Abstract
The photosynthetic water oxidase is composed of ˜15 polypeptides which are grouped around two functional parts: photosystem II and the catalytic manganese centre. Photochemically driven vectorial electron transfer between the manganese centre and bound plastoquinone causes deprotonation–protonation reactions at opposite sides of the thylakoid membrane. Thereby the water oxidase acts as a proton pump. Incubation of stacked thylakoids with N,N'-dicyclohexylcarbodiimide (DCCD) short-circuited its proton pumping activity. Under flashing light, the extent of both proton release into the lumen by water oxidation and of proton uptake from the medium by reduced quinone was diminished. Instead there was a rapid electrogenic backreaction with a strong H/D-isotope effect. Apparently protons which were produced by water oxidation were channelled across the transmembrane protein to the bound quinone. A more rapid protonation of the reduced quinone was evident from a shortening of the time lag for the reduction of photosystem I. These effects were paralleled by the preferential labelling with [14C]DCCD in stacked thylakoids of two polypeptides with 20 and 24 kd apparent molecular mass. These may be capping the oxidizing and the reducing terminus of the water oxidase to control proton extrusion and proton uptake respectively.
Keywords: photosynthesis, photosystem II, proton pump, water oxidase, DCCD
Full text
PDF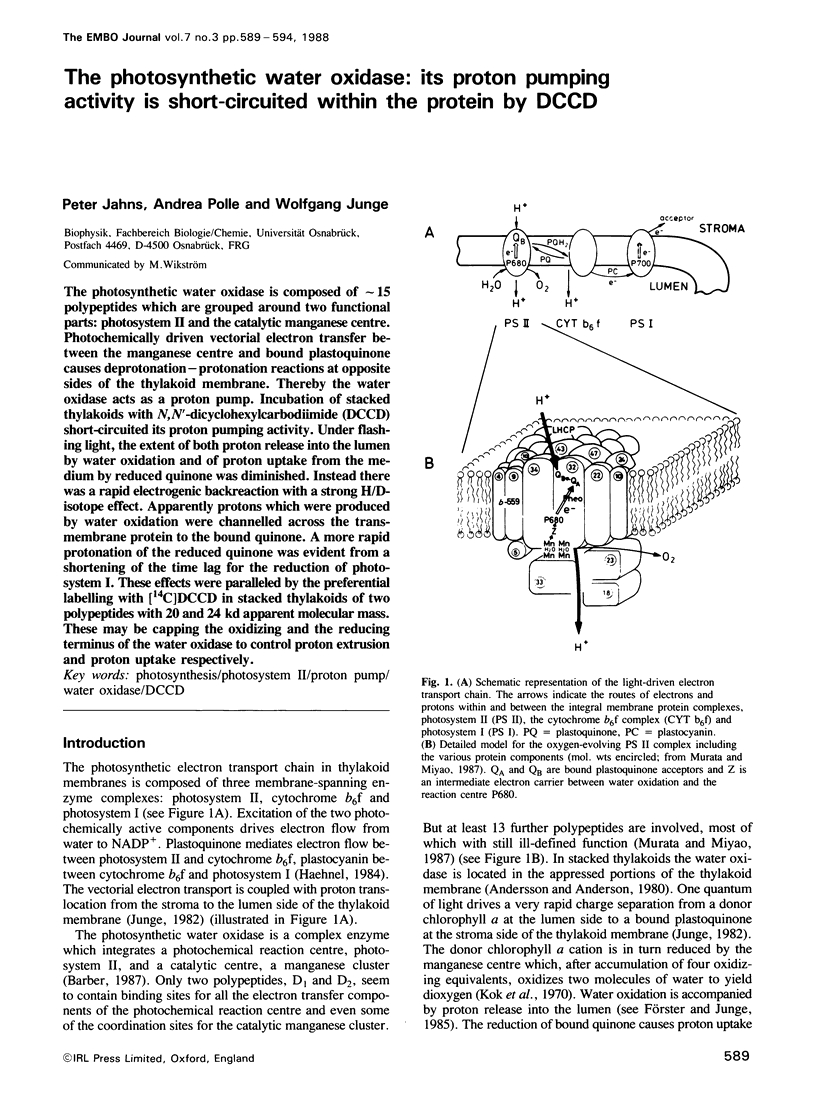


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B., Anderson J. M. Lateral heterogeneity in the distribution of chlorophyll-protein complexes of the thylakoid membranes of spinach chloroplasts. Biochim Biophys Acta. 1980 Dec 3;593(2):427–440. doi: 10.1016/0005-2728(80)90078-x. [DOI] [PubMed] [Google Scholar]
- Azzi A., Casey R. P., Nałecz M. J. The effect of N,N'-dicyclohexylcarbodiimide on enzymes of bioenergetic relevance. Biochim Biophys Acta. 1984 Dec 17;768(3-4):209–226. doi: 10.1016/0304-4173(84)90017-x. [DOI] [PubMed] [Google Scholar]
- Casey R. P., Thelen M., Azzi A. Dicyclohexylcarbodiimide binds specifically and covalently to cytochrome c oxidase while inhibiting its H+-translocating activity. J Biol Chem. 1980 May 10;255(9):3994–4000. [PubMed] [Google Scholar]
- Dunahay T. G., Staehelin L. A. Isolation and Characterization of a New Minor Chlorophyll a/b-Protein Complex (CP24) from Spinach. Plant Physiol. 1986 Feb;80(2):429–434. doi: 10.1104/pp.80.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelbrecht S., Lill H., Junge W. Reconstitution of CF1-depleted thylakoid membranes with complete and fragmented chloroplast ATPase. The role of the delta subunit for proton conduction through CF0. Eur J Biochem. 1986 Nov 3;160(3):635–643. doi: 10.1111/j.1432-1033.1986.tb10085.x. [DOI] [PubMed] [Google Scholar]
- Haehnel W. The reduction kinetics of chlorophyll aI as an indicator for proton uptake between the light reactions in chloroplasts. Biochim Biophys Acta. 1976 Sep 13;440(3):506–521. doi: 10.1016/0005-2728(76)90038-4. [DOI] [PubMed] [Google Scholar]
- Junge W., Ausländer W., McGeer A. J., Runge T. The buffering capacity of the internal phase of thylakoids and the magnitude of the pH changes inside under flashing light. Biochim Biophys Acta. 1979 Apr 11;546(1):121–141. doi: 10.1016/0005-2728(79)90175-0. [DOI] [PubMed] [Google Scholar]
- Junge W., Witt H. T. On the ion transport system of photosynthesis--investigations on a molecular level. Z Naturforsch B. 1968 Feb;23(2):244–254. doi: 10.1515/znb-1968-0222. [DOI] [PubMed] [Google Scholar]
- Kok B., Forbush B., McGloin M. Cooperation of charges in photosynthetic O2 evolution-I. A linear four step mechanism. Photochem Photobiol. 1970 Jun;11(6):457–475. doi: 10.1111/j.1751-1097.1970.tb06017.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liveanu V., Yocum C. F., Nelson N. Polypeptides of the oxygen-evolving photosystem II complex. Immunological detection and biogenesis. J Biol Chem. 1986 Apr 25;261(12):5296–5300. [PubMed] [Google Scholar]
- Pototsky NYa, Remennikov V. G., Kotova E. A., Samuilov V. D. The inhibition of photosynthetic electron transfer in Rhodospirillum rubrum by N ,N' -dicyclohexylcarbodiimide. Biochim Biophys Acta. 1981 Feb 12;634(2):266–270. doi: 10.1016/0005-2728(81)90145-6. [DOI] [PubMed] [Google Scholar]
- Prochaska L. J., Fink P. S. On the role of subunit III in proton translocation in cytochrome c oxidase. J Bioenerg Biomembr. 1987 Apr;19(2):143–166. doi: 10.1007/BF00762722. [DOI] [PubMed] [Google Scholar]
- Sane P. V., Johanningmeier U., Trebst A. The inhibition of photosynthetic electron flow by DCCD. An indication for proton channels. FEBS Lett. 1979 Dec 1;108(1):136–140. doi: 10.1016/0014-5793(79)81195-3. [DOI] [PubMed] [Google Scholar]
- Shoshan V., Selman B. R. The interaction of N,N'-dicyclohexylcarbodiimide with chloroplast coupling factor 1. J Biol Chem. 1980 Jan 25;255(2):384–389. [PubMed] [Google Scholar]




