Abstract
We have characterized the U2 snRNA gene family in the higher plant Arabidopsis thaliana. It consists of 10-15 genes which do not appear to be closely clustered. Six of the U2 genes were sequenced and the structure of the Arabidopsis U2 RNA termini was determined in order to define the coding regions. Each of the genes codes for a distinct RNA differing from the others by 2-13 point mutations, localized in the 3' part of the 196 nt-long RNA. The upstream non-coding regions of all genes show strong sequence similarity in positions −81 to −1 and contain three highly conserved sequence elements: GTCCCACATCG (positions −78 to −68; 100% conservation), GTAGTATAAATA (−37 to −26) and CAANTC (−6 to −1). The coding regions are followed by the sequence CAN7-9AGTNNAA, a putative termination signal. The expression of three of the genes was studied in electroporated Orychophragmus violaceus and Nicotiana tabacum protoplasts. The genes, one of which contains a T → C change in the Sm antigen binding site, were actively transcribed and processed into U2 RNAs of the expected size and containing trimethylguanosine caps. Deletion analysis indicates that sequences upstream of the conserved −80 to −1 region are not important for transcription in protoplasts. The 5'-terminal parts of U2 RNAs from several monocot and dicot plants were sequenced. This region, containing the sequence implicated in base-pairing with the branch point in pre-mRNA introns, is identical in all U2 RNAs examined.
Keywords: Arabidopsis, plant gene expression, protoplast transfection, snRNA genes, U2 RNA
Full text
PDF

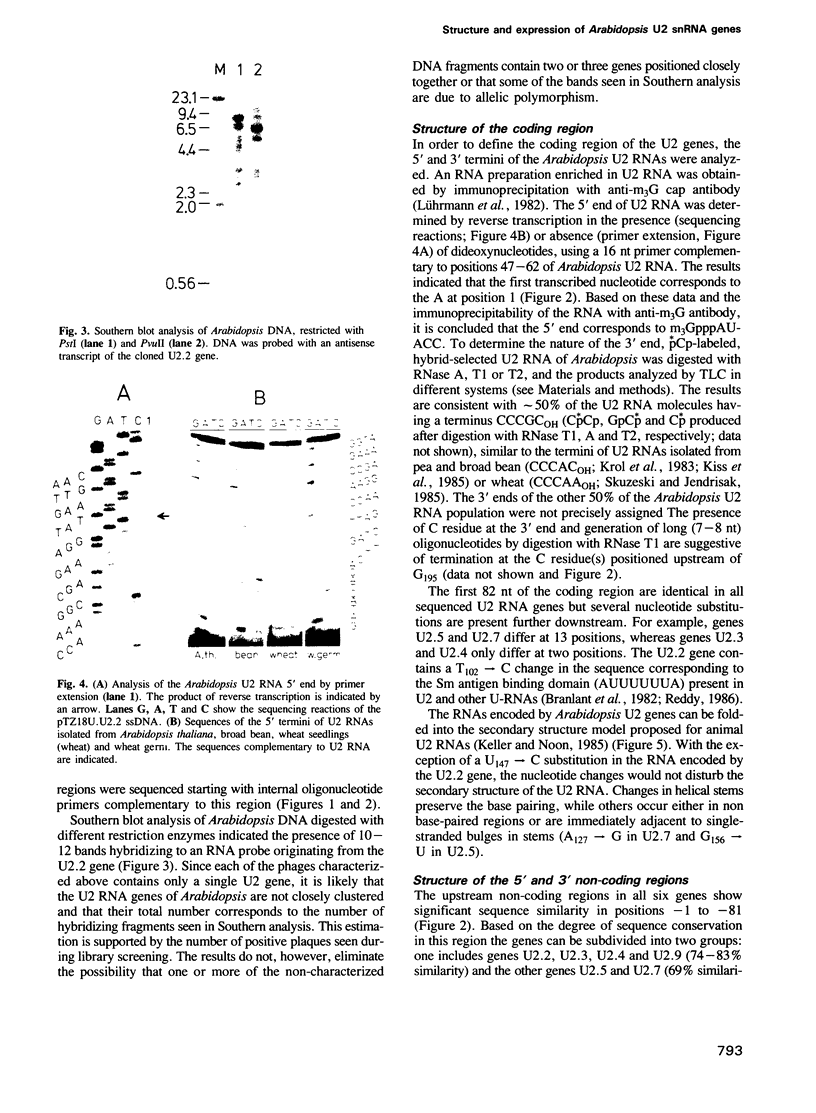






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso A., Beck E., Jorcano J. L., Hovemann B. Divergence of U2 snRNA sequences in the genome of D. melanogaster. Nucleic Acids Res. 1984 Dec 21;12(24):9543–9550. doi: 10.1093/nar/12.24.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ares M., Jr U2 RNA from yeast is unexpectedly large and contains homology to vertebrate U4, U5, and U6 small nuclear RNAs. Cell. 1986 Oct 10;47(1):49–59. doi: 10.1016/0092-8674(86)90365-x. [DOI] [PubMed] [Google Scholar]
- Black D. L., Chabot B., Steitz J. A. U2 as well as U1 small nuclear ribonucleoproteins are involved in premessenger RNA splicing. Cell. 1985 Oct;42(3):737–750. doi: 10.1016/0092-8674(85)90270-3. [DOI] [PubMed] [Google Scholar]
- Branlant C., Krol A., Ebel J. P., Lazar E., Haendler B., Jacob M. U2 RNA shares a structural domain with U1, U4, and U5 RNAs. EMBO J. 1982;1(10):1259–1265. doi: 10.1002/j.1460-2075.1982.tb00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabot B., Black D. L., LeMaster D. M., Steitz J. A. The 3' splice site of pre-messenger RNA is recognized by a small nuclear ribonucleoprotein. Science. 1985 Dec 20;230(4732):1344–1349. doi: 10.1126/science.2933810. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes D. J., Kirschner M. W., Caput D., Dahlberg J. E., Lund E. Differential expression of multiple U1 small nuclear RNAs in oocytes and embryos of Xenopus laevis. Cell. 1984 Oct;38(3):681–689. doi: 10.1016/0092-8674(84)90263-0. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Fromm M., Taylor L. P., Walbot V. Expression of genes transferred into monocot and dicot plant cells by electroporation. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5824–5828. doi: 10.1073/pnas.82.17.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hernandez N., Weiner A. M. Formation of the 3' end of U1 snRNA requires compatible snRNA promoter elements. Cell. 1986 Oct 24;47(2):249–258. doi: 10.1016/0092-8674(86)90447-2. [DOI] [PubMed] [Google Scholar]
- Jensen E. G., Hellung-Larsen P., Frederiksen S. Synthesis of low molecular weight RNA components A, C and D by polymerase II in alpha-amanitin-resistant hamster cells. Nucleic Acids Res. 1979 Jan;6(1):321–330. doi: 10.1093/nar/6.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi C. P. An inspection of the domain between putative TATA box and translation start site in 79 plant genes. Nucleic Acids Res. 1987 Aug 25;15(16):6643–6653. doi: 10.1093/nar/15.16.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmaier M., Tebb G., Mattaj I. W. Functional characterization of X. laevis U5 snRNA genes. EMBO J. 1987 Oct;6(10):3071–3078. doi: 10.1002/j.1460-2075.1987.tb02614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith B., Chua N. H. Monocot and dicot pre-mRNAs are processed with different efficiencies in transgenic tobacco. EMBO J. 1986 Oct;5(10):2419–2425. doi: 10.1002/j.1460-2075.1986.tb04516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller E. B., Noon W. A. Intron splicing: a conserved internal signal in introns of Drosophila pre-mRNAs. Nucleic Acids Res. 1985 Jul 11;13(13):4971–4981. doi: 10.1093/nar/13.13.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T., Antal M., Solymosy F. Plant small nuclear RNAs. III. The complete primary and secondary structure of broad bean U2 RNA: phylogenetic and functional implications. Nucleic Acids Res. 1987 Feb 11;15(3):1332–1332. doi: 10.1093/nar/15.3.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T., Tóth M., Solymosy F. Plant small nuclear RNAs. Nucleolar U3 snRNA is present in plants: partial characterization. Eur J Biochem. 1985 Oct 15;152(2):259–266. doi: 10.1111/j.1432-1033.1985.tb09192.x. [DOI] [PubMed] [Google Scholar]
- Konarska M., Filipowicz W., Domdey H., Gross H. J. Formation of a 2'-phosphomonoester, 3',5'-phosphodiester linkage by a novel RNA ligase in wheat germ. Nature. 1981 Sep 10;293(5828):112–116. doi: 10.1038/293112a0. [DOI] [PubMed] [Google Scholar]
- Krol A., Ebel J. P., Rinke J., Luhrmann R. U1, U2 and U5 small nuclear RNAs are found in plants cells. Complete nucleotide sequence of the U5 RNA family from pea nuclei. Nucleic Acids Res. 1983 Dec 20;11(24):8583–8594. doi: 10.1093/nar/11.24.8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutwiler L. S., Meyerowitz E. M., Tobin E. M. Structure and expression of three light-harvesting chlorophyll a/b-binding protein genes in Arabidopsis thaliana. Nucleic Acids Res. 1986 May 27;14(10):4051–4064. doi: 10.1093/nar/14.10.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhrmann R., Appel B., Bringmann P., Rinke J., Reuter R., Rothe S., Bald R. Isolation and characterization of rabbit anti-m3 2,2,7G antibodies. Nucleic Acids Res. 1982 Nov 25;10(22):7103–7113. doi: 10.1093/nar/10.22.7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E. Differential accumulation of U1 and U4 small nuclear RNAs during Xenopus development. Genes Dev. 1987 Mar;1(1):39–46. doi: 10.1101/gad.1.1.39. [DOI] [PubMed] [Google Scholar]
- Lund E., Kahan B., Dahlberg J. E. Differential control of U1 small nuclear RNA expression during mouse development. Science. 1985 Sep 20;229(4719):1271–1274. doi: 10.1126/science.2412294. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Reed R. The role of small nuclear ribonucleoprotein particles in pre-mRNA splicing. Nature. 1987 Feb 19;325(6106):673–678. doi: 10.1038/325673a0. [DOI] [PubMed] [Google Scholar]
- Mattaj I. W. Cap trimethylation of U snRNA is cytoplasmic and dependent on U snRNP protein binding. Cell. 1986 Sep 12;46(6):905–911. doi: 10.1016/0092-8674(86)90072-3. [DOI] [PubMed] [Google Scholar]
- Mattaj I. W., Zeller R. Xenopus laevis U2 snRNA genes: tandemly repeated transcription units sharing 5' and 3' flanking homology with other RNA polymerase II transcribed genes. EMBO J. 1983;2(11):1883–1891. doi: 10.1002/j.1460-2075.1983.tb01675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M., Pettersson I., Hinterberger M., Karmas A., Steitz J. A. The U1 small nuclear RNA-protein complex selectively binds a 5' splice site in vitro. Cell. 1983 Jun;33(2):509–518. doi: 10.1016/0092-8674(83)90432-4. [DOI] [PubMed] [Google Scholar]
- Murphy J. T., Burgess R. R., Dahlberg J. E., Lund E. Transcription of a gene for human U1 small nuclear RNA. Cell. 1982 May;29(1):265–274. doi: 10.1016/0092-8674(82)90111-8. [DOI] [PubMed] [Google Scholar]
- Murphy J. T., Skuzeski J. T., Lund E., Steinberg T. H., Burgess R. R., Dahlberg J. E. Functional elements of the human U1 RNA promoter. Identification of five separate regions required for efficient transcription and template competition. J Biol Chem. 1987 Feb 5;262(4):1795–1803. [PubMed] [Google Scholar]
- Murray M. G., Thompson W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R. Compilation of small RNA sequences. Nucleic Acids Res. 1986;14 (Suppl):r61–r72. doi: 10.1093/nar/14.suppl.r61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short K. C., Torrey J. G. Cytokinins in seedling roots of pea. Plant Physiol. 1972 Feb;49(2):155–160. doi: 10.1104/pp.49.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strub K., Galli G., Busslinger M., Birnstiel M. L. The cDNA sequences of the sea urchin U7 small nuclear RNA suggest specific contacts between histone mRNA precursor and U7 RNA during RNA processing. EMBO J. 1984 Dec 1;3(12):2801–2807. doi: 10.1002/j.1460-2075.1984.tb02212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani T., Watanabe-Nagasu N., Okada N., Ohshima Y. Molecular cloning and characterization of a gene for rat U2 small nuclear RNA. J Mol Biol. 1983 Aug 15;168(3):579–594. doi: 10.1016/s0022-2836(83)80303-9. [DOI] [PubMed] [Google Scholar]
- de Vegvar H. E., Lund E., Dahlberg J. E. 3' end formation of U1 snRNA precursors is coupled to transcription from snRNA promoters. Cell. 1986 Oct 24;47(2):259–266. doi: 10.1016/0092-8674(86)90448-4. [DOI] [PubMed] [Google Scholar]