr Clin North Am 2005;52:1553–1578 [DOI] [PubMed] [Google Scholar]
- 4.Liu E, Eisenbarth GS. Accepting clocks that tell time poorly: fluid-phase versus standard ELISA autoantibody assays. Clin Immunol 2007;125:120–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bingley PJ, Williams AJK. Validation of autoantibody assays in type 1 diabetes: workshop programme. Autoimmunity 2004;37:257–260 [DOI] [PubMed] [Google Scholar]
- 6.Achenbach P, Schlosser M, Williams AJ, et al. Combined testing of antibody titer and affinity improves insulin autoantibody measurement: Diabetes Antibody Standardization Program. Clin Immunol 2007;122:85–90 [DOI] [PubMed] [Google Scholar]
- 7.Schlosser M, Mueller PW, Achenbach P, Lampasona V, Bingley PJ; Participating Laboratories . Diabetes Antibody Standardization Program: first evaluation of assays for autoantibodies to IA-2β. Diabetes Care 2011;34:2410–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lampasona V, Schlosser M, Mueller PW, et al. Diabetes Antibody Standardization Program: first proficiency evaluation of assays for autoantibodies to zinc transporter 8. Clin Chem 2011;57:1693–1702 [DOI] [PubMed] [Google Scholar]
- 9.Törn C, Mueller PW, Schlosser M, Bonifacio E, Bingley PJ; Participating Laboratories . Diabetes Antibody Standardization Program: evaluation of assays for autoantibodies to glutamic acid decarboxylase and islet antigen-2. Diabetologia 2008;51:846–852 [DOI] [PubMed] [Google Scholar]
- 10.Bingley PJ, Bonifacio E, Mueller PW. Diabetes Antibody Standardization Program: first assay proficiency evaluation. Diabetes 2003;52:1128–1136 [DOI] [PubMed] [Google Scholar]
- 11.Bonifacio E, Yu L, Williams AK, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for National Institute of Diabetes and Digestive and Kidney Diseases consortia. J Clin Endocrinol Metab 2010;95:3360–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonifacio E, Lampasona V, Bernasconi L, Ziegler AG. Maturation of the humoral autoimmune response to epitopes of GAD in preclinical childhood type 1 diabetes. Diabetes 2000;49:202–208 [DOI] [PubMed] [Google Scholar]
- 13.Achenbach P, Guo LH, Gick C, et al. A simplified method to assess affinity of insulin autoantibodies. Clin Immunol 2010;137:415–421 [DOI] [PubMed] [Google Scholar]
- 14.Curnock RM, Reed CR, Rokni S, Broadhurst JW, Bingley PJ, Williams AJ. ’Insulin autoantibody affinity measurement using a single concentration of unlabelled insulin competitor discriminates risk in relatives of patients with type 1 diabetes. Clin Exp Immunol 2012;167:67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richter W, Shi Y, Baekkeskov S. Autoreactive epitopes defined by diabetes-associated human monoclonal antibodies are localized in the middle and C-terminal domains of the smaller form of glutamate decarboxylase. Proc Natl Acad Sci U S A 1993;90:2832–2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoppu S, Ronkainen MS, Kulmala P, Akerblom HK, Knip M; Childhood Diabetes in Finland Study Group . GAD65 antibody isotypes and epitope recognition during the prediabetic process in siblings of children with type I diabetes. Clin Exp Immunol 2004;136:120–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayr A, Schlosser M, Grober N, et al. GAD autoantibody affinity and epitope specificity identify distinct immunization profiles in children at risk for type 1 diabetes. Diabetes 2007;56:1527–1533 [DOI] [PubMed] [Google Scholar]
- 18.Fenalti G, Hampe CS, Arafat Y, et al. COOH-terminal clustering of autoantibody and T-cell determinants on the structure of GAD65 provide insights into the molecular basis of autoreactivity. Diabetes 2008;57:1293–1301 [DOI] [PubMed] [Google Scholar]
- 19.Greenbaum CJ, Palmer JP, Kuglin B, Kolb H. Insulin autoantibodies measured by radioimmunoassay methodology are more related to insulin-dependent diabetes mellitus than those measured by enzyme-linked immunosorbent assay: results of the Fourth International Workshop on the Standardization of Insulin Autoantibody Measurement. J Clin Endocrinol Metab 1992;74:1040–1044 [DOI] [PubMed] [Google Scholar]
- 20.Hampe CS, Kockum I, Landin-Olsson M, et al. GAD65 antibody epitope patterns of type 1.5 diabetic patients are consistent with slow-onset autoimmune diabetes. Diabetes Care 2002;25:1481–1482 [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi T, Tanaka S, Okubo M, Nakanishi K, Murase T, Lernmark A. Unique epitopes of glutamic acid decarboxylase autoantibodies in slowly progressive type 1 diabetes. J Clin Endocrinol Metab 2003;88:4768–4775 [DOI] [PubMed] [Google Scholar]
- 22.Butler MH, Solimena M, Dirkx R Jr, Hayday A, De Camilli P. Identification of a dominant epitope of glutamic acid decarboxylase (GAD-65) recognized by autoantibodies in stiff-man syndrome. J Exp Med 1993;178:2097–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J, Namchuk M, Bugawan T, et al. Higher autoantibody levels and recognition of a linear NH2-terminal epitope in the autoantigen GAD65, distinguish stiff-man syndrome from insulin-dependent diabetes mellitus. J Exp Med 1994;180:595–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daw K, Ujihara N, Atkinson M, Powers AC. Glutamic acid decarboxylase autoantibodies in stiff-man syndrome and insulin-dependent diabetes mellitus exhibit similarities and differences in epitope recognition. J Immunol 1996;156:818–825 [PubMed] [Google Scholar]
- 25.Piquer S, Belloni C, Lampasona V, et al. Humoral autoimmune responses to glutamic acid decarboxylase have similar target epitopes and subclass that show titer-dependent disease association. Clin Immunol 2005;117:31–35 [DOI] [PubMed] [Google Scholar]
- 26.Brooking H, Ananieva-Jordanova R, Arnold C, et al. A sensitive non-isotopic assay for GAD65 autoantibodies. Clin Chim Acta 2003;331:55–59 [DOI] [PubMed] [Google Scholar]
- 27.Burbelo PD, Hirai H, Issa AT, et al. Comparison of radioimmunoprecipitation with luciferase immunoprecipitation for autoantibodies to GAD65 and IA-2beta. Diabetes Care 2010;33:754–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miao D, Guyer KM, Dong F, et al. GAD65 autoantibodies detected by electrochemiluminescence assay identify high risk for type 1 diabetes. Diabetes 2013;62:4174–4178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bender C, Schlosser M, Christen U, Ziegler AG, Achenbach P. GAD autoantibody affinity in schoolchildren from the general population. Diabetologia 2014;57:1911–1918 [DOI] [PubMed] [Google Scholar]
- 30.De Grijse J, Asanghanwa M, Nouthe B, et al.; Belgian Diabetes Registry . Predictive power of screening for antibodies against insulinoma-associated protein 2 beta (IA-2beta) and zinc transporter-8 to select first-degree relatives of type 1 diabetic patients with risk of rapid progression to clinical onset of the disease: implications for prevention trials. Diabetologia 2010;53:517–524 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.