Abstract
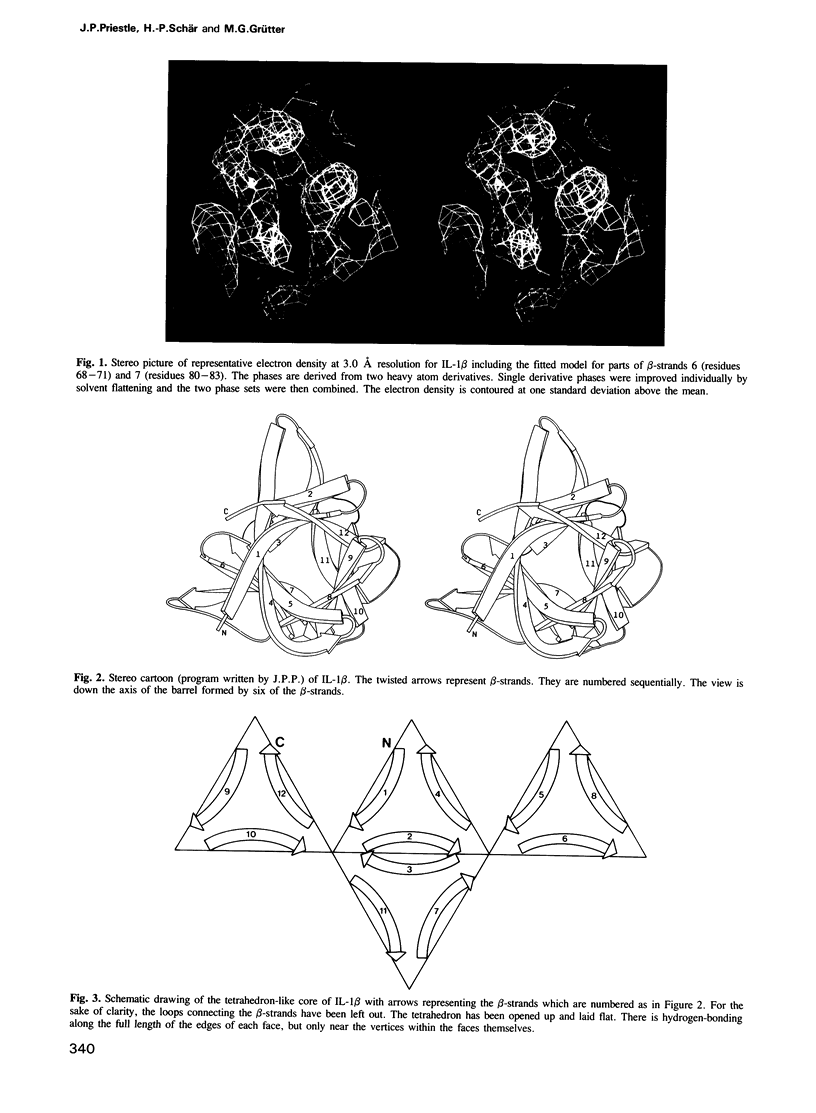
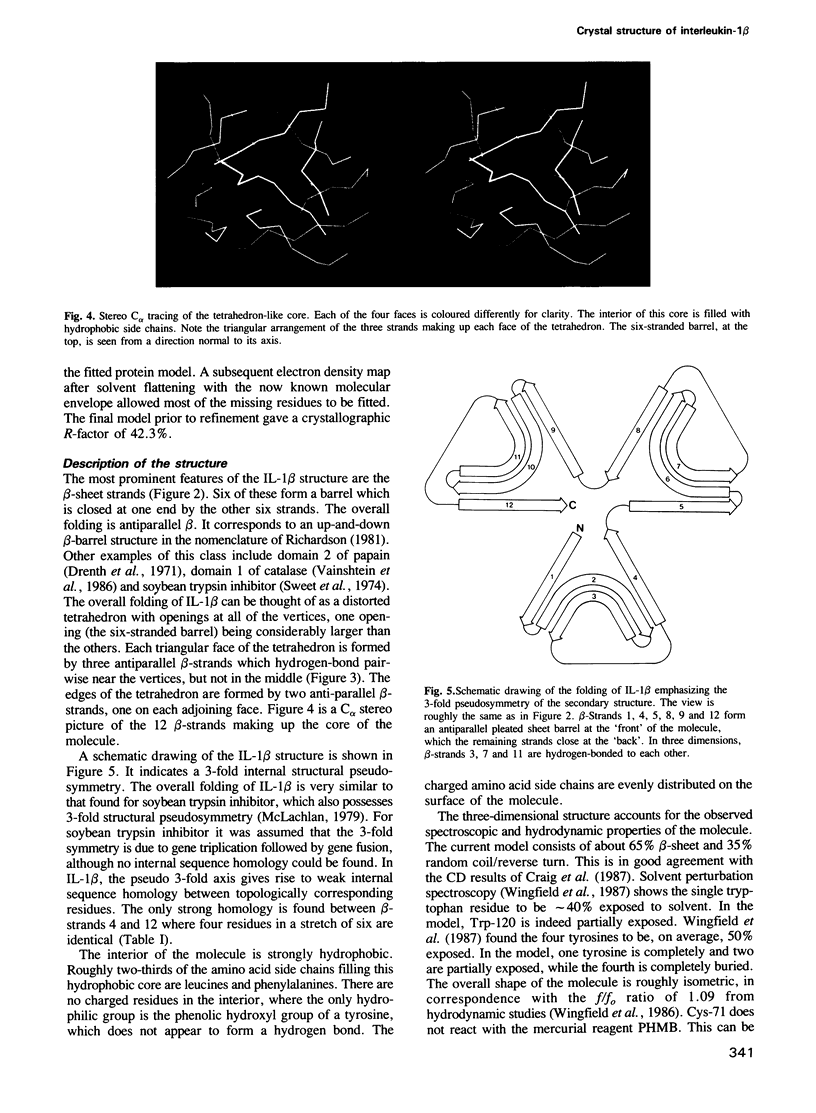
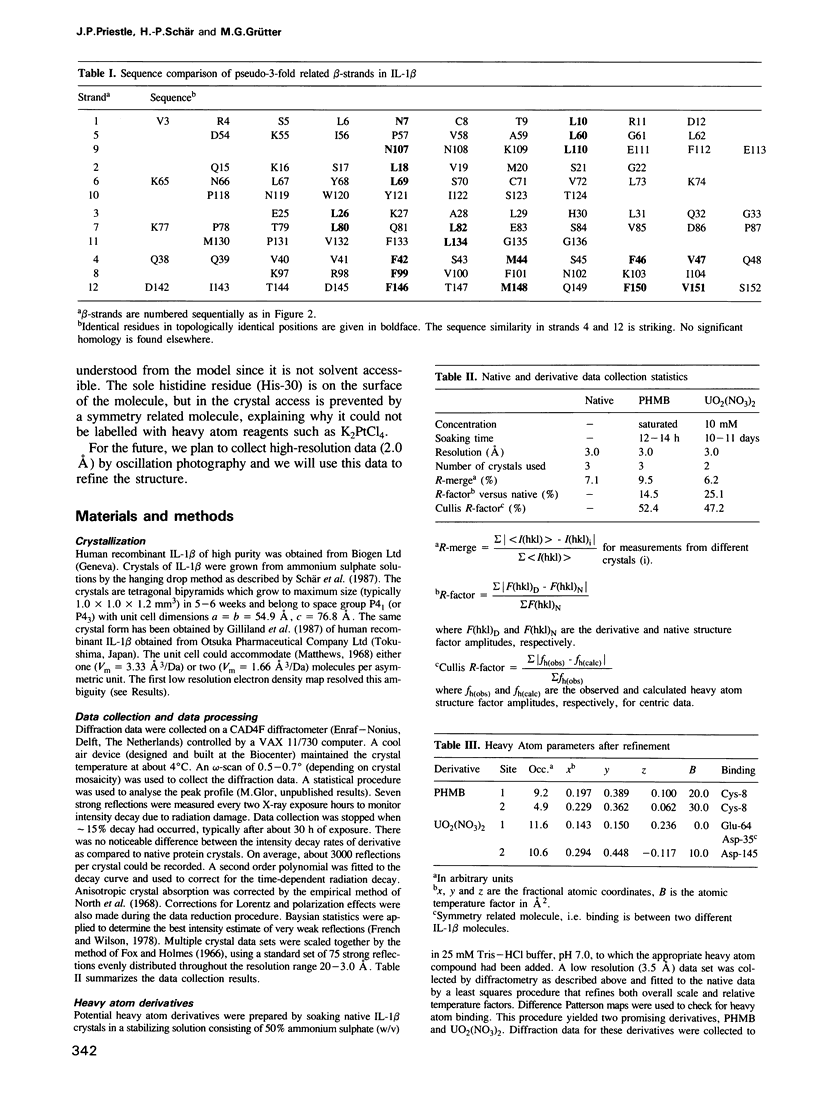
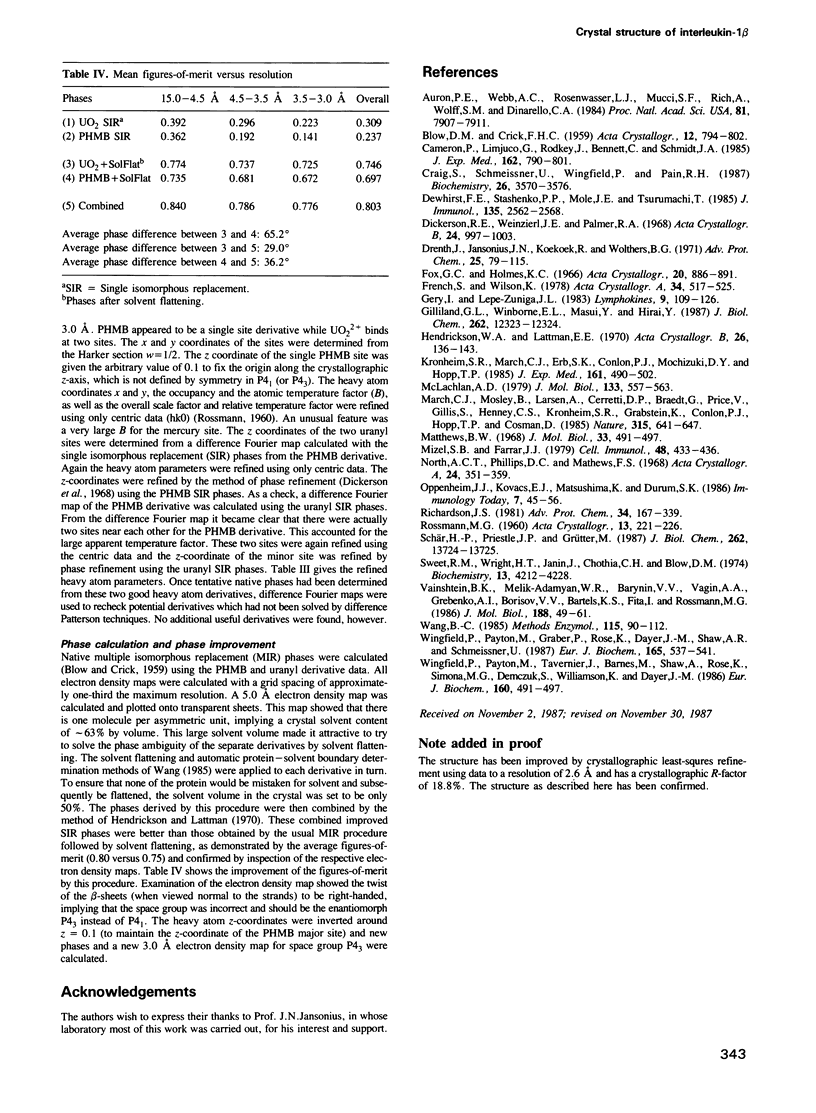
The crystal structure of human recombinant interleukin-1 beta has been determined at 3.0 A resolution by the isomorphous replacement method in conjunction with solvent flattening techniques. The model prior to refinement has a crystallographic R-factor of 42.3%. The structure is composed of 12 beta-strands forming a complex network of hydrogen bonds. The core of the structure can best be described as a tetrahedron whose edges are each formed by two antiparallel beta-strands. The interior of this structure is filled with hydrophobic side chains. There is a 3-fold repeat in the folding of the polypeptide chain. Although this folding pattern suggests gene triplication, no strong internal sequence homology between topologically corresponding residues exists. The folding topology of interleukin-1 beta is very similar to that described by McLachlan (1979) J. Mol. Biol., 133, 557-563, for soybean trypsin inhibitor.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auron P. E., Webb A. C., Rosenwasser L. J., Mucci S. F., Rich A., Wolff S. M., Dinarello C. A. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7907–7911. doi: 10.1073/pnas.81.24.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron P., Limjuco G., Rodkey J., Bennett C., Schmidt J. A. Amino acid sequence analysis of human interleukin 1 (IL-1). Evidence for biochemically distinct forms of IL-1. J Exp Med. 1985 Sep 1;162(3):790–801. doi: 10.1084/jem.162.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig S., Schmeissner U., Wingfield P., Pain R. H. Conformation, stability, and folding of interleukin 1 beta. Biochemistry. 1987 Jun 16;26(12):3570–3576. doi: 10.1021/bi00386a048. [DOI] [PubMed] [Google Scholar]
- Dewhirst F. E., Stashenko P. P., Mole J. E., Tsurumachi T. Purification and partial sequence of human osteoclast-activating factor: identity with interleukin 1 beta. J Immunol. 1985 Oct;135(4):2562–2568. [PubMed] [Google Scholar]
- Drenth J., Jansonius J. N., Koekoek R., Wolthers B. G. The structure of papain. Adv Protein Chem. 1971;25:79–115. doi: 10.1016/s0065-3233(08)60279-x. [DOI] [PubMed] [Google Scholar]
- Gilliland G. L., Winborne E. L., Masui Y., Hirai Y. A preliminary crystallographic study of recombinant human interleukin 1 beta. J Biol Chem. 1987 Sep 5;262(25):12323–12324. [PubMed] [Google Scholar]
- Kronheim S. R., March C. J., Erb S. K., Conlon P. J., Mochizuki D. Y., Hopp T. P. Human interleukin 1. Purification to homogeneity. J Exp Med. 1985 Mar 1;161(3):490–502. doi: 10.1084/jem.161.3.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March C. J., Mosley B., Larsen A., Cerretti D. P., Braedt G., Price V., Gillis S., Henney C. S., Kronheim S. R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985 Jun 20;315(6021):641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. Solvent content of protein crystals. J Mol Biol. 1968 Apr 28;33(2):491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D. Three-fold structural pattern in the soybean trypsin inhibitor (Kunitz). J Mol Biol. 1979 Oct 9;133(4):557–563. doi: 10.1016/0022-2836(79)90408-x. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Farrar J. J. Revised nomenclature for antigen-nonspecific T-cell proliferation and helper factors. Cell Immunol. 1979 Dec;48(2):433–436. doi: 10.1016/0008-8749(79)90139-4. [DOI] [PubMed] [Google Scholar]
- Richardson J. S. The anatomy and taxonomy of protein structure. Adv Protein Chem. 1981;34:167–339. doi: 10.1016/s0065-3233(08)60520-3. [DOI] [PubMed] [Google Scholar]
- Schär H. P., Priestle J. P., Grütter M. Crystallization and preliminary x-ray diffraction studies of recombinant human interleukin-1 beta. J Biol Chem. 1987 Oct 5;262(28):13724–13725. [PubMed] [Google Scholar]
- Sweet R. M., Wright H. T., Janin J., Chothia C. H., Blow D. M. Crystal structure of the complex of porcine trypsin with soybean trypsin inhibitor (Kunitz) at 2.6-A resolution. Biochemistry. 1974 Sep 24;13(20):4212–4228. doi: 10.1021/bi00717a024. [DOI] [PubMed] [Google Scholar]
- Vainshtein B. K., Melik-Adamyan W. R., Barynin V. V., Vagin A. A., Grebenko A. I., Borisov V. V., Bartels K. S., Fita I., Rossmann M. G. Three-dimensional structure of catalase from Penicillium vitale at 2.0 A resolution. J Mol Biol. 1986 Mar 5;188(1):49–61. doi: 10.1016/0022-2836(86)90479-1. [DOI] [PubMed] [Google Scholar]
- Wang B. C. Resolution of phase ambiguity in macromolecular crystallography. Methods Enzymol. 1985;115:90–112. doi: 10.1016/0076-6879(85)15009-3. [DOI] [PubMed] [Google Scholar]
- Wingfield P., Payton M., Graber P., Rose K., Dayer J. M., Shaw A. R., Schmeissner U. Purification and characterization of human interleukin-1 alpha produced in Escherichia coli. Eur J Biochem. 1987 Jun 15;165(3):537–541. doi: 10.1111/j.1432-1033.1987.tb11472.x. [DOI] [PubMed] [Google Scholar]
- Wingfield P., Payton M., Tavernier J., Barnes M., Shaw A., Rose K., Simona M. G., Demczuk S., Williamson K., Dayer J. M. Purification and characterization of human interleukin-1 beta expressed in recombinant Escherichia coli. Eur J Biochem. 1986 Nov 3;160(3):491–497. doi: 10.1111/j.1432-1033.1986.tb10066.x. [DOI] [PubMed] [Google Scholar]