Key Points
In MM patients, stringent CR criteria, in particular the sFLC ratio, do not predict significantly better outcome among MM patients in conventional CR.
Abstract
Stringent complete response (sCR) criteria are used in multiple myeloma as a deeper response category compared with CR, but prospective validation is lacking, it is not always clear how evaluation of clonality is performed, and is it not known what the relative clinical influence is of the serum free light chain ratio (sFLCr) and bone marrow (BM) clonality to define more sCR. To clarify this controversy, we focused on 94 patients that reached CR, of which 69 (73%) also fulfilled the sCR criteria. Patients with sCR displayed slightly longer time to progression (median, 62 vs 53 months, respectively; P = .31). On analyzing this contribution to the prognosis of sFLCr or clonality, it was found that the sFLCr does not identify patients in CR at distinct risk; by contrast, low-sensitive multiparametric flow cytometry (MFC) immunophenotyping (2 colors), which is equivalent to immunohistochemistry, identifies a small number of patients (5 cases) with high residual tumor burden and dismal outcome; nevertheless, using traditional 4-color MFC, persistent clonal BM disease was detectable in 36% of patients, who, compared with minimal residual disease-negative cases, had a significantly inferior outcome. These results show that the current definition of sCR should be revised.
Introduction
Achieving deeper levels of tumor debulking in multiple myeloma (MM) represents a surrogate marker for survival.1-4 To discriminate different outcomes among patients in conventional complete response (CR), the International Myeloma Working Group (IMWG) introduced more stringent CR (sCR) criteria5 by adding a normal free-light chain ratio (sFLCr) plus the absence of clonal plasma cells (PCs) in bone marrow (BM) by immunohistochemistry (IHC) to the preexisting European Society for Blood and Marrow Transplantation CR criteria.6 In 2011, the evaluation of BM clonality by low-sensitivity multiparametric flow cytometry (MFC) was included as an alternative methodology to IHC to define sCR.7 Despite its wide use as a clinical end point, only 1 study8 has reported a benefit of sCR over CR, whereas other studies suggested that the κ/λ values do not provide additional prognostication.9-11 Furthermore, the term sCR is widely used without a clear description of how BM clonality was evaluated, nor the individual influence of the sFLC and BM clonality to define sCR criteria. Here, we report on the value of achieving sCR among patients in conventional CR included in 2 consecutive Grupo Español de Mieloma Múltiple/Programa para el Estudio de la Terapéutica en Hemopatías Malignas (GEM/PETHEMA) clinical trials. We also studied the individual contribution to the prognosis of patients in CR of each of these parameters: sFLCr, BM clonality by low-sensitivity MFC, and minimal residual disease (MRD) monitoring by conventional 4-color MFC.
Study design
This study focuses on 94 patients in CR: 50 who were transplant eligible and were treated according to the GEM2005MENOS65 (median follow-up, 70 months), and 44 elderly MM patients included in the GEM2005MAS65 (median follow-up, 65 months) trials.12,13 After 6 induction cycles or after transplantation in younger patients, all were in CR as strictly defined according to the EBMT criteria.6 In all cases, sFLC (FREELITE assay; Binding Site Ltd.) was measured by immune-nephelometry, and sFLC κ/λ ratios were classified as normal (0.26-1.65) or abnormal (<0.26 if the patient was λ; >1.65 if the patient was κ, following the IMWG guidelines).5 BM clonality was defined by IHC when the κ/λ ratio was >4:1 or <1:2 for κ and λ patients, respectively, after counting ≥100 PCs. Here, we used an alternative method to IHC based on a low-sensitivity MFC approach to define clonality. Thus, for patients with a κ isotype, a 4:1 ratio of clonal/polyclonal PCs was defined by the presence of 80% phenotypically aberrant clonal PCs within the BM PC compartment. For patients with the λ isotype, a ratio of 1:2 polyclonal/clonal PCs was defined by the presence of 50% clonal PCs within the BM PC compartment. The low-sensitivity MFC-based assessment of clonality adapted to the IHC ratios as proposed by the IMWG criteria was also compared with MRD monitoring using conventional 4-color MFC as described elsewhere14,15: >20 clonal PCs after measuring ≥200 000 nucleated cells, at a sensitivity level of 10−4.
Briefly, erythrocyte-lysed whole BM samples were immunophenotyped using the 4-color antibody combination CD38-fluorescein isothiocyanate/CD56-phycoerythrin/CD19-PerCP-Cy5.5/CD45-allophycocyanin, with the exception of selected cases in which other antigens rather than CD56 (e.g., CD28, CD81, and/or CD117) were more useful to discriminate clonal from normal PCs (patient-specific approach). Data acquisition was performed in FACSCalibur and FACSCantoII flow cytometers (Becton-Dickinson, San Jose, CA); Infinicyt software (Cytognos, Salamanca, Spain) was used to analyze flow data.16 Time to progression (TTP) and overall survival (OS) curves were plotted by the Kaplan-Meier method, and the log-rank test was used to estimate the statistical significance of differences observed between curves.
Results and discussion
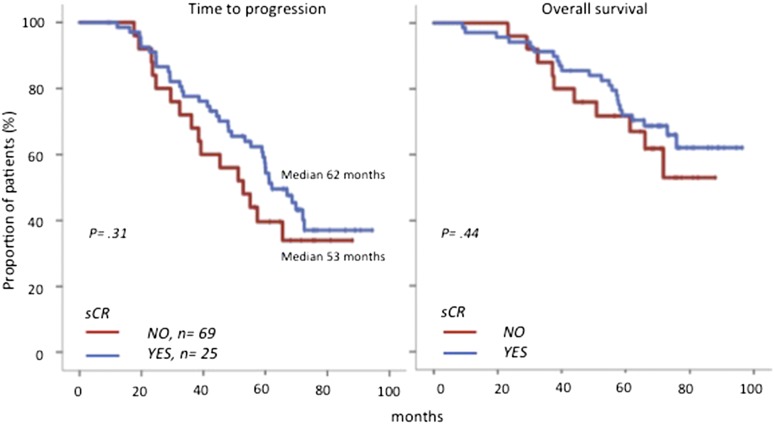
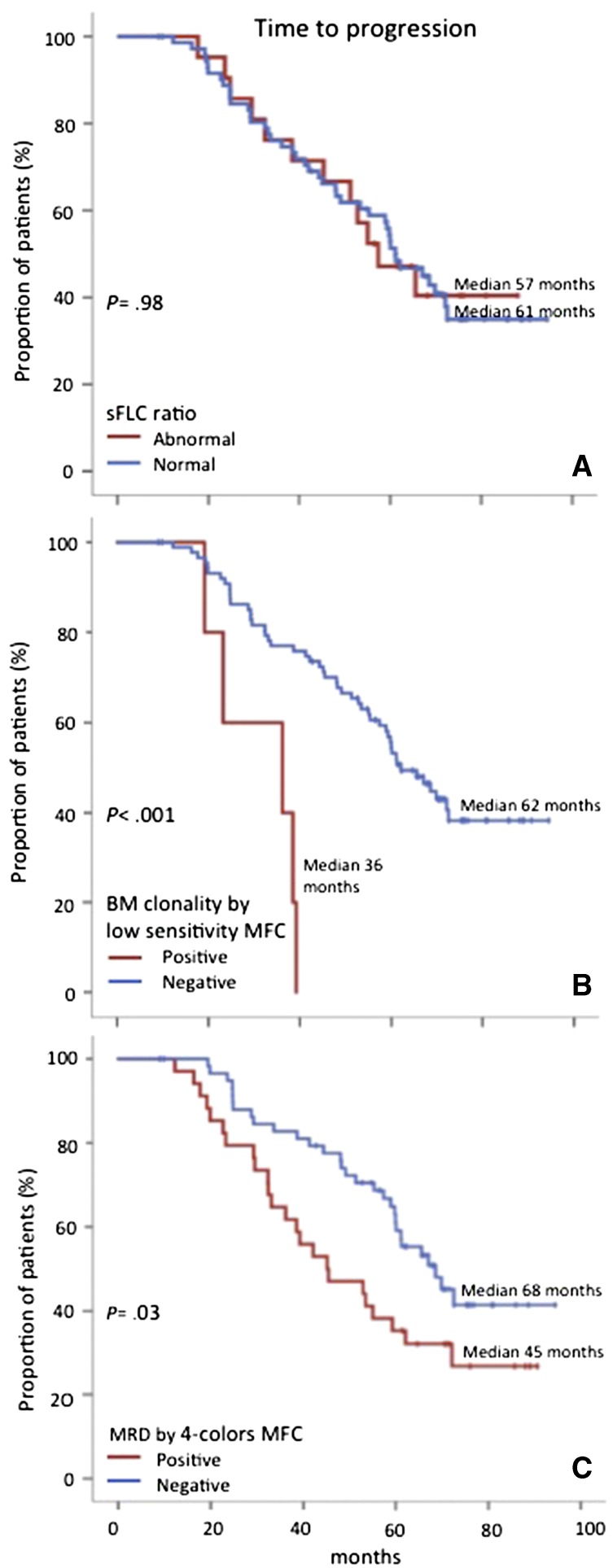
Patients achieving CR showed superior outcome compared with those failing to reach CR, regardless of the induction therapy or patients’ age (data not shown).12,13,17 The rate of sCR was of 73% in transplant-eligible patients and 79% in elderly cases; overall, 69 (73%) of 94 cases in CR fulfilled the sCR criteria, whereas the remaining 25 cases were not considered in sCR because they failed to accomplish 1 of the 2 criteria: abnormal sFLCr (n = 21; 84%) or BM PC clonality (n = 5; 20%); 1 patient had both abnormal sFLCr and BMPC clonality. On comparing the 69 patients in sCR with the 25 in CR, the former showed a nonsignificantly longer TTP (median, 62 vs 53 months, respectively; P = .31) and OS (both medians not reached [NR]; P = .44; Figure 1). Interestingly, patients with abnormal vs normal sFLCr showed superimposable TTP (median, 57 vs 61 months; P = .98; Figure 2A) and OS (both medians, NR; P = .90). By contrast, the few patients (n = 5) in whom BM clonality was detected by the low-sensitivity IHC-adapted MFC method had significantly shorter TTP (median, 36 vs 62 months, respectively; P < .001; Figure 2B) and OS (44 months vs NR; P = .002) than patients in whom BM clonality was undetectable or detected at levels below the threshold proposed for IHC assessment (i.e., MRD). On using our traditional MRD method (that albeit limited at the time by 4 colors, was 2-log more sensitive than IHC), persistent MRD was detectable among 34 of the 94 (36%) patients who, compared with MRD-negative cases, had significantly inferior TTP (median, 45 vs 68 months, respectively; P = .03; Figure 2C) and OS (median, 76 months vs NR, respectively; P = .07). The prognostic value of MRD was equally observed among patients in sCR (data not shown; P = .03). As expected, the outcome of MRD-positive patients by MFC was not as dismal compared with cases with high residual disease by low-sensitivity MFC, because the former method also included patients with low MRD levels; nevertheless, sensitive and quantitative MRD monitoring can also discriminate the high-risk population by stratifying patients into 3 risk categories: high, intermediate, and low risk according to MRD levels (>0.1%, 0.1-0.01%, and <0.01%, respectively).1,18
Figure 1.
Time to progression and overall survival of patients in conventional CR according to their status for the sCR criteria.
Figure 2.
Survival regarding every component of sCR definition. Time to progression of patients in conventional CR according to (A) normal vs abnormal sFLC ratios; (B) BM clonality by low-sensitivity MFC; and (C) conventional MRD monitoring by 4-color MFC.
Because the sFCL test is insensitive to the monoclonal or polyclonal nature of light chains, and the ratio κ/λ is frequently altered by the oligoclonal bands19 that emerged in the context of immune regeneration,20 the lack of clinical relevance of κ/λ ratios reported herein is not surprising and agrees with previous observations.9-11,21 However, the absence of significant differences for TTP and OS between patients in stringent vs conventional CR differs from that reported by Kapoor et al,8 which showed highly significantly survival benefit for patients in sCR compared with those in conventional CR. Although the number of patients in this study and follow-up of both series are similar, unfortunately, the Kapoor et al study does not mention the individual contribution of the sFLC ratios or BM clonality assessments to understand the origin of the discordant results. In the present study, only the IHC-adapted MFC-based BM clonality (low-sensitivity MFC) assessment (and not the sFLCr) identified patients in CR with a different outcome; however, it should be noted that only 5% of the patients (probably with a nonsecretory high tumor burden) showed residual disease by this method. Because BM biopsies are not the standard of care to monitor the response in the GEM/PETHEMA clinical trials, we cannot perform a direct comparison between IHC and low-sensitivity MFC; however, it is likely that the multicolor (4-color instead of single or 2-color staining) and higher cellular input (≥200 000 nucleated cells) of the IHC-adapted MFC method should render higher sensitivity and specificity compared with IHC. Conversely, using MRD monitoring by conventional MFC on the same population revealed that the percentage of MRD positivity increased to 36%, and these patients had significantly inferior outcomes. These results highlight the limitations of IHC when low numbers of clonal PCs are masked by polyclonal (κ and λ) normal PCs and confirm that attaining deeper levels of remission does translate into prolonged survival.1
In summary, our results show that for MM patients in CR, response assessment according to the stringent CR criteria does not predict a different outcome. In particular, the sFLCr does not identify patients in CR at distinct risk, whereas low-sensitivity MFC immunophenotyping only identifies a small number of patients with high residual tumor burden and dismal outcome; MRD monitoring using conventional MFC identifies a complementary group of patient with shorter survival. These results should stimulate the scientific community to perform a large (meta)-analysis and corroborate the exact role of the sCR criteria in MM.
Acknowledgments
The authors thank Arturo Touchard for providing data management support and all participating members of the GEM/PETHEMA.
This research project was supported by grants PI06339, PS09/01370, PI12/01761, Sara Borrell (CD13/00340), and Juan Rodes (JR14/00016) from the Fondo de Investigación Sanitaria, the Red Temática de Investigación Cooperativa en Cáncer of the Instituto de Salud Carlos III (Ministry of Economy and Competitivity, Madrid, Spain)–Federación Española de Enfermedades Raras (RD12/0036/0061, RD12/0036/0048, RD12/0036/0058 and RD12/0036/0046) from Fondo de Investigaciones Sanitarias, Asociación Española Contra el Cáncer (GCB120981SAN), and the Cancer Research Innovation Spain foundation.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Contributor Information
Collaborators: Carmen Menchaca, José María Guinea, María Blanca Villarubia, Abelardo Bárez, Jorge Groiss, Inmaculada Fuentes, Carlos López, Rafael Ramos, Joan Besalduch, Antonia Sampol, Joan Bargay, Eugenia Abella, Joan Bladé, Miguel Granell, Albert Oriol, Elena Cabezudo, Germán las Heras, Juan Alfonso Soler, Elena Rámila, Antonio Asensio, Mercedes Gironella, Dolors Badenes, Rosa María López, Juan Miguel Bergua, María Luisa Martín, María Mas, José Luis Bello, Yolanda González, Enrique Bengoechea, María Asunción Echeveste, Anastasia Aulés, Carmen Aguilera, Jesús Arias, Nicolás Díaz, Dolores Hernández, Raquel de Paz, María Jesús Blanchard, Juan José Lahuerta, Isabel Krsnik Castello, Joaquín Díaz-Mediavilla, Rafael Martínez, Adrián Alegre, Elena Prieto, Rafael Flores, Esther Jaro, Nieves Somolinos, Francisco Javier Peñalver, José Francisco Tomás, Felipe de Arriba, Jerónima Ibáñez, Julio Esteban, María Ángeles Goñi, José María Arguiñano, Jesús F. San Miguel, Felipe Prósper, Fernando Ortega, Alexia Suárez, Santiago Jiménez, José Manuel Calvo, María José Allegue, Carmen Albó, Concha Poderós, Manuel Constenla, Marivi Mateos, Miguel Teodoro Hernández, Bernardo J. González, Eulogio Conde, José Mariano Hernández, Lourdes Escoda, Marta Cervera, Javier de la Rubia, Paz Ribas, Aurelio López, Ana Isabel Teruel, Amparo Avaria, María Ángeles Ruíz, María José Fernández, Isabel Navarro Gonzalo, Javier García-Frade, Elena Fernández, Alfonso García de Coca, Rebeca Cuello, Fernando Marco, José María Beltrán de Heredia, Juan Carlos García-Ruíz, Jesús María Ojanguren, Monserrat Pérez, Luis Palomera, Araceli Rubio, Luis López, and Vicente Carrasco
Authorship
Contribution: J.M.-L., B.P. A.Orfao, J.B, J.F.S.M, and J.J.L. provided the concept and design; J.M.-L., B.P., M.-V.M., T.C., M.-B.V., M.A.S.-G., L.P., A.Oriol, F.d.A., J.B., J.F.S.F., and J.J.L. provided the study material and patients; J.M.-L., B.P., and L.L.-A., collected and assembled the data; B.P., T.C., L.B., and A.Orfao provided data analysis and interpretation; J.M.-L., B.P., L.L.-A., J.F.S.M., and J.J.L. wrote the manuscript; and all authors provided final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joaquín Martínez López, Servicio de Hematología, Hospital Universitario 12 de Octubre, Av de Córdoba s/n, 28041 Madrid Spain; e-mail: jmarti01@ucm.es.
Appendix: study group members
The members of GEM/PETHEMA are: Carmen Menchaca and José María Guinea, Hospital Txagorritxu; María Blanca Villarubia, Hospital General Universitario de Alicante; Abelardo Bárez, Hospital Nuestra Señora de Sonsoles - Ávila; Jorge Groiss and Inmaculada Fuentes, Complejo Hospitalario Universitario de Badajoz; Carlos López and Rafael Ramos, Hospital de Mérida; Joan Besalduch and Antonia Sampol, Complejo Asistencial Son Dureta - Palma de Mallorca; Joan Bargay, Hospital Son Llatzer - Palma de Mallorca; Eugenia Abella, Hospital del Mar - Barcelona; Joan Bladé, Hospital Clinic I Provincial de Barcelona; Miguel Granell, Hospital de la Santa Creu I Sant Pau - Barcelona; Albert Oriol, Hospital Universitari Germans Trias I Pujol de Badalona; Elena Cabezudo, Hospital de Sant Joan de Déu - Manresa; Germán las Heras, Fundació Hospital Sant Joan de Déu - Martorell; Juan Alfonso Soler and Elena Rámila, Hospital Parc Taulí - Sanbadell; Antonio Asensio, Hospital Residencia Sant Camil - Sant Pere de Ribes; Mercedes Gironella, Hospital Vall D'hebron - Barcelona; Dolors Badenes, Clínica Mútua de Terrasa; Rosa María López, Hospital Virgen del Puerto - Plasencia; Juan Miguel Bergua and María Luisa Martín, Complejo Hospitalario de Cáceres; María Mas, Hospital General de Castellón; José Luis Bello, Complejo Hospitalario Universitario de Santiago; Yolanda González, Hospital Universitari Dr. Josep Trueta de Girona; Enrique Bengoechea and María Asunción Echeveste, Hospital Donostia; Anastasia Aulés, Hospital General San Jorge - Huesca; Carmen Aguilera, Hospital El Bierzo - Ponferrada; Jesús Arias and Nicolás Díaz, Complejo Hospitalario Xeral-Calde - Lugo; Dolores Hernández and Raquel de Paz, Hospital Universitario La Paz - Madrid; María Jesús Blanchard, Hospital Ramón y Cajal- Madrid; Juan José Lahuerta, Hospital Universitario 12 de Octubre - Madrid; Isabel Krsnik Castello, Hospital Universitario Puerta de Hierro - Mahadahonda; Joaquín Díaz-Mediavilla and Rafael Martínez, Hospital Clínico San Carlos - Madrid; Adrián Alegre, Hospital Universitario de la Princesa - Madrid; Elena Prieto, Fundación Jiménez Díaz/Ute; Rafael Flores and Esther Jaro, Hospital de Fuenlabrada; Nieves Somolinos, Hospital Universitario de Getafe; Francisco Javier Peñalver, Hospital Universitario Fundación Alcorcón; José Francisco Tomás, Centro Oncológico MD Anderson International España - Madrid; Felipe de Arriba, Hospital J.M. Morales Meseguer - Murcia; Jerónima Ibáñez, Hospital General Universitario Santa María del Rosell- Cartagena; Julio Esteban, Hospital Virgen del Castillo - Yecla; María Ángeles Goñi and José María Arguiñano, Hospital Virgen del Camino- Pamplona; Jesús F. San Miguel y Felipe Prósper, Clinica Universidad de Navarra - Pamplona; Fernando Ortega, Hospital General Rio Carrión- Palencia; Alexia Suárez and Santiago Jiménez , Hospital de Gran Canaria Doctor Negrín - Las Palmas de Gran Canaria; José Manuel Calvo, Hospital Doctor José Molina Orasa (HG Lanzarote) - Arrecife; María José Allegue, Hospital Montecelo - Pontevedra; Carmen Albó and Concha Poderós, Complejo Hospitalario Xeral-Cies - Vigo; Manuel Constenla, Complejo Hospitalario de Pontevedra; Marivi Mateos, Hospital Universitario de Salamanca; Miguel Teodoro Hernández and Bernardo J. González, Hospital Universitario de Canarias - San Cristóbal de la Laguna; Eulogio Conde, Hospital Universitario Marqués de Valdecilla - Santander; José Mariano Hernández, Hospital General de Segovia; Lourdes Escoda, Hospital Universitari Joan Xxiii de Tarragona; Marta Cervera, Hospital Universitari Joan Xxiii de Tarragona; Javier de la Rubia, Hospital Universitario La Fe de Valencia; Paz Ribas, Hospital Universitario Dr. Peset Aleixandre; Aurelio López , Hospital Arnau de Vilanova - Valencia; Ana Isabel Teruel, Hospital Clínico Universitario Valencia; Amparo Avaria, Fundación Instituto Valenciano de Oncología - Valencia; María Ángeles Ruíz and María José Fernández, Hospital Francesc de Borja de Gandia; Isabel Navarro Gonzalo, Hospital de Sagunto; Javier García-Frade and Elena Fernández, Hospital Universitario del Rio Hortega - Valladolid; Alfonso García de Coca and Rebeca Cuello, Hospital Clínico Universitario de Valladolid; Fernando Marco and José María Beltrán de Heredia, Hospital de Basurto - Bilbao; Juan Carlos García-Ruíz, Hospital de Cruces - Barakaldo; Jesús María Ojanguren, Hospital Galdakao-Usansolo; Monserrat Pérez, Hospital Virgen de la Concha - Zamora; Luis Palomera, Hospital Clínico Universitario Lozano Blesa - Zaragoza; Araceli Rubio, Hospital Universitario Miguel Servet - Zaragoza; and Luis López and Vicente Carrasco, Hospital Royo Villanova - Zaragoza.
References
- 1.Martinez-Lopez J, Lahuerta JJ, Pepin F, et al. Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood. 2014;123(20):3073–3079. doi: 10.1182/blood-2014-01-550020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harousseau JL, Attal M, Avet-Loiseau H. The role of complete response in multiple myeloma. Blood. 2009;114(15):3139–3146. doi: 10.1182/blood-2009-03-201053. [DOI] [PubMed] [Google Scholar]
- 3.van de Velde HJ, Liu X, Chen G, Cakana A, Deraedt W, Bayssas M. Complete response correlates with long-term survival and progression-free survival in high-dose therapy in multiple myeloma. Haematologica. 2007;92(10):1399–1406. doi: 10.3324/haematol.11534. [DOI] [PubMed] [Google Scholar]
- 4.Munshi NC, Anderson KC. Minimal residual disease in multiple myeloma. J Clin Oncol. 2013;31(20):2523–2526. doi: 10.1200/JCO.2013.49.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durie BG, Harousseau JL, Miguel JS, et al. International Myeloma Working Group. International uniform response criteria for multiple myeloma. Leukemia. 2006;20(9):1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 6.Bladé J, Samson D, Reece D, et al. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Br J Haematol. 1998;102(5):1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 7.Rajkumar SV, Harousseau JL, Durie B, et al. International Myeloma Workshop Consensus Panel 1. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117(18):4691–4695. doi: 10.1182/blood-2010-10-299487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapoor P, Kumar SK, Dispenzieri A, et al. Importance of achieving stringent complete response after autologous stem-cell transplantation in multiple myeloma. J Clin Oncol. 2013;31(36):4529–4535. doi: 10.1200/JCO.2013.49.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hari PPM, Logan BR, et al. Immunoglobulin Free Light Chain and Heavy Chain/Light Chain assays: comparison with electrophoretic responses in multiple myeloma (MM). Blood. 2011;118:2877. [Google Scholar]
- 10.Giarin MM, Giaccone L, Sorasio R, et al. Serum free light chain ratio, total kappa/lambda ratio, and immunofixation results are not prognostic factors after stem cell transplantation for newly diagnosed multiple myeloma. Clin Chem. 2009;55(8):1510–1516. doi: 10.1373/clinchem.2009.124370. [DOI] [PubMed] [Google Scholar]
- 11.Ludwig H, Milosavljevic D, Zojer N, et al. Immunoglobulin heavy/light chain ratios improve paraprotein detection and monitoring, identify residual disease and correlate with survival in multiple myeloma patients. Leukemia. 2013;27(1):213–219. doi: 10.1038/leu.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosiñol L, Oriol A, Teruel AI, et al. Programa para el Estudio y la Terapéutica de las Hemopatías Malignas/Grupo Español de Mieloma (PETHEMA/GEM) group. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood. 2012;120(8):1589–1596. doi: 10.1182/blood-2012-02-408922. [DOI] [PubMed] [Google Scholar]
- 13.Mateos MV, Oriol A, Martínez-López J, et al. GEM2005 trial update comparing VMP/VTP as induction in elderly multiple myeloma patients: do we still need alkylators? Blood. 2014;124(12):1887–1893. doi: 10.1182/blood-2014-05-573733. [DOI] [PubMed] [Google Scholar]
- 14.San Miguel JF, Almeida J, Mateo G, et al. Immunophenotypic evaluation of the plasma cell compartment in multiple myeloma: a tool for comparing the efficacy of different treatment strategies and predicting outcome. Blood. 2002;99(5):1853–1856. doi: 10.1182/blood.v99.5.1853. [DOI] [PubMed] [Google Scholar]
- 15.Mateo Manzanera G, San Miguel Izquierdo JF, Orfao de Matos A. Immunophenotyping of plasma cells in multiple myeloma. Methods Mol Med. 2005;113:5–24. doi: 10.1385/1-59259-916-8:5. [DOI] [PubMed] [Google Scholar]
- 16.Paiva B, Vídriales MB, Rosiñol L, et al. Grupo Español de MM/Programa para el Estudio de la Terapéutica en Hemopatías Malignas Cooperative Study Group. A multiparameter flow cytometry immunophenotypic algorithm for the identification of newly diagnosed symptomatic myeloma with an MGUS-like signature and long-term disease control. Leukemia. 2013;27(10):2056–2061. doi: 10.1038/leu.2013.166. [DOI] [PubMed] [Google Scholar]
- 17.Mateos MV, Richardson PG, Schlag R, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol. 2010;28(13):2259–2266. doi: 10.1200/JCO.2009.26.0638. [DOI] [PubMed] [Google Scholar]
- 18.Rawstron AC, Gregory WM, de Tute RM, et al. Minimal residual disease in myeloma by flow cytometry: independent prediction of survival benefit per log reduction. Blood. 2015;125(12):1932–1935. doi: 10.1182/blood-2014-07-590166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Larrea CF, Cibeira MT, Elena M, et al. Abnormal serum free light chain ratio in patients with multiple myeloma in complete remission has strong association with the presence of oligoclonal bands: implications for stringent complete remission definition. Blood. 2009;114(24):4954–4956. doi: 10.1182/blood-2009-06-224832. [DOI] [PubMed] [Google Scholar]
- 20.Tovar N, de Larrea CF, Aróstegui JI, et al. Natural history and prognostic impact of oligoclonal humoral response in patients with multiple myeloma after autologous stem cell transplantation: long-term results from a single institution. Haematologica. 2013;98(7):1142–1146. doi: 10.3324/haematol.2013.084350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paiva B, Martinez-Lopez J, Vidriales MB, et al. Comparison of immunofixation, serum free light chain, and immunophenotyping for response evaluation and prognostication in multiple myeloma. J Clin Oncol. 2011;29(12):1627–1633. doi: 10.1200/JCO.2010.33.1967. [DOI] [PubMed] [Google Scholar]