Abstract
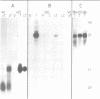
In Drosophila spermatogenesis transcription occurs only premeiotically while translation can be detected also in postmeiotic spermatids. To analyse the underlying processes mst(3)gl-9, a gene specifically expressed in the male germ cells of Drosophila melanogaster, was studied. The putative protein encoded by mst(3)gl-9 is mostly composed of repetitive Cys-Gly-Pro motifs. The transcriptional and translational control of expression of mst(3)gl-9 has been investigated by P-mediated transformation. Only 102 bp of 5' upstream sequences and the first 201 bp of the gene are sufficient to maintain the gene specific characteristics of expression, namely premeiotic transcription and postmeiotic translation separated by 3 days of development.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boswell R. E., Mahowald A. P. tudor, a gene required for assembly of the germ plasm in Drosophila melanogaster. Cell. 1985 Nov;43(1):97–104. doi: 10.1016/0092-8674(85)90015-7. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Glaser R. L., Wolfner M. F., Lis J. T. Spatial and temporal pattern of hsp26 expression during normal development. EMBO J. 1986 Apr;5(4):747–754. doi: 10.1002/j.1460-2075.1986.tb04277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hess O., Meyer G. F. Genetic activities of the Y chromosome in Drosophila during spermatogenesis. Adv Genet. 1968;14:171–223. doi: 10.1016/s0065-2660(08)60427-7. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Klemenz R., Gehring W. J. Translational and transcriptional control elements in the untranslated leader of the heat-shock gene hsp22. Cell. 1986 Feb 14;44(3):429–438. doi: 10.1016/0092-8674(86)90464-2. [DOI] [PubMed] [Google Scholar]
- Karess R. E., Rubin G. M. Analysis of P transposable element functions in Drosophila. Cell. 1984 Aug;38(1):135–146. doi: 10.1016/0092-8674(84)90534-8. [DOI] [PubMed] [Google Scholar]
- Kiefer B. I. Ultrastructural Abnormalities in Developing Sperm of X/0 DROSOPHILA MELANOGASTER. Genetics. 1966 Dec;54(6):1441–1452. doi: 10.1093/genetics/54.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R., Hultmark D., Gehring W. J. Selective translation of heat shock mRNA in Drosophila melanogaster depends on sequence information in the leader. EMBO J. 1985 Aug;4(8):2053–2060. doi: 10.1002/j.1460-2075.1985.tb03891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley D. L., Grell E. H. Spermiogenesis without chromosomes in Drosophila melanogaster. Genetics. 1969;61(1 Suppl):69–78. [PubMed] [Google Scholar]
- Lis J. T., Simon J. A., Sutton C. A. New heat shock puffs and beta-galactosidase activity resulting from transformation of Drosophila with an hsp70-lacZ hybrid gene. Cell. 1983 Dec;35(2 Pt 1):403–410. doi: 10.1016/0092-8674(83)90173-3. [DOI] [PubMed] [Google Scholar]
- Logan J., Shenk T. Adenovirus tripartite leader sequence enhances translation of mRNAs late after infection. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3655–3659. doi: 10.1073/pnas.81.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry T. J., Lindquist S. The preferential translation of Drosophila hsp70 mRNA requires sequences in the untranslated leader. Cell. 1985 Oct;42(3):903–911. doi: 10.1016/0092-8674(85)90286-7. [DOI] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Mismer D., Rubin G. M. Analysis of the promoter of the ninaE opsin gene in Drosophila melanogaster. Genetics. 1987 Aug;116(4):565–578. doi: 10.1093/genetics/116.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P. P., Hinnebusch A. G. Multiple upstream AUG codons mediate translational control of GCN4. Cell. 1986 Apr 25;45(2):201–207. doi: 10.1016/0092-8674(86)90384-3. [DOI] [PubMed] [Google Scholar]
- Olivieri G., Olivieri A. Autoradiographic study of nucleic acid synthesis during spermatogenesis in Drosophila melanogaster. Mutat Res. 1965 Aug;2(4):366–380. doi: 10.1016/0027-5107(65)90072-2. [DOI] [PubMed] [Google Scholar]
- Poole S. J., Kauvar L. M., Drees B., Kornberg T. The engrailed locus of Drosophila: structural analysis of an embryonic transcript. Cell. 1985 Jan;40(1):37–43. doi: 10.1016/0092-8674(85)90306-x. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Raghavan K. V., Crosby M. A., Mathers P. H., Meyerowitz E. M. Sequences sufficient for correct regulation of Sgs-3 lie close to or within the gene. EMBO J. 1986 Dec 1;5(12):3321–3326. doi: 10.1002/j.1460-2075.1986.tb04646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Vectors for P element-mediated gene transfer in Drosophila. Nucleic Acids Res. 1983 Sep 24;11(18):6341–6351. doi: 10.1093/nar/11.18.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer U. The regulation of male-specific transcripts by sex determining genes in Drosophila melanogaster. EMBO J. 1986 Dec 20;5(13):3579–3582. doi: 10.1002/j.1460-2075.1986.tb04685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J. A., Lis J. T. A germline transformation analysis reveals flexibility in the organization of heat shock consensus elements. Nucleic Acids Res. 1987 Apr 10;15(7):2971–2988. doi: 10.1093/nar/15.7.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982 Oct 22;218(4570):341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Steller H., Pirrotta V. P transposons controlled by the heat shock promoter. Mol Cell Biol. 1986 May;6(5):1640–1649. doi: 10.1128/mcb.6.5.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]