Summary
Lower levels of CXCL11 and TARC/CCL17 are associated with occupational formaldehyde exposure.
Abstract
Background.
Formaldehyde has been classified as a human myeloid leukemogen. However, the mechanistic basis for this association is still debated.
Objectives.
We aimed to evaluate whether circulating immune/inflammation markers were altered in workers occupationally exposed to formaldehyde.
Methods.
Using a multiplexed bead-based assay, we measured serum levels of 38 immune/inflammation markers in a cross-sectional study of 43 formaldehyde-exposed and 51 unexposed factory workers in Guangdong, China. Linear regression models adjusting for potential confounders were used to compare marker levels in exposed and unexposed workers.
Results.
We found significantly lower circulating levels of two markers among exposed factory workers compared with unexposed controls that remained significant after adjusting for potential confounders and multiple comparisons using a false discovery rate of 10%, including chemokine (C-X-C motif) ligand 11 (36.2 pg/ml in exposed versus 48.4 pg/ml in controls, P = 0.0008) and thymus and activation regulated chemokine (52.7 pg/ml in exposed versus 75.0 pg/ml in controls, P = 0.0028), suggesting immunosuppression among formaldehyde-exposed workers.
Conclusions.
Our findings are consistent with recently emerging understanding that immunosuppression might be associated with myeloid diseases. These findings, if replicated in a larger study, may provide insights into the mechanisms by which formaldehyde promotes leukemogenesis.
Introduction
Formaldehyde (CH2O) is one of the most produced and economically important chemicals globally, due to its diverse industrial and commercial uses. Approximately 21 million tons of formaldehyde is produced annually worldwide (1). Formaldehyde use occurs mainly in the construction, automobile, textile and furniture markets and especially in the production of thermosetting resins, which accounted for ~63% of global demand in 2011 and generated a total revenue of USD 11.7 billion in 2012 (2,3). The International Agency for Research on Cancer has classified formaldehyde as a Group 1 carcinogen. This classification is based primarily on sufficient evidence in humans for an association with nasopharyngeal cancers and leukemia, predominantly myeloid leukemia (4). Multiple review groups have also concluded that formaldehyde is a leukemogen and the National Academy of Sciences has recently confirmed the listing of formaldehyde as ‘known to be a human carcinogen’ in the National Toxicology Program 12th Report on Carcinogens, based on sufficient evidence of an association with nasopharyngeal cancer, sinonasal cancer and myeloid leukemia in humans (5).
Occupational formaldehyde exposure has been shown to elicit cytogenetic and immunological effects (6). Possible underlying mechanisms driving formaldehyde carcinogenesis that have been proposed include inflammation, oxidative stress and apoptosis (7–10), either via direct damage to the bone marrow or indirect damage via hematopoietic stem or early progenitor cells located in the peripheral blood or nasal passages before they are transported to the bone marrow (11). Studies have also demonstrated immunological effects of formaldehyde by immune alterations of T lymphocytes (CD3+), natural killer (NK) cells (CD56+), tumor necrosis factor alpha (TNF-α) and B-cells in formaldehyde-exposed workers (6,12). There is also suggestive evidence of a link between immunosuppression and increased risk of acute myeloid leukemia (AML) and nasopharyngeal cancers (13,14).
However, limited data are available on the immunologic effects and leukemogenic mechanisms of formaldehyde. We have shown previously lower peripheral blood cell counts, lymphocyte subsets and myeloid blood progenitor cell counts in formaldehyde-exposed factory workers compared with unexposed individuals, suggesting possible hematotoxic effects of formaldehyde (15,16). Conversely, a recent study by Jia et al. (17) reported higher percentage of B-cells (CD19+) and NK cells (CD56+) among formaldehyde-exposed workers compared with the unexposed workers. Jia et al. also evaluated serum levels of five cytokines (interferon-c [IFN-c], interleukin-4 [IL-4] and IL-10, IL-8 and TNF-α) in formaldehyde-exposed and unexposed workers and found significantly higher levels of IL-10 and IL-4 but lower levels of IL-8 and IFN-c in the exposed group than in the controls.
In this cross-sectional study, we aimed to evaluate the levels of 38 markers from a large panel of circulating cytokines, chemokines and growth factors (immune/inflammation markers) among formaldehyde-exposed factory workers and unexposed controls in Guangdong, China.
Materials and methods
Study population
This study has been described in detail previously (15). Briefly, we recruited 43 formaldehyde-exposed workers in two factories in Guangdong, China in June and July of 2006. One of the factories produced formaldehyde-melamine resins and the other factory used formaldehyde-melamine resins to manufacture plastic utensils. Fifty-one unexposed workers were selected as controls from three workplaces located in the same geographic region as the two factories with formaldehyde exposure but did not have occupational exposures to formaldehyde or any other known hematotoxic or genotoxic chemicals. Controls were frequency-matched to cases by sex and age (±5 years), had comparable demographic and socioeconomic characteristics as the exposed workers and were engaged primarily in manufacturing. We excluded workers with prior history of cancer, chemotherapy, radiotherapy, or previous occupations with notable exposures to benzene, butadiene, styrene, and/or ionizing radiation. The participation rates for exposed workers (92%) and controls (95%) were comparable. Questionnaires were used to collect information on each individual’s demographic, lifestyle and occupational history. The study was approved by Institutional Review Boards at the National Cancer Institute in the USA and the Guangdong Poison Control Center in China. All participants gave written informed consent.
Exposure assessment
Full-shift formaldehyde exposure for each worker was measured using UMEx 100 diffusion samplers (SKC, Eighty Four, PA) and has been described previously (15). Briefly, samplers were worn in the breathing zone by the exposed workers in the workplaces for a full shift (>4h) over a 3-week period on at least two occasions. A subset of unexposed workers was monitored for formaldehyde exposure on a single day. Organic vapor monitors (3M 3500 OVM, St. Paul, MN) were used to measure each worker’s exposure to other organic compounds. The monitors were analysed for chloroform, methylene chloride, tetrachloroethylene, trichloroethylene and benzene, and no hydrocarbons were detected in any of the selected samples. The analysis laboratory was blinded to the source of the samplers.
Measurement of circulating immune/inflammation markers
Serum samples were collected from each participant at the end of the exposure assessment period and the same biological samples were collected from the same subjects as Zhang et al. (15) and Hosgood et al. (16). Samples were delivered to the processing laboratories and frozen within 4h of collection. To ensure consistency, all biological samples (exposed and unexposed) were collected, stored, transported and processed the same way using standardized protocol (15). A total of 86 immune/inflammation markers were measured (38 markers remained for subsequent analysis after quality control) in 420 µl of serum that had undergone a single freeze-thaw cycle using Millipore’s multiplexed bead-based assay. Serum samples were assayed in duplicate and average concentrations were calculated. Samples were randomized on plates by exposure status. Each plate included the same quality control (QC) sample to assess batch-to-batch variation and blinded duplicate QC samples were included on each plate to assess within-batch variation. Markers with <20% of sample measurements above the limit of detection (LOD) (in either exposed, controls or overall; 32 out of 86 markers) were excluded from the analyses. In addition, we excluded markers which did not fulfill our quality control criteria: an intra-class correlation coefficient cutoff of 60% if the coefficient of variation (CV) was <5%, and an intra-class correlation coefficient of 80% if the CV was >5% but <30%. This excluded an additional 16 markers from the analysis (Supplementary Table S1, available at Carcinogenesis Online). A total of 38 markers remained for subsequent analyses with an average intra-class correlation coefficient of 65.0% and average CV of 6.0%, based on natural log-transformed data.
Statistical analysis
Demographics of exposed factory workers and unexposed controls were compared using the Wilcoxon rank sum test for continuous variables and Fisher’s exact test for categorical variables. Marker levels were natural log-transformed due to right-skewness. Levels below LOD were assigned half the LOD value (18). The Wilcoxon rank sum test was first used to compare the marker levels between exposed workers and controls. All markers were subsequently tested using multiple linear regression, adjusting for continuous age, sex and potential confounding by current smoking status (yes or no), current alcohol consumption (yes or no), recent infection (yes or no), recent medication use (yes or no) and body mass index (BMI; continuous) if they were significant predictors of each marker with P value <0.05 or variables that changed the regression coefficient of formaldehyde exposure by >15%. We also did a sensitivity analysis by further adjusting for blood cell counts (white blood cells, lymphocytes, monocytes and granulocytes). All markers were analysed as continuous outcomes and formaldehyde exposure status as a dichotomous exposure variable (exposed or unexposed) due to limited variation in exposure levels among the exposed. For markers (N = 4; IL-10, sCD40L, Fractalkine (CX3CL1) and Amylin) with >50% of values below LOD, we dichotomized the marker levels (above or below LOD) and used logistic regression for sensitivity analysis, adjusting for potential confounders. Multiple comparisons were adjusted for by calculating the false discovery rate (FDR) using the Benjamini-Hochberg method (19). All statistical analyses were conducted using R version 3.0.2 (20) and SAS version 9.3 (Cary, NC). All tests were conducted as two-sided and considered significant with a P-value <0.05 and FDR <20%.
Results
The formaldehyde-exposed workers and unexposed controls were frequency-matched by age and sex. Additionally, exposed and unexposed workers were not significantly different in their current smoking status, current alcohol consumption, BMI, recent infection status and recent medication use (Table 1). The exposed group had a median formaldehyde exposure of 1.28 p.p.m. (range: 0.32–5.61) and the unexposed group had a median of 0.026 p.p.m. (range: 0.015–0.026).
Table 1.
Characteristics of formaldehyde-exposed factory workers and non-exposed controls in Guangdong, China
| Characteristic | Exposed (N = 43) | Control (N = 51) | P value |
|---|---|---|---|
| Age, years (Mean ± SD) | 31.3±5.9 | 29.6±7.0 | 0.25 |
| Sex (%) | |||
| Male | 37 (86.0) | 44 (86.3) | 1.00 |
| Female | 6 (14.0) | 7 (13.7) | |
| Current smoking status (%) | |||
| No | 25 (58.1) | 28 (54.9) | 0.84 |
| Yes | 18 (41.9) | 23 (45.1) | |
| Current alcohol consumption (%) | |||
| No | 32 (74.4) | 30 (58.8) | 0.13 |
| Yes | 11 (25.6) | 21 (41.2) | |
| Recent infections (%) | |||
| No | 26 (60.5) | 36 (70.6) | 0.38 |
| Yes | 17 (39.5) | 15 (29.4) | |
| Recent use of medication (%) | |||
| No | 17 (58.6) | 16 (64.0) | 0.78 |
| Yes | 12 (41.4) | 9 (36.0) | |
| BMI, kg/m2 (Mean ± SD) | 21.5±2.5 | 22.2±3.2 | 0.37 |
| FA exposure, ppm (Median (IQR) [min, 10th, 90th percentile, max]) | 1.28 (1.1) [0.32, 0.65, 2.47, 5.61] | 0.026 (0.012) [0.015, 0.015, 0.026, 0.026] | NA |
BMI, body mass index; FA, formaldehyde; SD, standard deviation.
a P values were obtained from Fisher’s exact test for categorical variables and Wilcoxon rank sum test for continuous variables.
Using Wilcoxon rank sum test to compare all 38 markers, the median levels of 10 (26.3%) immune/inflammation markers were significantly different in formaldehyde-exposed workers, compared with unexposed controls. Eight out of these 10 significant markers [chemokine (C-X-C motif) ligand 11 (CXCL11), thymus and activation regulated chemokine—TARC (CCL17), TNF-related apoptosis-inducing ligand (TRAIL), SAP, sCD40L, EGF, MCP-1(CCL2), MCP-4 (CCL13)] were lower in exposed workers whereas two markers (PP, sIL-6R) were significantly higher in exposed workers compared with unexposed controls (Supplementary Table S1, available at Carcinogenesis Online).
After adjusting for covariates using multiple linear regression, concentrations of four markers [CXCL11, TARC (CCL17), C-reactive protein (CRP), TRAIL] were significantly lower in formaldehyde-exposed workers than unexposed controls (Table 2), which is significantly more markers than expected by chance (Fisher’s exact test P value < 0.05). All four markers survived the adjustment for multiple comparisons with a FDR cutoff of 20%. The largest decline in marker levels in exposed workers was observed for CRP (74.3% decrease), followed by TRAIL (46.0% decrease) and TARC (29.7% decrease) (Figure 1). The markers that did not survive adjustment for covariates include SAP, sCD40L, EGF, MCP-1 (CCL2), MCP-4 (CCL13), PP and sIL-6R. Using a stringent FDR cutoff of 10%, the two markers that remained significant were CXCL11 (median = 36.2 pg/ml in exposed versus 48.4 pg/ml in controls, P = 0.0008) and TARC (median = 52.7 pg/ml in exposed versus 75.0 pg/ml in controls, P = 0.0028). Spearman correlation between the two significant markers among controls was 0.082 (P = 0.57). Adjusting for blood cell counts (white blood cells, monocytes and lymphocytes) by including them in the model did not alter our findings (Supplementary Table S2, available at Carcinogenesis Online).
Table 2.
Selecteda immune/inflammation markers that were significantly different between formaldehyde-exposed factory workers and non-exposed controls
| Median concentration (IQR), pg/ml | |||||||
|---|---|---|---|---|---|---|---|
| Marker | Controls (IQR) | FA-exposed (IQR) | % Change | P valueb | FDRc | P valued | FDRc |
| CXCL11 | 48.4 (39.9) | 36.2 (27.9) | −25.2 | 0.014 | 0.11 | 0.00080e | 0.030 |
| TARC (CCL17) | 75.0 (41.9) | 52.7 (39.6) | −29.7 | 0.0024 | 0.09 | 0.0028f | 0.053 |
| CRP | 3269100.0 (6632240.9) | 840617.6 (1398687.4) | −74.3 | 0.060 | 0.20 | 0.015g | 0.19 |
| TRAIL | 22.1 (14.5) | 12.0 (11.9) | −46.0 | 0.017 | 0.11 | 0.021h | 0.20 |
FA, formaldehyde; IQR, interquartile range.
a P value < 0.05 and FDR < 20%.
b P values were obtained from Wilcoxon rank sum tests comparing between exposed workers and controls.
cMultiple comparisons were controlled for with FDR using the Benjamini-Hochberg method.
d P values were obtained from linear regression models, adjusting for age, sex and (i) significant variables in univariate models or (ii) variables that change the coefficient of formaldehyde by at least 15%.
eModels adjusted for age, sex and alcohol consumption.
fModels adjusted for age, sex, smoking status and alcohol consumption.
gModels adjusted for age, sex, alcohol consumption and recent medication use.
hModels adjusted for age, sex and BMI.
Figure 1.
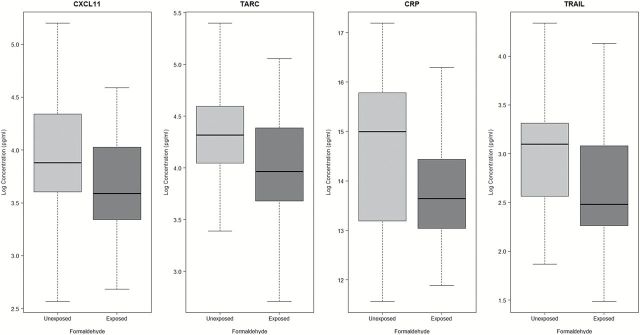
Concentrations of selected significant markers (natural log-transformed) by formaldehyde exposure status with P-value < 0.05 and FDR < 20%. Formaldehyde-exposed workers have lower concentrations of four immune/inflammatory markers as compared with unexposed workers.
Discussion
We successfully quantified 38 immune/inflammation markers from a large panel and found that formaldehyde-exposed factory workers had significantly lower levels of four markers compared with unexposed controls, and two of these markers remained statistically significant after adjusting for multiple comparisons using a stringent FDR cutoff of 10%. This is the first study to link these markers with formaldehyde exposure among occupationally exposed workers.
Previous reports of the same population have shown lower peripheral blood cell counts, including decreased counts of total lymphocytes and specific lymphocyte subsets such as NK cells, regulatory T cells and CD8+ effector memory T cells, as well as lower myeloid blood progenitor cell counts in formaldehyde-exposed factory workers compared with unexposed controls (15,16). Consistent with our previous observations in this population, our results indicate pan-immunosuppression, with lower levels of 57.9% (22/38) of the total detected immune/inflammation markers and all (4/4) of the significant markers were lower in formaldehyde-exposed factory workers after adjusting for multiple comparisons using a FDR of 20%, of which two remained significantly lower in formaldehyde-exposed factory workers compared with unexposed controls after adjusting for multiple comparisons using a stringent FDR cutoff of 10%. These significant immune/inflammation markers are mainly produced or produced in response to factors released by white blood cell types such as neutrophils during immune responses (21). However, there was only weak to moderate correlation (range from 0.01 to 0.42) between various blood cell counts and levels of the top two significant markers in unexposed controls only, exposed workers only and overall (Supplementary Table S3, available at Carcinogenesis Online). The highest correlation was observed between TARC and monocyte (r = 0.42, P = 0.0057) among exposed workers only. TARC and monocyte are also marginally significantly correlated among unexposed controls only (rho = 0.28, P = 0.05). Therefore, the alterations observed in immune/inflammation markers were unlikely to be explained substantially by blood cell count differences. Taken together, our results suggest that formaldehyde exposure may result in subtle alterations in immune activity.
However, we were unable to compare our IL-8, IL-4 and IFNg findings with that of Jia et al. (17) because these three markers had either poor CV (IL-8) or >80% of samples below the LOD (IL-4 and IFNg) and were excluded from the analysis. However, we observed IL-10 to be lower among formaldehyde-exposed workers than unexposed controls after adjusting for covariates (model estimate = 1.08, higher odds of <LOD in exposed group), but 55.3% of the samples’ IL-10 were below detectable levels (58.1% in cases and 52.9% in controls) and there was no difference in the median concentrations. Similar to Jia et al., we found no significant differences in TNF-α levels between formaldehyde-exposed and unexposed workers. A possible reason for the differences between our findings and that of Jia et al. is the higher formaldehyde exposure in our study population compared with Jia et al. The range of formaldehyde concentration in our study was 0.32–5.61 p.p.m. (mean = 1.46 p.p.m.) and the high exposure group in Jia et al. was exposed to lower formaldehyde concentrations that ranged between 0.36 and 1.53 p.p.m. (mean = 0.63 p.p.m.). Therefore, the maximum formaldehyde exposure in Jia et al.’s highest exposed group corresponds to our mean exposure levels in the exposed workers. Therefore, it could be that immunosuppression via lowering immune/inflammatory marker levels only occur with high formaldehyde exposures. Other differences between our studies include the study inclusion criteria, 95% of our workers has worked in the study factories for at least a year and they had to have had formaldehyde exposure levels of ~1–2 p.p.m. on most days during the initial screening, whereas Jia et al. included workers who have been exposed to formaldehyde for at least 6 months without specifying the magnitude of exposure. Moreover, the factory type is different in both studies. Jia et al. enrolled workers from a plywood factory where there could have been co-exposure to wood dust, glue and other chemicals such as chloroform, methylene chloride, tetrachloroethylene, trichloroethylene and benzene, none of which were detected in our study. A recent study by Maiellaro et al. (22) found that low-dose exposure to formaldehyde during rat pregnancy induced decreased IL-6 and TNF-α secretion but elevated levels of IL-10, which were also not replicated in our study, although the median TNF-α levels were 1.53% lower in exposed workers than unexposed workers in our study.
It has been shown that immunosuppression increases AML risk in patients receiving immunosuppressive medications after organ transplantation (13,23). In addition, immunosuppression has been demonstrated to be positively associated with risk of nasopharyngeal cancers (14). Decreased levels of immune/inflammation markers may therefore indicate loss of immune surveillance that is commonly observed in AML patients to inhibit antitumor immune responses (24,25). However, the exact mechanisms by which AML evade the immune system and suppress immune response are still undefined. A recent study of 15 AML patients showed that AML blasts can modify the immune microenvironment by suppressing T-cell proliferation through enhanced arginine metabolism (24). Hence, our findings suggest that global immunosuppression may be a possible contributing mechanism via which formaldehyde leads to pancytopenia as shown in our previous report and eventually carcinogenesis, but future longitudinal studies are needed to investigate this hypothesis.
Animal studies of formaldehyde-exposed rats have shown various dose-dependent alterations of cytokines and inflammatory protein PLUNC (palate, lung and nasal epithelial clone), which plays a role in the innate immune response in the upper airways and nasopharynx (26–28). A study conducted among Hungarian nurses measured immune alteration by using ratio of lymphocyte subpopulations (T, helper T cell, cytotoxic T cell, B and NK cells), Th/Tc ratio and the activation (receptor for IL-2 expression) of T cells, and found increased immune alterations in nurses exposed to formaldehyde from sterilizing gases compared with unexposed controls (29). These are consistent with our findings of altered levels of circulating immune/inflammation markers in formaldehyde-exposed workers.
Interestingly, the two significant cytokines from our study have previously been shown to be altered in plasma of humans and in vitro with leukemia and nasopharyngeal carcinoma (30–33). CXCL11, also known as the interferon-inducible T-cell alpha chemoattractant (I-TAC), is a chemotactic for activated T-cells and is highly expressed in peripheral blood leukocytes (34). CXCL11 has also been shown in vivo to elicit antitumor immune response in T-cell lymphoma cells (35), which supports that lower CXCL11 levels may increase cancer risk. Decreased serum levels of TARC, a Th2 chemokine also known as CCL17, have been found in patients with untreated AML (31).
Other significant immune markers with levels at least 45% lower among exposed workers include CRP and TRAIL. CRP belongs to the protein family of pentraxins and it is a marker of downstream acute-phase inflammatory response commonly associated with cardiovascular disease (36–38). Contrary to our findings, higher levels of CRP has been reported to be positively associated with acute leukemia and mortality in patients with primary or post-polycythemia vera/essential thrombocythemia myelofibrosis (39). However, the factory workers in our study are chronically exposed to hematotoxic toxins that are less prevalent in the general population and as a result may have an increased risk of leukemia, albeit possibly via an alternate mechanism. TRAIL is a member of the TNF family of cytokines and has been demonstrated to induce apoptosis in human AML cells, both in vitro and in vivo (40). It has also been shown to exert an antitumor effect on nasopharyngeal carcinoma cancer stem cells when used in combination with Smac (second mitochondria-derived activator of caspase) mimetics (41) and therefore, lower levels of TRAIL may reduce apoptosis and increase the risk of AML and nasopharyngeal cancer.
Limitations of this study include poor performance and detectability of some markers, which limited our ability to evaluate the association with these markers. In addition, the markers analysed are a small subset of all immune/inflammation markers. This study was originally designed to compare the differences between formaldehyde-exposed and unexposed workers and therefore, we were unable to evaluate the dose–response of formaldehyde and the immune/inflammation markers. However, our study measured a large panel of immune/inflammation markers, which is by far the largest panel to be measured in formaldehyde-exposed workers.
In conclusion, we found significantly lower levels of four immune/inflammation markers in formaldehyde-exposed factory workers compared with unexposed controls, suggesting that formaldehyde may have inhibitory effects on various soluble immune markers. Our results provide potential evidence for the association between formaldehyde and immunosuppression. Replication of these findings in studies with larger sample size and additional studies linking these markers to AML and nasopharyngeal cancer as well as future studies to understand the link between down-regulation of these markers in circulation, formaldehyde exposure and cancer development are warranted.
Supplementary material
Supplementary and Tables S1–S3 can be found at http://carcin.oxfordjournals.org/
Funding
National Cancer Institute (Intramural fund) and National Institute of Environmental Health Sciences (R01ES017452 to L.Z. and P42ES004705 to M.T.S.)
Conflict of interest statement: None declared.
Supplementary Material
Glossary
Abbreviations
- AML
acute myeloid leukemia
- CRP
C-reactive protein
- CXCL11
chemokine (C-X-C motif) ligand 11
- IFN
interferon
- IL
interleukin
- LOD
limit of detection
- NK
natural killer
- TARC
thymus and activation regulated chemokine
- TNF
tumor necrosis factor
References
- 1. International Agency for Research on Cancer. (2006) Formaldehyde, 2-Butoxyethanol and 1-tert-Butoxypropan-2-ol. IARC monographs on the evaluation of carcinogenic risks to humans, 88, 39–325. [PMC free article] [PubMed] [Google Scholar]
- 2. IHS Chemical. (2012) Directory of Chemical Producers, Formaldehyde. Chemical Economics Handbook. http://www.ihs.com/products/chemical/planning/ceh/formaldehyde.aspx (accessed October 6, 2014).
- 3. Transparency Market Research. (2013) Formaldehyde Market for UF Resins, PF Resins, MF Resins, Polyacetal Resins, Pentaerythritol, MDI, 1, 4-Butanediol, and Other Applications—Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2012–2018. Albany, NY, Transparency Market Research. [Google Scholar]
- 4. Baan R., et al. (2009) Special report: Policy. A review of human carcinogens-Part F: chemical agents and related occupations. Lancet Oncol., 10, 1143–1144. [DOI] [PubMed] [Google Scholar]
- 5. National Research Council. (2014) Review of the Formaldehyde Assessment in the National Toxicology Program 12th Report on Carcinogens. The National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- 6. Costa S., et al. (2013) Cytogenetic and immunological effects associated with occupational formaldehyde exposure. J. Toxicol. Environ. Health. A, 76, 217–229. [DOI] [PubMed] [Google Scholar]
- 7. Rager J.E., et al. (2013) Formaldehyde and epigenetic alterations: microRNA changes in the nasal epithelium of nonhuman primates. Environ. Health Perspect., 121, 339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Romanazzi V., et al. (2013) 15-F-2t isoprostane as biomarker of oxidative stress induced by tobacco smoke and occupational exposure to formaldehyde in workers of plastic laminates. Sci. Total Environ., 442, 20–25. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Y.C., et al. (2013) Bone marrow injury induced via oxidative stress in mice by inhalation exposure to formaldehyde. Plos One, 8(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morgan K.T. (1997) A brief review of formaldehyde carcinogenesis in relation to rat nasal pathology and human health risk assessment. Toxicol. Pathol., 25, 291–307. [DOI] [PubMed] [Google Scholar]
- 11. Zhang L.P., et al. (2009) Formaldehyde exposure and leukemia: a new meta-analysis and potential mechanisms. Mutat. Res., 681, 150–168. [DOI] [PubMed] [Google Scholar]
- 12. Aydin S., et al. (2013) Assessment of immunotoxicity and genotoxicity in workers exposed to low concentrations of formaldehyde. Arch. Toxicol., 87, 145–153. [DOI] [PubMed] [Google Scholar]
- 13. Morton L.M., et al. (2014) Risk of myeloid neoplasms after solid organ transplantation. Leukemia, 28, 2317–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shebl F.M., et al. (2010) Salivary gland and nasopharyngeal cancers in individuals with acquired immunodeficiency syndrome in United States. Int. J. Cancer, 126, 2503–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang L.P., et al. (2010) Occupational exposure to formaldehyde, hematotoxicity, and leukemia-specific chromosome changes in cultured myeloid progenitor cells. Cancer Epidemiol. Biomarkers Prev., 19, 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hosgood H.D., et al. (2013) Occupational exposure to formaldehyde and alterations in lymphocyte subsets. Am. J. Ind. Med., 56, 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jia X., et al. (2014) Effects of formaldehyde on lymphocyte subsets and cytokines in the peripheral blood of exposed workers. PLoS One, 9(8), e104069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hornung W.R., et al. (1990) Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg., 5, 46–51. [Google Scholar]
- 19. Benjamini Y., et al. (1995) Controlling the false discovery rate—a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol., 57, 289–300. [Google Scholar]
- 20. R Core Team. (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 21. Cassatella M.A. (2006) On the production of TNF-related apoptosis-inducing ligand (TRAIL/Apo-2L) by human neutrophils. J. Leukoc. Biol., 79, 1140–1149. [DOI] [PubMed] [Google Scholar]
- 22. Maiellaro M., et al. (2014) Exposure to low doses of formaldehyde during pregnancy suppresses the development of allergic lung inflammation in offspring. Toxicol. Appl. Pharmacol., 278, 266–274. [DOI] [PubMed] [Google Scholar]
- 23. Gale R.P., et al. (2012) Commentary: does immune suppression increase risk of developing acute myeloid leukemia? Leukemia, 26, 422–423. [DOI] [PubMed] [Google Scholar]
- 24. Teague R.M., et al. (2013) Immune evasion in acute myeloid leukemia: current concepts and future directions. J. Immunother. Cancer, 1:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lion E., et al. (2012) Natural killer cell immune escape in acute myeloid leukemia. Leukemia, 26, 2019–2026. [DOI] [PubMed] [Google Scholar]
- 26. Im H., et al. (2006) Evaluation of toxicological monitoring markers using proteomic analysis in rats exposed to formaldehyde. J. Proteome Res., 5, 1354–1366. [DOI] [PubMed] [Google Scholar]
- 27. Ahn K.H., et al. (2010) Proteomic analysis of bronchoalveolar lavage fluid obtained from rats exposed to formaldehyde. J. Health Sci., 56, 287–295. [Google Scholar]
- 28. Bingle C.D., et al. (2002) PLUNC: a novel family of candidate host defence proteins expressed in the upper airways and nasopharynx. Hum. Mol. Genet., 11, 937–943. [DOI] [PubMed] [Google Scholar]
- 29. Tompa A., et al. (2006) Chemical safety and health conditions among Hungarian hospital nurses. Ann. N. Y. Acad. Sci., 1076, 635–648. [DOI] [PubMed] [Google Scholar]
- 30. Yan X.J., et al. (2011) Identification of outcome-correlated cytokine clusters in chronic lymphocytic leukemia. Blood, 118, 5201–5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Olsnes A.M., et al. (2006) T lymphocyte chemotactic chemokines in acute myelogenous leukemia (AML): local release by native human AML blasts and systemic levels of CXCL10 (IP-10), CCL5 (RANTES) and CCL17 (TARC). Cancer Immunol. Immunother., 55, 830–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ghia P., et al. (2001) Chemoattractants MDC and TARC are secreted by malignant B-cell precursors following CD40 ligation and support the migration of leukemia-specific T cells. Blood, 98, 533–540. [DOI] [PubMed] [Google Scholar]
- 33. Xia W.X., et al. (2013) A prognostic model predicts the risk of distant metastasis and death for patients with nasopharyngeal carcinoma based on pre-treatment serum C-reactive protein and N-classification. Eur. J. Cancer, 49, 2152–2160. [DOI] [PubMed] [Google Scholar]
- 34. Cole K.E., et al. (1998) Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J. Exp. Med., 187, 2009–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hensbergen P.J., et al. (2005) The CXCR3 targeting chemokine CXCL11 has potent antitumor activity in vivo involving attraction of CD8(+) T lymphocytes but not inhibition of angiogenesis. J. Immunother., 28, 343–351. [DOI] [PubMed] [Google Scholar]
- 36. Agrawal A., et al. (2009) Pattern recognition by pentraxins. Adv. Exp. Med. Biol., 653, 98–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jenny N.S., et al. (2007) Serum amyloid P and cardiovascular disease in older men and women—results from the Cardiovascular Health Study. Arterioscler. Thromb. Vasc. Biol., 27, 352–358. [DOI] [PubMed] [Google Scholar]
- 38. Hirschfield G.M., et al. (2003) C-reactive protein and cardiovascular disease: new insights from an old molecule. QJM, 96, 793–807. [DOI] [PubMed] [Google Scholar]
- 39. Barbui T., et al. (2013) Elevated C-reactive protein is associated with shortened leukemia-free survival in patients with myelofibrosis. Leukemia, 27, 2084–2086. [DOI] [PubMed] [Google Scholar]
- 40. Nieda M., et al. (2001) TRAIL expression by activated human CD4(+)V alpha 24NKT cells induces in vitro and in vivo apoptosis of human acute myeloid leukemia cells. Blood, 97, 2067–2074. [DOI] [PubMed] [Google Scholar]
- 41. Wu M.S., et al. (2013) Smac mimetics in combination with TRAIL selectively target cancer stem cells in nasopharyngeal carcinoma. Mol. Cancer Ther., 12, 1728–1737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


