Abstract
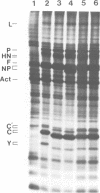
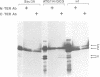
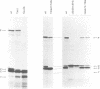
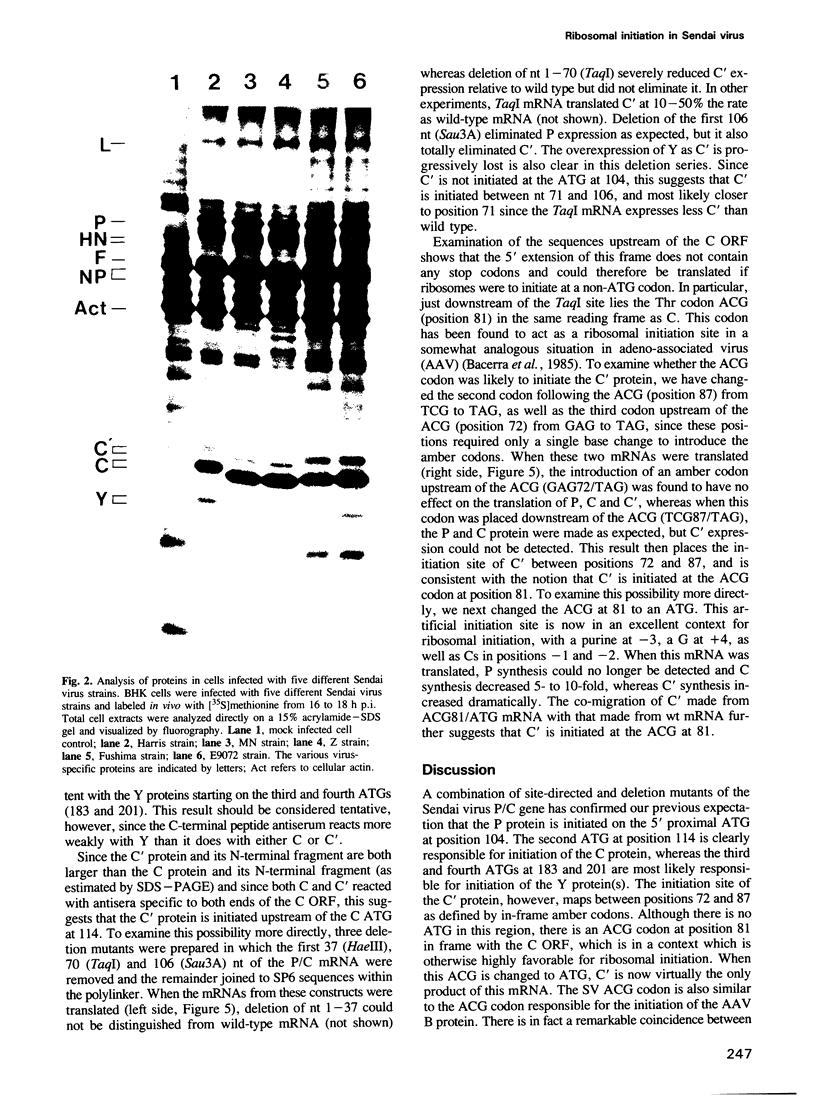
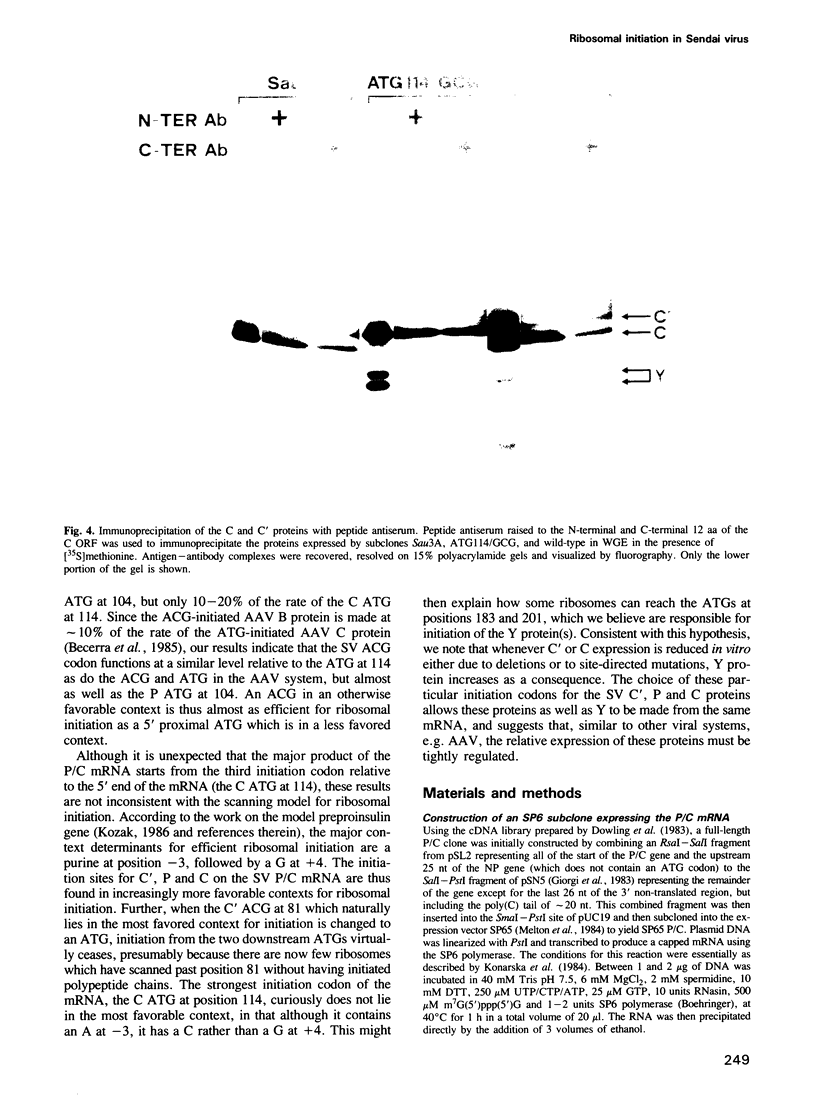
The Sendai virus P/C mRNA expresses the P and C proteins from alternate reading frames. The C reading frame of this mRNA, however, is responsible for three proteins, C', C and Y, none of which appear to be precursors to each other in vivo. Using site-directed and deletion mutagenesis of the P/C gene cloned in SP6 and in vitro translation of the mRNAs, we show that the 5' most proximal initiation codon of the mRNA is an ACG at position 81, responsible for C' synthesis. The succeeding initiation codons, all ATGs, are responsible for the P protein (position 104), the C protein (position 114) and the Y protein(s) (either positions 183 or 201). Examination of the relative molar amounts of the C', P and C proteins found in vivo suggests that an ACG in an otherwise favorable context is almost as efficient for ribosome initiation as an ATG in a less favored context, but only 10-20% as efficient as an ATG in a more favored context. The judicious choice of increasingly more favorable initiation codons in the P/C gene allows multiple proteins to be made from a single mRNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Buzash-Pollert E. Can ACG serve as an initiation codon for protein synthesis in eucaryotic cells? Mol Cell Biol. 1985 Dec;5(12):3621–3624. doi: 10.1128/mcb.5.12.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra S. P., Rose J. A., Hardy M., Baroudy B. M., Anderson C. W. Direct mapping of adeno-associated virus capsid proteins B and C: a possible ACG initiation codon. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7919–7923. doi: 10.1073/pnas.82.23.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J. A., Kolakofsky D. Identification of an additional Sendai virus non-structural protein encoded by the P/C mRNA. J Gen Virol. 1987 Sep;68(Pt 9):2515–2519. doi: 10.1099/0022-1317-68-9-2515. [DOI] [PubMed] [Google Scholar]
- Curran J. A., Richardson C., Kolakofsky D. Ribosomal initiation at alternate AUGs on the Sendai virus P/C mRNA. J Virol. 1986 Feb;57(2):684–687. doi: 10.1128/jvi.57.2.684-687.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefsen L., Kolakofsky D. In vitro synthesis of the nonstructural C protein of Sendai virus. J Virol. 1983 Apr;46(1):321–324. doi: 10.1128/jvi.46.1.321-324.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling P. C., Giorgi C., Roux L., Dethlefsen L. A., Galantowicz M. E., Blumberg B. M., Kolakofsky D. Molecular cloning of the 3'-proximal third of Sendai virus genome. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5213–5216. doi: 10.1073/pnas.80.17.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkind P. R., Cross R. K., Lamb R. A., Merz D. C., Choppin P. W. In vitro synthesis of structural and nonstructural proteins of Sendai and SV5 viruses. Virology. 1980 Jan 15;100(1):22–33. doi: 10.1016/0042-6822(80)90548-6. [DOI] [PubMed] [Google Scholar]
- Giorgi C., Blumberg B. M., Kolakofsky D. Sendai virus contains overlapping genes expressed from a single mRNA. Cell. 1983 Dec;35(3 Pt 2):829–836. doi: 10.1016/0092-8674(83)90115-0. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltiner M., Kempe T., Tjian R. A novel strategy for constructing clustered point mutations. Nucleic Acids Res. 1985 Feb 11;13(3):1015–1025. doi: 10.1093/nar/13.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W. The molecular biology of paramyxoviruses. Med Microbiol Immunol. 1974;160(2-3):73–83. doi: 10.1007/BF02121714. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D., Boy de la Tour E., Delius H. Molecular weight determination of Sendai and Newcastle disease virus RNA. J Virol. 1974 Feb;13(2):261–268. doi: 10.1128/jvi.13.2.261-268.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarska M. M., Padgett R. A., Sharp P. A. Recognition of cap structure in splicing in vitro of mRNA precursors. Cell. 1984 Oct;38(3):731–736. doi: 10.1016/0092-8674(84)90268-x. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kramer W., Drutsa V., Jansen H. W., Kramer B., Pflugfelder M., Fritz H. J. The gapped duplex DNA approach to oligonucleotide-directed mutation construction. Nucleic Acids Res. 1984 Dec 21;12(24):9441–9456. doi: 10.1093/nar/12.24.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Determination by peptide mapping of the unique polypeptides in Sendai virions and infected cells. Virology. 1978 Feb;84(2):469–478. doi: 10.1016/0042-6822(78)90263-5. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Mahy B. W., Choppin P. W. The synthesis of sendai virus polypeptides in infected cells. Virology. 1976 Jan;69(1):116–131. doi: 10.1016/0042-6822(76)90199-9. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody D. S. Translation initiation at an ACG triplet in mammalian cells. J Biol Chem. 1987 Aug 25;262(24):11847–11851. [PubMed] [Google Scholar]