Abstract
Chemotherapy-induced cognitive impairment (CICI) is a quality of life-altering consequence of chemotherapy experienced by a large percentage of cancer survivors. Approximately half of FDA-approved anti-cancer drugs are known to produce ROS. Doxorubicin (Dox), a prototypical ROS-generating chemotherapeutic agent, generates superoxide (O2−•) via redox cycling. Our group previously demonstrated that Dox, which does not cross the BBB, induced oxidative damage to plasma proteins leading to TNF-α elevation in the periphery and, subsequently, in brain following cancer chemotherapy. We hypothesize that such processes play a central role in CICI. The current study tested the notion that O2−• is involved and likely responsible for Dox-induced plasma protein oxidation and TNF-α release. Addition of O2−• as the potassium salt (KO2) to plasma resulted in significantly increased oxidative damage to proteins, indexed by protein carbonyl (PC) and protein-bound HNE levels. We then adapted this protocol for use in cell culture. Incubation of J774A.1 macrophage culture using this KO2-18crown6 protocol with 1 and 10 μM KO2 resulted in dramatically increased levels of TNF-α produced. These findings, together with our prior results, provide strong evidence that O2−• and its resulting reactive species are critically involved in Dox-induced plasma protein oxidation and TNF-α release.
Keywords: Superoxide free radical and doxorubicin induced oxidative stress, tumor necrosis factor-alpha, macrophage, chemotherapy induced cognitive impairment, cancer chemotherapy
1. Introduction
More than half of the FDA approved anti-cancer drugs are known to cause reactive oxygen species (ROS) production [1]. Doxorubicin (Dox) is a quinone containing antineoplastic anthracycline used commonly in multi-drug chemotherapy regimens primarily to treat solid tumors [2]. Dox, a prototypical ROS-producing chemotherapeutic agent, in the presence of molecular oxygen, generates the reactive superoxide radical anion (O2−•) via redox cycling of the quinone moiety [3–7]. Our group has demonstrated that Dox-induced oxidative damage to plasma proteins in vivo induces the elevation of the inflammatory cytokine, tumor necrosis factor-alpha (TNF-α), in the periphery [1, 2, 8]. TNF-α crosses the blood-brain barrier (BBB) via receptor-mediated endocytosis resulting in central nervous system toxicities including further TNF-α elevation in brain, oxidative and nitrosative damage to key biomolecules, mitochondrial dysfunction, and neuronal death [8–13].
O2−• is considered a key reactive radical generated within the cell leading to protein oxidation, lipid peroxidation, and hydrogen peroxide (H2O2) and hydroxyl radical (•OH) production that can further damage biomolecules [14–17]. The goal of this study was to determine if O2−• produces oxidative protein damages in plasma and TNF-α elevation in macrophages similar to that observed following Dox administration in order to further elucidate the mechanisms by which Dox may cause chemotherapy-induced cognitive impairment (CICI), despite the inability of Dox or its major metabolite to cross the BBB. Previously, our laboratory demonstrated that Dox-induced oxidation of apolipoprotein A-I (ApoA-I) in J774.A1 macrophage culture led to increased TNF-α production [2].
To accomplish this goal, O2−• was added to plasma samples from wild-type (WT) mice in the form of potassium superoxide salt (KO2) in an appropriate solvent including an 18crown6 stabilizer [14, 18, 19], and oxidative stress parameters, protein carbonyl (PC) and protein-bound 4-hydroxy-2-trans-nonenal (HNE), were measured. PC levels serve as a measure of protein oxidation, while protein-bound HNE is a lipid peroxidation product that damages proteins [20, 21]. The O2−• protocol we developed for our plasma experiments was then adapted for use in macrophage cell culture to determine if O2−• induces Dox-like TNF-α consequences.
2. Methods and Materials
2.1 Chemicals
Chemicals, proteases, protease inhibitors, and antibodies used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise noted. Precision Plus Protein™ All Blue Standards, BCA reagents, and nitrocellulose membranes were purchased from Bio-RAD (Hercules, CA, USA).
2.2 Statistical analysis
All data are presented as mean±SEM. Statistical analyses were performed using ANOVA (and Bonferroni’s multiple comparison post-test) followed by a two-tailed Student’s t-test to make individual comparisons between groups where appropriate, with p<0.05 considered significant. Normality of data sets was tested using the D’Agostino & Pearson omnibus normality test where appropriate.
2.3 Animals
All procedures using animals were performed according to the protocols approved by the University of Kentucky Animal Care and Use Committee. Wild-type, male, SKH1 hairless, albino mice (2–3 months old) were purchased from the Jackson Laboratory. Mice were kept under standard conditions housed in the University of Kentucky Animal Facility, and all experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Kentucky. These animals were euthanized and blood and tissues collected for molecular or biochemical analysis. Whole blood collected by cardiac puncture, was immediately collected in EDTA tubes and plasma immediately separated by centrifugation.
2.4 Sample preparation
Protein estimation was performed using the bicinchoninic acid (BCA, Pierce) assay. Homogenized plasma samples were diluted according to initial protein estimation results using 20 ug sample in isolation buffer [0.32 M sucrose, 2 mM EDTA, 2mM EGTA, and 20mM HEPES pH 7.4 with protease inhibitors, 0.2 mM PMSF, 20ug/mL trypsin inhibitor, 4 μg/ml leupeptin, 4 μg/ml pepstatin A, and 5 μg/ml aprotinin].
2.5 Slot blot assay
The slot-blot method was used to determine levels of protein carbonyl and protein-bound HNE in plasma as previously described [2, 22]. For protein carbonyl determination, samples were derivatized with 2,4-dinitrophenylhydrazine (DNPH). For protein-bound HNE, samples were solubilized in Laemmli buffer. Protein (250 ng) from each sample was loaded onto a nitrocellulose membrane in respective wells in a slot-blot apparatus (Bio-Rad) under vacuum. Nitrocellulose membranes were blocked in 3% bovine serum albumin (BSA) in PBS with 0.2% (v/v) Tween-20 for 1.5 h and then incubated in primary antibody (anti-dinitrophenylhydrazone primary or anti-protein-bound HNE, respectively, each produced in rabbit, Sigma-Aldrich) for 2 h, washed three times in PBS with 0.2% (v/v) Tween-20 and then incubated for 1 h with secondary antibody (goat anti-rabbit secondary linked to alkaline phosphatase). Nitrocellulose membranes were developed with 5-bromo-4-chloro-3-indolyl-phosphate (BCIP) dipotassium and nitro blue tetrazolium (NBT) chloride in alkaline phosphatase activity (ALP) buffer, dried, and scanned for analysis. Image analysis was performed using Scion Image (Scion Corporation, Frederick, MD).
2.6 Solvent selection and potassium superoxide solution preparation
KO2 is a yellow solid that reacts readily with water and decomposes if exposed to water vapor or carbon dioxide in air. To avoid this, a saturated solution of KO2 was prepared fresh, according to the method previously described [14, 18, 19], in a solvent of anhydrous dimethyl sulfoxide (DMSO) containing 200mM crown ether (18crown6) to aid in solubility. To the prepared solvent, excess KO2 was added and the KO2 concentration estimated by UV-vis absorbance and using Beer’s law [18, 23]. A saturated solution of KO2 was approximately 250 μM under the stated conditions. Serial dilutions of this saturated solution were performed using the DMSO+18crown6 solvent to obtain the desired O2−• concentrations.
2.7 Plasma oxidation with potassium superoxide
KO2 in a solvent of DMSO containing 18crown6 was added to plasma from WT mice (2–3 months old WT, male, SKH1 hairless, albino mice purchased from the Jackson Laboratory) and incubated at 37°C for 0, 15, 30, and 90 min. Concentrations of 0, 0.1, 1.0, or 10 μM KO2 made using serial dilution were added to plasma to broadly encompass Dox concentrations used in previous studies. The solvent, DMSO containing 18crown6, was added to all control incubations.
2.8 Macrophage stimulation with potassium superoxide
Cell culture experiments were carried out using mouse BALB/c monocyte macrophage cell line (J774A.1) collected from murine blood. The mouse macrophage cell line J774A.1 (American Type Culture Collection) was cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% (v/v) fetal bovine serum, streptomycin (100μg/ml), and penicillin (100U/ml). All cultures were incubated at 37 °C in a humidified atmosphere with 5% CO2. J774A.1 macrophage cells were plated at a density of 5 × 105 cells/well in 48-well plates. J774.A1 macrophages were seeded onto a 48-well plate at 5×105 cells/well and allowed to grow overnight under standard culture conditions. KO2 was prepared as described above. Preincubation of solvent, lipopolysaccharide (LPS; 1 μg/ml), KO2 (0.1μM; 1μM; 10μM) for 1 h was performed before their addition to J774.A1 macrophages. Lipopolysaccharide (LPS; 1 μg/ml) or KO2 (0.1μM; 1μM; 10μM) was added and the cells were incubated for 24 h and compared with cells incubated in media only and cells incubated in media containing DMSO + 18crown6 vehicle. The supernatant was collected and levels of TNF-α (pg/ml) were determined with a specific ELISA kit for mouse TNF-α (R&D Systems).
3. Results
3.1 Protocol development
A variety of solvents and combinations were explored to make a stable solution of KO2. KO2 releases O2−• upon addition to an aqueous environment [14]. O2−• then reacts rapidly with water present in any solvent combination forming H2O2. In this study, the reaction of KO2 with water was more rapid and vigorous than expected. In fact, KO2 reacted with water vapor in the air during any attempt at weighing KO2, contrary to some methods describing standard weighing preparation or preparation in a water-based solution [24, 25]. Transition metals are known to influence the reactivity of dioxygen radicals including those present in O2−• [26]. Chelex removal of metal ions did not prevent this problem [27], and prior addition of catalase to the solvent only accelerated the reaction of KO2 with water presumably by reacting away the formed H2O2 and shifting the reaction equilibrium toward product [28–30]. This prompted the pursuit of a suitable anhydrous solvent for KO2. KO2 is slightly soluble in anhydrous DMSO [31]. A crown ether, 18crown6, was used to enhance solubility and stability [19].
3.2 Plasma oxidation from potassium superoxide
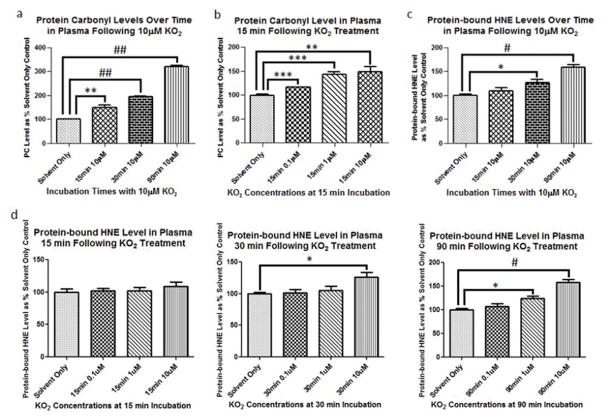
Plasma samples were treated with 0, 0.1, 1.0, or 10 μM KO2 for 0, 15, 30, and 90 min and analyzed via slot blot to determine relative levels of PC and protein-bound HNE as measures of protein oxidation and lipid peroxidation, respectively [20, 21]. PC damage to protein in plasma following incubation with KO2 was rapid. Using 10 μM KO2, PC levels for each successive time point were significantly elevated over the previous one indicated by Bonferroni’s Multiple Comparison Test. After 15 min incubation at 37°C, significant increases in PC were observed at 0.1, 1, and 10 μM KO2 (Fig. 1a, ***p<0.005, ***p<0.005, and **p<0.01, respectively). Significant increases in PC in plasma were also observed at each concentration, 0.1, 1, and 10 μM, of KO2 measured after 15 min incubation at 37°C (Fig. 1b, ***p<0.005, ***p<0.005, and **p<0.01, respectively). Similar experiments were performed to assess KO2-induced protein-bound HNE in plasma. Protein-bound HNE was significantly elevated after 30 and 90 min incubations with 10 μM KO2 at 37°C (*p<0.05, #p<0.001, respectively (Fig. 1c). Protein-bound HNE levels were not significantly elevated at the KO2 concentrations tested for the 15 min incubation at 37°C. Significant increases in protein-bound HNE levels were seen after 30 min incubation at 37°C with the 10 μM KO2 concentration ( *p<0.05) and after 90 min incubation at 37°C with the 1 uM and 10 μM KO2 concentrations (*p<0.05, #p<0.001, respectively) (Fig. 1d). The higher concentrations of KO2 and longer incubation times were required to reach significant increases in protein-bound HNE following KO2 addition (Fig. 1c, Fig. 1d). Decisions for moving forward were based on these preliminary KO2 dose-response results with varied incubation times.
Fig. 1.
Protein carbonyl (PC) and protein-bound HNE (HNE) are measures of protein oxidation and lipid peroxidation. (a) PC levels were assessed at 15, 30, and 90 min incubations with 10 μM KO2 at 37°C. All incubation times tested at 10 μM KO2 and 37°C, 15, 30, and 90 min, resulted in significantly increased PC compared to solvent alone (**p<0.01, ##p<0.0001, ##p<0.0001, respectively). PC for each successive time point at 10 μM KO2 was significantly elevated over the previous one indicated by Bonferroni’s Multiple Comparison Test. (b), Significant increases in PC were observed at 0.1, 1, and 10 μM KO2 after 15 min incubation at 37°C (***p<0.005, ***p<0.005, and **p<0.01, respectively). (c) Protein-bound HNE was significantly elevated after 30 and 90 min incubations with 10 μM KO2 at 37°C (*p<0.05, #p<0.001, respectively). (d) Protein-bound HNE levels were not significantly elevated at the KO2 concentrations tested for the 15 min incubation at 37°C. Significant increases in protein-bound HNE levels were seen after 30 min incubation at 37°C with the 10 μM KO2 concentration ( *p<0.05) and after 90 min incubation at 37°C with the 1 uM and 10 μM KO2 concentrations (*p<0.05, #p<0.001, respectively).
3.3 Superoxide induces TNF-α elevation in macrophage culture similar to that seen following doxorubicin administration
Previously, we reported TNF-α elevation in plasma or macrophage culture following Dox treatment and O2−• produced through redox cycling of Dox as the likely cause [2, 10]. Our group also demonstrated in a cross-over human clinical study that TNF-α and soluble TNF-α receptor levels are elevated in human plasma following i.v. Dox administration [32]. Macrophages are a principal source of TNF-α production in vivo [33–35], and microglial activation in brain following Dox-induced TNF-α elevation leads to the previously mentioned central nervous system toxicities [24, 33]. Here, we test our hypothesis that O2−•, administered as KO2, will lead to TNF-α elevation in macrophage culture similar to that observed following Dox administration [2]. Significantly increased TNF-α elevation in J774.A1 macrophage culture after incubation with KO2 for 24 h was observed. TNF-α was increased in these cell lines following incubation with 1 and 10 μM KO2 (p<*** 0.0001) (Fig. 2). Incubation of these macrophages with the 10 μM KO2 concentration resulted in TNF-α greater than treatment of the cells with lipopolysaccharide (LPS; 1 μg/ml), a known initiator of TNF-α transcription via nuclear factor κ-light-chain enhancer of activated B cells (NF-κB) (Fig. 2) [36–41].
Fig. 2.
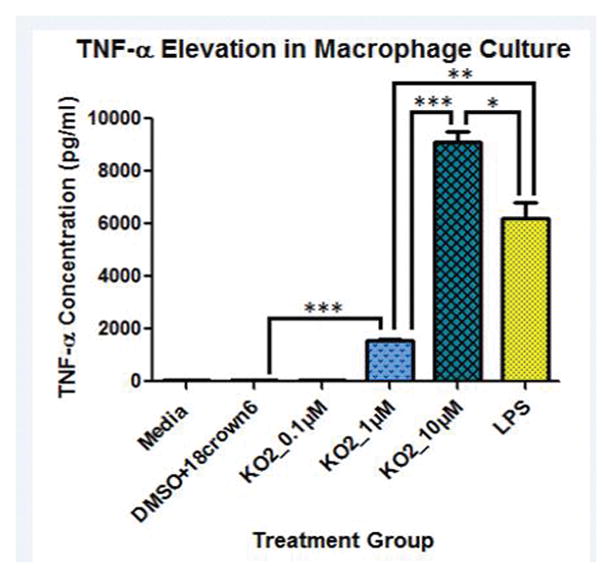
Superoxide (O2−•) induces TNF-α production in J774.A1 macrophages. J774.A1 macrophages were seeded onto a 48-well plate at 5×105 cells/well and allowed to grow overnight. Preincubation of solvent, lipopolysaccharide (LPS; 1 μg/ml), KO2 (0.1μM; 1μM; 10μM) for 1 h was performed before their addition to J774.A1 macrophages. Following 24 h incubation, supernatants were collected and analyzed for TNF-α concentration. Values are means±SEM (n=3). (*p<0.05, **p<0.005, ***p<0.0001 compared to solvent alone). One-way ANOVA (p<0.0001) with Bonferroni’s Multiple Comparison Test also demonstrated these significant differences between groups.
4. Discussion
Prior studies by our laboratory implicated O2−•, produced in vivo via the redox cycling of the chemotherapeutic agent Dox, in increased oxidative damage to plasma proteins, elevation of TNF-α in the periphery, followed by transfer of TNF-α to brain and further TNF-α elevation in the parenchyma. Subsequent CNS toxicity including mitochondrial dysfunction and neuronal death were observed, and we suggest such processes are involved in CICI [1, 2, 8–13, 42]. The quinone moiety within the molecular structure of Dox cycles between the quinone and semi-quinone, producing superoxide free radical from molecular oxygen as it cycles back to the quinone [2–5, 42]. The current study was undertaken to test the plausibility of our hypothesis that O2−• from Dox is responsible for oxidative protein damage, TNF-α elevation, and cognitive consequences observed following chemotherapy with ROS-producing anti-cancer drugs, like Dox, that do not cross the BBB but result in these unwanted clinical signs and symptoms.
In the current study, superoxide caused oxidative damage to plasma proteins in vitro rapidly and at small concentrations of KO2, similar to damage caused by Dox. All incubation times tested, 15, 30, and 90 min, resulted in significantly increased plasma protein oxidative damage as indicated by PC elevation with PC at each successive time point significantly increased over the previous one (Fig. 1a) [20]. Significant PC elevation was observed in plasma even at the lowest concentrations of KO2 tested (Fig. 1b). Evidence of lipid damage was also found in KO2-treated plasma in the form of protein-bound HNE, a product of lipid peroxidation [21] (Fig. 1c). Significant increases in protein-bound HNE was observed after 30 min incubation at 10 uM KO2 (Fig. 1c, Fig. 1d) and at 1 and 10 uM KO2 (Fig. 1d). Higher concentrations of KO2 and longer incubation times tested were required to reach significant increases in protein-bound HNE following KO2 addition (Fig. 1c) which might reflect the negative charge of O2−• being slow to enter a hydrophobic environment.
In biological systems, O2−• is produced enzymatically during reactions catalyzed by oxidases and non-enzymatically during inefficient actions of the mitochondrial electron transport chain. The damaging effects of O2−• to biomolecules may be limited due to the rapid rate of radical-radical reactions, the limited reactivity of O2−• with non-free radical targets, and the diffusion-limited efficiency of superoxide dismutase (SOD) enzymes [43–45]. Reaction of O2−• with SOD produces a less reactive H2O2 that can be converted to water and molecular oxygen by peroxidase enzymes. Reaction of However, when the chemotherapeutic agent Dox is present in vivo, continued redox cycling of the quinone moiety creates a continued source of O2−• as Dox travels into the cell and into the nucleus [2, 32]. O2−• reacts with other free radicals including nitric oxide (NO•) rapidly, at approximately diffusion-limited rates. The reaction of O2−• with NO• produces the even more reactive, peroxynitrite (ONOO−) whose downstream reactivity damages SOD, thereby limiting natural defenses against these free radicals [9, 46]. O2−• has been shown to be highly reactive with iron-sulfur clusters producing H2O2 and, in a subsequent reaction, iron II (Fe2+). Increased production of H2O2, produced via the actions of SOD or reaction of O2−• with iron-sulfur clusters, through Fenton Chemistry can lead to the production of the highly reactive •OH, the strongest oxidant in biological systems [47]. Reactive oxygen species (ROS), including O2−•, NO•, •OH, H2O2, and reactive aldehydes (i.e., HNE) can oxidize intracellular proteins and other biomolecules [48]. In a series of reactions outlined by Stadtman, 1997 under conditions where only O2−• and •OH were formed, radical-mediated protein oxidation lead to oxidation of amino acid side chains, fragmentation of the peptide backbone, and protein-protein cross links [48]. These provide supporting evidence for a plausible chemical mechanism for the oxidation of proteins by O2−• and its reaction products.
This study directly addresses a critical aspect of our proposed mechanism of CICI, the question of whether superoxide, produced via redox cycling of Dox, is an oxidant capable of inducing oxidative damage to plasma protein and TNF-α elevation in macrophages, the proposed cytokine culprit of CICI [2, 32]. These results are consistent with our previous results demonstrating increased protein oxidation and lipid peroxidation markers in plasma and subsequent TNF-α elevation following Dox administration in vivo and in macrophage culture. These results demonstrate that O2−•, when added to plasma in the form of KO2 salt, stabilized by the molecular cage of a crown ether, 18crown6, and incubated at physiologic 37°C, results protein oxidation in plasma and TNF-α elevation in macrophage culture similar to that observed following Dox administration in vivo. Together, these results are compelling evidence supporting the notion that O2−• production as a result of Dox administration is the likely initiating event in the neurotoxicity associated with Dox and provides useful insights into our hypothesized mechanism of CICI caused by cancer chemotherapy with ROS-producing chemotherapeutic agents like Dox.
HIGHLIGHTS.
We showed earlier doxorubicin produces O2−• and leads to TNF elevation in plasma
We showed earlier that elevated plasma TNF translocated to brain to cause apoptosis
Here KO2 added to plasma or macrophages elevated oxidative stress and TNF, respectively
We conclude that O2−• from Dox is involved in processes that lead to brain cell death
Acknowledgments
The authors gratefully acknowledge insights from Dr. A.F. Miller, University of Kentucky, Department of Chemistry for her knowledge and insights into working with potassium superoxide.
This work was supported in part by a grant from NIH [CA-148341] and the Markey Cancer Center Research Funds.
Abbreviations
- ROS
reactive oxygen species
- Dox
doxorubicin
- O2−•
superoxide
- TNF-α
tumor necrosis factor-alpha
- BBB
blood-brain barrier
- H2O2
hydrogen peroxide
- •OH
hydroxyl radical
- CICI
chemotherapy-induced cognitive impairment
- ApoA-I
apolipoprotein A-I
- WT
wild-type
- KO2
potassium superoxide salt
- PC
protein carbonyl
- HNE
4-hydroxy-2-trans-nonenal
- BCA
bicinchoninic acid
- DNPH
2,4-dinitrophenylhydrazine
- BSA
bovine serum albumin
- BCIP
5-bromo-4-chloro-3-indolyl-phosphate dipotassium
- NBT
nitro blue tetrazolium chloride
- ALP
alkaline phosphatase activity buffer
- DMSO
dimethylsulfoxide
- 18crown6
crown ether, 18-crown-6
- LPS
lipopolysaccharide
- NF-κB
nuclear factor κ-light-chain enhancer of activated B cells
- SOD
superoxide dismutase
- NO•
nitric oxide
- ROS
reactive oxygen species
Footnotes
Conflict of interest
There was no conflict of interest in this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen Y, Jungsuwadee P, Vore M, Butterfield DA, St Clair DK. Collateral damage in cancer chemotherapy: oxidative stress in nontargeted tissues. Molecular interventions. 2007;7:147–156. doi: 10.1124/mi.7.3.6. [DOI] [PubMed] [Google Scholar]
- 2.Aluise CD, Miriyala S, Noel T, Sultana R, Jungsuwadee P, Taylor TJ, Cai J, Pierce WM, Vore M, Moscow JA, St Clair DK, Butterfield DA. 2-Mercaptoethane sulfonate prevents doxorubicin-induced plasma protein oxidation and TNF-alpha release: implications for the reactive oxygen species-mediated mechanisms of chemobrain. Free radical biology & medicine. 2011;50:1630–1638. doi: 10.1016/j.freeradbiomed.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Bachur NR, Gordon SL, Gee MV. Anthracycline antibiotic augmentation of microsomal electron transport and free radical formation. Molecular pharmacology. 1977;13:901–910. [PubMed] [Google Scholar]
- 4.Cummings J, Anderson L, Willmott N, Smyth JF. The molecular pharmacology of doxorubicin in vivo. Eur J Cancer. 1991;27:532–535. doi: 10.1016/0277-5379(91)90209-v. [DOI] [PubMed] [Google Scholar]
- 5.Handa K, Sato S. Generation of free radicals of quinone group-containing anti-cancer chemicals in NADPH-microsome system as evidenced by initiation of sulfite oxidation. Gann = Gan. 1975;66:43–47. [PubMed] [Google Scholar]
- 6.Vasquez-Vivar J, Martasek P, Hogg N, Masters BS, Pritchard KA, Jr, Kalyanaraman B. Endothelial nitric oxide synthase-dependent superoxide generation from adriamycin. Biochemistry. 1997;36:11293–11297. doi: 10.1021/bi971475e. [DOI] [PubMed] [Google Scholar]
- 7.Konorev EA, Kennedy MC, Kalyanaraman B. Cell-permeable superoxide dismutase and glutathione peroxidase mimetics afford superior protection against doxorubicin-induced cardiotoxicity: the role of reactive oxygen and nitrogen intermediates. Archives of biochemistry and biophysics. 1999;368:421–428. doi: 10.1006/abbi.1999.1337. [DOI] [PubMed] [Google Scholar]
- 8.Joshi G, Sultana R, Tangpong J, Cole MP, St Clair DK, Vore M, Estus S, Butterfield DA. Free radical mediated oxidative stress and toxic side effects in brain induced by the anti cancer drug adriamycin: insight into chemobrain. Free radical research. 2005;39:1147–1154. doi: 10.1080/10715760500143478. [DOI] [PubMed] [Google Scholar]
- 9.Tangpong J, Sompol P, Vore M, St Clair W, Butterfield DA, St Clair DK. Tumor necrosis factor alpha-mediated nitric oxide production enhances manganese superoxide dismutase nitration and mitochondrial dysfunction in primary neurons: an insight into the role of glial cells. Neuroscience. 2008;151:622–629. doi: 10.1016/j.neuroscience.2007.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tangpong J, Cole MP, Sultana R, Joshi G, Estus S, Vore M, St Clair W, Ratanachaiyavong S, St Clair DK, Butterfield DA. Adriamycin-induced, TNF-alpha-mediated central nervous system toxicity. Neurobiology of disease. 2006;23:127–139. doi: 10.1016/j.nbd.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Tangpong J, Cole MP, Sultana R, Estus S, Vore M, St Clair W, Ratanachaiyavong S, St Clair DK, Butterfield DA. Adriamycin-mediated nitration of manganese superoxide dismutase in the central nervous system: insight into the mechanism of chemobrain. Journal of neurochemistry. 2007;100:191–201. doi: 10.1111/j.1471-4159.2006.04179.x. [DOI] [PubMed] [Google Scholar]
- 12.Joshi G, Aluise CD, Cole MP, Sultana R, Pierce WM, Vore M, St Clair DK, Butterfield DA. Alterations in brain antioxidant enzymes and redox proteomic identification of oxidized brain proteins induced by the anti-cancer drug adriamycin: implications for oxidative stress-mediated chemobrain. Neuroscience. 2010;166:796–807. doi: 10.1016/j.neuroscience.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joshi G, Hardas S, Sultana R, St Clair DK, Vore M, Butterfield DA. Glutathione elevation by gamma-glutamyl cysteine ethyl ester as a potential therapeutic strategy for preventing oxidative stress in brain mediated by in vivo administration of adriamycin: Implication for chemobrain. Journal of neuroscience research. 2007;85:497–503. doi: 10.1002/jnr.21158. [DOI] [PubMed] [Google Scholar]
- 14.McPherson DB, Kilker RP, Foley TD. Superoxide activates constitutive nitric oxide synthase in a brain particulate fraction. Biochemical and biophysical research communications. 2002;296:413–418. doi: 10.1016/s0006-291x(02)00897-5. [DOI] [PubMed] [Google Scholar]
- 15.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 4. Oxford University Press; Oxford; New York: 2007. [Google Scholar]
- 16.Gutteridge JM. Lipid peroxidation initiated by superoxide-dependent hydroxyl radicals using complexed iron and hydrogen peroxide. FEBS letters. 1984;172:245–249. doi: 10.1016/0014-5793(84)81134-5. [DOI] [PubMed] [Google Scholar]
- 17.Lopresti AL, Maker GL, Hood SD, Drummond PD. A review of peripheral biomarkers in major depression: the potential of inflammatory and oxidative stress biomarkers. Progress in neuro-psychopharmacology & biological psychiatry. 2014;48:102–111. doi: 10.1016/j.pnpbp.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Miller AF. Superoxide processing. In: Meyer TJ, McCleverty JA, editors. Comprehensive Coordination Chemistry II. Elsevier Science; Oxford: 2003. pp. 479–506. [Google Scholar]
- 19.Valentine JS, Curtis AB. A convenient preparation of solutions of superoxide aniom and the reaction of superoxide anion with a copper (II) complex. Journal of the American Chemical Society. 1975;97:224–226. doi: 10.1021/ja00834a058. [DOI] [PubMed] [Google Scholar]
- 20.Butterfield DA, Stadtman ER. Protein Oxidation Processes in Aging Brain. Adv Cell Aging and Gerontol. 1997;2:161–191. [Google Scholar]
- 21.Subramaniam R, Roediger F, Jordan B, Mattson MP, Keller JN, Waeg G, Butterfield DA. The lipid peroxidation product, 4-hydroxy-2-trans-nonenal, alters the conformation of cortical synaptosomal membrane proteins. Journal of neurochemistry. 1997;69:1161–1169. doi: 10.1046/j.1471-4159.1997.69031161.x. [DOI] [PubMed] [Google Scholar]
- 22.Sultana R, Butterfield DA. Slot-blot analysis of 3-nitrotyrosine-modified brain proteins. Methods in enzymology. 2008;440:309–316. doi: 10.1016/S0076-6879(07)00820-8. [DOI] [PubMed] [Google Scholar]
- 23.Kim S, Di Cosimo R, San Filippo J. Spectrometric and chemical characterization of superoxide. Anal Chem. 1979;51:679–681. [Google Scholar]
- 24.Olojo RO, Xia RH, Abramson JJ. Spectrophotometric and fluorometric assay of superoxide ion using 4-chloro-7-nitrobenzo-2-oxa-1,3-diazole. Analytical biochemistry. 2005;339:338–344. doi: 10.1016/j.ab.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 25.Maioli NA, Zarpelon AC, Mizokami SS, Calixto-Campos C, Guazelli CF, Hohmann MS, Pinho-Ribeiro FA, Carvalho TT, Manchope MF, Ferraz CR, Casagrande R, Verri WA., Jr The superoxide anion donor, potassium superoxide, induces pain and inflammation in mice through production of reactive oxygen species and cyclooxygenase-2. Braz J Med Biol Res. 2015;48:321–331. doi: 10.1590/1414-431X20144187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aust SD, Morehouse LA, Thomas CE. Role of metals in oxygen radical reactions. Journal of free radicals in biology & medicine. 1985;1:3–25. doi: 10.1016/0748-5514(85)90025-x. [DOI] [PubMed] [Google Scholar]
- 27.Halliwell B, Gutteridge JM. Iron and free radical reactions: two aspects of antioxidant protection. Trends in biochemical sciences. 1986;11:372–375. [Google Scholar]
- 28.Lardinois OM. Reactions of bovine liver catalase with superoxide radicals and hydrogen peroxide. Free radical research. 1995;22:251–274. doi: 10.3109/10715769509147544. [DOI] [PubMed] [Google Scholar]
- 29.Aksenov MY, Tucker HM, Nair P, Aksenova MV, Butterfield DA, Estus S, Markesbery WR. The expression of key oxidative stress-handling genes in different brain regions in Alzheimer’s disease. Journal of molecular neuroscience: MN. 1998;11:151–164. doi: 10.1385/JMN:11:2:151. [DOI] [PubMed] [Google Scholar]
- 30.Stadtman ER, Berlett BS. Fenton chemistry. Amino acid oxidation. The Journal of biological chemistry. 1991;266:17201–17211. [PubMed] [Google Scholar]
- 31.Weber WP, Gokel GW. Reactions of Superoxide Ions. Springer; Berlin Heidelberg: 1977. [Google Scholar]
- 32.Hayslip J, Dressler EV, Weiss H, Taylor TJ, Chambers M, Noel T, Miriyala S, Keeney JT, Ren X, Sultana R, Vore M, Butterfield DA, St Clair D, Moscow JA. Plasma TNF-alpha and Soluble TNF Receptor Levels after Doxorubicin with or without Co-Administration of Mesna-A Randomized, CrossOver Clinical Study. PloS one. 2015;10:e0124988. doi: 10.1371/journal.pone.0124988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furtado JL, Oliveira GA, Pontes AS, da Setubal SS, Xavier CV, Lacouth-Silva F, Lima BF, Zaqueo KD, Kayano AM, Calderon LA, Stabeli RG, Soares AM, Zuliani JP. Activation of J77A.1 macrophages by three phospholipases A2 isolated from Bothrops atrox snake venom. Bio Med research international. 2014;2014:683123. doi: 10.1155/2014/683123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raffa RB. A proposed mechanism for chemotherapy-related cognitive impairment (‘chemo-fog’) Journal of clinical pharmacy and therapeutics. 2011;36:257–259. doi: 10.1111/j.1365-2710.2010.01188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim S, Dicosimo R, Filippo JS. Spectrometric and Chemical Characterization of Superoxide. Anal Chem. 1979;51:679–681. [Google Scholar]
- 36.Keeney JT, Forster S, Sultana R, Brewer LD, Latimer CS, Cai J, Klein JB, Porter NM, Butterfield DA. Dietary vitamin D deficiency in rats from middle to old age leads to elevated tyrosine nitration and proteomics changes in levels of key proteins in brain: Implications for low vitamin D-dependent age-related cognitive decline. Free radical biology & medicine. 2013;65C:324–334. doi: 10.1016/j.freeradbiomed.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schreck R, Albermann K, Baeuerle PA. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review) Free radical research communications. 1992;17:221–237. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- 38.Chandel NS, Trzyna WC, McClintock DS, Schumacker PT. Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J Immunol. 2000;165:1013–1021. doi: 10.4049/jimmunol.165.2.1013. [DOI] [PubMed] [Google Scholar]
- 39.Griscavage JM, Wilk S, Ignarro LJ. Inhibitors of the proteasome pathway interfere with induction of nitric oxide synthase in macrophages by blocking activation of transcription factor NF-kappa B. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:3308–3312. doi: 10.1073/pnas.93.8.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell metabolism. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stigger F, Lovatel G, Marques M, Bertoldi K, Moyses F, Elsner V, Siqueira IR, Achaval M, Marcuzzo S. Inflammatory response and oxidative stress in developing rat brain and its consequences on motor behavior following maternal administration of LPS and perinatal anoxia. International journal of developmental neuroscience: the official journal of the International Society for Developmental Neuroscience. 2013;31:820–827. doi: 10.1016/j.ijdevneu.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Aluise CD, Sultana R, Tangpong J, Vore M, St Clair D, Moscow JA, Butterfield DA. Chemo brain (chemo fog) as a potential side effect of doxorubicin administration: role of cytokine-induced, oxidative/nitrosative stress in cognitive dysfunction. Advances in experimental medicine and biology. 2010;678:147–156. doi: 10.1007/978-1-4419-6306-2_19. [DOI] [PubMed] [Google Scholar]
- 43.Bielski BHJ, Richter HW. Study of Superoxide Radical Chemistry by Stopped-Flow Radiolysis and Radiation-Induced Oxygen-Consumption. J Am Chem Soc. 1977;99:3019–3023. [Google Scholar]
- 44.Sheng Y, Abreu IA, Cabelli DE, Maroney MJ, Miller AF, Teixeira M, Valentine JS. Superoxide dismutases and superoxide reductases. Chem Rev. 2014;114:3854–3918. doi: 10.1021/cr4005296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller AF. Superoxide dismutases: ancient enzymes and new insights. FEBS letters. 2012;586:585–595. doi: 10.1016/j.febslet.2011.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Surmeli NB, Litterman NK, Miller AF, Groves JT. Peroxynitrite mediates active site tyrosine nitration in manganese superoxide dismutase. Evidence of a role for the carbonate radical anion. J Am Chem Soc. 2010;132:17174–17185. doi: 10.1021/ja105684w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Augusto O, Miyamoto S. Oxygen Radicals and Related Species. In: Pantopoulos K, Schipper HM, editors. Principles of free radical biomedicine. Nova Science Publishers; New York, NY: 2011. p. v. [Google Scholar]
- 48.Stadtman ER. Protein oxidation and aging. Free Radic Res. 2006;40:1250–1258. doi: 10.1080/10715760600918142. [DOI] [PubMed] [Google Scholar]