Abstract
Although the root system is indispensable for absorption of nutrients and water, it is poorly studied in maize owing to the difficulties of direct measurement of roots. Here, 103 maize lines were used to compare root architectures under well-watered and water-stressed conditions. Significant genetic variation, with medium to high heritability and significant correlations, was observed for root traits. Total root length (TRL) and total root surface area (TSA) had high phenotypical diversity, and TRL was positively correlated with TSA, root volume, and root forks. The first two principal components explained 94.01% and 91.15% of total root variation in well-watered and water-stressed conditions, respectively. Thus, TRL and TSA, major contributors to root variation, can be used as favorable selection criteria at the seedling stage. We found that stiff stalk and non-stiff stalk groups (temperate backgrounds) showed relatively higher mean values for root morphological diversity than the TST group (tropical/subtropical background). Of the tested lines, 7, 42, 45, and 9 were classified as drought sensitive, moderately sensitive, moderately drought tolerant, and highly drought tolerant, respectively. Seven of the 9 extremely drought tolerant lines were from the TST group, suggesting that TST germplasms harbor valuable genetic resources for drought tolerance that could be used in breeding to improve abiotic stress tolerance in maize.
Keywords: maize, drought tolerance, root system, heterotic groups
Introduction
Maize is one of the most important cultivated grain crops around the world and is widely used to provide food, forage, and industrial raw materials. Due to rapid changes in populations, society, and economies, the demand for maize is expected to be higher than for wheat or rice by 2020 (Pingali 2001). The productivity and yield of maize are frequently limited by various biotic and abiotic stress factors, such as drought, salinity, high and low temperatures, nutrient deficiencies, disease, and insect pests. Drought stress can affect yield through different mechanisms across the whole life cycle of the maize plant (Leach et al. 2011). Therefore, drought is one of the most serious causes of productivity loss. Many studies have pinpointed flowering as the most drought sensitive stage, although seedling establishment is also important because of its influence on plant stand establishment (Bänziger et al. 2000, Leach et al. 2011).
In addition to anthesis-silking interval (ASI), new traits and methods have been used to help identify drought tolerant genotypes at different developmental stages and to further the creation of new cultivars (Bruce et al. 2002, Meeks et al. 2013). Drought can damage a field at any time throughout the season. The fate of seedlings will determine the structure and dynamics of most plant populations according to the “stress gradient hypothesis” (De La Cruz et al. 2008, Kitajima and Fenner 2000). Thus, phenotypical evaluation at the seedling stage is regarded as an attractive approach because it is a high-throughput and low cost method that saves space and time (Meeks et al. 2013). This approach has been successfully used to develop drought tolerant varieties in cowpea (Singh et al. 1999, Singh and Matsui 2002), cotton (Longenberger et al. 2006), wheat (Tomar and Kumar 2004), and maize (Meeks et al. 2013, Pace et al. 2014, Ruta et al. 2010). Another advantage of using seedling drought screens, where young seedlings undergo cycles of water stress in the greenhouse, is that phenotypical variation caused by experimental errors can be controlled better because the plants are much more uniform at an early seeding stage, compared to other periods of plant development (Wang et al. 2015). In cowpea, traits related to drought tolerance at the seedling stage have been shown to be associated with productivity and yield of adult plants (Meeks et al. 2013, Singh et al. 1999, Singh and Matsui 2002). In maize, significant correlations between seminal root traits and adult field traits have been reported, and for shoot weight versus adult plant height, and lateral root length versus brace root development (Landi et al. 1998, 2001, Pace et al. 2014).
Under drought stress, plants seek to reduce the impact of the lack of water by reducing the transpiration rate and by increasing the efficiency of water acquisition from the soil (Végh 2013). Plants have developed numerous adaptive mechanisms for better growth under drought conditions such as modification of the root system, osmotic adjustments, stomatal regulation, chemical production, and accumulation. The root system not only supports the above ground organs of the plant but also plays a crucial role in obtaining water by accessing sources far down in the soil profile. The roots are the first organs to sense a water shortage (Trachsel et al. 2010). The root system is therefore generally considered as the most important organ with respect to improving crop adaptation to water stress (Vadez 2014). Maize responds to drought stress by redirecting root growth and dry matter accumulation away from the shoot to the root (Ribaut et al. 2009, Sharp et al. 2004). In maize, this shift involves an increase in root cell wall extensibility that is mediated by increased levels of xyloglucan endotransglucosylases/hydrolases and other cell wall-loosening factors at the root tip. These modifications result in sustained growth of the root and inhibited growth of the shoot in the face of decreased water potential (Ober and Sharp 2007).
Root morphology is a poorly studied maize characteristic due to the difficulties of making direct measurements under the soil and also of observing or removing roots of plants grown under agronomic conditions. Genetic improvement to produce deep-rooted plants is considered an important strategy for improving water capture and yield stability (Kondo et al. 2003). Variation in root system architecture can be explored to improve plant vigor by improving water use efficiency and nutrient extraction under difficult growing conditions (Malamy and Benfey 1997). Primary root elongation rate and ABA accumulation under different water conditions have been studied in 12 maize inbred lines to assess the relationship between root growth and hormonal conditions (Leach et al. 2011). Differences in morphological root traits have been characterized at the seedling stage in 74 maize inbred lines to quantify the phenotypic and genotypic coefficient of variation, heritability, and interrelationships between these traits (Kumar et al. 2012). Recently, 384 inbred lines from the Ames panel were genotyped with high density single nucleotide polymorphism markers using genotyping-by-sequencing technology to study phenotypic variation of 22 seedling root architecture traits (Pace et al. 2015). This analysis identified SNP markers throughout the genome that are associated with root architecture traits and the locations of associated SNP markers for possible candidate genes or functional markers for effects on root development have been determined (Pace et al. 2015).
Currently, digital image analysis enables a more accurate and less subjective approach to the analysis of plant root systems; the technology is also quite time- and labor-saving (Bouma et al. 2000, Himmelbauer 2004). Automated phenotypic analysis by digital image software is an innovative approach and there are several software frameworks that extract root morphology traits in two-dimensions in various hierarchies that have been widely used to characterize maize root architecture (Kumar et al. 2012, Pace et al. 2014, 2015, Ruta et al. 2010). In the present study, we aimed to (i) characterize the phenotypic variation for morphological root traits at the seedling stage in 103 maize inbred lines using digital image analysis, (ii) identify root related traits accounting for most of the variation among the tested maize lines, and (iii) compare root phenotypic diversity among different heterotic groups, and evaluate drought tolerance of maize germplasms under well-watered and water-stressed conditions.
Materials and Methods
Plant materials
One hundred and three maize inbred lines from three different heterotic groups, including stiff stalk (SS), non-stiff stalk (NSS), and tropical/subtropical (TST), were selected for identification of root architecture characteristics. Thirty-four of the lines from China were provided by Sichuan Agricultural University of China, 38 maize lines were from the International Maize and Wheat Improvement Center (CIMMYT), 30 maize inbred lines were from the USA, and 1 line was from Nigeria. This panel includes diverse maize germplasms including 45 temperate lines and 58 tropical/subtropical lines, and 23 of the lines were used to establish the Nested Association Mapping populations developed by Buckler et al. (2009). Detailed information on each line is given in Supplemental Table 1.
Plant growth conditions
All the test lines were planted in Jinghong, Yunnan province, China, between October 11, 2011 and February 15, 2012 for seed production. Experiments were performed in a climate-controlled chamber with a 16 h light/8 h dark photoperiod, a temperature cycle of 25°C/18°C (day/night), and 65% relative humidity in June, 2012 and repeated in January, 2013. All tested lines were grown in vermiculite with normal water potential after germination on moist filter paper for 72 h. Seedlings of uniform size at the 2-leaf stage and without visible root injuries were transferred to plastic containers holding nutrient solution (10 L solution/container) with minor modifications of the previously described basal composition (Hoagland and Arnon 1950). The nutrient solution was aerated continuously and renewed every two days. Distilled water was added regularly to maintain the volume. Six plants for each inbred line grown under well-watered (WW) or water-stressed (WS) conditions (induced by adding the osmolyte 20% polyethylene glycol (w/v) PEG8000; Sigma-Aldrich) were selected for measurement of their root parameters at the 5-leaf stage. The concentration of polyethylene glycol and the stress time of four days were based on a previous report (Trachsel et al. 2010).
Root measurements
Measurements were made on seedlings after cultivation in control or low water potential conditions for four days. The seedlings were assigned to the two water regimes using a randomized experimental design, and two replicate experiments were performed. The complete root system was isolated from each plant and placed on a tray with no overlapping of any roots. The WinRhizo Pro 2007a (Regent Instrument Inc., Quebec, Canada) root analysis system was used to investigate root morphology based on images (400 DPI) captured using the EPSON professional scanner (Bouma et al. 2000, Magalhães et al. 2011). The following root parameters were measured: root length (RL, cm), root surface area (RSA, cm2), root average diameter (RAD, cm), root volume (RV, cm3), and the total number of root tips (TRT). More details of each measured parameter are given in Table 1.
Table 1.
Description of the tested traits in the study
| Abbreviated name | Full trait name | Description |
|---|---|---|
| TRL | Total root length (cm) | The average root length of six plants |
| RL | Root length (cm) | RL1–3: The average root length of six plants in diameter between 0.0 and 0.5 mm, 0.5 and 2.0 mm, and greater than 2.0 mm, respectively. |
| TSA | Total root surface area (cm2) | The average root surface area of six plants |
| SA | Root surface area (cm2) | SA1–3: The average root surface area of six plants in diameter between 0.0 and 0.5 mm, 0.5 and 2.0 mm, and greater than 2.0 mm, respectively. |
| TRT | Total root tips | The average number of root tips of six plants |
| RT | Root tips | RT1–3: The average number of root tips of six plants in diameter between 0.0 and 0.5 mm, 0.5 and 2.0 mm, and greater than 2.0 mm, respectively. |
| TRV | Total root volume (cm3) | The average root volume of six plants |
| RV | Root volume (cm3) | RV1–3: The average root volume of six plants in diameter between 0.0 and 0.5 mm, 0.5 and 2.0 mm, and greater than 2.0 mm, respectively. |
| RAD | Root average diameter (cm) | The average root diameter of six plants |
| RF | Root forks | The average root forks of six plants |
Statistical analysis
The data from all measurements of root traits were recorded and compiled in Microsoft Excel 2007. Descriptive analyses including mean, standard deviation (SD), coefficient of variation (CV), analysis of variance (ANOVA), Pearson’s correlation, and heritability (H2) estimates were calculated for the tested traits under WW and WS treatments using the SAS program (v9.3). The estimate of heritability is defined by the formula H = VG/(VG + VE), where VG and VE represent estimates of genetic and environmental variances, respectively (Smith et al. 1998). The estimated values of phenotypes for each inbred line were calculated based on best linear unbiased prediction (BLUP) (Piepho et al. 2008). The BLUP values were then used to classify lines into three different categories according to their performance: (i) low performing lines with non-desirable root characteristics [≤X̄ − SD], (ii) lines with medium performance [≥X̄ − SD] to [≤X̄ + SD], and (iii) high performing genotypes with desirable traits [≥X̄ + SD] (Abdel-Ghani et al. 2012, Zar 2010). A polymorphic diversity index, the Shannon-Weaver diversity index (H′), was calculated for each trait (Hutcheson 1970). A principal component analysis (PCA) was performed to identify the major traits accounting for most of the variation in tested maize inbred lines using the SAS program (v9.3). A comprehensive drought resistance measurement value (D value) was introduced to estimate the tolerance capability of all tested lines. The D value was calculated across traits to evaluate maize drought tolerance using the formulas described below (Xu et al. 2009, Zhang and Xu 2009). The drought resistance coefficient (DRC) represents different drought tolerance in the various maize inbred lines. We use Pj to represent the DRC of the jth trait below:
Fuzzy subordinate function analysis was used to decrease the one-sidedness of a simple individual trait to evaluate drought tolerance in various traits. μ(Xj) stands for the subordinative function value that indicates a positive correlation between trait and drought resistance. By contrast, 1 − μ(Xj) represents a negative correlation between trait and drought resistance.
where
μ(Xj) is the subordinative function value of DRC of the jth trait;
Xj is the DRC of the jth trait;
Xmin is the minimum value of the DRC of the jth trait;
Xmax is the maximum value of the DRC of the jth trait;
Comprehensive drought resistance measurement was made using the formula:
where
D is the comprehensive drought resistance measurement of each maize inbred line under WS condition;
Pj is the DRC of the jth trait in each maize inbred line.
Results
Genetic variation analysis
The BLUP values and descriptive statistics for each root trait for the 103 inbred lines across two repeat experiments (excluding five lines without observed values in both biological repeats) are summarized in Table 2. Phenotypic variation among genotypes for each trait was confirmed by the mean, range, standard deviation, and coefficient of variation. Compared to the WW condition, the mean values of most traits were substantially decreased under the WS condition. A wide range of variation was observed for root traits in the two water treatments. Root length, root surface, and root volume (root diameter greater than 2.5 mm) had relatively higher coefficients of variation (>50%) in both water treatments while average root diameter had relatively lower coefficients of variation (around 15%).
Table 2.
Means, coefficients of variation and heritability estimates for the tested traits under well-watered (WW) and water-stressed (WS) conditions
| Traits | Mean | Standard derivation (SD) | Coefficient of variation (CV%) | Heritability | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| WW | WS | WW | WS | WW | WS | WW | WS | |
| TRL | 470.05 | 316.04 | 174.16 | 122.76 | 37.05 | 38.84 | 0.41 | 0.42 |
| TSA | 81.10 | 55.71 | 31.06 | 22.99 | 38.30 | 41.26 | 0.38 | 0.54 |
| RAD | 0.56 | 0.57 | 0.09 | 0.08 | 15.62 | 14.39 | 0.64 | 0.59 |
| TRV | 1.16 | 0.80 | 0.54 | 0.39 | 46.78 | 48.12 | 0.43 | 0.64 |
| TRT | 699.67 | 549.14 | 269.13 | 208.23 | 38.46 | 37.92 | 0.38 | 0.41 |
| RF | 3184.46 | 1911.93 | 1595.42 | 941.71 | 50.10 | 49.25 | 0.58 | 0.48 |
| RL1 | 309.00 | 197.76 | 129.31 | 83.33 | 41.85 | 56.79 | 0.47 | 0.39 |
| RL2 | 50.51 | 36.08 | 18.72 | 14.86 | 37.07 | 43.19 | 0.31 | 0.51 |
| RL3 | 1.56 | 1.04 | 0.96 | 0.65 | 61.48 | 59.99 | 0.40 | 0.54 |
| SA1 | 21.31 | 7.76 | 8.50 | 4.41 | 39.89 | 38.27 | 0.43 | 0.43 |
| SA2 | 13.87 | 9.41 | 5.56 | 4.07 | 40.12 | 46.29 | 0.35 | 0.58 |
| SA3 | 1.55 | 1.05 | 0.95 | 0.63 | 60.89 | 59.37 | 0.38 | 0.50 |
| RV1 | 0.15 | 0.10 | 0.06 | 0.04 | 39.65 | 38.39 | 0.42 | 0.43 |
| RV2 | 0.35 | 0.23 | 0.15 | 0.11 | 44.10 | 36.05 | 0.40 | 0.61 |
| RV3 | 0.15 | 0.10 | 0.09 | 0.06 | 62.05 | 55.36 | 0.35 | 0.33 |
TRL: total root length; TSA: total root surface area; RAD: root average diameter; TRV: total root volume; TRT: total root tips; RF: root forks; RL1–3, SA1–3 and RV1–3 indicate average root length, root surface area and root volume in diameter between 0.0 and 0.5 mm, 0.5 and 2.0 mm and greater than 2.0 mm, respectively.
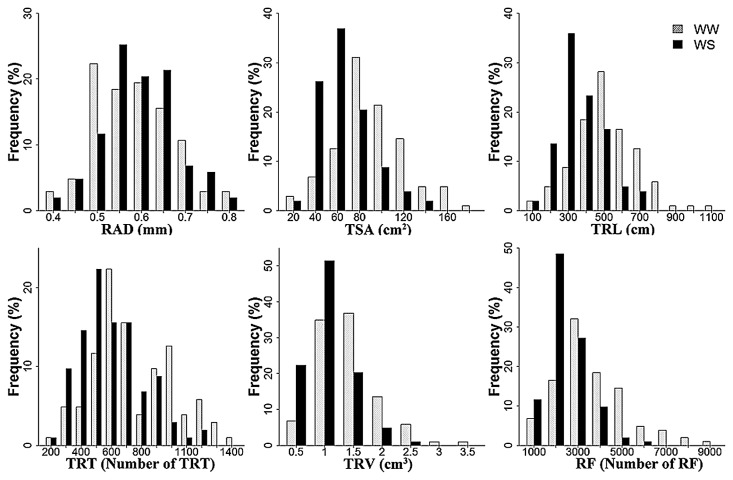
ANOVA revealed significant genetic variation among genotypes for nine traits (Table 3). Genotypic variation was significant for six traits at P < 0.001 and eight traits showed significant variation under different water regimes, the exception being average root diameter. The results indicate that these traits were significantly affected by water supply in the tested maize lines. The level of variation was also reflected by the distribution of traits representing different drought resistance criteria (Fig. 1). Histograms of frequency distribution of root traits showed approximately normal distributions. Under the WS condition, the values for seven traits (TRL, TSA, RAD, TRV, TRT, and RF) decreased significantly.
Table 3.
Analysis of variance for the tested traits under two water regimes
| Variables | Type III Sum of squares | Mean square | F value | Significance | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Genotype | Treatment | Genotype | Treatment | Genotype | Treatment | Genotype | Treatment | |
| df | 102 | 1 | 102 | 1 | 102 | 1 | 102 | 1 |
| TRL | 6,305,553.64 | 2,280,575.16 | 61,218.97 | 2,280,575.16 | 1.94 | 72.15 | *** | *** |
| TSA | 219,795.56 | 62,480.89 | 2,133.94 | 62,480.89 | 2.63 | 77.12 | *** | *** |
| RAD | 2.35 | 0.00 | 0.02 | 0.00 | 2.73 | 0.45 | *** | NS |
| TRV | 67.66 | 12.48 | 0.66 | 12.48 | 3.21 | 60.89 | *** | *** |
| TRT | 15,746,306.45 | 2,084,110.65 | 152,876.76 | 2,084,110.65 | 1.14 | 15.53 | NS | *** |
| RF | 481,303,981.69 | 154,999,703.12 | 4,672,854.19 | 154,999,703.12 | 3.33 | 110.38 | *** | *** |
| RL1 | 3,312,588.26 | 1,176,337.89 | 32,161.05 | 1,176,337.89 | 1.59 | 58.22 | *** | *** |
| RL2 | 85,641.90 | 20,168.48 | 831.47 | 20,168.48 | 2.62 | 63.66 | *** | *** |
| RL3 | 197.11 | 26.94 | 1.91 | 26.94 | 1.68 | 23.61 | *** | *** |
| SA1 | 14,044.77 | 5,048.83 | 136.36 | 5,048.83 | 1.70 | 62.79 | *** | *** |
| SA2 | 7,046.01 | 1,918.43 | 68.41 | 1,918.43 | 2.68 | 75.23 | *** | *** |
| SA3 | 187.12 | 25.17 | 1.82 | 25.17 | 1.41 | 19.50 | * | *** |
| RV1 | 0.66 | 0.23 | 0.01 | 0.23 | 1.86 | 67.85 | *** | *** |
| RV2 | 5.18 | 1.40 | 0.05 | 1.40 | 2.80 | 77.90 | *** | *** |
| RV3 | 1.68 | 0.19 | 0.02 | 0.19 | 1.12 | 13.36 | NS | *** |
df: degree of freedom; TRL: total root length; TSA: total root surface area; RAD: root average diameter; TRV: total root volume; TRT: total root tips; RF: root forks; RL1–3, SA1–3 and RV1–3 indicate average root length, root surface area and root volume in diameter between 0.0 and 0.5 mm, 0.5 and 2.0 mm and greater than 2.0 mm, respectively.
significant at P < 0.05, P < 0.01 and P < 0.001, respectively. NS, not significant.
Fig. 1.
Frequency distribution of variation for 9 traits in 103 maize lines. TRL: total root length; TSA: total root surface area; RAD: root average diameter; TRV: total root volume; TRT: total root tips; RF: root forks. WW: well-watered condition; WS: water-stressed condition.
Heritability estimates
Heritability estimates for the traits are shown in Table 2. The traits ranged from 0.315 (RL2 under WW) to 0.636 (RAD under WW). RAD showed relatively high heritability (0.636 for WW and 0.593 for WS), as did RF (0.581 for WW and 0.480 for WS) and TRV (0.429 for WW and 0.637 for WS). TRL (0.409 for WW and 0.417 for WS), TSA (0.383 for WW and 0.539 for WS), and TRT (0.376 for WW and 0.406 for WS) showed lower heritabilities. The heritability estimates for RF and RAD were higher under WW than WS conditions, but for other traits were lower under WW than WS conditions. These results indicate that heritability of root characteristics was generally lower than other agronomic traits, and that the root morphology of maize seedlings was strongly influenced by the environment. The widely used indicator, RAD was highly heritable, suggesting that it is a reliable indicator for drought tolerance.
Genetic correlations among tested traits
Pearson correlations among the traits were calculated, and significant correlations (P < 0.01) were observed between all pairs of traits (Table 4). Relatively high positive correlations were found for root-related traits under the two water regimes, for example, TRL and TSA (r = 0.894 and r = 0.891 for WW and WS, respectively), and TRV and TSA (r = 0.930 and r = 0.948 for WW and WS, respectively). RAD showed significant negative correlations with TRL, TRT, RF, RL1, RV1, and RSA1 under the two water regimes.
Table 4.
Genetic correlation among tested traits under well-watered (WW) and water-stressed (WS) conditions
| TRL | TSA | RAD | TRV | TRT | RF | |
|---|---|---|---|---|---|---|
| WW | ||||||
| TRL | 1 | .894** | −.127 | .670** | .764** | .941** |
| TSA | 1 | .304** | .930** | .561** | .833** | |
| RAD | 1 | .606** | −.358** | −.134 | ||
| TRV | 1 | .304** | .613** | |||
| TRT | 1 | .755** | ||||
| RF | 1 | |||||
| WS | ||||||
| TRL | 1 | .891** | −.065 | .739** | .704** | .913** |
| TSA | 1 | .287** | .948** | .605** | .849** | |
| RAD | 1 | .541** | −.231* | .005 | ||
| TRV | 1 | .432** | .724** | |||
| TRT | 1 | .587** | ||||
| RF | 1 | |||||
TRL: total root length; TSA: total root surface area; RAD: root average diameter; TRV: total root volume; TRT: total root tips; RF: root forks; WW: well water; WS: water stress;
significant at P < 0.05, P < 0.01 and P < 0.001, respectively.
Diversity patterns with respect to heterotic groups
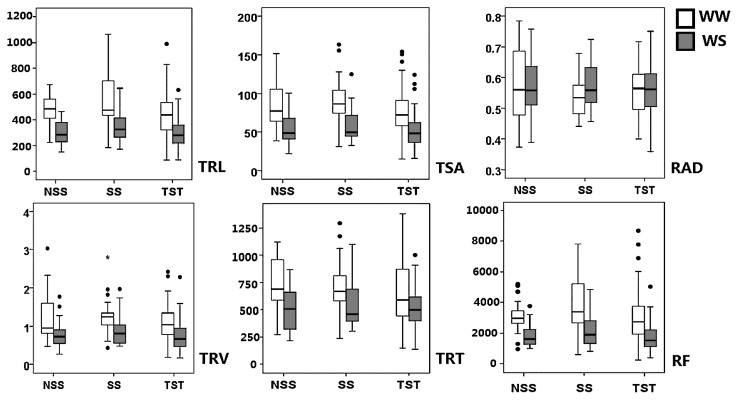
A comparison of the root characteristics of the different heterotic groups identified clear variation for all traits in the three groups. For most root traits, the SS and NSS groups (temperate maize backgrounds) showed higher mean values, while the TST group (tropical and subtropical backgrounds) displayed relatively lower mean values. Remarkable variation was found for total root length, and the mean values in the three heterotic groups, NSS, SS, and TST, were 501.8 cm, 526.8 cm, and 472.9 cm, respectively. Similar results were also observed for other traits including the number of root forks and tips, total root surface area and total root volume. The mean, median, and range of phenotypic variation in NSS, SS, and TST groups are shown in Fig. 2.
Fig. 2.
Box plot showing the medians and ranges of phenotypic variation in three heterotic groups of maize. TRL: total root length; TSA: total root surface area; RAD: root average diameter; TRV: total root volume; TRT: total root tips; RF: root forks. WW: well-watered condition; WS: water-stressed condition. NSS: non-Stiff Stalk; SS: Stiff Stalk; TST: subpopulation including tropical/subtropical lines.
The maize lines in the NSS, TST, and SS groups were classified into three categories, namely high, medium, and low performance (Table 5). Under the WS condition, 6 (21%), 5 (9%), and 2 (11%) lines from NSS, TST, and SS groups, respectively, showed large total root volume (TRV) [≥X̄ + SD]. Under the WW condition, 5 (18%), 11 (19%), and 2 (11%) lines from NSS, TST, and SS groups, respectively showed large TRV. For all the traits except TRV, the TST group had a lower proportion of lines with [≥X̄ + SD] than either the NSS or SS groups under the two water regimes (Table 5). The results indicated that the NSS and SS groups (temperate maize backgrounds) contained more lines with desirable root characteristics than the TST group (tropical/subtropical germplasms) under the two water regimes.
Table 5.
The Shannon-Weaver diversity index (H′) and performance categories under drought stress
| Traits | Treatment | All maize lines | NSS group | TST group | SS group | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||||||
| Low | Medium | High | H′ | Low | Medium | High | H′ | Low | Medium | High | H′ | Low | Medium | High | H′ | ||
| TRL | WW | 13 | 83 | 13 | 0.72 | 3 | 23 | 2 | 0.59 | 8 | 43 | 6 | 0.73 | 1 | 14 | 3 | 0.65 |
| TRL | WS | 16 | 73 | 21 | 0.87 | 6 | 16 | 6 | 0.98 | 7 | 41 | 10 | 0.80 | 2 | 14 | 2 | 0.68 |
| TSA | WW | 12 | 82 | 15 | 0.73 | 2 | 22 | 4 | 0.66 | 8 | 42 | 7 | 0.76 | 3 | 11 | 4 | 0.93 |
| TSA | WS | 14 | 76 | 20 | 0.83 | 3 | 20 | 5 | 0.79 | 7 | 41 | 10 | 0.80 | 2 | 14 | 2 | 0.68 |
| RAD | WW | 19 | 71 | 19 | 0.89 | 5 | 17 | 6 | 0.94 | 8 | 41 | 8 | 0.79 | 5 | 9 | 4 | 1.04 |
| RAD | WS | 17 | 77 | 16 | 0.82 | 6 | 19 | 3 | 0.83 | 9 | 40 | 9 | 0.83 | 2 | 13 | 3 | 0.78 |
| TRV | WW | 10 | 86 | 13 | 0.66 | 3 | 19 | 6 | 0.83 | 4 | 48 | 5 | 0.54 | 2 | 14 | 2 | 0.68 |
| TRV | WS | 11 | 82 | 17 | 0.74 | 3 | 20 | 5 | 0.79 | 7 | 40 | 11 | 0.83 | 0 | 16 | 2 | 0.35 |
| TRT | WW | 12 | 80 | 17 | 0.76 | 2 | 22 | 4 | 0.66 | 6 | 42 | 9 | 0.75 | 1 | 14 | 3 | 0.65 |
| TRT | WS | 18 | 74 | 18 | 0.86 | 5 | 18 | 5 | 0.90 | 8 | 43 | 7 | 0.75 | 2 | 13 | 3 | 0.78 |
| RF | WW | 9 | 87 | 13 | 0.64 | 2 | 24 | 2 | 0.51 | 5 | 46 | 6 | 0.62 | 1 | 14 | 3 | 0.65 |
| RF | WS | 12 | 79 | 19 | 0.78 | 4 | 17 | 7 | 0.93 | 5 | 43 | 10 | 0.74 | 2 | 13 | 3 | 0.78 |
TRL: total root length; TSA: total root surface area; RAD: root average diameter; TRV: total root volume; TRT: total root tips; RF: root forks; NSS: non-Stiff Stalk; SS: Stiff Stalk; TST: subpopulation including tropical/subtropical lines; H′: Shannon-Weaver diversity index; WW: well-watered condition; WS: water-stressed condition.
The Shannon-Weaver diversity index (H′) was calculated for all the lines and the three heterotic groups (Table 5). The H′ values varied in the traits TRL, TSA, RAD, TRV, TRT, and RF with an average of 0.770, 0.784, 0.745, and 0.721 for all the maize lines, NSS, TST, and SS groups, respectively. Under the two water regimes, RAD and TRL showed relatively higher levels of variation. RF was less variable in all lines and across the three heterotic groups. The variation in root morphology among the three heterotic groups indicated that the NSS group had the highest average H′ value, while the SS and TST groups had almost similar values. All the root traits except RAD showed higher H′ values and higher diversity under the WS condition than the WW condition. For three traits, TRL, TRT, and RF, higher diversity was present in the TST group under the WW condition than under the WS condition.
Principal component analysis
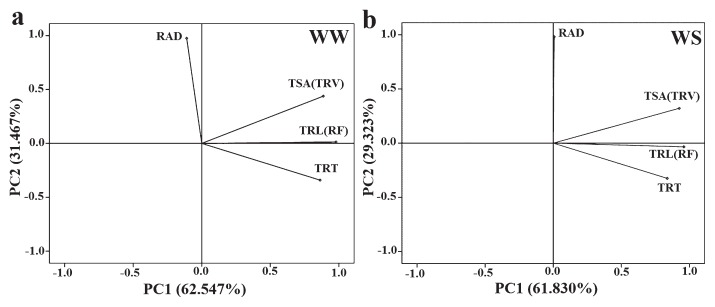
Based on the genetic correlations among different root traits, two traits with correlation coefficients higher than 0.9 were combined in our principal components analysis. The first two principal components (PCs) explained about 94.01% and 91.15% of the total variation among the maize lines under WW and WS conditions, respectively (Fig. 3a, 3b). The first PC, which explained more than 61% of the total variation, revealed that TSA and TRL, and their highly correlated traits TRV and RF, were the most important contributing traits. The most important trait in the second principal component, which contributed nearly 30% of the total variation, was RAD.
Fig. 3.
Principal component analysis of four traits under WW (a) and WS (b) condition. TRL: total root length; TSA: total root surface area; RAD: root average diameter; TRT: total root tips; TRV: total root volume; RF: root forks. WW: well-watered condition; WS: water-stressed condition.
Germplasm resources for root morphology improvement under drought stress
The maize lines were classified into three categories, high, medium, and low performance with respect to each trait (Table 5). Most maize lines were found to fall into the medium performance category, and this was the case for all three heterotic groups. The proportion of maize lines with high performance ranged from 10 to 15%, that with medium performance ranged from 70 to 75%, and that with low performance ranged from 14 to 17%. The D value, as a synthetic index, was used to evaluate root morphology among the maize lines under drought stress (Supplemental Table 1). Based on D values, the lines could be classified into four groups. Group 1 with 7 lines, including 5003, Ji853, and Zheng22, showed sensitivity to drought with D values lower than 0.20. Group 2, with 42 lines, including Dan598 and ES40, showed moderate sensitivity to drought stress with D values between 0.20 and 0.40. Group 3 with 45 lines showed moderate tolerance with D values between 0.40 and 0.70. Group 4, with 9 lines, including CML247 and Mo17, showed drought tolerance with D values greater than 0.70. Interestingly, 7 of the 9 extremely drought tolerant lines came from the TST group (tropical genetic background). The maize lines that were extremely sensitive or extremely tolerant under drought stress are listed in Supplemental Table 1.
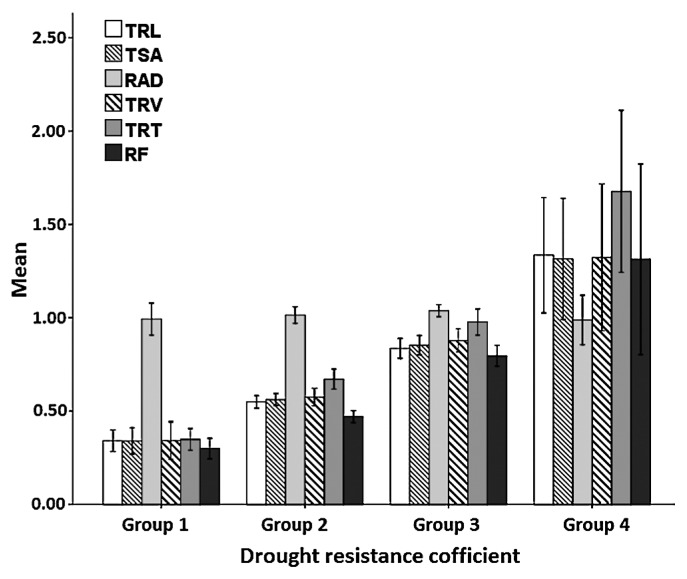
The mean values and standard deviations of the drought resistance coefficient (DRC) for each trait in the four groups with different levels of drought tolerance are shown in Fig. 4. The mean values of DRC for all root traits were lowest in group 1, moderate in groups 2 and 3, and highest in group 4, except for RAD. This result indicates that drought tolerant maize lines with higher D values also had higher drought resistance coefficients, and consistent results were obtained when these two indices were calculated based on the root morphology traits used for drought tolerance screening.
Fig. 4.
The mean values and standard deviations of drought resistance coefficient for 9 traits in three groups classified for drought tolerance. Groups 1, 2, 3 and 4 represent inbred lines identified with drought sensitivity, moderate sensitivity, moderate drought tolerance and high tolerance ability under drought stress. N = 7, 42, 45 and 9 for groups 1, 2, 3 and 4, respectively. Bars are the standard deviation. TRL: total root length; TSA: total root surface area; RAD: root average diameter; TRV: total root volume; TRT: total root tips; RF: root forks.
Discussion
Drought stress affects many of the processes required for plant growth and development, for example, it inhibits cell elongation, reduces cell division, decreases the photosynthetic rate, and modifies root morphology (Kumar et al. 2004). Maize is susceptible to drought stress throughout its life cycle, and water deficits during mid to late vegetative development and flowering significantly affect root morphology, reproductive tissues, biomass production, and gain yield (Chen et al. 2012, Heiniger 2001, Kumar et al. 2004, Svačina et al. 2014). The root system is an indispensable organ for absorption of nutrients and water in plants and its physiological characteristics largely determine the rates of absorption of nutrients and water (Hodge et al. 2009). Roots, embedded in the soil, are the first plant organs sensing water shortage (Trachsel et al. 2010). In the present investigation, the integrity of the studied root systems was maintained by growing the tested inbred lines in solution culture. It may seem counter-intuitive to conduct a water-stressed experiment in a nutrition solution. However, drought stress is manifested in multiple ways in plants grown in soil including mechanical impedance and desiccation (Trachsel et al. 2010). PEG with a molecular weight above 8000 Da is an osmolyte that does not enter the root system; it can been used to modify the osmotic potential of nutrient solutions and induce desiccation stress in a relatively controlled manner (Trachsel et al. 2010). A previous study examined the effect of growing plants under PEG8000 stress and quantified the ability of three tropical maize inbred lines to proliferate roots under conditions of adequate water supply compared to desiccation stress (Trachsel et al. 2010). Drought condition can be successfully mimicked by PEG treatment, which reduces the external free water concentration without altering the ionic composition of the cell and causes reduction in leaf water potentials (Claes et al. 1990, Ruta et al. 2010). Thus, root system responses to drought stress can be tested without root damage in solution cultures by controlling access to water.
Enhanced root growth, as evaluated by root dry weight and root-to-shoot ratios in moderate and high P-efficiency maize lines, is connected to the search for nutrients and water. Under water-stressed conditions, maize lines with different genetic backgrounds and origins displayed different drought tolerance capabilities and showed varied root architecture traits at the seedling stage (Kumar et al. 2012, Liang et al. 2013). Characterization of maize germplasm with better stress tolerance traits and screening for drought tolerant maize lines are essential to the success of breeding programs.
Traditionally, root length density and depth are considered as ideal criteria for evaluating drought tolerance of a plant root system (Kashiwagi et al. 2006). The ratio of root weight to shoot weight has been used as an index for drought resistance because large deep-rooted systems are able to extract more water while relatively smaller shoots transpire less (Srividya et al. 2011). Total root length represents the sum of the primary, crown, seminal, and lateral roots. The various components of the root system have also been selected as important traits for root morphology improvement under drought stress. In this study, we investigated the influence of water stress on the root morphology in 103 maize lines and examined various root traits, including TRL, TSA, TRT, RAD, and RV. We identified significant variation, medium to high heritability, and significant correlations for these root traits. The PCA showed that root traits such as TRL, TSA, and RF were responsible for most of the phenotypic variation at the seedling stage in the tested maize lines. In this study, H′ values were calculated to compare the levels of diversity among the tested traits. A low H′ value indicates an unbalanced frequency distribution for a trait with a lack of genetic diversity, while a high H′ value indicates an even frequency distribution and a wide range of variation. All recorded traits showed high H′ values, with TRL and RAD showing the relatively highest levels of phenotypic diversity. TRL was positively correlated with TSA, RV, and RF. In combination with the PCA analysis, we found that TRL and TSA were sufficient to explain the variation and that they could be used as favorable selection criteria for drought tolerance at the seedling stage. Moreover, TRL and TSA showed much higher drought tolerance coefficients in drought-tolerant maize lines. This result is in agreement with previous reports. For example, Kumar et al. (2012) reported that total root length and root dry weight (DW) provide the largest contribution to total phenotypic variation and might be sufficient to improve other root traits. Root surface area has been shown to have a close relationship to nutrient absorption rates (Imada et al. 2008). Thus, the larger TSA present in maize lines with high phosphorous absorbance efficiency can help the plants to overcome nutrient deficiencies (Zhang et al. 2014). However, there are some conflicting results in the literature regarding the role of root development at the seedling stage and subsequent grain yield. Manavalan et al. (2011) concluded that variation in root characteristics among parents of NAM lines is inherent but is not related to variations in kernel size. However, other researchers drew the opposite conclusion and stated that deep rooting was positively associated with seed yield and crop growth (Eghball and Maranville 1993, Pandey et al. 2000a, 2000b). There is a very weak correlation between kernel weight and TRL and root DW, indicating a weak influence of root morphology on kernel seed size (Kumar et al. 2012). Therefore, a vigorous plant root system not only contributes to improving stand establishment but also enables the plant to survive in stressful conditions.
Diversity in root morphology can be exploited to improve nutrient and water use efficiency under abiotic stresses. A combination of stress resistance ability and the heterotic patterns of maize germplasm will be valuable to breeding and yield improvement. A comparison of the root characteristics of different heterotic groups indicated that SS and NSS groups (temperate maize backgrounds) showed relatively higher diversity of root morphologies than the TST group (tropical maize background). TST lines showed the highest level of diversity in TRL, TRT, and RF under the WW condition but showed lower diversity under the WS condition. Most importantly, 7 of the 9 extremely drought tolerant lines were from the TST group, indicating that the TST germplasm harbors more valuable genetic resources for drought tolerance. This result provides important information for maize hybrid breeding and improving resistance to abiotic stresses. Based on the synthetic index of D values, 7, 42, 45, and 9 maize lines were classified as drought sensitive, moderately sensitive, moderately drought tolerant, and highly drought tolerant, respectively. Drought resistance coefficients for all tested traits, except RAD, were highest in group 4, intermediate in groups 2/3, and lowest in group 1. As a result, 9 maize lines with a well-developed root system were selected for their extreme drought tolerance and could be used in maize breeding for further improvement of tolerance to abiotic stresses. Some of these lines with well-developed root systems have been selected and reported previously in drought tolerance screening experiments based on different selection criteria such as ASI and NDVI in different stages (Lu et al. 2011), while others are newly identified drought tolerant inbred lines and offer the possibility of new drought-responsive genes.
In conclusion, we found a range of responses to drought stress among various maize lines at the seedling stage as revealed by analysis of the root systems and physiological characteristics. We show that selection criteria based on the use of TRL and TSA at the seedling stage might be successful predictors of nutrient and water-use efficiencies in the field. The tested maize lines need be further investigated for their performance at the adult stage under WW and WS conditions. Additionally, the association of adult stage traits with seedling root traits needs to be further examined.
Supplementary Material
Acknowledgements
This work was supported by National Excellent Doctoral Dissertation of China (201358), Foundation for Sichuan Youth Science and Technology Innovative Research Team (2013TD0014), and National High Technology Research and Development Program of China (2012AA101104), and the National Natural Science Foundation of China (31471514).
Literature Cited
- Abdel-Ghani, A.H., Kumar, B., Reyes-Matamoros, J., Gonzalez-Portilla, P.J., Jansen, C., Martin, J.P.S., Lee, M. and Lübberstedt, T. (2012) Genotypic variation and relationships between seedling and adult plant traits in maize (Zea mays L.) inbred lines grown under contrasting nitrogen levels. Euphytica 189: 123–133. [Google Scholar]
- Bänziger, M., Edmeades, G.O., Beck, D. and Bellon, M. (2000) Breeding for drought and nitrogen stress tolerance in maize: from theory to practice. CIMMYT, Mexico D.F., p. 68. [Google Scholar]
- Bouma, T.J., Nielsen, K.L. and Koutstaal, B. (2000) Sample preparation and scanning protocol for computerised analysis of root length and diameter. Plant and Soil 218: 185–196. [Google Scholar]
- Bruce, W.B., Edmeades, G.O. and Barker, T.C. (2002) Molecular and physiological approaches to maize improvement for drought tolerance. J. Exp. Bot. 53: 13–25. [PubMed] [Google Scholar]
- Buckler, E.S., Holland, J.B., Bradbury, P.J., Acharya, C.B., Brown, P.J., Browne, C., Ersoz, E., Flint-Garcia, S., Garcia, A., Glaubitz, J.C.et al. (2009) The genetic architecture of maize flowering time. Science 325: 714–718. [DOI] [PubMed] [Google Scholar]
- Chen, J., Xu, W., Velten, J., Xin, Z. and Stout, J. (2012) Characterization of maize inbred lines for drought and heat tolerance. Journal of Soil and Water Conservation 67: 354–364. [Google Scholar]
- Claes, B., Dekeyser, R., Villarroel, R., Van den Bulcke, M., Bauw, G., Van Montagu, M. and Caplan, A. (1990) Characterization of a rice gene showing organ-specific expression in response to salt stress and drought. Plant Cell 2: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Cruz, M., Romao, R.L., Escudero, A. and Maestre, F.T. (2008) Where do seedlings go? A spatio-temporal analysis of seedling mortality in a semi-arid gypsophyte. Ecography 31: 720–730. [Google Scholar]
- Eghball, B. and Maranville, J.W. (1993) Root development and nitrogen influx of corn genotypes grown under combined drought and nitrogen stresses. Agron. J. 85: 147–152. [Google Scholar]
- Heiniger, R.W. (2001) The impact of early drought on corn yield. NC: North Carolina. [Google Scholar]
- Himmelbauer, M.L., Loiskandl, W. and Kastanek, F. (2004) Estimating length, average diameter and surface area of roots using two different image analyses systems. Plant and Soil 260: 111–120. [Google Scholar]
- Hoagland, D.R. and Arnon, D.I. (1950) The water-culture method for growing plants without soil. Circular. California Agricultural Experiment Station 347: 32. [Google Scholar]
- Hodge, A., Berta, G., Doussan, C., Merchan, F. and Crespi, M. (2009) Plant root growth, architecture and function. Plant and Soil 321: 153–187. [Google Scholar]
- Hutcheson, K.. (1970) A test for comparing diversities based on the Shannon formula. J. Theor. Biol. 29: 151–154. [DOI] [PubMed] [Google Scholar]
- Imada, S., Yamanaka, N. and Tamai, S. (2008) Water table depth affects Populus alba fine root growth and whole plant biomass. Functional Ecology 22: 1018–1026. [Google Scholar]
- Kashiwagi, J., Krishnamurthy, L., Crouch, J.H. and Serraj, R. (2006) Variability of root length density and its contributions to seed yield in chickpea (Cicer arietinum L.) under terminal drought stress. Field Crops Res. 95: 171–181. [Google Scholar]
- Kitajima, K. and Fenner, M. (2000) Ecology of seedling regeneration. In: Fenner, M. (ed.) Seeds: the ecology of regeneration in plant communities, CABI Publishing, Wallingford, pp. 331–359. [Google Scholar]
- Kondo, M., Pablico, P., Aragones, D., Agbisit, R., Abe, J., Morita, S. and Courtois, B. (2003) Genotypic and environmental variations in root morphology in rice genotypes under upland field conditions. In: Abe, J. (ed.) Roots: The Dynamic Interface between Plants and the Earth, Kluwer Academic Publisher, The Netherlands, pp. 189–200. [Google Scholar]
- Kumar, B., Abdel-Ghani, A.H., Reyes-Matamoros, J., Hochholdinger, F. and Lübberstedt, T. (2012) Genotypic variation for root architecture traits in seedlings of maize (Zea mays L.) inbred lines. Plant Breed. 131: 465–478. [Google Scholar]
- Kumar, R., Malaiya, S. and Srivastava, M.N. (2004) Evaluation of morphophysiological traits associated with drought tolerance in rice. Indian J. Plant Physiol. 9: 305–307. [Google Scholar]
- Landi, P., Albrecht, B., Giuliani, M.M. and Sanguineti, M.C. (1998) Seedling characteristics in hydroponic culture and field performance of maize genotypes with different resistance to root lodging. Maydica 43: 111–116. [Google Scholar]
- Landi, P., Giuliani, M.M., Darrah, L.L., Tuberosa, R., Conti, S. and Sanguineti, M.C. (2001) Variability for root and shoot traits in a maize population grown in hydroponics and in the field and their relationships with vertical root pulling resistance. Maydica 46: 177–182. [Google Scholar]
- Leach, K.A., Hejlek, L.G., Hearne, L.B., Nguyen, H.T., Sharp, R.E. and Davis, G.L. (2011) Primary root elongation rate and abscisic acid levels of maize in response to water stress. Crop Sci. 51: 157–172. [Google Scholar]
- Liang, Y.S., Zhan, X.D., Wang, H.M., Gao, Z.Q., Lin, Z., Chen, D.B., Shen, X.H., Cao, L.Y. and Cheng, S.H. (2013) Locating QTLs controlling several adult root traits in an elite Chinese hybrid rice. Gene 526: 331–335. [DOI] [PubMed] [Google Scholar]
- Longenberger, P.S., Smith, C.W., Thaxton, P.S. and McMichael, B.L. (2006) Development of a screening method for drought tolerance in cotton seedlings. Crop Sci. 46: 2104–2110. [Google Scholar]
- Lu, Y., Hao, Z., Xie, C., Crossa, J., Araus, J.-L., Gao, S., Vivek, B.S., Magorokosho, C., Mugo, S., Makumbi, D.et al. (2011) Large-scale screening for maize drought resistance using multiple selection criteria evaluated under water-stressed and well-watered environments. Field Crops Res. 124: 37–45. [Google Scholar]
- Magalhães, P.C., de Souza, T.C. and Cantão, F.R.O. (2011) Early evaluation of root morphology of maize genotypes under phosphorus deficiency. Plant Soil Environ. 57: 135–138. [Google Scholar]
- Malamy, J.E. and Benfey, P.N. (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44. [DOI] [PubMed] [Google Scholar]
- Manavalan, L.P., Musket, T. and Nguyen, H.T. (2011) Natural genetic variation for root traits among diversity lines of maize (Zea Mays L.). Maydica 56: 1707. [Google Scholar]
- Meeks, M., Murray, S.C., Hague, S. and Hays, D. (2013) Measuring maize seedling drought response in search of tolerant germplasm. Agronomy 3: 135–147. [Google Scholar]
- Ober, E.S. and Sharp, R.E. (2007) Regulation of root growth responses to water deficit. In: Jenks, M.A., Hasegawa P.M. and Jain S.M. (eds.) Advances in Molecular Breeding Toward Drought and Salt Tolerant Crops, Springer, The Netherlands, pp. 33–54. [Google Scholar]
- Pace, J., Lee, N., Naik, H.S., Ganapathysubramanian, B. and Lübberstedt, T. (2014) Analysis of maize (Zea mays L.) seedling roots with the high-throughput image analysis tool ARIA (Automatic Root Image Analysis). PLoS One 9: e108255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace, J., Gardner, C., Romay, C., Ganapathsybrumanian, B. and Lübberstedt, T. (2015) Genome-wide association analysis of seedling root development in maize (Zea mays L.). BMC Genomics 16: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey, R.K., Maranville, J.W. and Admou, A. (2000a) Deficit irrigation and nitrogen effects on maize in a Sahelian environment: I. Grain yield and yield components. Agricultural Water Management 46: 1–13. [Google Scholar]
- Pandey, R.K., Maranville, J.W. and Chetima, M.M. (2000b) Deficit irrigation and nitrogen effects on maize in a Sahelian environment: II. Shoot growth, nitrogen uptake and water extraction. Agricultural Water Management 46: 15–27. [Google Scholar]
- Piepho, H.P., Möhring, J., Melchinger, A.E. and Büchse, A. (2008) BLUP for phenotypic selection in plant breeding and variety testing. Euphytica 161: 209–228. [Google Scholar]
- Pingali, P.L. (2001) CIMMYT 1999–2000 World Maize Facts and Trends. Meeting World Maize Needs: Technological Opportunities and Priorities for the Public Sector. CIMMYT; Mexico, DF. [Google Scholar]
- Ribaut, J.-M., Betran, J., Monneveux, P. and Setter, T. (2009) Drought tolerance in maize. In: Bennetzen, J.L. and Hake S.C. (eds.) Handbook of Maize: Its Biology, Springer, New York, pp. 311–344. [Google Scholar]
- Ruta, N., Stamp, P., Liedgens, M., Fracheboud, Y. and Hund, A. (2010) Collocations of QTLs for seedling traits and yield components of tropical maize under water stress conditions. Crop Sci. 50: 1385–1392. [Google Scholar]
- Sharp, R.E., Poroyko, V., Hejlek, L.G., Spollen, W.G., Springer, G.K., Bohnert, H.J. and Nguyen, H.T. (2004) Root growth maintenance during water deficits: physiology to functional genomics. J. Exp. Bot. 55: 2343–2351. [DOI] [PubMed] [Google Scholar]
- Singh, B.B., Mai-Kodomi, Y. and Terao, T. (1999) A simple screening method for drought tolerance in cowpea. Indian J. Genet. Plant Breed. 59: 211–220. [Google Scholar]
- Singh, B.B. and Matsui, T. (2002) Cowpea varieties for drought tolerance. Challenges and Opportunities for Enhancing Sustainable Cowpea Production: 287–300. [Google Scholar]
- Smith, S.E., Kuehl, R.O., Ray, I.M., Hui, R. and Soleri, D. (1998) Evaluation of simple methods for estimating broad-sense heritability in stands of randomly planted genotypes. Crop Sci. 38: 1125–1129. [Google Scholar]
- Srividhya, A., Vemireddy, L.R., Ramanarao, P.V., Sridhar, S., Jayaprada, M., Anuradha, G., Srilakshmi, B., Reddy, H.K., Hariprasad, A.S. and Siddiq, E.A. (2011) Molecular mapping of QTLs for drought related traits at seedling stage under PEG induced stress conditions in rice. Am. J. Plant Sci. 2: 190–201. [Google Scholar]
- Svačina, P., Středa, T. and Chloupek, O. (2014) Uncommon selection by root system size increases barley yield. Agron. Sustain. Dev. 34: 545–551. [Google Scholar]
- Tomar, S.M.S. and Kumar, G.T. (2004) Seedling survivability as a selection criterion for drought tolerance in wheat. Plant Breed. 123: 392–394. [Google Scholar]
- Trachsel, S., Stamp, P. and Hund, A. (2010) Growth of axile and lateral roots of maize: Response to desiccation stress induced by polyethylene glycol 8000. Maydica 55: 101–109. [Google Scholar]
- Végh, K.R. (2013) Root and leaf traits, water use and drought tolerance of maize genotypes. Biologia 68: 1123–1127. [Google Scholar]
- Vadez, V.. (2014) Root hydraulics: The forgotten side of roots in drought adaptation. Field Crops Res. 165: 15–24. [Google Scholar]
- Wang, L., Liu, K., Mao, S., Li, Z., Lu, Y., Wang, J., Liu, Y., Wei, Y. and Zheng, Y. (2015) Large-scale screening for Aegilops tauschii tolerant genotypes to phosphorus deficiency at seedling stage. Euphytica 204: 571–586. [Google Scholar]
- Xu, G.F., Zhang, Z.Y. and Xiang, Z.X. (2009) Comprehensive evaluation of cold resistance on four Lysimachia plants by subordinate function values analysis. Journal of Northwest Forestry University 24: 24–26. [Google Scholar]
- Zar, J.H. (2010) Biostatistical analysis, 5th edn Practice Hall, New Jersey, p. 944. [Google Scholar]
- Zhang, L.T., Li, J., Rong, T.Z., Gao, S.B., Wu, F.K., Xu, J., Li, M.L., Cao, M.J., Wang, J., Hu, E.L.et al. (2014) Large-scale screening maize germplasm for low-phosphorus tolerance using multiple selection criteria. Euphytica 197: 435–446. [Google Scholar]
- Zhang, Z.Y. and Xu, G.F. (2009) Comprehensive evaluation of heat tolerance of four ground covering plants by subordinate function values analysis. Pratacultural Science 26: 57–60. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.