Antiretroviral therapy (ART)–naive human immunodeficiency virus-infected individuals with concurrent anemia and inflammation are at particularly high risk of failing treatment. Future therapeutics and interventions beyond ART that can reduce inflammation or identify occult infections are needed to improve outcomes.
Keywords: HIV, anemia, inflammation, CRP, antiretroviral therapy
Abstract
Background. Anemia is a known risk factor for clinical failure following antiretroviral therapy (ART). Notably, anemia and inflammation are interrelated, and recent studies have associated elevated C-reactive protein (CRP), an inflammation marker, with adverse human immunodeficiency virus (HIV) treatment outcomes, yet their joint effect is not known. The objective of this study was to assess prevalence and risk factors of anemia in HIV infection and to determine whether anemia and elevated CRP jointly predict clinical failure post-ART.
Methods. A case-cohort study (N = 470 [236 cases, 234 controls]) was nested within a multinational randomized trial of ART efficacy (Prospective Evaluation of Antiretrovirals in Resource Limited Settings [PEARLS]). Cases were incident World Health Organization stage 3, 4, or death by 96 weeks of ART treatment (clinical failure). Multivariable logistic regression was used to determine risk factors for pre-ART (baseline) anemia (females: hemoglobin <12.0 g/dL; males: hemoglobin <13.0 g/dL). Association of anemia as well as concurrent baseline anemia and inflammation (CRP ≥10 mg/L) with clinical failure were assessed using multivariable Cox models.
Results. Baseline anemia prevalence was 51% with 15% prevalence of concurrent anemia and inflammation. In analysis of clinical failure, multivariate-adjusted hazard ratios were 6.41 (95% confidence interval [CI], 2.82–14.57) for concurrent anemia and inflammation, 0.77 (95% CI, .37–1.58) for anemia without inflammation, and 0.45 (95% CI, .11–1.80) for inflammation without anemia compared to those without anemia and inflammation.
Conclusions. ART-naive, HIV-infected individuals with concurrent anemia and inflammation are at particularly high risk of failing treatment, and understanding the pathogenesis could lead to new interventions. Reducing inflammation and anemia will likely improve HIV disease outcomes. Alternatively, concurrent anemia and inflammation could represent individuals with occult opportunistic infections in need of additional screening.
The global prevalence of HIV in 2013 was 29.2 million, with 1.3 million deaths [1]. Despite availability of antiretroviral therapy (ART), clinical ART failure is greatest in low- and middle-income countries due to lack of ART access as well as other factors, including a higher burden of malnutrition and anemia [2–4]. Studies from diverse areas of the world have shown that baseline (pre-ART) anemia and ongoing anemia during ART predict HIV progression and adverse clinical outcomes, including morbidity and mortality [5–11].
Anemia is defined by sex-specific hemoglobin cutoffs and has multiple known causes, including infectious diseases, inflammation, and deficiencies of iron, vitamin A, or vitamin B12 [12, 13]. However, data are lacking on whether any of these types of anemia contribute to adverse HIV outcomes. A plausible biological explanation is also missing on the association of all-cause anemia and HIV outcomes, with suggestions that this association is not causal but that anemia might represent individuals with advanced HIV disease [14–16]. As studies have suggested that iron status might not predict HIV disease progression [17], and as data from our cohort suggest low prevalence of deficiencies of vitamin A and vitamin B12, we hypothesize a major role for inflammation-associated anemia.
Inflammation, a well-known cause of anemia referred to as anemia of inflammation or anemia of chronic disease (ACD) [18], may be particularly relevant in the setting of HIV. However, without an established definition, clinicians and researchers have used various, nonoverlapping criteria to define ACD, such as the exclusion of other causes of anemia, ferritin or soluble transferrin receptor concentrations, the ratio of soluble transferrin receptor to log ferritin (TfR-F index), plasma cytokine levels, or a profile based on a combination of parameters. Notably, C-reactive protein (CRP) is another marker of inflammation that has not been used to define ACD, but is already commonly measured in many clinical settings, including some resource-limited settings, as a point-of-care assay [19] and may be particularly useful in the setting of anemia and HIV.
Given the interrelationship between anemia and inflammation and the independent associations of both anemia and elevated CRP with adverse outcomes in HIV [5, 20, 21], it is of great interest and potential significance to determine if anemia and elevated CRP contribute jointly to the risk of adverse HIV treatment outcomes. To distinguish from ACD, we will refer to the presence of anemia and CRP-defined inflammation as “concurrent anemia and inflammation.” The association of concurrent anemia and inflammation with HIV treatment outcomes has not been explored.
This study aims to assess the prevalence and risk factors of anemia among ART-naive HIV-infected adults, and to determine whether baseline anemia and baseline concurrent anemia and inflammation are associated with adverse treatment outcomes post-ART initiation. To address our objectives, we analyzed a case-cohort sample nested within the multinational Prospective Evaluation of Antiretrovirals in Resource Limited Settings (PEARLS) trial [21], a randomized trial of ART efficacy in ART-naive HIV-infected adults from 9 countries.
METHODS
Design Overview
We performed a case-cohort study nested within PEARLS (ClinicalTrials.gov identifier NCT00084136), a randomized trial of ART efficacy conducted among 1571 ART-naive HIV-infected adults between May 2005 and May 2010 [22]. The nested case-cohort design allows for efficient assessment of multiple outcomes and allows prevalence estimates from the subcohort [23, 24]. The effect estimates and standard errors obtained from the case-cohort design tend to be very similar to those of the full cohort when cohorts are >1250 participants and the sampling fraction is >10% [23–25].
Study Population
The parent PEARLS trial recruited participants from Brazil (n = 231), Haiti (n = 100), India (n = 255), Malawi (n = 221), Peru (n = 134), South Africa (n = 210), Thailand (n = 100), United States (n = 210), and Zimbabwe (n = 110). Inclusion criteria included age >18 years and CD4+ count <300 cells/µL. Individuals with acute illness, abnormal neutrophil, creatinine clearance, aspartate or alanine aminotransferase (as defined in parent study), and hemoglobin concentration <7.5 g/dL, as well as pregnant women, were excluded from recruitment [21]. Participants were randomized with equal probability to receive 1 of 3 ART regimens: (1) efavirenz plus twice-daily lamivudine/zidovudine; (2) atazanavir plus didanosine enteric coated and emtricitabine, all given once daily; or (3) efavirenz plus emtricitabine/tenofovir disoproxil fumarate once daily. Participants were followed for 96 weeks following ART initiation; study endpoints included clinical failure, virologic failure, and incident tuberculosis.
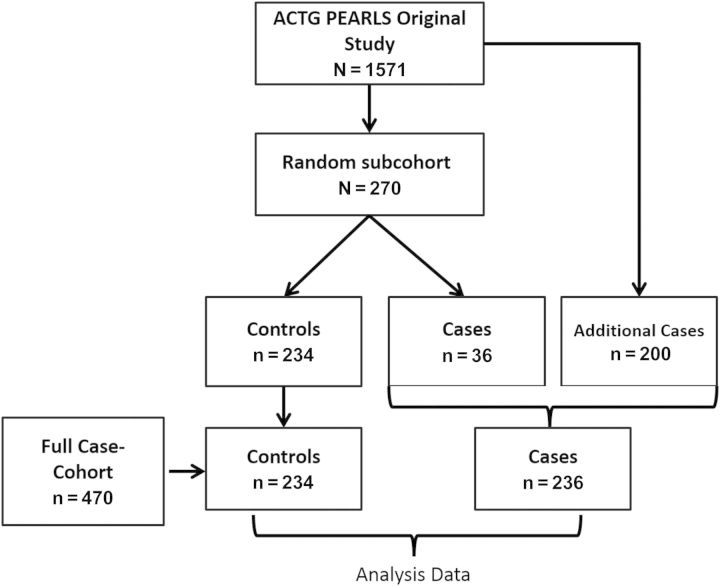
Our full case cohort (n = 470) consisted of a stratified random subcohort of 270 PEARLS participants (30 per country) and all additional cases in PEARLS outside of the random subcohort that met criteria for our outcomes of interest (Figure 1). There were no significant differences between the random subcohort and the full parent cohort in baseline characteristics [26]. Our primary outcome was clinical failure, defined as incident World Health Organization (WHO) stage III or IV event or death within 96 weeks post–ART initiation (236 cases [clinical failure] and 234 controls) (Figure 1). Secondary outcomes were virologic failure, defined as 2 successive plasma HIV type 1 (HIV-1) RNA levels >1000 copies/mL at or after 16 weeks post–ART initiation [26] and incident tuberculosis, defined as pulmonary or extrapulmonary tuberculosis by 96 weeks on ART (Figure 1).
Figure 1.
Case-cohort study. A random subcohort of 270 individuals and any additional cases from the original full cohort (AIDS Clinical Trials Group [ACTG] Prospective Evaluation of Antiretrovirals in Resource Limited Settings [PEARLS]) were part of the full case cohort. For the clinical failure case cohort, the outcome was clinical failure, which was defined as incident World Health Organization stage 3 or 4 events or death within 96 weeks after antiretroviral therapy (ART) (n = 234 controls and n = 236 cases). For the virologic failure case cohort, the outcome was virologic failure, which was defined as 2 successive plasma human immunodeficiency virus type 1 RNA levels >1000 copies/mL at or after 16 weeks post-ART (n = 246 controls and n = 165 cases). For the tuberculosis case cohort, the outcome was incident tuberculosis by 96 weeks post-ART initiation (n = 255 controls and n = 76 cases).
Ethics Statement
This study was approved by ethics committees and institutional review boards at Johns Hopkins University and participating site institutions. US Department of Health and Human Services guidelines for human experimentation were followed.
Data Collection and Laboratory Analysis
Body mass index (BMI) was measured at baseline. We assessed baseline clinical history and following ART initiation at 2, 4, and 8 weeks and every 4 weeks thereafter through 24 weeks, and every 8 weeks thereafter through 96 weeks. Baseline serum and plasma samples obtained from the participants were stored at −80°C. Each site laboratory measured CD4+ T-cell count, serum hemoglobin, and serum albumin; these measurements were externally quality assured according to the National Institutes of Health Division of AIDS and AIDS Clinical Trials Group (ACTG) Network laboratory quality assurance [22]. The Roche Amplicor Monitor Assay (version 1.5, Branchburg, New Jersey) was used to measure plasma HIV-1 RNA load. An enzyme-linked immunosorbent assay (ELISA) kit was used to measure plasma CRP (R&D Systems) at Johns Hopkins University.
Iron deficiency was evaluated by measuring serum ferritin (ALPCO) and serum soluble transferrin receptor (R&D Systems) concentrations using ELISA kits. Concentrations of serum retinol (vitamin A) and serum vitamin B12 were measured using high-performance liquid chromatography–ultraviolet and an immunochemical automated analyzer, Abbott AxSYM (Abbott Laboratories), respectively.
Definitions
Anemia was defined based on WHO hemoglobin cutoffs (males: <13.0 g/dL; nonpregnant women: <12.0 g/dL) [27]; a separate study assessed prevalence of pre-ART anemia and factors for the full PEARLS trial cohort (N = 1571) but used a different definition for anemia (hemoglobin ≤10.0 g/dL) [28].
ACD was defined based on the TfR-F index as previously described with index values <1 indicating ACD [18, 29]. Inflammation was defined as plasma CRP concentrations ≥10 mg/L, based on previous HIV studies [30]. Concurrent anemia and inflammation was defined as anemic individuals having inflammation.
Iron deficiency was defined by either ferritin or soluble transferrin receptor concentrations. As serum ferritin levels can be influenced by inflammation, 2 different cutoffs were used for defining iron deficiency: for CRP <5 mg/L, ferritin <12 µg/L was used to define iron deficiency, and for CRP ≥5 mg/L, ferritin <30 µg/L was used as previously described for HIV-infected populations [17]. The cutoff for soluble transferrin receptor for defining iron deficiency was >8.3 mg/L [31]. Prevalence of iron deficiency was similar when using soluble transferrin receptor-ferritin (sTfR), another index of iron deficiency. Based on established cutoffs, the following values were used to define deficiencies: ≤1.05 µmol/L for retinol [32], ≤148 pmol/L for vitamin B12 [33], and ≤3.5 g/dL for hypoalbuminemia [34].
Statistical Analyses
The random subcohort was used to estimate baseline anemia prevalence and assess differences in anemia by covariates. Fisher exact test was used to calculate P values of differences in categorical variables. Univariable and multivariable logistic regression models were used to calculate the odds ratio (OR) and 95% confidence interval (CI) for anemia by covariates. Race was not used in multivariable models due to co-linearity with country. Stata software version 13 was used for data analysis; GraphPad Prism Software version 5 was used to create forest plots for ORs and CIs.
S-plus Tibco Spotfire software version 7.2 was used to conduct univariable and multivariable Cox proportional hazards analysis to determine the association of baseline anemia (model 1) and baseline concurrent anemia and inflammation (model 2) with study outcomes in the full case cohort. In model 2, the case cohort was divided into 4 categories based on anemia and inflammation status: (1) no anemia and low inflammation (used as referent); (2) no anemia and high inflammation; (3) anemia and low inflammation; and (4) concurrent anemia and inflammation. All Cox models were stratified by treatment and country; to account for the stratification in our study, we used Barlow weighting and robust standard errors in regression models [23]. Adjusting for race and removing country from the multivariable models yielded similar estimates (data not shown).
RESULTS
Study Population Characteristics
Prevalence of all-cause baseline anemia at study entry was 51%. The prevalence of concurrent baseline anemia and inflammation (based on CRP cutoffs of 10 mg/L) was 15% (Table 1). The prevalence of ACD, based on TfR-F index, was 37%.
Table 1.
Prevalence of Types of Anemia and Concurrent Anemia and Inflammation in the PEARLS (Prospective Evaluation of Antiretrovirals in Resource Limited Settings) Subcohort
| Anemia Type | No. (%) |
|---|---|
| All-cause anemiaa | 138 (51.0) |
| Concurrent anemia and inflammationb | 37 (14.7) |
| TfR-F indexc | |
| <1 (ACD) | 69 (36.7) |
| 1–2 | 41 (15.9) |
| >2 (ACD + IDA) | 17 (6.6) |
Data are presented as No. and prevalence (%) of anemic people with concurrent micronutrient deficiency or inflammation.
Abbreviations: ACD, anemia of chronic disease; IDA, iron deficiency anemia; TfR-F, soluble transferrin receptor-log10 ferritin.
a Anemia was defined based on hemoglobin cutoffs for males (<13.0 g/dL) and nonpregnant females (<12.0 g/dL).
b Concurrent anemia and inflammation was defined as having anemia as well as C-reactive protein values >10 mg/L.
c Soluble TfR-F index was calculated using the ratio of soluble transferrin receptor to log10 ferritin.
Risk Factors for Anemia
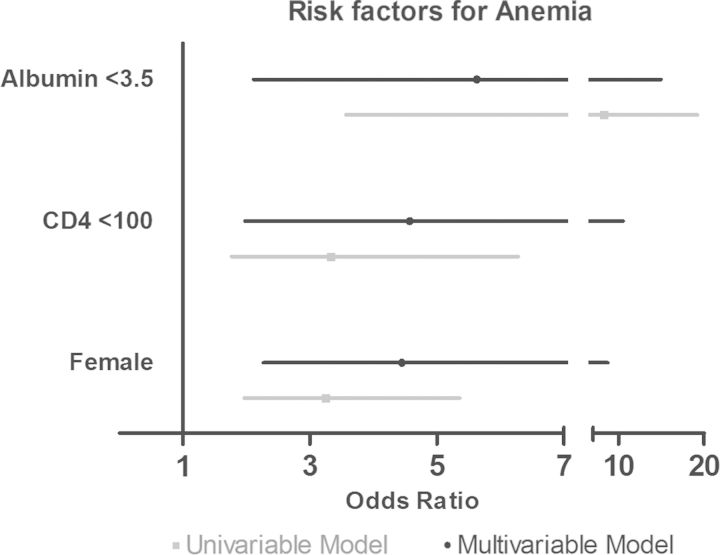
Risk factors for baseline anemia, assessed using the subcohort, were female sex (P < .001), race (P = .001), lower BMI (P = .009), lower CD4 count (P = .001), higher viral load (P = .01), and hypoalbuminemia (P < .001) (Table 2). Among these, female sex relative to male sex (adjusted OR [aOR], 4.45 [95% CI, 2.27–8.73]), hypoalbuminemia (aOR, 5.63 [95% CI, 2.12–14.97]), and CD4 count <100 cells/µL (aOR, 4.57 [95% CI, 1.98–10.53]) remained independent risk factors for baseline anemia in multivariable regression models adjusted for viral load, country, BMI, and CRP (Figure 2).
Table 2.
Characteristics of Subcohort Population by Baseline Pre–Antiretroviral Therapy Anemia Status
| Characteristic | All (n = 269) | Anemiaa (n = 138 [51%]) | Normal (n = 131 [49%]) | P Valueb |
|---|---|---|---|---|
| Sex | ||||
| Male | 135 (50) | 50 (37) | 85 (63) | <.001 |
| Female | 134 (50) | 88 (66) | 46 (34) | |
| Age, y | ||||
| <30 | 68 (25) | 31 (46) | 37 (54) | .56 |
| 30–40 | 119 (44) | 63 (53) | 56 (47) | |
| >40 | 82 (30) | 44 (54) | 38 (46) | |
| Country | ||||
| Brazil | 30 (11) | 10 (33) | 20 (67) | .07 |
| Haiti | 30 (11) | 20 (67) | 10 (33) | |
| India | 30 (11) | 17 (57) | 13 (43) | |
| Malawi | 30 (11) | 20 (67) | 10 (33) | |
| Peru | 30 (11) | 11 (37) | 19 (63) | |
| South Africa | 30 (11) | 18 (60) | 12 (40) | |
| Thailand | 30 (11) | 14 (47) | 16 (53) | |
| United States | 30 (11) | 13 (43) | 17 (57) | |
| Zimbabwe | 29 (11) | 15 (52) | 14 (49) | |
| Race | ||||
| White | 15 (6) | 3 (20) | 12 (80) | .001 |
| Black | 134 (50) | 82 (61) | 52 (39) | |
| Hispanic | 58 (22) | 21 (36) | 37 (64) | |
| Asian | 61 (23) | 32 (42) | 29 (48) | |
| Body mass index, kg/m2 | ||||
| <18.5 | 25 (9) | 20 (80) | 5 (20) | .009 |
| 18.5–24.9 | 174 (65) | 85 (49) | 89 (51) | |
| ≥25 | 70 (26) | 33 (47) | 37 (53) | |
| Prior tuberculosis diagnosis | ||||
| No | 233 (87) | 114 (49) | 119 (51) | .05 |
| Yes | 36 (13) | 24 (67) | 12 (33) | |
| Treatment arm | ||||
| A | 99 (37) | 54 (55) | 45 (45) | .71 |
| B | 88 (33) | 44 (50) | 44 (50) | |
| C | 82 (30) | 40 (49) | 42 (51) | |
| CD4 count, cells/µL | ||||
| <100 | 72 (27) | 50 (69) | 22 (31) | .001 |
| 100–200 | 91 (34) | 45 (49) | 46 (51) | |
| >200 | 106 (31) | 43 (41) | 63 (59) | |
| Log viral load, copies/mL | ||||
| <4 | 29 (11) | 12 (41) | 17 (59) | .01 |
| 4–5 | 96 (36) | 40 (42) | 56 (58) | |
| >5 | 144 (54) | 86 (60) | 58 (40) | |
| Albumin, g/dL | ||||
| <3.5 | 51 (19) | 44 (86) | 7 (14) | <.001 |
| ≥3.5 | 218 (81) | 94 (43) | 124 (57) | |
| Log C-reactive protein, mg/L | ||||
| <10 | 186 (69) | 93 (50) | 93 (50) | .60 |
| ≥10 | 83 (31) | 45 (54) | 38 (46) | |
Data are presented as No. (%) of the subcohort.
a Anemia is defined based on hemoglobin cutoffs for males (<13.0 g/dL) and nonpregnant females (<12.0 g/dL).
b Fisher exact test.
Figure 2.
Independent factors associated with pre–antiretroviral therapy anemia in the subcohort. Forest plot showing the odds ratio (OR) of anemia by covariates using data from the subcohort. Univariable (gray line) and multivariable (black line) logistic regression were used to calculate the OR of being anemic. Multivariable models were adjusted for sex (reference: male), country (reference: Brazil), body mass index (BMI) (reference: normal defined as 18–25 kg/m2), prior tuberculosis (reference: no prior tuberculosis), CD4 count (reference: >200 cells/µL), log viral load (reference: <4 copies/mL), albumin (reference: ≥3.5 g/dL), and C-reactive protein (reference: >10 mg/L). Country, log viral load, BMI, prior tuberculosis, and C-reactive protein were not associated with anemia in multivariable models and are not shown in this figure.
Association of Anemia With Clinical Failure
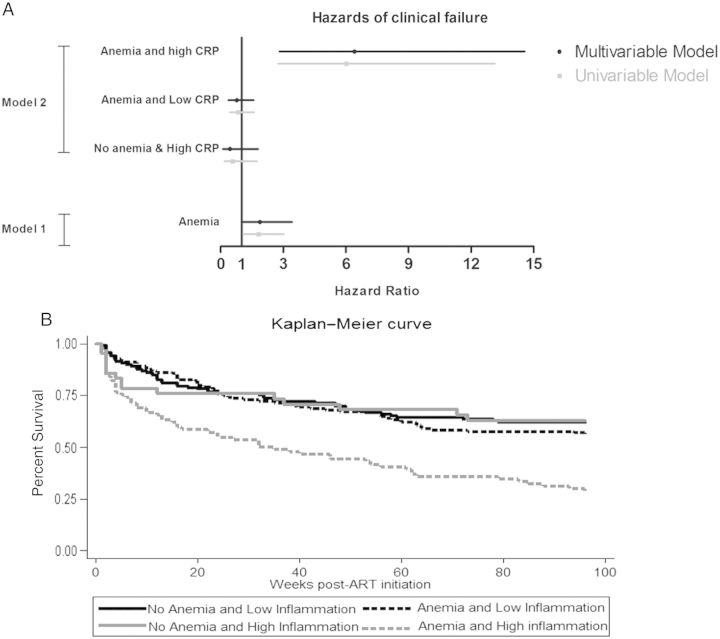
The clinical failure case cohort (N = 470) comprised 234 controls and 236 cases, where cases were defined as WHO stage 3 (n = 127, including 45 pulmonary tuberculosis), stage 4 (n = 48, including 12 extrapulmonary tuberculosis), and death (n = 15) (Figure 1). Using the clinical failure case cohort (Figure 1), baseline anemia was associated with increased hazards of clinical failure in univariable (hazard ratio [HR], 1.81 [95% CI, 1.09–3.03]) and multivariable (adjusted HR [aHR], 1.88 [95% CI, 1.03–3.42]) Cox regression models, which adjusted for sex, age, BMI, CD4 count, viral load, and CRP (see model 1 in Figure 3A).
Figure 3.
Association of baseline anemia with clinical treatment failure using full case cohort. A, Using the full case cohort (n = 470), hazard ratios of clinical treatment failure by baseline anemia and baseline concurrent anemia and inflammation were calculated using univariable and multivariable Cox proportional hazards regression models. In multivariable model 1, the variables adjusted for were sex, age, body mass index (BMI), CD4 count, viral load, and C-reactive protein (CRP), and the reference group was people with no anemia. In multivariable model 2, categories were based on combination of anemia and CRP. Hazard ratio of clinical failure in nonanemic people with high inflammation (CRP ≥10 mg/L) (labeled No anemia and High CRP), anemic people with low inflammation (CRP <10 mg/L) (labeled Anemia and Low CRP), and anemic people with high inflammation (concurrent anemia and inflammation) were compared to the reference group of nonanemic people with low inflammation. Multivariable model 2 adjusted for sex, age, BMI, CD4 count, and viral load. Variables were categorized using the same definitions in Table 1. B, Kaplan–Meier curve is shown by the 4 categories. Abbreviation: ART, antiretroviral therapy.
Association of Concurrent Anemia and Inflammation With Outcome
We next assessed if baseline anemia with and without inflammation was associated with clinical failure (model 2) post–ART initiation. Using no anemia and low inflammation (prevalence, 37%) as referent in model 2 (Figure 3A), concurrent anemia and inflammation (prevalence, 15%) was associated with increased hazards of clinical failure in both univariable (HR, 6.01 [95% CI, 2.75–13.14]) and multivariable models (aHR, 6.41 [95% CI, 2.82–14.57]), which adjusted for sex, age, BMI, CD4 count, and viral load; this significant association was confirmed in models that also adjusted for iron deficiency and hypoalbuminemia (data not shown). In contrast, anemia and low inflammation (prevalence, 37%; aHR, 0.77 [95% CI, .37–1.58]) and no anemia and high inflammation (prevalence, 11%; aHR, 0.45 [95% CI, .11–1.80]) had similar hazards of clinical failure relative to no anemia and low inflammation (Figure 3A and 3B). Seven percent of cases with concurrent anemia and inflammation died, 36% developed WHO stage IV (13% had extrapulmonary tuberculosis), and 57% developed WHO stage III disease (23% had pulmonary tuberculosis) (Table 3). As shown in Table 3, the major events associated with concurrent anemia and inflammation were stage IV events, notably, extrapulmonary tuberculosis and AIDS opportunistic infections.
Table 3.
Causes of Clinical Failure by World Health Organization Disease Stage
| Disease Stage | No Concurrent Anemia and Inflammation (n = 129), No. (%) | Concurrent Anemia and Inflammation (n = 61), No. (%) |
|---|---|---|
| WHO stage III | 92 (71) | 35 (57) |
| Pulmonary TB | 31 (24) | 14 (23) |
| Oral candidiasis | 18 (14) | 5 (8) |
| Bacterial infections | 8 (6) | 8 (13) |
| Other | 35 (27) | 8 (13) |
| WHO stage IV | 26 (20) | 22 (36) |
| Extrapulmonary TB | 4 (3) | 8 (13) |
| AIDS opportunistic infectionsa | 15 (12) | 11 (18) |
| Other | 7 (5) | 3 (5) |
| Death | 11 (9) | 4 (7) |
Abbreviations: TB, tuberculosis; WHO, World Health Organization.
a Toxoplasmic encephalitis, cytomegalovirus retinitis, non-Hodgkin lymphoma, Pneumocystis jirovecii pneumonia, disseminated cryptococcosis, cryptococcal meningitis, coccidioidal meningitis, cryptosporidiosis, isosporiasis, Kaposi sarcoma, progressive multifocal leukoencephalopathy, and human immunodeficiency virus wasting (weight loss >10%).
Although our hypothesis and analysis were focused on concurrent anemia and inflammation, we also had data available for other causes of anemia. The prevalence were low (<10%) for iron deficiency anemia, vitamin B12 deficiency anemia, and vitamin A deficiency anemia, and none of these deficiencies were associated with clinical failure in multivariable models. Similarly, there was no association of ACD with clinical failure (data not shown).
Compared to no anemia and low inflammation, concurrent anemia and inflammation was associated with increased hazards of incident tuberculosis post-ART in both univariable (aHR, 15.27 [95% CI, 5.98–38.97]) and multivariable (aHR, 15.52 [95% CI, 4.31–53.77]) Cox models adjusted for sex, age, BMI, CD4 count, viral load, and prior tuberculosis diagnosis. No association with virologic failure was observed in either univariable or multivariable models (data not shown).
Characteristics of Individuals With Concurrent Anemia and Inflammation
Among those with concurrent anemia and inflammation, 50% also met criteria for ACD (TfR-F index <1), whereas 6% had an index value >2. In addition, 53% of those with concurrent anemia and inflammation also had vitamin A deficiency, 5% had iron deficiency, and 3% had vitamin B12 deficiency. Concurrent anemia and inflammation had higher serum vitamin B12 concentrations and lower serum vitamin A concentrations compared to those without concurrent anemia and inflammation (Supplementary Appendix Table 1).
DISCUSSION
Studying a case-cohort sample of ART-naive, HIV-infected adults from diverse areas of the world, we observed that both anemia and inflammation were common in the setting of HIV. In addition, to our knowledge, this is the first study to show that concurrent pre-ART anemia and elevated CRP has a 6-fold higher hazard of clinical failure post-ART; neither anemia without inflammation nor inflammation without anemia was associated with increased risk. Identifying concurrent anemia and inflammation in HIV patients prior to ART initiation and focusing on reducing their inflammation levels or thoroughly investigating for occult infections would likely reduce adverse ART outcomes for a particularly high-risk group.
Pre-ART anemia prevalence was >50%, which is consistent with high rates documented in other studies of HIV-infected adults [5]. Our identified risk factors for anemia—namely, female sex, lower CD4 count, and hypoalbuminemia—are also supported by findings from other studies [13, 34–39]. In addition, although we were unable to document causal relationships due to the cross-sectional nature of our assessment, our data suggested that inflammation might be contributing to anemia, whereas deficiencies of vitamin A, vitamin B12, and iron were less common. We did not, however, have information on folic acid deficiency anemia as we did not measure red blood cell folate levels. Regarding baseline anemia and ART outcomes, our analysis is consistent with many studies that have shown an association between pre-ART anemia and clinical failure post-ART [8–11]. Our analysis, however, is among the first to find that concurrent anemia and inflammation, but not anemia or inflammation alone, is a predictor of clinical failure post-ART; further studies are needed to elucidate the mechanism for this relationship, as this association has not been previously described in the setting of HIV. Elevated CRP alone has been previously associated with adverse treatment outcomes [20, 21, 30]. One potential mechanism for this association is that inflammation can lead to metabolic (oxidative stress, weight loss, and wasting) and nutritional (depletion of vitamins and minerals) changes that have been associated with adverse HIV outcomes [30, 40, 41]. However, biological explanations for the association of anemia with HIV outcomes are less clear, with suggestions that anemia might not directly lead to disease progression but is an indicator of an advanced disease stage [14–16]. Our data show that individuals with anemia but no inflammation do not have an increased risk of disease progression, supporting this proxy role of anemia in the setting of inflammation.
We postulate that concurrent anemia and inflammation in HIV represents a condition where uncontrolled inflammation is a direct contributor to anemia (through inhibition of erythropoiesis and suppression of bone marrow [42]) representing a more advanced disease stage, which then results in adverse disease outcomes despite initiation of ART. An alternate explanation for this association is that the concurrent presence of anemia and inflammation may represent the early stages of an occult opportunistic infection, such as tuberculosis. These individuals with concurrent anemia and inflammation are at highest risk for adverse outcomes and may benefit most from clinical therapies (ie, anti-inflammatory drugs or thorough investigation for occult infections). Due to the relatively high treatment adherence of our study population [43], the lack of adherence is not a likely explanation for the observed increase in clinical failure risk for this group. As inflammation may be multifactorial, it seems possible that individuals with inflammation but without anemia are not at increased risk because they have transient inflammation or have less advanced disease stage; we observe that they have a longer median time to disease progression and have lower median CRP levels (data not shown) than the group with concurrent anemia and inflammation. Thus, concurrent anemia might help us distinguish if a patient's elevated CRP is cause for concern (due to its advanced stage or occult infection). Future studies are needed in which anti-inflammatory drugs are administered in a randomized trial of HIV-infected individuals with high inflammation to determine if reducing inflammation alone is enough to reduce adverse outcomes. Alternatively, if this represents occult infections, then changes in clinical practice, including a more thorough investigation for occult infections and closer monitoring after ART initiation, are needed. In addition, future studies are needed to determine if concomitant parasitic infections, known to cause anemia and inflammation, might explain the increased clinical failure [44]. Although there are no established criteria to define ACD, previous studies have suggested using TfR-F index values <1 [18, 29]. Interestingly, our CRP-based definition identified an overlapping yet distinct population with better prognostic value than the TfR-F index. Given that our CRP-based definition also describes a population where concurrent inflammation and anemia is contributing to adverse outcomes, it is possible that the definition of ACD based on TfR-F index alone, due to its lack of prognostic value, might not be as valid for HIV-infected populations.
Strengths of our study include the prospective design to determine associations and the use of a random subcohort for prevalence estimates of anemia among HIV-infected patients initiating ART. A limitation of our study was the exclusion of participants with severe anemia (hemoglobin <7.5 g/dL), which may have underestimated the effect of concurrent anemia and inflammation on clinical failure. The parent study excluded individuals with CD4 counts >300 cells/µL; thus, future studies are needed to determine if these findings are generalizable to HIV-infected individuals with higher CD4 counts. Our study lacked adequate power to detect effect on mortality as an independent endpoint. Nonetheless, we were able to observe an association with outcome even when only considering tuberculosis as an outcome (40% of the cases), which suggests that this association is clinically important.
In conclusion, concurrent anemia and CRP-defined inflammation could be a useful marker in HIV-infected individuals initiating ART to identify people at high risk for clinical treatment failure. Whether these individuals have a more advanced HIV disease state or occult opportunistic infections, future therapeutics and interventions beyond ART that can reduce inflammation or identify occult infections are needed to improve treatment outcomes.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors thank the The Prospective Evaluation of Antiretrovirals in Resource-Limited Settings study participants for volunteering their time and efforts.
Author contributions. R. S. conducted the data analysis and wrote the primary version of the manuscript. W.-T. Y., N. G., P. C., R. C. B., J. S. C., and A. M. T. contributed to data interpretation and manuscript review. S. B., A. L. R., S. W. C., N. M., C. K., S. P., W. S., C. R., P. S., B. S., S. P., and S. T. contributed to data collection and manuscript review. R. D. S. contributed to study design, laboratory testing, and review of the manuscript. T. B. C. contributed to study design, data collection, oversight of study implementation, and manuscript review. A. G. obtained funding and contributed to study design, manuscript writing, and review.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funders had no role in study design, data collection, analysis, publication decision, or manuscript preparation.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grant numbers UM1 AI068634, UM1 AI068636, UM1 AI106701, and R01 AI080417). The parent trial A5175 was also supported in part by Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, and GlaxoSmithKline.
Potential conflicts of interest. T. B. C. is an advisory board member for Gilead Sciences. A. G. has received grant funding from Gilead Foundation. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Murray CJ, Ortblad KF, Guinovart C, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384:1005–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moh R, Danel C, Messou E, et al. Incidence and determinants of mortality and morbidity following early antiretroviral therapy initiation in HIV-infected adults in West Africa. AIDS 2007; 21:2483–91. [DOI] [PubMed] [Google Scholar]
- 3.Moore D, Liechty C, Ekwaru P, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS 2007; 21:713–9. [DOI] [PubMed] [Google Scholar]
- 4.Paton NI, Sangeetha S, Earnest A, Bellamy R. The impact of malnutrition on survival and the CD4 count response in HIV-infected patients starting antiretroviral therapy. HIV Med 2006; 7:323–30. [DOI] [PubMed] [Google Scholar]
- 5.Belperio PS, Rhew DC. Prevalence and outcomes of anemia in individuals with human immunodeficiency virus: a systematic review of the literature. Am J Med 2004; 116(suppl 7A):27S–43. [DOI] [PubMed] [Google Scholar]
- 6.Gupta A, Nadkarni G, Yang WT, et al. Early mortality in adults initiating antiretroviral therapy (ART) in low- and middle-income countries (LMIC): a systematic review and meta-analysis. PLoS One 2011; 6:e28691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan PS, Hanson DL, Chu SY, Jones JL, Ward JW. Epidemiology of anemia in human immunodeficiency virus (HIV)-infected persons: results from the multistate adult and adolescent spectrum of HIV disease surveillance project. Blood 1998; 91:301–8. [PubMed] [Google Scholar]
- 8.Russell EC, Charalambous S, Pemba L, Churchyard GJ, Grant AD, Fielding K. Low haemoglobin predicts early mortality among adults starting antiretroviral therapy in an HIV care programme in South Africa: a cohort study. BMC Public Health 2010; 10:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah S, Smith CJ, Lampe F, et al. Haemoglobin and albumin as markers of HIV disease progression in the highly active antiretroviral therapy era: relationships with gender. HIV Med 2007; 8:38–45. [DOI] [PubMed] [Google Scholar]
- 10.Duong T, Jourdain G, Ngo-Giang-Huong N, et al. Laboratory and clinical predictors of disease progression following initiation of combination therapy in HIV-infected adults in Thailand. PLoS One 2012; 7:e43375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fregonese F, Collins IJ, Jourdain G, et al. Predictors of 5-year mortality in HIV-infected adults starting highly active antiretroviral therapy in Thailand. J Acquir Immune Defic Syndr 2012; 60:91–8. [DOI] [PubMed] [Google Scholar]
- 12.Balarajan Y, Ramakrishnan U, Ozaltin E, Shankar AH, Subramanian SV. Anaemia in low-income and middle-income countries. Lancet 2011; 378:2123–35. [DOI] [PubMed] [Google Scholar]
- 13.Volberding PA, Levine AM, Dieterich D, Mildvan D, Mitsuyasu R, Saag M. Anemia in HIV infection: clinical impact and evidence-based management strategies. Clin Infect Dis 2004; 38:1454–63. [DOI] [PubMed] [Google Scholar]
- 14.Berhane K, Karim R, Cohen MH, et al. Impact of highly active antiretroviral therapy on anemia and relationship between anemia and survival in a large cohort of HIV-infected women: Women's Interagency HIV Study. J Acquir Immune Defic Syndr 2004; 37:1245–52. [DOI] [PubMed] [Google Scholar]
- 15.Santiago-Rodriguez EJ, Mayor AM, Fernandez-Santos DM, Ruiz-Candelaria Y, Hunter-Mellado RF. Anemia in a cohort of HIV-infected Hispanics: prevalence, associated factors and impact on one-year mortality. BMC Res Notes 2014; 7:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris RJ, Sterne JA, Abgrall S, et al. Prognostic importance of anaemia in HIV type-1-infected patients starting antiretroviral therapy: collaborative analysis of prospective cohort studies. Antivir Ther 2008; 13:959–67. [PMC free article] [PubMed] [Google Scholar]
- 17.Kupka R, Msamanga GI, Mugusi F, Petraro P, Hunter DJ, Fawzi WW. Iron status is an important cause of anemia in HIV-infected Tanzanian women but is not related to accelerated HIV disease progression. J Nutr 2007; 137:2317–23. [DOI] [PubMed] [Google Scholar]
- 18.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med 2005; 352:1011–23. [DOI] [PubMed] [Google Scholar]
- 19.Yoon C, Davis JL, Huang L, et al. Point-of-care C-reactive protein testing to facilitate implementation of isoniazid preventive therapy for people living with HIV. J Acquir Immune Defic Syndr 2014; 65:551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell TB, Smeaton LM, Kumarasamy N, et al. Efficacy and safety of three antiretroviral regimens for initial treatment of HIV-1: a randomized clinical trial in diverse multinational settings. PLoS Med 2012; 9:e1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol 1999; 52:1165–72. [DOI] [PubMed] [Google Scholar]
- 24.Onland-Moret NC, van der AD, van der Schouw YT, et al. Analysis of case-cohort data: a comparison of different methods. J Clin Epidemiol 2007; 60:350–5. [DOI] [PubMed] [Google Scholar]
- 25.Cai J, Zeng D. Sample size/power calculation for case-cohort studies. Biometrics 2004; 60:1015–24. [DOI] [PubMed] [Google Scholar]
- 26.Havers F, Smeaton L, Gupte N, et al. 25-Hydroxyvitamin D insufficiency and deficiency is associated with HIV disease progression and virological failure post-antiretroviral therapy initiation in diverse multinational settings. J Infect Dis 2014; 210:244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr 2009; 12:444–54. [DOI] [PubMed] [Google Scholar]
- 28.Firnhaber C, Smeaton L, Saukila N, et al. Comparisons of anemia, thrombocytopenia, and neutropenia at initiation of HIV antiretroviral therapy in Africa, Asia, and the Americas. Int J Infect Dis 2010; 14:e1088–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Punnonen K, Irjala K, Rajamaki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood 1997; 89:1052–7. [PubMed] [Google Scholar]
- 30.Drain PK, Kupka R, Msamanga GI, Urassa W, Mugusi F, Fawzi WW. C-reactive protein independently predicts HIV-related outcomes among women and children in a resource-poor setting. AIDS 2007; 21:2067–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grant FK, Martorell R, Flores-Ayala R, et al. Comparison of indicators of iron deficiency in Kenyan children. Am J Clin Nutr 2012; 95:1231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semba RD, Graham NM, Caiaffa WT, Margolick JB, Clement L, Vlahov D. Increased mortality associated with vitamin A deficiency during human immunodeficiency virus type 1 infection. Arch Intern Med 1993; 153:2149–54. [PubMed] [Google Scholar]
- 33.Jiang T, Christian P, Khatry SK, Wu L, West KP., Jr Micronutrient deficiencies in early pregnancy are common, concurrent, and vary by season among rural Nepali pregnant women. J Nutr 2005; 135:1106–12. [DOI] [PubMed] [Google Scholar]
- 34.Sudfeld CR, Isanaka S, Aboud S, et al. Association of serum albumin concentration with mortality, morbidity, CD4 T-cell reconstitution among Tanzanians initiating antiretroviral therapy. J Infect Dis 2013; 207:1370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SW, Kang YA, Yoon YS, et al. The prevalence and evolution of anemia associated with tuberculosis. J Korean Med Sci 2006; 21:1028–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levine AM, Berhane K, Masri-Lavine L, et al. Prevalence and correlates of anemia in a large cohort of HIV-infected women: Women's Interagency HIV Study. J Acquir Immune Defic Syndr 2001; 26:28–35. [DOI] [PubMed] [Google Scholar]
- 37.Masaisa F, Gahutu JB, Mukiibi J, Delanghe J, Philippe J. Anemia in human immunodeficiency virus-infected and uninfected women in Rwanda. Am Trop Med Hyg 2011; 84:456–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Semba RD, Shah N, Klein RS, Mayer KH, Schuman P, Vlahov D. Prevalence and cumulative incidence of and risk factors for anemia in a multicenter cohort study of human immunodeficiency virus-infected and -uninfected women. Clin Infect Dis 2002; 34:260–6. [DOI] [PubMed] [Google Scholar]
- 39.Subbaraman R, Devaleenal B, Selvamuthu P, et al. Factors associated with anaemia in HIV-infected individuals in southern India. Int J STD AIDS 2009; 20:489–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rimaniol AC, Zylberberg H, Zavala F, Viard JP. Inflammatory cytokines and inhibitors in HIV infection: correlation between interleukin-1 receptor antagonist and weight loss. AIDS 1996; 10:1349–56. [DOI] [PubMed] [Google Scholar]
- 41.Ford ES, Liu S, Mannino DM, Giles WH, Smith SJ. C-reactive protein concentration and concentrations of blood vitamins, carotenoids, and selenium among United States adults. Eur J Clin Nutr 2003; 57:1157–63. [DOI] [PubMed] [Google Scholar]
- 42.Steinvil A, Rogowski O, Banai S, et al. Anemia and inflammation have an additive value in risk stratification of patients undergoing coronary interventions. J Cardiovasc Med (Hagerstown) 2015; 16:106–11. [DOI] [PubMed] [Google Scholar]
- 43.Safren SA, Biello KB, Smeaton L, et al. Psychosocial predictors of non-adherence and treatment failure in a large scale multi-national trial of antiretroviral therapy for HIV: data from the ACTG A5175/PEARLS trial. PLoS One 2014; 9:e104178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.King CH, Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic Illn 2008; 4:65–79. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.