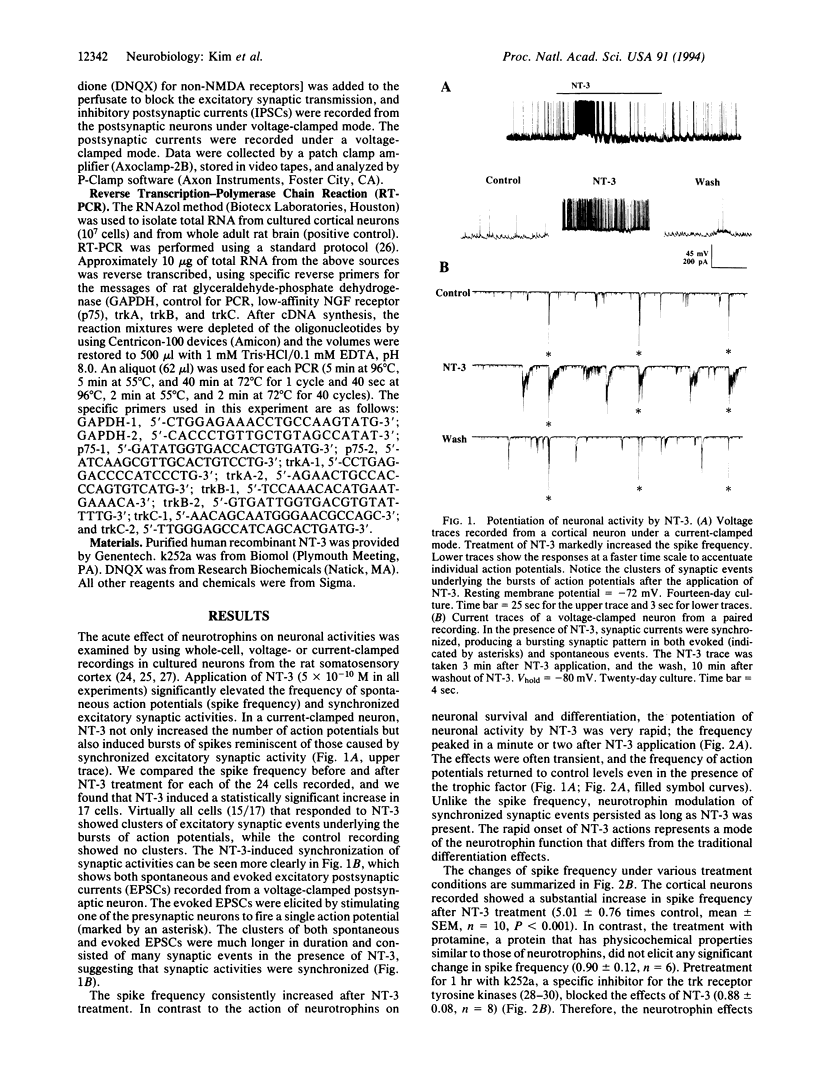
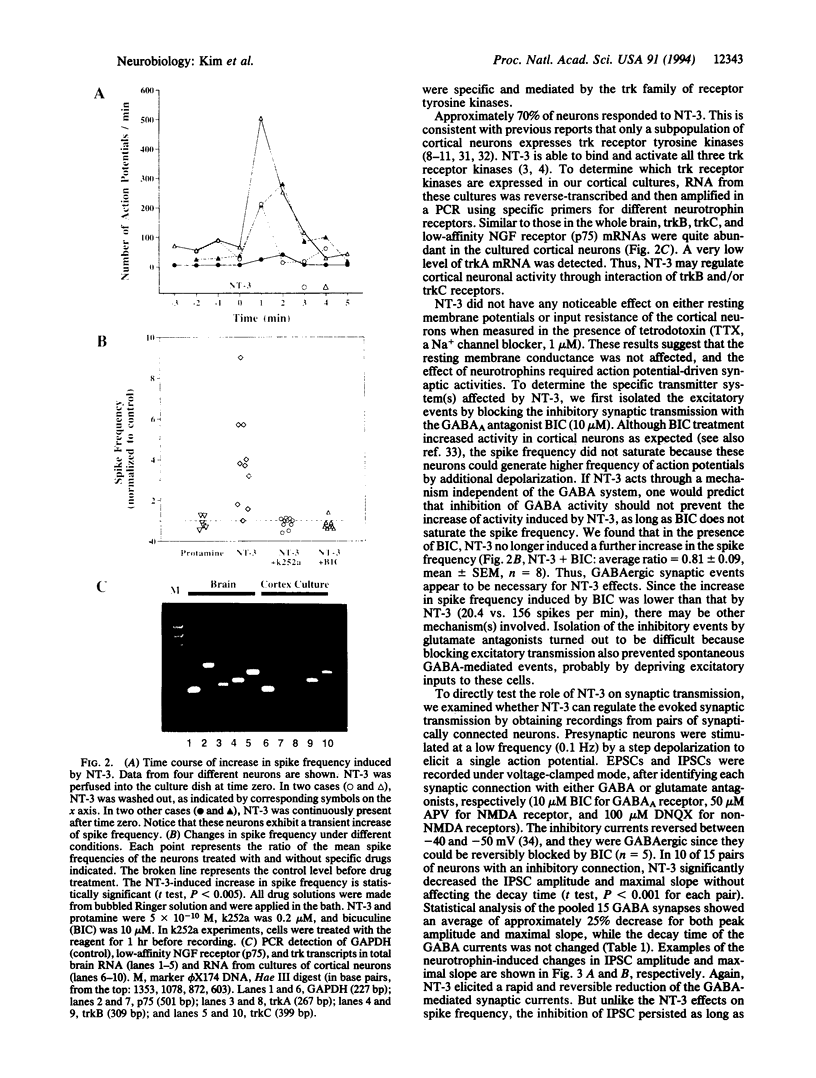
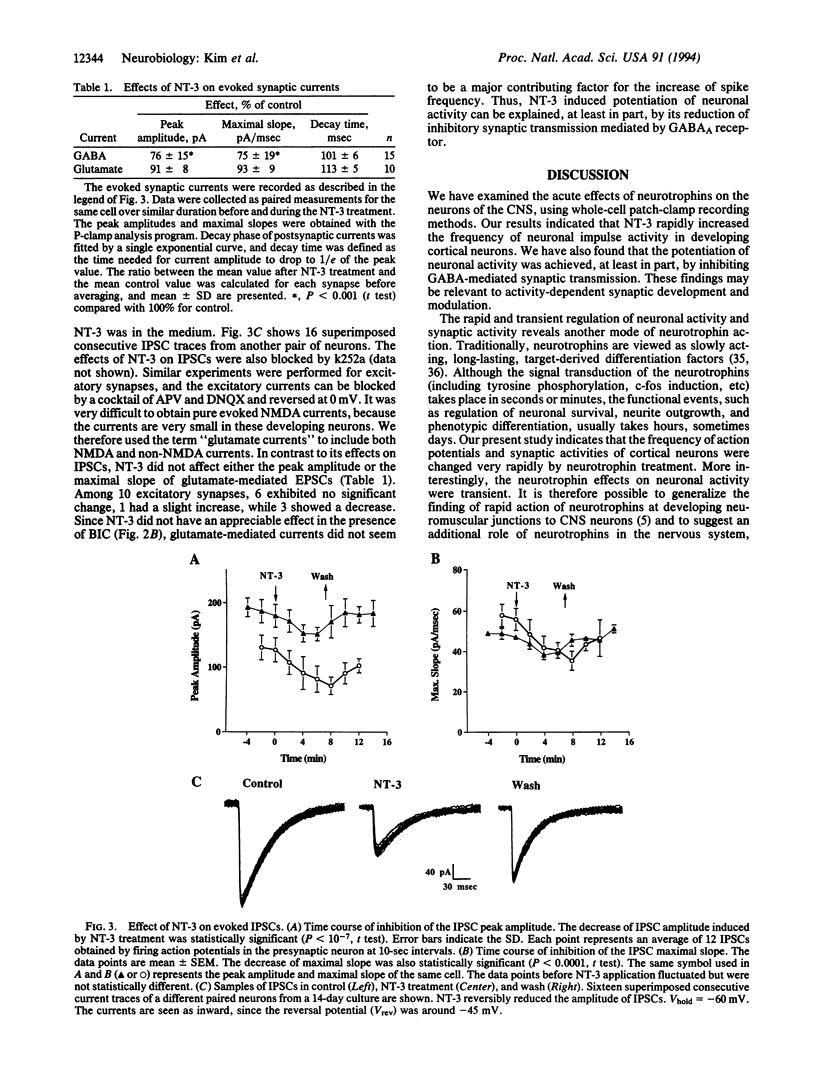
Abstract
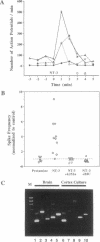
Neurotrophins have traditionally been regarded as slowly acting signals essential for neuronal survival and differentiation. However, brain-derived neurotrophic factor and neurotrophin 3 (NT-3) have recently been reported to exert an acute potentiation of synaptic activity at the amphibian neuromuscular junction. Little is known about the role of neurotrophins on functional synapses in the central nervous system. Here we show that NT-3 rapidly increased the frequency of spontaneous action potentials, and it synchronized excitatory synaptic activities in developing cortical neurons. Moreover, the inhibitory synaptic transmission mediated by gamma-aminobutyric acid (GABA) subtype A receptors was found to be reduced by NT-3. Thus, the excitatory effects of NT-3 on spontaneous action potentials were attributable to a reduction of GABAergic transmission. Our findings, together with previous reports of rapid regulation of central nervous system neurotrophin expression by neuronal activity and of the role of GABAergic transmission in cortical plasticity, suggest a mechanism for modulation of synaptic transmission and activity-dependent synaptic modulation in cortical neurons.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altar C. A., Criden M. R., Lindsay R. M., DiStefano P. S. Characterization and topography of high-affinity 125I-neurotrophin-3 binding to mammalian brain. J Neurosci. 1993 Feb;13(2):733–743. doi: 10.1523/JNEUROSCI.13-02-00733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M. Nerve growth factor: a tale of two receptors. Oncogene. 1993 Aug;8(8):2033–2042. [PubMed] [Google Scholar]
- Barde Y. A. Trophic factors and neuronal survival. Neuron. 1989 Jun;2(6):1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- Bear M. F., Press W. A., Connors B. W. Long-term potentiation in slices of kitten visual cortex and the effects of NMDA receptor blockade. J Neurophysiol. 1992 Apr;67(4):841–851. doi: 10.1152/jn.1992.67.4.841. [DOI] [PubMed] [Google Scholar]
- Berg M. M., Sternberg D. W., Parada L. F., Chao M. V. K-252a inhibits nerve growth factor-induced trk proto-oncogene tyrosine phosphorylation and kinase activity. J Biol Chem. 1992 Jan 5;267(1):13–16. [PubMed] [Google Scholar]
- Castrén E., Pitkänen M., Sirviö J., Parsadanian A., Lindholm D., Thoenen H., Riekkinen P. J. The induction of LTP increases BDNF and NGF mRNA but decreases NT-3 mRNA in the dentate gyrus. Neuroreport. 1993 Jul;4(7):895–898. doi: 10.1097/00001756-199307000-00014. [DOI] [PubMed] [Google Scholar]
- Castrén E., Zafra F., Thoenen H., Lindholm D. Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao M. V. Neurotrophin receptors: a window into neuronal differentiation. Neuron. 1992 Oct;9(4):583–593. doi: 10.1016/0896-6273(92)90023-7. [DOI] [PubMed] [Google Scholar]
- Ernfors P., Bengzon J., Kokaia Z., Persson H., Lindvall O. Increased levels of messenger RNAs for neurotrophic factors in the brain during kindling epileptogenesis. Neuron. 1991 Jul;7(1):165–176. doi: 10.1016/0896-6273(91)90084-d. [DOI] [PubMed] [Google Scholar]
- Ernfors P., Wetmore C., Olson L., Persson H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron. 1990 Oct;5(4):511–526. doi: 10.1016/0896-6273(90)90090-3. [DOI] [PubMed] [Google Scholar]
- Ernfors Patrik, Merlio Jean-Phillipe, Persson Håkan. Cells Expressing mRNA for Neurotrophins and their Receptors During Embryonic Rat Development. Eur J Neurosci. 1992 Oct;4(11):1140–1158. doi: 10.1111/j.1460-9568.1992.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Gall C. M., Isackson P. J. Limbic seizures increase neuronal production of messenger RNA for nerve growth factor. Science. 1989 Aug 18;245(4919):758–761. doi: 10.1126/science.2549634. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Holtzman D. M., Li Y., Parada L. F., Kinsman S., Chen C. K., Valletta J. S., Zhou J., Long J. B., Mobley W. C. p140trk mRNA marks NGF-responsive forebrain neurons: evidence that trk gene expression is induced by NGF. Neuron. 1992 Sep;9(3):465–478. doi: 10.1016/0896-6273(92)90184-f. [DOI] [PubMed] [Google Scholar]
- Isackson P. J., Huntsman M. M., Murray K. D., Gall C. M. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: temporal patterns of induction distinct from NGF. Neuron. 1991 Jun;6(6):937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- Jacobs K. M., Donoghue J. P. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991 Feb 22;251(4996):944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- Klein R., Martin-Zanca D., Barbacid M., Parada L. F. Expression of the tyrosine kinase receptor gene trkB is confined to the murine embryonic and adult nervous system. Development. 1990 Aug;109(4):845–850. doi: 10.1242/dev.109.4.845. [DOI] [PubMed] [Google Scholar]
- Korsching S. The neurotrophic factor concept: a reexamination. J Neurosci. 1993 Jul;13(7):2739–2748. doi: 10.1523/JNEUROSCI.13-07-02739.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamballe F., Smeyne R. J., Barbacid M. Developmental expression of trkC, the neurotrophin-3 receptor, in the mammalian nervous system. J Neurosci. 1994 Jan;14(1):14–28. doi: 10.1523/JNEUROSCI.14-01-00014.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor: thirty-five years later. Biosci Rep. 1987 Sep;7(9):681–699. doi: 10.1007/BF01116861. [DOI] [PubMed] [Google Scholar]
- Lohof A. M., Ip N. Y., Poo M. M. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993 May 27;363(6427):350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- Lu B., Greengard P., Poo M. M. Exogenous synapsin I promotes functional maturation of developing neuromuscular synapses. Neuron. 1992 Mar;8(3):521–529. doi: 10.1016/0896-6273(92)90280-q. [DOI] [PubMed] [Google Scholar]
- Lu B., Yokoyama M., Dreyfus C. F., Black I. B. Depolarizing stimuli regulate nerve growth factor gene expression in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6289–6292. doi: 10.1073/pnas.88.14.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B., Yokoyama M., Dreyfus C. F., Black I. B. NGF gene expression in actively growing brain glia. J Neurosci. 1991 Feb;11(2):318–326. doi: 10.1523/JNEUROSCI.11-02-00318.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonpierre P. C., Belluscio L., Friedman B., Alderson R. F., Wiegand S. J., Furth M. E., Lindsay R. M., Yancopoulos G. D. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990 Oct;5(4):501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- Nye S. H., Squinto S. P., Glass D. J., Stitt T. N., Hantzopoulos P., Macchi M. J., Lindsay N. S., Ip N. Y., Yancopoulos G. D. K-252a and staurosporine selectively block autophosphorylation of neurotrophin receptors and neurotrophin-mediated responses. Mol Biol Cell. 1992 Jun;3(6):677–686. doi: 10.1091/mbc.3.6.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson S. L., Grover L. M., Schwartzkroin P. A., Bothwell M. Neurotrophin expression in rat hippocampal slices: a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron. 1992 Dec;9(6):1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- Ringstedt T., Lagercrantz H., Persson H. Expression of members of the trk family in the developing postnatal rat brain. Brain Res Dev Brain Res. 1993 Mar 19;72(1):119–131. doi: 10.1016/0165-3806(93)90165-7. [DOI] [PubMed] [Google Scholar]
- Snider W. D. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994 Jun 3;77(5):627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Steininger T. L., Wainer B. H., Klein R., Barbacid M., Palfrey H. C. High-affinity nerve growth factor receptor (Trk) immunoreactivity is localized in cholinergic neurons of the basal forebrain and striatum in the adult rat brain. Brain Res. 1993 May 28;612(1-2):330–335. doi: 10.1016/0006-8993(93)91681-h. [DOI] [PubMed] [Google Scholar]
- Tapley P., Lamballe F., Barbacid M. K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene. 1992 Feb;7(2):371–381. [PubMed] [Google Scholar]
- Thoenen H. The changing scene of neurotrophic factors. Trends Neurosci. 1991 May;14(5):165–170. doi: 10.1016/0166-2236(91)90097-e. [DOI] [PubMed] [Google Scholar]
- Zafra F., Castrén E., Thoenen H., Lindholm D. Interplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10037–10041. doi: 10.1073/pnas.88.22.10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafra F., Hengerer B., Leibrock J., Thoenen H., Lindholm D. Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J. 1990 Nov;9(11):3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]