Abstract
Immunoglobulin G (IgG)-based drugs are arguably the most successful class of protein therapeutics due in part to their remarkably long blood circulation. This arises from IgG interaction with the neonatal Fc receptor, FcRn. FcRn is the central regulator of IgG and albumin homeostasis throughout life and is increasingly being recognized as an important player in autoimmune disease, mucosal immunity, and tumor immune surveillance. Various engineering approaches that hijack or disrupt the FcRn-mediated transport pathway have been devised to develop long-lasting and non-invasive protein therapeutics, protein subunit vaccines, and therapeutics for treatment of autoimmune and infectious disease. In this review, we highlight the diverse biological functions of FcRn, emerging therapeutic opportunities, as well as the associated challenges of targeting FcRn for drug delivery and disease therapy.
Keywords: Albumin, Immunoglobulin G, FcRn, nanoparticle, protein engineering
1.1 Introduction
It has now been 50 years since the remarkable foresight by F.W.R. Brambell, who put forth the hypothesis that there exists a specific receptor responsible for the salvage of IgG from catabloism1, eventually identified as the neonatal Fc receptor (FcRn). Originally discovered in the rat as the receptor responsible for the transmission of maternal antibodies from mother to pup2–5, FcRn is now known to be involved in a multitude of critical biological functions throughout human life6. The most recognized of these functions is the FcRn-mediated recycling and transcytosis process that results in the extraordinarily long, ~ 21 day serum persistence of IgG and albumin in humans7. In addition to regulating IgG and albumin homeostasis, FcRn participates in a plethora of immunological processes critical to the maintenance of human health8. Thus, FcRn has become an attractive target for drug delivery and disease therapy. In this review, we highlight the aspects of FcRn biology that have enabled the development of FcRn targeted therapeutics for the treatment of autoimmune and infection disease, as well as the expanding drug delivery approaches that hijack FcRn functions (Table 1). For more information regarding basic FcRn biology, we direct readers to a number of excellent past6,9–12 and recent7,13,14 reviews. We conclude this review by putting forth a hypothesis regarding the FcRn-dependent regulation of IgG homeostasis and echoing the words of a recent opinion by Clark Anderson15; ‘we may still have much to learn about the FcRn’.
Table 1.
Summary of drug delivery approaches and therapeutic modalities that target the FcRn
| PROTEIN HALF-LIFE EXTENSION | |||
|---|---|---|---|
| Strategy | Mechanism | Effect | Reference |
| 1. Fc-fusion | Hijacking FcRn recycling, increased molecular weight (MW) | Half-life extension by reducing catabolism and renal clearance | 14, 77 |
| 2. Albumin fusion | Hijacking FcRn recycling, increased MW | Same as Fc-fusion | 136 |
| 3. IgG-Fc or albumin engineering | Increased mAb/albumin affinity for FcRn at pH 6 | Half-life extension by reducing catabolism | 39, 40, 63, 64, 66, 70, 71, 72, 73, 74, 78, 81, 139 |
| 4. Monomeric Fc and CH domains | Hijacking FcRn recycling, increased MW | Increased half-life by reducing catabolism and potentially renal clearance | 146, 147, 148, 149, 150 |
| 5. Fusion to alternate FcRn binding ligands | Hijacking FcRn recycling | Half-life extension by reducing catabolism (only validated in vivo in ref. 147) | 151, 152 |
| NON-INVASIVE PROTEIN DELIVERY | |||
|---|---|---|---|
| Strategy | Mechanism | Effect | Reference |
| 1. Fc-fusion | FcRn-mediated epithelial transcytosis | Increased pulmonary/oral protein absorption | 101, 102, 103, 112, 118 |
| 2. Fc-decorated protein nanocontainers | FcRn-mediated epithelial transcytosis, protection of protein cargo from degradation by encapsulation | Oral insulin delivery that improves glucose tolerance dependent on FcRn | 123 |
| MUCOSAL VACCINATION | |||
|---|---|---|---|
| Strategy | Mechanism | Effect | Reference |
| 1. Fc-antigen fusion | FcRn-mediated transcytosis, FcγR- mediated delivery to phagocytic cells, FcRn potentiation of antigen presentation | Improved innate and adaptive immune response against protein antigens | 128, 129, 131 |
| 2. bnAb-Fc engineering | Increased FcRn-dependent deposition and/or retention of engineered IgG in mucosal tissues | Enhanced protection against intrarectal HIV challenge | 133 |
| INHIBITORS OF THE IgG-FcRn INTERACTION | |||
|---|---|---|---|
| Strategy | Mechanism | Effect | Reference |
| 1. IVIG | Saturation of FcRn | Accelerated clearance of endogenous IgG | 83, 85 |
| 2. FcRn antagonists (α-β2M mAbs, α-FcRn mAbs, Abdegs, synthetic peptides, small molecules) | Blocking IgG binding site on FcRn and/or competing with endogenous IgG for binding to FcRn | Accelerated clearance of endogenous IgG | 87, 88, 89, 91, 93 |
| 3. IgG antagonists | Blocking FcRn binding site on IgG | Inhibits FcRn-IgG interaction in vitro. Not tested in vivo. May accelerate IgG clearance. | 30, 97 |
| INHIBITORS OF THE IgG-ALBUMIN INTERACTION | |||
|---|---|---|---|
| Strategy | Mechanism | Effect | Reference |
| 1. α-FcRn mAbs | Blocking albumin binding site on FcRn | Not determined. May enhance clearance of albumin or albumin bound toxins | 40 |
| INCREASED TUMOR/TISSUE ACCUMULATION, DISTRIBUTION, and/or RETENTION | |||
|---|---|---|---|
| Strategy | Mechanism | Effect | Reference |
| 1. IgG-Fc engineering | Presumably increased FcRn transport via increased affinity for FcRn | Improved anti-tumor effect in mice potentially mediated by tumor cell FcRn expression, increased amount of IgG in bronchio-alveolar lavage of monkeys | 64, 79 |
1.2 FcRn structure, biology, and function
1.2.1 FcRn structure and mechanism of IgG and albumin binding
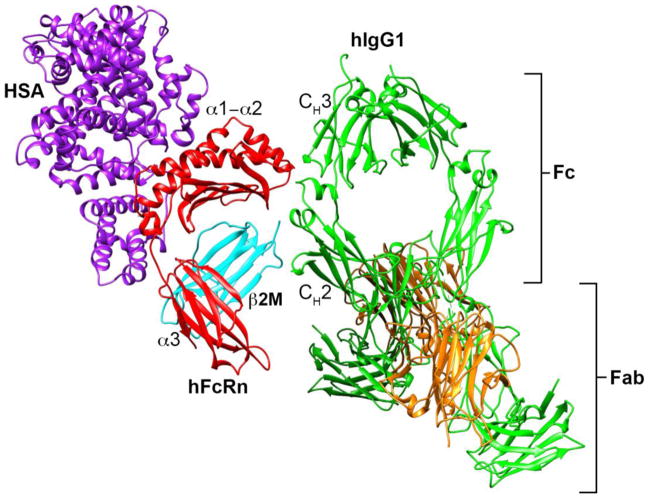
FcRn is structurally homologous to the MHC Class I heterodimeric receptor family16 consisting of a type I transmembrane heavy chain that non-covalently associates with the soluble light chain, β2-microglobulin (β2m). β2m is absolutely necessary for the proper folding, transport, and function of FcRn, as well as other MHC Class I homologs, both in vitro and in vivo17–20. The FcRn heavy chain contains three soluble domains (α1, α2, and α3), a single transmembrane helix, and a cytoplasmic tail (Figure 1). Unlike MHC Class I molecules, FcRn does not directly present antigen to T-cells due to point mutations on the top face of FcRn that disrupt peptide binding.
Figure 1. Structure of human FcRn in contact with hIgG1 and human serum albumin.
Human IgG1 (green) contacts the α2 domain of human FcRn (red) and N-terminus of β2M (cyan) at the intersection of the CH2-CH3 domains within the Fc portion of IgG. Human serum albumin (purple) contacts a distinctly different binding site that spans the α1-α2 domains of FcRn and β2M. The HSA/hFcRn/hIgG1 model in this figure is based upon the HSA/hFcRn/Fc-YTE structure35 (PDB code 4N0U) and the full length human IgG1 structure (PDB code 1HZH). As there is no crystal structure of the full length hIgG1/hFcRn complex, full length hIgG1 was aligned to human FcRn based upon the human Fc-YTE/FcRn structure using Chimera178 v1.6.1 (UCSF, San Francisco, CA).
The ability of FcRn to protect IgG from intracellular catabolism is the result of a specific, pH-dependent interaction with the Fc portion of IgG (Figure 1). IgG binds FcRn in a strictly pH-dependent manner at acidic (< 6.5) but not neutral pH (> 7) mediated by electrostatics between titratable histidine residues in the Fc CH2-CH3 domains of IgG and acidic residues on the α2-domain of FcRn21–25 (Figure 2). Binding is further stabilized by a series of hydrophobic interactions and hydrogen bonds between Fc and residues within the FcRn α2-domain and the β2m light chain N-terminus26. One IgG molecule can simultaneous bind two FcRn molecules due to the homodimeric nature of IgG27,28 resulting in a high affinity interaction between FcRn and IgG at pH 6 due to avidity. The 2:1 interaction between FcRn and IgG is critical for efficient binding, recycling, and transcytosis in vitro29 and to preserve the long serum persistence of IgG in vivo22.
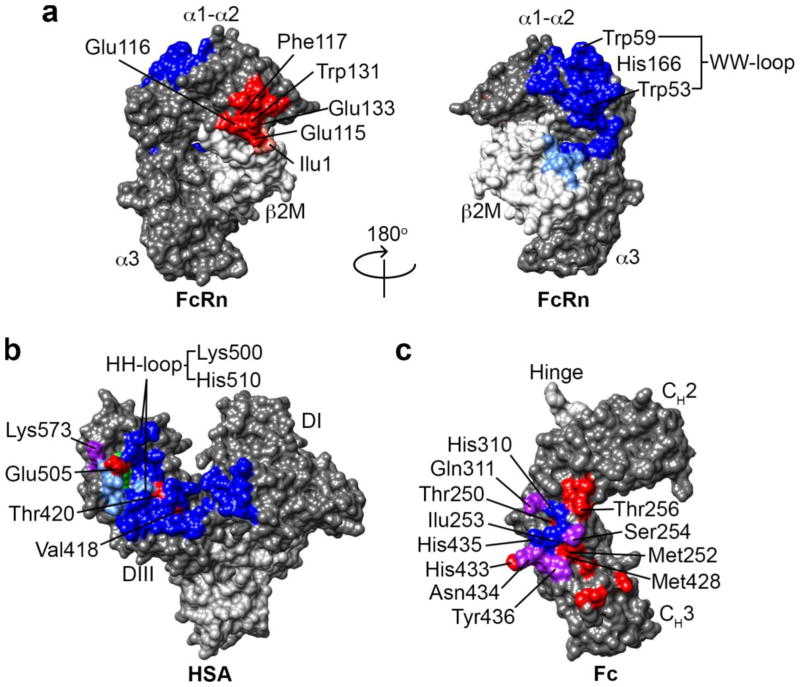
Figure 2. Amino acid residues on albumin and the IgG1 Fc-domain involved in binding to human FcRn and that have been mutated to alter albumin and IgG half-life.
(a) The human FcRn heavy chain (HC) and β2M light chain (LC) residues involved in the IgG (red, HC; salmon, LC) and albumin (blue, HC; light blue, LC) interaction. Residues involved in the IgG-FcRn interaction are restricted to the α-2 domain of the HC and Ilu1 on the β2M LC, whereas the residues involved in the albumin-FcRn interaction span the α-1 and α-2 HC domains and include a number of residues on the β2M LC24,27,35,39. (b) Human serum albumin residues involved in pH-dependent binding to human FcRn. Residues that interact with the hFcRn HC are colored blue, β2M LC are colored light blue, and those that interact with both the HC and LC are colored green. The residues are mostly located within domain III (DIII) with a smaller contribution from domain I (DI). The critical HH-loop residues on albumin that result in pH-dependent binding to the WW-loop on hFcRn are located in DIII. Amino acid residues that have been mutated to increase the albumin-FcRn affinity at pH 6 are labeled red and cluster in DIII39,139. Residues that contribute to the endogenous albumin-FcRn interaction and also have been mutated to increase affinity are colored purple. (c) Human IgG1 Fc-domain residues involved in pH-dependent binding to human FcRn are colored blue and purple. The residues predominately cluster at the CH2-CH3 domain interface and include the key protonatable histidine residues H310 and H435. The residues in the Fc-domain of hIgG1 that have been mutated to alter the binding affinity to human FcRn are colored red. Again the residues cluster at the CH2-CH3 domain interface and most are distinct from the FcRn contact residues shown in blue. The residues colored purple are both involved in the endogenous IgG-FcRn interaction and have been mutated to alter IgG affinity for FcRn.
The FcRn binding site on IgG overlaps with the staphylococcal protein A (SpA), streptococcal protein G (SpG), and rheumatoid factor binding site30, but is distinct from the classical Fcγ receptors25 and the C1q component of complement31,32 that bind near the upper CH2 domain and hinge region. IgG binding to FcRn is independent of glycosylation33 in contrast to glycan dependent IgG binding to classical Fcγ receptors34.
The amino acid residues involved in the IgG-FcRn interaction have been determined by mutagenesis25, molecular modeling26, and crystallography21,24,27,35 (Figure 2). The IgG-FcRn interface is composed of three subsites: a hydrophobic core and two electrostatic sites. Ile253 on IgG is conserved across species and is critical for interaction with FcRn making numerous hydrophobic contacts with α2 domain amino acids Leu112, Phe117, and Trp131 on FcRn26. The hallmark pH-dependent binding is driven by a key histidine residue, His310, on IgG that becomes positively charged as the pH approaches 6.0–6.5 resulting in the formation of a salt bridge with Glu115 on FcRn. His435 on IgG also interacts with Asp130 on FcRn and may also contribute to the pH-dependent binding. Indeed, certain IgG3 allotypes contain an Arg at position 435, which contributes to the significantly shorter half-life of the IgG3 subclass compared to IgG1, whereas the His435-containing IgG3 allotype half-life is comparable to IgG136. Thus, both His310 and His435 are hot spot residues on IgG that contribute to pH-dependent binding and FcRn dependent half-life. Ile1 is the only known residue on β2m that contributes to IgG binding as mutation to Ala abrogates rat IgG binding to rat FcRn37.
In addition to IgG, FcRn also binds to albumin in a pH-dependent manner. As a result, albumin is salvaged by FcRn, which results in it’s long half-life in humans. Molecular modeling, structure guided mutagenesis, and the recent crystal structure of the albumin-FcRn complex indicates that albumin binds to a distinct site on FcRn spanning the α1 and α2-domains, opposite that of IgG35,38–40 (Figure 1 and Figure 2). IgG and albumin can simultaneously and non-cooperatively bind FcRn in vitro41,42 in agreement with their distinctly different binding sites; however, whether a single FcRn molecule can simultaneously transport both IgG and albumin has not been determined. It is also unknown if concomitant or sequential albumin binding to FcRn is critical to IgG salvage by FcRn in vivo, or vice versa.
Although IgG and albumin share the key pH-dependent FcRn binding mechanism, the 1:1 stoichiometry of the FcRn-albumin interaction41 results in a significantly lower equilibrium binding affinity of albumin-FcRn compared to the 2:1 stoichiometry of the FcRn-IgG interaction, which increases the apparent affinity of IgG for FcRn due to avidity. However, under conditions that do not permit bivalent IgG binding to FcRn, the albumin-FcRn interaction (2–5 μM) and IgG-FcRn interaction (1–4 μM) at pH 6 have similar affinities39,41,43–45. The IgG-FcRn interaction is predominately driven by electrostatics whereas the albumin-FcRn interaction is predominantly hydrophobic35,39. The albumin interaction with FcRn spans a very large surface area and includes residues located within the α1 and α2 domains of the FcRn heavy chain as well as a number of residues on the β2m light chain (Figure 2). In addition, pH-dependent binding between albumin and FcRn is the result of pH-dependent conformational changes within the HH-loop of albumin enabling numerous hydrophobic interactions with the WW-loop of FcRn (Figure 2a,b), whereas the IgG-FcRn interaction is due to the formation of pH-dependent salt bridges between protonated histidine residues on IgG-Fc and acidic residues on FcRn (Figure 2a,c).
Both IgG and albumin exhibit significant cross-species differences in their interaction with mouse and human FcRn42. Human FcRn is selective for evolutionarily related IgG species but ignores rodent IgGs while mouse FcRn is promiscuous in its interaction with IgG from various species46. FcRn is less restrictive towards albumin across species as both mouse and human albumin bind mouse and human FcRn, albeit with species-specific kinetics and affinity42. The cross-species binding specificities of IgG and albumin are important considerations when choosing pre-clinical animal models to study the in vivo properties of FcRn ligands.
1.2.2 The sites of FcRn protection of IgG in vivo
The major cells types involved in the FcRn-mediated protection of IgG from degradation have been deduced in the mouse. IgG clearance is accelerated in irradiated wild-type mice reconstituted with bone marrow from FcRn α-chain null mice47, indicating the hematopoietic compartment is a major contributor to IgG homeostasis. FcRn is expressed in human intestinal macrophages, peripheral blood monocytes, and dendritic cells48 as well as in mouse splenic macrophages, B cells, dendritic cells, and monocytes47,49; however, it is unknown if a particular hematopoietic cell type or many are important in maintaining serum IgG levels.
Conditional deletion of FcRn in mouse vascular endothelial cells, as well as essentially complete deletion in hematopoietic cells, results in rapid clearance of IgG and reduced steady state levels of IgG and albumin, indicating that the vascular endothelium is also a major contributor to IgG homeostasis49 in the mouse, as was expected given the close contact between the vascular endothelium and IgG in blood. Thus, endothelial cells and hematopoietic cells including monocytes, macrophages, dendritic cells, and possibly B cells are important regulators of IgG clearance in the mouse.
FcRn expression at the mRNA level in mice is highest in the liver followed by kidneys, skin, lungs, spleen, and muscle50. The relative IgG tissues-to-plasma area under the curve ratios in wild type and FcRn α-chain null mice indicate that FcRn contributes significantly to IgG exposure in the skin and muscle51, which are presumed to be the major sites of IgG catabolism in humans.
FcRn is also expressed in a wide variety of human tissues; however, the contribution of human endothelial cells to the FcRn-mediated protection of IgG is a point of debate as there are conflicting reports on the extent of FcRn expression in human endothelial cell lines52 versus human tissues53. Regardless, expression of FcRn in human tissue resident dendritic cells, macrophages, and neutrophils, muscle endothelial cells, and a range of epithelial cells6,53 is consistent with data in the mouse49,54. Therefore, the sites of FcRn function in humans is likely tissue specific endothelial cells, such as in the muscle and/or skin, hematopoietic cells such as peripheral blood monocytes and intravascular or tissue resident macrophages, and tissue specific epithelial cells. The specific human cell types and tissues that express FcRn and contribute to IgG and albumin homeostasis warrants further investigation.
1.2.3 The mechanism of FcRn-mediated recycling and transcytosis of IgG
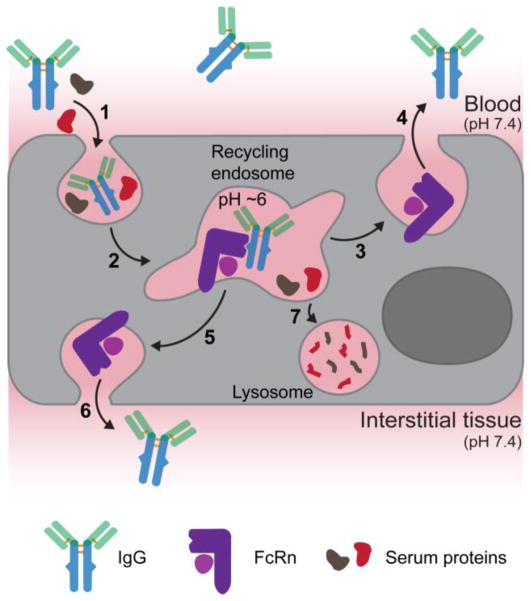
The ability of FcRn to protect IgG from intracellular catabolism has been deduced through a series of in vitro cellular trafficking studies52,55–58. The albumin-FcRn salvage pathway has not been studied in vitro but it is widely believed to follow a fate similar to IgG. In almost all cell types FcRn is localized predominantly to intracellular vesicles such as early and recycling endosomes and sorting tubules59. FcRn expression on the cell surface is limited and the pH of the extracellular environment is not favorable for an IgG-FcRn interaction; therefore, IgG is believed to enter cells through non-specific, fluid-phase pinocytosis (Figure 3). Endocytosed IgG is trafficked along the endosomal pathway and encounters FcRn in the early endosome where the acidic microenvironment (pH ≅ 6) favors a productive IgG-FcRn interaction56. The FcRn-IgG complex is trafficked away from the lysosomal pathway and back to the plasma membrane, where upon membrane fusion the FcRn-IgG complex disassociates due to the elevated extracellular pH55, returning IgG to the extracellular space, such as the blood, thus extending the serum half-life of IgG. Serum proteins that are not associated with a recycling receptor or IgGs that do not dissociate from FcRn57 are destined for lysosomal degradation, either because they are not salvaged from transport to the lysosome or are catabolized during receptor turnover, respectively. In addition to recycling, FcRn can transcytosis IgG across polarized cell monolayers via a presumably similar molecular mechanism delivering IgG from the blood into tissue interstitial space and vice versa (Figure 3).
Figure 3. The FcRn-mediated recycling and transcytosis model.
FcRn-mediated recycling initiates upon non-specific fluid-phase pinocytosis of serum IgG into FcRn expressing cells (1). As IgG is trafficked along the endosomal pathway (2) the pH decreases to 6 resulting in association with endosomal FcRn. The IgG-FcRn complex is recycled back to the plasma membrane (3) where IgG is released into blood due to its weak affinity for FcRn at blood pH (4). FcRn-mediated transcytosis of IgG across polarized epithelial cells, such as in the gut or lung, follows a similar cellular trafficking mechanism that directs the FcRn-IgG complex to the opposing cell membrane (5) where IgG can be released into the interstitial tissue space (6) due to the elevated pH. Serum proteins that do not bind a salvage receptor are trafficked to the lysosome and catabolized (7).
1.3 Modulating the IgG-FcRn interaction
Because FcRn contributes significantly to the half-life of IgG and its transport across cellular barriers, a number of macromolecular engineering approaches have been devised to modulate the IgG-FcRn interaction (21). The principle approaches have involved mutations of Fc-domain amino acid residues in proximity to the FcRn binding site. Modulating the IgG-FcRn interaction to increase antibody half-life could enable less frequent dosing while still maintaining efficacy. Conversely, reducing the half-life of antibodies used for tumor imaging may improve signal-to-noise by enabling antibody accumulation in the tumor but rapid clearance from the blood60,61. Finally, inhibiting the endogenous IgG-FcRn interaction has therapeutic potential for the treatment of IgG-mediated autoimmune disease.
1.3.1 Fc-engineering to increase the half-life of therapeutic antibodies
The identification of the amino acid residues involved in the regulation of the catabolism and transcytosis of IgG indicated a strong correlation between serum half-life and affinity for FcRn at pH 622,23,62. This suggested that increasing the affinity of the IgG-FcRn interaction at pH 6 would result in an engineered IgG with increased serum persistence. Ghetie, Ward and colleagues randomly mutated three residues in close proximity to the IgG-FcRn binding interface and selected Fc variants that bound FcRn with increasing stringency by phage display63. One mouse Fc mutant (T252L/T254S/T256F) with an ~ 3.5-fold increase in affinity for mouse FcRn at pH 6 while still maintaining pH-dependent binding had a modest but significantly increased half-life in mice63. This seminal study was the first to demonstrate that it is possible to increase the serum persistence of Fc, and likely IgG, by increasing affinity toward FcRn at pH 6.
Mutation of the same amino acid residues (M252Y/S254T/T256E) in the human IgG1 anti-respiratory syncytial virus (RSV) antibody motavizumab results in an ~ 10-fold increase in affinity for human FcRn at pH 6 without increasing affinity at pH 7.4 and an ~ 4-fold increase in half-life in monkeys64. Importantly, the M252Y/S254T/T256E human IgG1 mutant also has an increased half-life in healthy adult humans65. This is a major validation of engineering efforts to increase IgG affinity for FcRn at pH 6 as a means to increase serum persistence in humans.
A separate set of IgG1 mutations (M428L/N434S) that resulted in an ~ 11-fold improvement in affinity for human FcRn at pH 6 and wild-type IgG like binding at pH 7.4 also resulted in an ~ 4-fold and ~ 3-fold increase in half-life in human FcRn transgenic mice (Tg276) and monkeys, respectively66. In this case the IgG1 mutations were introduced into the anti-vascular endothelial growth factor (VEGF) antibody bevacizumab and the anti-epidermal growth factor receptor (EGFR) antibody cetuximab. This was the first study to demonstrate that an FcRn-dependent increase in half-life in mice also translates to an improvement in anti-tumor activity in vivo, indicating that at least in these particular mouse cancer models increased drug exposure through half-life extension preserves efficacy and enables less frequent dosing.
Unfortunately, the IgG-FcRn affinity to half-life relationship observed in animal models may not be readily translated to humans. An alternative IgG mutant (N434H) with an ~ 3-fold increase in affinity for human FcRn at pH 6 and an ~ 2-fold increase in half-life in baboons did not show an increased half-life in diseased humans67. Thus, disease state and antibody target resulting in FcRn-independent IgG clearance mechanisms are potential confounding factors when translating engineered IgGs from pre-clinical models into the clinic.
A number of additional mutations to the Fc-domain of IgG that alter pH-dependent binding to FcRn (Figure 2) have now been described and are reviewed extensively in references14,50,68. For a detailed analysis of the IgG-FcRn affinity to half-life relationship including a table summarizing the IgG-Fc mutants, their respective affinity for FcRn, and resulting half-life in various animal models, see reference69. Increasing IgG affinity toward FcRn at pH 6 while maintaining little to no binding at pH 7.4 is a validated mechanism to increase antibody half-life. A high-level evaluation of all pre-clinical animal models used to evaluate IgG half-life indicates that a ~ 5–10 fold increase in affinity at pH 6 typically translates to an ~ 2–4-fold increase in half-life69. However, the FcRn affinity to half-life correlation is not always clear in pre-clinical animal models, as was also observed in humans.
Reports that either support64,66,70–79 or a refute78,80,81 the IgG-FcRn affinity to half-life relationship are in the literature. In all cases, the extent of IgG characterization with FcRn varies making it difficult to draw useful comparisons between studies. To address these issues, future studies evaluating the impact of FcRn affinity on IgG pharmacokinetics should consider numerous factors in addition to simply measuring equilibrium binding affinity at pH 6 and pH 7.4 such as: 1) binding kinetics including on and off-rates, 2) species matched FcRn binding affinities, and 3) alternative FcRn-independent clearance mechanisms. Despite the conflicting reports, it is clear that modulating the IgG-FcRn interaction via Fc mutation results in the generation of antibodies with altered in vivo properties and that maintaining pH-dependent binding to FcRn is critical for half-life extension.
In addition to altering half-life, engineering FcRn binding may be a strategy to improve the tissue distribution of antibodies or alternative drugs and drug carriers. An engineered variant of the anti-VEGF antibody bevacizumab with increased affinity for both human and mouse FcRn at pH 6 and pH 7.4 had an increased tumor accumulation and improved anti-tumor response in mice bearing human tumor xenografts compared to the wild-type antibody, especially at low doses, despite the similar pharmacokinetic properties of the two mAbs79. It was suggested that the improved anti-tumor effect may be due to a FcRn-mediated increase in antibody retention or distribution within the tumor, as alternative factors, such as systemic clearance, FcγR binding, and non-specific binding were the same for both wild-type and variant antibody. Similarly, Dall’Acqua and coworkers observed an increase in the bronchoalveolar lavage concentration of the anti-RSV variant described above64. Collectively, these observations suggest that FcRn contributes to tissue-specific drug accumulation and may be a useful target for improving drug/nanoparticle accumulation or penetration within tumors and/or peripheral organs. Engineering FcRn transport capabilities into nanoparticles and the impact on tumor penetration has yet to be explored.
1.3.2 Inhibitors of the IgG-FcRn interaction
Pathogenic IgGs that are reactive toward a self antigen is a hallmark of numerous autoimmune disorders82. Because FcRn contributes to the serum persistence of IgG, therapeutics that block the IgG-FcRn interaction represent a potential treatment modality for IgG-mediated autoimmunity. Mice deficient in FcRn are protected against IgG-mediated disease83,84 indicating that FcRn contributes, at least in part, to autoimmunity by maintaining pathogenic IgGs in circulation. High dose intravenous immunoglobulin (IVIg) therapy is FDA-approved for the treatment of immune diseases and provides a therapeutic benefit in IgG-mediated autoimmunity in part by saturation of FcRn thereby increasing the catabolism of pathogenic IgG85. However, IVIg therapy is expensive requiring IgG extraction from the plasma of many blood donors, thus recombinant approaches may provide alternative cost-effective treatment options.
IgG based antagonists have been developed to inhibit the endogenous IgG-FcRn interaction (Figure 4). One approach to interfere with the IgG-FcRn interaction is the use of monoclonal antibodies directed against FcRn or β2m via the classical antibody:antigen binding mechanism. One such antibody, 1G386, binds the rat FcRn heavy chain with an affinity of 1.9 nM and 5.8 nM at pH 6 and pH 7.4, respectively, inhibits IgG binding to FcRn in vitro, accelerates the clearance of endogenous serum IgG in rats, and reduces disease severity in rat models of myasthenia gravis87. In a similar approach, the anti-rat β2m antibody, 4C986, also inhibits IgG binding to FcRn and accelerates the clearance of a model IgG autoantibody in rats with ~ 50-fold increased potency compared to IVIg88. However, as β2m is the common light chain of numerous MHC Class I like molecules16 the off target effect of 4C9 will need to be carefully monitored.
Figure 4. Inhibitors of the FcRn-IgG interaction.
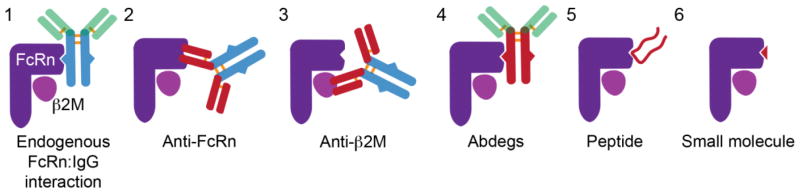
From left to right: 1) High dose IVIg can saturate FcRn and accelerate the clearance of endogenous IgG, 2) anti-FcRn heavy chain antibodies and 3) anti-β2m light chain antibodies bind FcRn epitopes that overlap with the IgG-Fc domain binding site, thereby inhibiting FcRn function, 4) Fc-engineered IgGs that that have increased, pH-independent affinity for FcRn (Abdegs), and 5) peptides and 6) small molecules that compete with IgG for binding to FcRn.
An alternative antibody engineering approach to reduce IgG levels in serum is to engineer the Fc-domain of IgG to have high affinity for FcRn at both pH 6 and pH 7.4, termed antibodies that enhance IgG degradation (Abdegs)89. A series of five Fc mutations located at the IgG-FcRn binding interface resulted in an engineered IgG with a 1.2 nM and 7.4 nM affinity for mouse FcRn at pH 6 and pH 7.4, respectively, similar to 1G3. Abdegs, inhibit FcRn-mediated recycling of wild-type IgG in vitro, accelerate the clearance of exogenous IgG, and reduce endogenous IgG concentrations in mice89. The high affinity at pH 7.4 presumably enables Abdegs to bind FcRn transiently present on the surface of cells during exocytosis55 preventing any subsequent interaction with endogenous IgG. In addition, the increased affinity at pH 6 enables Abdegs to compete more efficiently with endogenous IgG for binding to FcRn in acidified endosomes. Both properties prevent or disrupt the endogenous IgG-FcRn interaction resulting in accelerated IgG clearance. Abdegs are effective in treating a murine model of arthritis at 25- to 50-fold lower doses than IVIg therapy90 indicating that potent antagonists of the IgG-FcRn interaction is an alternative therapeutic intervention in autoimmunity. The Abdeg technology is in clinical development by the Netherlands-based biotech company, arGEN-X. The safety of Abdegs should be carefully monitored in the clinic as treatment regimens for autoimmune diseases commonly rely on the use of immunosuppressive drugs. Therefore, the combination of immune suppression and clearance of endogenous IgG may increase the risk of infection.
More recently, synthetic peptides that compete with IgG for binding to FcRn were identified from a phage library91. The phage identified FcRn binding peptides (FcBP) interacts with FcRn at the same position as the Fc-domain of IgG, despite the complete lack of sequence homology between the two FcRn binding molecules92. The FcBP is highly specific for human and monkey FcRn, with an approximate 1000-fold weaker binding to mouse and rat FcRn91. The phage identified FcBP monomer binds FcRn with micromolar affinity at pH 6 and pH 7.4 and inhibits IgG binding to FcRn in vitro using HEK293 cells engineered to express human FcRn, but is not effective at accelerating the clearance of IgG in mice presumably due to its weak affinity and rapid clearance. A chemically optimized peptide dimer (SYN1436) that bound FcRn with subnanomolar affinity at pH 6 and pH 7.4 was effective at increasing the clearance of exogenous IgG in mice as well as endogenous IgG in monkeys91, but has not been evaluated in a therapeutic model of IgG-mediated autoimmunity. Small molecule inhibitors of the IgG-FcRn interaction have also been reported93. Although less potent than IgG- and peptide-based inhibitors, these compounds represent a significant step toward the development of orally available IgG-FcRn inhibitors.
Additional molecules that bind Fc at the CH2-CH3 domain interface and may block the IgG-FcRn interaction have been described including: 1) a 13-amino acid cyclic peptide (FcIII) selected by phage display30, 2) a computationally designed IgG-Fc binding protein (FcBP6.1)94, and 3) an endogenous Fc receptor, TRIM2195,96. FcIII and TRIM21 bind Fc with nanomolar affinity at pH 6 and pH 7.4, whereas FcBP6.1 binds with micromolar affinity at pH 6 and nanomolar affinity at pH 7.4. In all cases, binding is predominantly driven by interaction with the Fc residues Met-252, Ile-253, Ser-254, Asn-434, and His-435. The same set of IgG-Fc domain amino acid residues is critical for pH-dependent binding to FcRn; therefore, it is likely that FcIII, FcBP6.1, and TRIM21 would inhibit IgG binding to FcRn. Indeed, we recently demonstrated that FcIII, when genetically fused to a model fluorescent, inhibits IgG binding to FcRn in vitro97; however, we have not explored the ability of FcIII to accelerate IgG clearance in vivo. The multitude of human and non-human ligands that bind at the CH2-CH3 domain interface on Fc indicates this region is naturally poised for interaction with distinct molecules with remarkable plasticity in binding mechanism and likely biological function30. Thus, it would not be surprising to discover additional endogenous human proteins that bind Fc at the CH2-CH3 domain interface.
The major limitation of current IgG-FcRn antagonists as drugs is their short half-life that necessitates frequent dosing to maintain therapeutic concentrations in blood. In addition, because IgG-based inhibitors (e.g. Abdegs, IVIg) can exert additional immunomodulatory functions through the engagement of Fcγ receptors and complement, it remains unknown whether inhibition of the IgG-FcRn interaction alone, as is the case for peptide and small molecule FcRn antagonists, is sufficient to produce a therapeutic benefit in IgG-mediated autoimmune disease.
1.4 Fc-fusion for half-life extension, non-invasive protein delivery, and as vaccines
The first Fc-fusion protein described in 1989 by Genentech98 came well before detailed knowledge of the Fc-FcRn interaction. The promising outcomes in this pioneering study demonstrated that Fc-fusion can endow a protein with unique effector functions mediated by Fc receptor binding and complement fixation. Additional molecular knowledge of the Fc domain and its biological functions has accelerated the use of Fc-fusion proteins in the clinic and as research reagents. Dissection of the Fc-FcRn structure in combination with a more detailed understanding of FcRn biology has resulted in numerous protein drug delivery strategies that take advantage of the unique biological properties of both the Fc domain and FcRn.
1.4.1 Fc-fusion as a versatile half-life extension platform
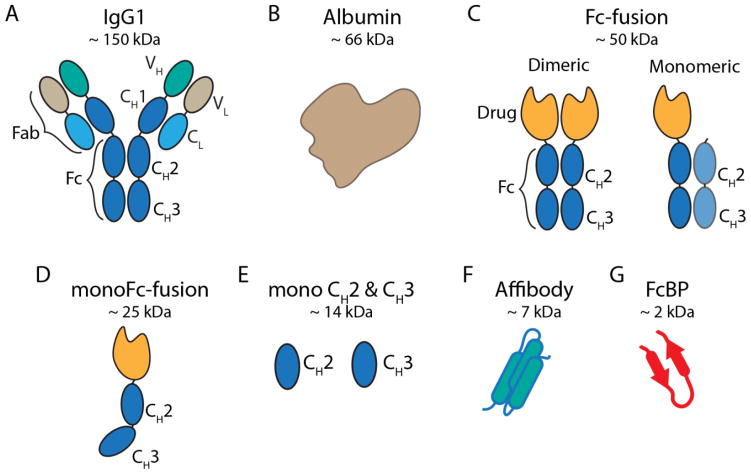
The Fc-domain of IgG alone is sufficient for a direct interaction with FcRn45,63. Genetic fusion of a protein to the Fc-domain of IgG, or Fc-fusion, results in half-life extension by hijacking the FcRn recycling and salvage pathway, and reducing renal clearance through the increase in molecular weight due to the size of the Fc-domain (~ 50 kDa). Typical Fc-fusions are constructed by fusing the C-terminus of an effector molecule to the N-terminus of the IgG hinge region followed by the Fc-domain of IgG (Figure 6c). A number of Fc-fusion proteins are approved for clinical use resulting, in some cases, in a half-life of up to 13 days in humans99. However, there are drawbacks to this approach including: production, which is preferred in eukaryotic expression systems due to the disulfide bond pattern and glycosylation of Fc, reduced stability of Fc-fusion proteins100, lower activity of the fusion partner101, and the large size of the Fc domain that may adversely affect tissue penetration of the fusion protein.
Figure 6. Schematic of various fusion protein domains that can hijack FcRn.
(a) Cartoon depiction of full length human IgG1 and (b) albumin are shown on the top. (c) The Fc-domain of hIgG1 used to construct Fc-fusion proteins. Typical Fc-fusions are constructed by fusing the C-terminus of a protein of interest to the N-terminus of the Fc hinge with either two drugs attached to a homodimeric Fc (left) or one drug attached to a heterodimeric Fc (right). (d) Monomeric Fc-domain fusion. Fc-domain residues are mutated to generate monoFc-fusions or residues are mutated to incorporate N-linked glycans resulting in a monomeric Fc. (e) Monomeric CH2 and CH3 derived from IgG1-Fc. (f) Engineered FcRn-binding affibody (cartoon derived from PDB 2M5A; a representative albumin binding affibody structure179). (g) FcBP fusion (cartoon derived from PDB code 3M17).
Second generation Fc-fusion formats have been devised to address these issues102. Monomeric Fc-fusions contain a monovalent effector molecule fused to the dimeric Fc-domain. Fc-fusion monomers are lower in molecular weight and can have an improved half-life and biological activity compared to traditional Fc-fusion proteins103. A monomeric Fc-factor IX fusion (Alprolix) developed by Biogen Idec recently won FDA approval for the treatment of hemophilia B validating the monomeric Fc-fusion platform for protein half-life extension104. Fc-fusion represents one of the most clinically successful protein half-life extension strategies to date and has been extensively reviewed recently14,102. Interestingly, Fc-fusion proteins in clinical use do not achieve the same ~ 21-day half-life as intact IgG. This may be due to a multitude of factors including steric hindrance between the Fc-domain and fusion partner that reduce protein stability or binding to FcRn, alternative clearance pathways dependent on the protein fused to Fc, contribution of the Fab domain to antibody half-life105–107, or molecular architecture.
1.4.2 Oral and pulmonary protein delivery via FcRn transcytosis
In addition to recycling, FcRn transports its ligands across cellular monolayers providing a feasible route for the transport of protein cargo across epithelial barriers and into the blood stream (Figure 3 and Figure 5a). Administration of protein therapeutics by the oral route remains the ‘holy grail’ of protein drug delivery; however, the low pH, harsh proteolytic environment of the GI tract, and low protein permeability of the intestinal epithelium are major hurdles. FcRn is expressed in the neonatal mouse17 and adult human intestine108,109 and can transport IgG across polarized intestinal epithelial cell monolayers in vitro110 and in mice111. Fc-fusion, in addition to prolonging circulation, can hijack FcRn transport for delivery of protein cargos across epithelial barriers, which has been evaluated by Syntonix Pharmaceuticals (now Biogen Idec) as a potential non-invasive protein delivery strategy. Oral delivery of a follicle stimulating hormone-Fc fusion (FSH-Fc) protein in a neonatal rat model resulted in systemic exposure of FSH-Fc that translated to an increase in testicular weight when compared to FSH or vehicle112. Although a valuable proof-of-concept study, the FSH-Fc bioavailability was not reported and the barrier properties of the neonatal rat intestine are a potential confounding factor as they do not mimic an adult rat or human113. Rodent FcRn expression in the intestine declines after weaning114,115; therefore, adult rodents may not be the preferred model to study FcRn-mediated intestinal IgG transport.
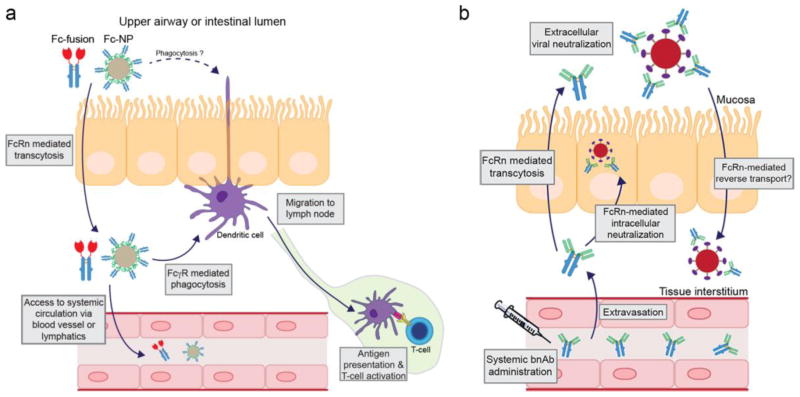
Figure 5. Proposed mechanisms of non-invasive protein delivery, mucosal vaccination, and mucosal viral neutralization.
(a) Fc-fusion proteins or Fc functionalized nanoparticles (Fc-NP) delivered to the lung airspace or intestinal luman transcytosis the polarized epithelial barrier mediated by FcRn. Once delivered to the tissue interstitial space, Fc-fusions or Fc-NPs may access the systemic circulation directly by crossing the capillary endothelial cell barrier or indirectly by first entering the lymphatic system prior to accessing the blood101,123. Alternatively, Fc-fusions or Fc-NPs may be phagocytosed by resident dendritic cells or macrophages expressing Fcγ receptors. Fc-fusions or Fc-NPs may be recycled by FcRn-expressing antigen presenting cells or be diverted to the lysosome where the proteolytic peptides can be loaded onto MHC molecules. APCs then migrate to the lymph node and stimulate a T-cell response resulting in immune induction129. (b) Broadly neutralizing antibodies (bnAbs) extravasate from the blood after systemic administration, accumulate in the tissue interstitial space, and are subsequently transported by FcRn across the epithelium, during which IgG may intercept and neutralize intracellular virus, or be released into the mucosa to neutralize invading pathogens, such as HIV or influenza132,133. IgG-opsonized virus may be degraded in the lysosome or be cleared from the mucosa via alternative mechanisms. Alternatively, IgG-opsonized virus may be reverse transcytosed by FcRn across the epithelial barrier and delivered to the tissue interstitial and initiate infection135. The latter has not been proven in vivo.
The lung represents an additional organ for the non-invasive delivery of proteins by inhalation. Compared to the intestine, the lung pH is neutral, the proteolytic activity is reduced, and the epithelium is naturally permeable to small peptides and proteins116. In fact, inhaled insulin was a clinical success resulting in FDA-approval in 2006, but was subsequently withdrawn from the market by Pfizer due to poor sales. Nonetheless, in June 2014, the FDA announced the approval of a second-generation inhaled insulin product, Afrezza, developed by California-based MannKind. To date, this is the only commercially available protein therapeutic approved for administration by non-parenteral routes, specifically via the lung.
FcRn is also expressed in bronchial epithelial cells of the adult human, non-human primate, and mouse lung117 and studies with Fc-fusion proteins confirmed that FcRn is a viable target for transporting protein cargos across the lung101,117 (Figure 5a). Pulmonary delivery of an erythropoietin Fc (EPO-Fc) fusion protein enables FcRn-specific transport from the lung air space and into systemic circulation with a bioavailability similar to subcutaneous administration (~ 35%) and an ~ 2-fold increase over EPO. An EPO-Fc monomer that contains a single EPO molecule fused to a Fc dimer exhibited higher pulmonary uptake and efficacy compared to the EPO-Fc dimer, suggesting that in addition to FcRn binding, alternative factors such as molecular weight or steric hindrance may also impact pulmonary absorption of Fc-fusion proteins. Absorption of EPO-Fc was higher in monkeys when deposited in the central airways compared to the deep lung, in agreement with the FcRn expression pattern in primates101. Studies with Fc-fusion proteins validate FcRn as a target to enable non-invasive protein delivery and the pulmonary route appears more promising than the oral route to achieve non-invasive protein delivery. Indeed, delivery of EPO-Fc fusion proteins by inhalation has been translated to humans118.
Although hijacking FcRn to enable non-invasive protein delivery appears promising, the transport capacity of FcRn in the human lung or intestine is unknown. The maximum serum concentration of EPO-Fc in humans increased from 0.2 ng/mL to 7.1 ng/mL as the dose increased from 3 μg/kg to 30 μg/kg118. Although sufficient to produce a biological response, EPO is an extremely potent drug. Typical intravenous or subcutaneous doses of EPO range from 1 μg/kg to 3 μg/kg resulting in plasma concentrations at steady state between 1 and 10 ng/mL119. Villasaliu and colleagues estimated the IgG-FcRn transport capacity in the lung to be ~ 6.5 μg/hr based upon transcytosis rates of monomeric IgG across a model Calu-3 cell monolayer and the assumed surface area of the human bronchi120. Extending this analysis and assuming that IgG has a maximum lifespan in the lung of about 24 hours116, and that distribution is limited to plasma (~3 L), approximately 150 μg of protein could be absorbed into systemic circulation resulting in maximum serum concentrations not exceeding 50 ng/mL. Monoclonal antibody doses are typically between ~ 2 – 10 mg/kg and require significantly higher serum concentrations in the μg/mL range to elicit a biological effect121,122; therefore, pulmonary/oral delivery of antibodies, and other low potency proteins, is likely constrained by the FcRn transport capacity. Nonetheless, FcRn transport may be a viable strategy for non-parenteral administration of potent proteins including EPO, human growth hormone, glucagon like peptide-1, and other potent cytokines and growth factors.
Packaging protein molecules into a carrier, such as a liposome or polymer, decorated with an FcRn binding ligand on the surface, such as Fc, has been proposed to increase the total amount of protein that can be delivered across an epithelial barrier by FcRn120 (Figure 5a). In fact, oral delivery of a polymer-based nanoparticle encapsulating insulin was recently shown to reduce blood glucose in fasting mice only when the Fc domain of IgG was present on the nanoparticle surface and mice expressed FcRn123.
However, care must be taken when designing multivalent FcRn-targeted nanoparticle systems. FcRn present at mucosal barriers serves a dual role in transporting IgG-immune complexes across epithelial barriers for subsequent phagocytosis by antigen presenting cells (APCs)111 and once internalized FcRn regulates immune complex sorting to the appropriate intracellular major histocompatibility complex (MHC) loading compartment124,125. Multivalent immune complexes that cross-link FcRn are typically sorted away from the recycling pathway and diverted to lysosomes58. Thus, Fc-decorated protein nanocontainers delivered to the lung or intestine may be phagocytosed by tissues resident APCs, degraded in the lysosome, and peptide antigens loaded onto MHC molecules for subsequent presentation to T-cells resulting in immune induction (Figure 5a). Nonetheless, this recent report by Pridgen and co-workers123 is an important proof-of-concept study that demonstrates the ability to hijack the FcRn to enable non-invasive delivery of protein-loaded nanoparticles resulting in a desired therapeutic effect. FcRn-targeted nanoparticles may also have utility in the development of oral or intranasal vaccines or to improve the efficacy of locally administered drugs for treatment of intestinal or pulmonary disease. Moving forward, it will be important to understand the cellular fate of Fc-modified nanoparticles delivered to the lung or intestine, and their potential for immune induction in vivo.
1.4.3 Fc-fusion proteins for vaccination
FcRn participates in immune surveillance at mucosal barriers, such as in the intestine and lung, through bidirectional transport of IgG from the tissue interstitial space to the lumen, and vice versa111. Monomeric IgG delivered to the lung airspace or intestinal lumen can neutralize antigen through the formation of IgG immune complexes (ICs), be transported back across the epithelial barrier by FcRn, and be delivered to tissues resident APCs111. FcRn is also expressed in professional APCs, such as macrophages and dendritic cells, and participates in MHC Class I and Class II antigen presentation to T-cells48,124,125, and was recently shown to contribute to tumor immunity against colorectal cancers and lung metastases by potentiating anti-tumor CD8+ T cell responses126. In polymorphonuclear neutrophils, FcRn facilitates the phagocytosis of IgG opsonized bacteria127. The immunologic functions of FcRn in phagocytes, epithelial cells, and antigen presenting cells at mucosal surfaces make it a potential target for protein subunit vaccination, mucosal vaccination, and pathogen neutralization strategies.
Ward and colleagues demonstrated that in vitro and in vivo there is a fine balance between the role of FcRn and Fcγ receptor engagement to induce antigen specific T-cell expansion128. Although in vitro Fc-antigen fusions that engage Fcγ receptors and have a high, pH-independent affinity to FcRn (Fc-mut) induce the most potent antigen specific T-cell response, Fc-mut has poor stimulatory capacity in vivo. In contrast, Fc-antigen fusions that retain pH-dependent binding to FcRn and engage Fcγ receptors (Fc-wt) induce weak T-cell responses in vitro, but are the most potent in vivo. The shorter in vivo half-life of Fc-mut compared to Fc-wt indicates that length of antigen exposure in vivo is more important than FcRn targeting, at least for antigen administered systemically. In all cases, Fcγ receptor binding is crucial to induce antigen specific T-cell responses against Fc-antigen fusions in vivo. The studies suggest that fine tuning the Fc-FcRn interaction (or Fc-FcγR interactions) to increase antigen processing by APCs without significantly altering serum persistence may be a viable strategy to improve antigen specific immune responses to Fc-fusions in vivo.
Typical vaccination strategies rely on intramuscular or subcutaneous injection yet most viral and bacterial infections occur at mucosal barriers. Thus, the ideal vaccination strategy would induce both systemic and mucosal immunity. Zhu and colleagues devised a FcRn-dependent mucosal vaccination strategy by fusing the herpes simplex virus type-2 glycoprotein gD to the Fc-domain of IgG (Fc-gD)129. Intranasal administration of Fc-gD to mice induced gD-specific local and peripheral T-cell responses resulting in the induction of gD-specific memory B-cells in the spleen, and most importantly protection against a subsequent intravaginal HSV-2 challenge that persisted for 6 months post immunization. Protection was both dependent on FcRn expression in mice and also Fc binding to FcRn. The role of Fcγ receptor engagement was not evaluated, but the mouse IgG isotype used (IgG2a) preferentially binds mouse activating Fcγ receptors (FcγRI, FcγRIII, FcγRIV) expressed on the surface of APCs34,130. Thus, a common theme of FcRn targeted vaccination strategies is the necessity for FcγR engagement on antigen presenting cells to facilitate antigen endocytosis and intracellular processing. Zhu and colleagues have also extended their Fc-fusion based mucosal vaccination strategy to induce protection against HIV infection with similar successes131, indicating this strategy may be general and not be restricted to a particular viral antigen.
Pathogen neutralization by IgG is a primary line of defense against invading viruses, such as influenza and HIV, and FcRn dependent deposition of neutralizing IgG in the mucosa is an important pathway in IgG-mediated host defense114,132. Fc-engineering to increase IgG affinity for FcRn at pH 6 and subsequently the in vivo half-life of IgG has been repeatedly demonstrated in the literature. Recently, this concept was extended to the broadly neutralizing (bnAb) anti-HIV antibody, VRC01, as a means to increase IgG deposition and/or retention in the rectal mucosa via increased FcRn-transport133 (Figure 5b). Indeed, VRC01 Fc-mutations that increase affinity for FcRn at both pH 6 and pH 7.4 increased VRC01 levels in rectal and vaginal tissue, resulting in greater protection against an intrarectal simian HIV challenge in macaques. These findings have important implications for the use of Fc-engineered bnAbs to prevent viral infections via prophylactic antibody administration.
In addition to extracellular pathogen neutralization by IgG, Zhu and colleagues identified an intracellular, FcRn-mediated viral neutralization mechanism134. An influenza hemagglutinin-specific monoclonal antibody, Y8-10C2, that neutralizes a particular influenza strain (PR8) only at acidic pH (e.g. only in acidic intracellular compartments) was used to demonstrate that FcRn expression in MDCK cells delivers Y8-10C2 to the early endosome where it can bind PR8 and divert the neutralized virus to the lysosome134. Importantly this was dependent on FcRn expression in MDCK cells and Y8-10C2 binding to PR8; however, a Y8-10C2 that lacks FcRn binding was not evaluated. Nonetheless, mice pre-treated with Y8-10C2 are protected from a subsequent lethal, intranasal PR8 challenge for up to 9 days, whereas FcRn knockout mice that received Y8-10C2 were only partially protected. This suggests that both FcRn dependent and independent mechanisms of presumably intracellular viral neutralization occur in vivo. Mice pre-treated with an irrelevant IgG control or vehicle died of infection within 6 days of viral challenge. Collectively, the studies by Ko et al.133 and Bai et al.134 shed new light on the role of FcRn in IgG-mediated viral neutralization; however, a recent study suggests that FcRn may also contribute to the transport of IgG-opsonized HIV across epithelial cell monolayers135, at least in vitro. Although this study raises an important consideration that deserves investigation, it is unclear if such a phenomena would occur in vivo, given the vast differences between an in vitro cell monolayer and the in vivo mucosal microenvironments.
1.5 Alternative strategies that target FcRn
Aside from Fc-fusion a number of additional engineering approaches have been devised to target proteins to FcRn. These strategies include fusion to albumin, engineered Fc domains, or synthetic peptides. To date, most of these strategies are not clinically validated, aside from albumin based drugs and a recently FDA-approved albumin fusion protein. Regardless, alternative strategies that target proteins to FcRn to enable half-life extension, non-invasive delivery, or immune modulation are likely to become important new reagents that expand our understanding of FcRn biology.
1.5.1 Albumin fusion
Albumin is the most abundant protein in human serum and has an extraordinarily long half-life as a result of its size and direct interaction with the FcRn. The interaction between albumin and FcRn has been exploited as a mechanism to increase the circulation time of proteins that are covalently or non-covalently bound to albumin136. Direct interaction with FcRn is achieved by genetic fusion or covalent attachment of a protein cargo to the terminus or single free cysteine, respectively, of recombinant serum albumin (Figure 7b) that in some cases results in half-lives ranging from 9–15 days in humans137. A GLP-1 albumin fusion (albiglutide) developed by Human Genome Sciences (acquired by GSK) extends the half-life of GLP-1 by ~ 3600-fold138 (~ 2 min to ~ 5 days) and won FDA-approval for the treatment of type 2 diabetes.
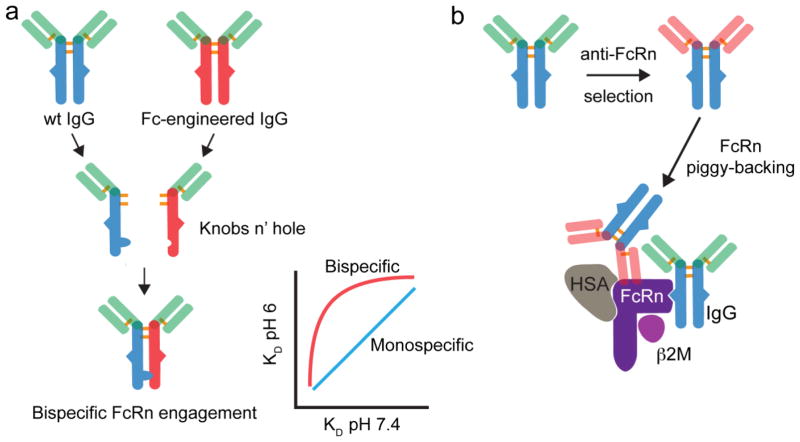
Figure 7. Potential IgG engineering strategies to alter Fc interactions with FcRn or hijack FcRn transport.
(a) Bi-specific IgG-Fc derived from various combination of wild-type or Fc-engineered IgGs may be constructed by various mechanism (e.g. Knobs n’ hole). Given the bivalent nature of the IgG-FcRn interaction, such bispecific IgG-Fc molecules may have interesting FcRn affinity relationships that may alter their recycling or transport properties. (b) The selection of IgGs (or alternative protein scaffolds) that bind an exosite on FcRn, which does not compete with endogenous IgG or albumin binding, may be a useful reagent to piggy-back on the FcRn an alter the biodistribtuion and/or clearance of fusion proteins and drug carriers.
Recently, human serum albumin (HSA) variants with increased affinity towards FcRn at pH 6 were isolated by yeast display39 or rational design139. The HSA variants maintain pH-dependent binding to FcRn, although some mutants bind weakly at pH 7.439. The ~ 300-fold increased affinity for FcRn at pH 6 translated to an ~ 1.5 fold increase in HSA half-life in monkeys. These studies validate the role of FcRn in regulating albumin homeostasis and engineering approaches to modify the albumin-FcRn interaction as a means to tailor the plasma half-life of albumin linked drugs.
Non-covalent binding of therapeutic proteins and peptides to albumin enables an indirect interaction with FcRn. Albumin binds a number of endogenous compounds including fatty acids. Conjugation of a C16 fatty acid to GLP-1 results in non-covalent attachment to albumin and half-life extension and is FDA-approved for the treatment of type 2 diabetes138. Fatty acids inhibit albumin binding to FcRn39, therefore fatty acid conjugated drugs bound to albumin may not be productively salvaged by FcRn in vivo. Alternative albumin binding molecules have been engineered such as peptides140, albumin binding domains from streptococcal protein G141, and antibody based scaffolds142. The immunogenicity of non-human albumin binding proteins is still a concern, especially if such therapeutics are intended for long-term use. Nonetheless, the use of albumin as a drug carrier is a clinically successful platform for half-life extension, and more details are presented in two recent reviews136,137.
1.5.2 Low molecular weight FcRn binding ligands
The molecular weight of protein therapeutics, or hydrodynamic radius, is inversely proportional to diffusivity and capillary permeability143. Monoclonal antibodies are proven anti-cancer agents but heterogeneous distribution in tumor tissues can result in restricted tumor cell exposure and poor efficacy. Antibody distribution through a tumor is the result of numerous factors including length of systemic exposure, extent of tumor vasculature, convection, diffusion, and antigen binding144. However, the concentration gradient between blood and tumor is a major driving force in protein accumulation within a tumor that can be controlled by modulating circulation time145. Low molecular weight proteins have excellent tumor penetration properties, but rapid clearance limits their use as therapeutics144. Strategies that increase serum persistence without substantially increasing molecular weight may improve the distribution of drugs in tumors (and other tissues) without compromising dosing frequency.
Towards this goal Dimitrov and colleagues generated a variety of engineered monomeric CH2, CH3, and Fc domains146–148 (Figure 6d,e). Although substantially smaller than dimeric Fc (~ 15 kDa vs 50 kDa), monomeric CH2 and CH3 bind weakly to FcRn, as expected given the contribution of amino acid residues that span both the CH2 and CH3 domains to FcRn binding22. Nonetheless, the terminal half-life of monomeric CH2 is extended 2–3 fold in mice compared to a mutant CH2 that does not bind FcRn149. Monomeric CH3 has not been evaluated in vivo. Monomeric CH2 and CH3 fusion proteins are unlikely to achieve circulation times comparable to Fc-fusion as they cannot make a bivalent, high-affinity interaction with FcRn. Additional mutations to increase affinity toward FcRn may improve the in vivo properties of monomeric CH2 and CH3 domains.
Fc is a dimer composed of two CH2-CH3 chains (Figure 6c). Fc dimerization is mainly mediated by hydrophobic interactions between two CH3 domains and stabilized by disulfide bonds in the hinge region preceding the CH2 domain. Fc dimerization empowers IgG with bivalent antigen binding as well as bivalent Fc receptor binding. Monomeric Fc domains have recently been described by either mutation of hydrophobic residues at the CH3-CH3 dimer interface146 or introduction of N-linked glycosylation sites on the CH3 domain150 (Figure 6d). Both formats retain pH-dependent binding to FcRn and a Fc monomer Fab fusion protein (Fab-monoFc) has a FcRn dependent half-life in mice; however, the half-life of Fab-monoFc compared to Fab alone was not determined. Nonetheless, monomeric Fc domains represent the smallest reported IgG domain (~ 25 kDa) that make a productive, pH-dependent interaction with FcRn. A more thorough investigation into the FcRn binding, circulating, and tumor penetrating properties of monomeric Fc compared to alternative formats (e.g. dimeric Fc fusion) is needed to understand the potential of this novel half-life extension platform.
We previously described an alternative strategy to engineer FcRn-mediated recycling and transcytosis in recombinant proteins by genetic fusion of short FcRn binding peptides (FcBP) to the termini of a model fluorescent protein151 (Figure 6g). Our approach was motivated by results of Mezo and colleagues who identified peptides that compete with IgG for binding to FcRn91. Proteins modified with FcBPs exhibit pH-dependent binding to FcRn with an affinity comparable to human IgG1, and are recycled and transcytosed across cell monolayers that express human FcRn. In vitro, FcBP fusion proteins are highly mimetic of the hIgG1-FcRn interaction. To date, FcBP fusion represents the smallest protein modification (~ 2 – 4 kDa) approach to target FcRn without compromising affinity or pH-dependent binding. However, we found that the plasma clearance of FcBP fusions proteins in vivo is independent of human FcRn binding, whereas the clearance of IgG and albumin is FcRn dependent (unpublished results). We speculate that alternative unknown factors are important regulators of IgG homeostasis in vivo, which cannot be replicated by FcBP fusion. We expand upon this hypothesis in section 1.7.
More recently, Seijsing et al. selected affibody molecules, a 3-helix bundle scaffold protein derived from staphylococcal protein A (Figure 6f), that exhibit pH-dependent binding to the FcRn152. The selected affibody variants (ZFcRn) bind human FcRn with low nanomolar affinity at pH 6 (10–50 nM), and weaker, yet functional binding at pH 7.4 (~ 100 – 1000 nM). Various ZFcRn affibodies were subsequently tethered to an albumin binding affibody (ABD) for evaluation in vivo. Fusion of ZFcRn to ABD resulted in a 2–3 fold increase in half-life (~ 60–90 hrs) of the ZFcRn-ABD fusion compared to the ABD (~ 33 hr) in mice. ZFcRn is an additional low molecular weight fusion molecule to endow recombinant proteins with FcRn-mediated recycling and transcytosis, however more research is required to elucidate the ZFcRn half-life extension mechanism. It is currently unknown whether ZFcRn can functionally hijack FcRn in the absence of albumin binding; the extended half-life of the ZFcRn-ABD fusion may be entirely dependent on albumin binding. To broaden the utility of the ZFcRn, it is important to determine if ZFcRn can extend the half-life of recombinant proteins that do not interact with FcRn. Nonetheless, ZFcRn represents an interesting new reagent to probe the in vitro and in vivo biology of the FcRn. Nanobodies that target FcRn have also been reported153; however, their utility in drug delivery or disease therapy has not been explored.
1.6 Future perspectives: the not so well characterized FcRn?
In the field of drug delivery, a deep understanding of underlying biological mechanisms that contribute to human physiology and disease is a prerequisite to the development of effective drug carriers. FcRn is a prime example of how basic biology and incremental discoveries can be translated into therapeutics. So what is next for FcRn? To conclude this review we provide a perspective on the future of FcRn as a therapeutic target for drug delivery and speculate that additional yet unknown biological mechanisms regulate FcRn function.
A number of unexplored drug delivery strategies may be useful when considering the known biology of FcRn. For instance, FcRn expressed in the lung and intestinal epithelia provides a route for the transport of Fc-fusion proteins across the epithelium and into the blood stream, enabling non-invasive protein administration via FcRn-mediated transcytosis112,118. However, FcRn also recycles its ligands and may act to increase lung retention of pulmonary administered therapeutics, which is hampered by rapid mucociliary and alveolar macrophage clearance116. It is unknown whether FcRn can increase the residence time of therapeutics delivered to the lung or what role alveolar macrophages play in the disposition of FcRn-targeted therapeutics. An investigation into these processes may identify useful FcRn-dependent strategies to improve the efficacy of pulmonary therapeutics.
Interestingly, intense expression of FcRn in human small intestine crypt cells was recently reported115. The cells also stained positive for chromagranin A, Glucagon-Like Peptide (GLP)-1, GLP-2 by immunohistochemistry suggesting their identity as enteroendocrine cells. Although the biology of FcRn in this specific cell type is unknown, the crosstalk between endocrine and immune cells in the gut154 suggests FcRn may play a specialized role in maintaining mucosal immunity in this cell type. Endocrine cells in the gut also play an important role in nutrient absorption and metabolism155. Thus, targeting therapeutics to FcRn expressing endocrine cells may be a strategy for the treatment of metabolic disease and gastrointestinal disorders but requires a significant amount of further research to comprehend the biology. It is provocative to speculate that at least part of the therapeutic effect elicited by the oral delivery of insulin loaded, FcRn targeted nanoparticles was mediated by a local FcRn dependent process in the gut rather than the systemic delivery of insulin via FcRn-transcytosis as suggested by the authors123.
In general, organ specific functions of FcRn outside of regulating IgG homeostasis have not been thoroughly investigated. Recently the expression of FcRn in the mouse and human eye was reported156. The expression pattern between human FcRn transgenic mice (Tg32 strain) and human tissues were similar although not identical. Interestingly, strong FcRn staining in monocytic cells co-expressing the Iba1 marker was observed in sections from the human eye156. We have observed a low frequency but consistent phenotype in the eye of the human FcRn transgenic mice72,157 associated with a partial or complete degeneration of predominately the left eye (unpublished observation). The expression of FcRn in the eye and the observed phenotype suggest that FcRn has specific, unknown functions in eye development and disease progression. Confirming the link between FcRn and eye disease, if any, may enable new ocular treatment opportunities using IgG/albumin based drugs.
There may also still be unexplored antibody engineering strategies that alter the biodistribution or pharmacokinetics of mAbs mediated by FcRn. Increasing the affinity the IgG-FcRn interaction at pH 6 can extend IgG half-life; however, there appears to be a direct correlation between affinity gains at pH 6 and pH 7.473. This correlation places a limit on increasing the affinity of the IgG-FcRn interaction at pH 6, as subsequent affinity gains at pH 7.4 decrease IgG serum persistence158. IgG can simultaneously bind two FcRn molecules due to the homodimeric nature of the Fc-domain27. Therefore, an intriguing idea may be to engineer a heterodimeric Fc with each subunit having a differential affinity for FcRn at either pH 6 or pH 7.4 (Figure 7a). Although heterodimeric Fc/FcRn interactions have been described, the hybrid Fc molecules reported all contain one Fc CH2-CH3 domain that lacks binding to FcRn21,22,29,159. An evaluation of hybrid Fc molecules constructed from two Fc CH2-CH3 domains with varying affinity for FcRn at pH 6 and/or pH 7.4 has not been conducted. Such engineered heterodimeric Fc molecules may alter the pH 6 to pH 7.4 affinity correlations, could be useful to study the role of FcRn affinity and dimerization on recycling, transcytosis, and half-life, and may result in IgGs with unusual in vivo properties. A number of methods now exist to readily create bispecific IgG-Fc160–162.
In addition, engineering mAb variable domains to bind FcRn in a pH-dependent (or independent) fashion at a site that does not interfere with albumin or IgG binding may be an efficient strategy to hijack FcRn-mediated recycling and transcytosis (Figure 7b). Presumably these engineered mAbs would not compete with the high concentrations of endogenous IgG and albumin for FcRn transport. Coupling engineered anti-FcRn mAbs or Fab-fragments to drugs or carriers may enable the non-invasive delivery of therapeutic agents or their accumulation in peripheral organs such as the lung or brain. A similar strategy that hijacks the transferrin receptor was recently reported by Yu and colleagues at Genentech to improve the brain accumulation of therapeutic antibodies163.
The role of FcRn in the protection and transport of albumin has lagged IgG. The recent crystal structure of an engineered human serum albumin variant in complex with human FcRn39 as well as the co-crystal structure of human albumin and human Fc in complex with human FcRn35 will certainly provide new insights into the albumin-FcRn-IgG interaction that should guide engineering approaches. Indeed, engineered albumin variants that have an increased affinity for FcRn at pH 6 and an extended half-life in mice and monkeys were recently described39,164. Compared to IgG, there is little knowledge of the FcRn-mediated recycling and transcytosis and intracellular fate of albumin. Albumin is a carrier of numerous endogenous compounds and exogenous drugs, thus engineering inhibitors of the albumin-FcRn interaction may be a viable strategy to enhance the clearance of albumin-bound molecules.
1.7 An alternative FcRn-IgG salvage hypothesis
As part of our efforts to improve the drug-like properties of proteins we developed a method, termed FcBP fusion, to engineer proteins to interact with the FcRn through short terminal peptide extensions. FcBP fusions are highly mimetic of the IgG-FcRn interaction and are recycled and transcytosed by FcRn in vitro. Similar in vitro assays formed the foundation of our knowledge of the FcRn recycling process and IgG salvage mechanism presumed to also occur in vivo. However, our attempts to hijack FcRn-recycling with a synthetic FcBP have not translated to a FcRn-dependent increase in half-life of FcBP modified cargos in vivo (unpublished results). This observation formed the initial basis of our hypothesis that the IgG-FcRn recycling pathway involves an additional component(s) that acts in collaboration with FcRn to salvage IgG but not FcBP fusions from catabolism.
We speculate that such a mechanism may involve a yet unknown receptor or known receptor with unknown/additional function, termed “Factor X.” In this conclusion, we outline three alternative IgG salvage mechanisms (Figure 8). Although we describe and justify our hypotheses based upon our experience with FcBP fusion proteins and relevant literature, the proposed mechanisms align with our current understanding of the IgG-FcRn interaction, as well as the caveats of the systems used to study the IgG-FcRn transport mechanism. The latter was the focus of a recent opinion article by Clark Anderson15.
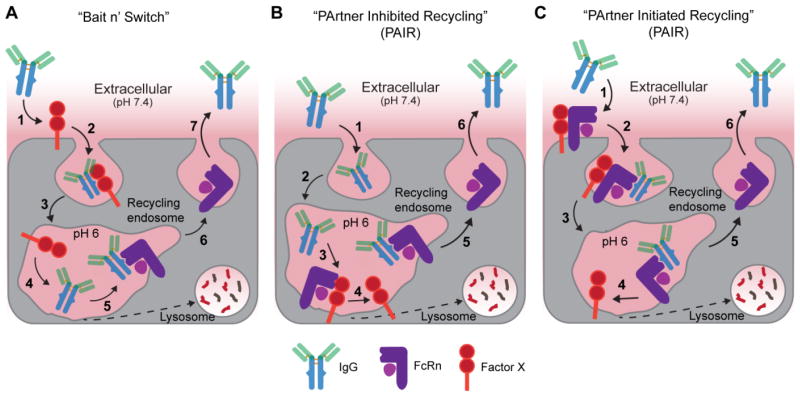
Figure 8. Alternative IgG salvage theories that act in combination with FcRn.
(a) In this “bait n’ switch” type mechanism an unknown cell-membrane anchored receptor, Factor X, binds IgG in serum (1) triggering receptor-mediated endocytosis (2). As IgG is trafficked along the endosomal pathway (3) the pH decreases to 6 resulting in dissociation of IgG from Factor X (4) and subsequent transfer to FcRn (5). FcRn then recycles IgG back to the plasma membrane (6) where IgG is released into blood due to its weak affinity for FcRn at blood pH (7). FcBP fusion proteins would not be readily endocytosed because they cannot bind Factor X. (b) In the PArtner Inhibited Recycling mechanism IgG is endocytosed into FcRn expressing cells by non-specific pinocytosis (1) and is trafficked to the endosome. The IgG binding site on FcRn is blocked by Factor X at steady state; however, IgG can effectively compete with the Factor X (2) resulting in dissociation of Factor X from FcRn and the formation of the FcRn-IgG complex (3). FcRn then recycles IgG back to the plasma membrane (4) where IgG is released into blood due to its weak affinity for FcRn at blood pH (5). FcBP fusion proteins cannot compete with the Factor X-FcRn interaction and are not salvaged by FcRn. (c) Alternatively, in a PArtner Initiated Recycling process an additional, unknown cell-membrane anchored FcRn binding partner increases the affinity of FcRn for IgG at physiological pH (1) resulting in FcRn-mediated endocytosis of IgG (2). As the complex is trafficked along the endosomal pathway (3) the pH decreases to 6 resulting in dissociation of Factor X from the FcRn-IgG complex (4). IgG stays bound to FcRn due to its high affinity at pH 6 and is trafficked back to the plasma membrane (5) where IgG is released into blood due to its weak affinity for FcRn at blood pH in the absence of Factor X (6). In all cases, IgG, FcBP fusion proteins, or additional serum components that do not bind FcRn or cannot compete with Factor X for binding FcRn are trafficked to the lysosome and degraded.
A number of observations in the literature support the potential of an additional IgG salvage process. First, and most relevant, is the observation that β2m deficient mice catabolize IgG faster than FcRn α-chain deficient mice leading Kim, Anderson and coworkers to suggest an alternative β2m-associated IgG salvage mechanism165. Unfortunately, β2m is required for FcRn function19,166 making it impossible with current mouse models to decouple the relative contribution of FcRn or an alternative β2m-dependent process that regulates IgG half-life. Also of relevance is the observation that the varying plasma clearance of IgG subclasses (1, 2, 3, and 4) and isotypes (λ or κ) cannot be explained by a simple FcRn-IgG affinity relationship80,167,168, suggesting alternative factors contribute to IgG serum persistence. Interestingly, albumin is not actively transported across the placental barrier even though IgG transport from mother to fetus is dependent on FcRn169. This suggests that the maternofetal transfer of IgG requires an additional FcRn-independent mechanism that is not replicated by albumin. Perhaps a similar step also regulates IgG circulation in blood?
In the first hypothesized mechanism, IgG binds Factor X at the cell surface. Factor X then shuttles IgG from the blood to FcRn-containing endosomes in a “bait and switch” type endocytosis and recycling mechanism (Figure 8a). This contrasts with the widely accepted mechanism of non-specific, fluid-phase uptake to deliver serum IgG to intracellular FcRn. In the “bait and switch” mechanism only serum proteins that bind both Factor X and FcRn complete the intracellular FcRn-mediated recycling process. A two-component “bait-n-switch” could regulate IgG salvage in parenchymal and hematopoietic cells as these cells both express known (or possibly unknown) IgG receptors (IgR)34 and are the major cell types involved in regulating IgG serum persistence in mice47,49. Even a low affinity interaction between IgG and an IgR could result in receptor-mediated endocytosis given the high concentration of IgG in serum while dissociation of IgG during endosomal transit, either due to weak or pH-dependent affinity, would enable capture by FcRn.
Alternative to the “bait and switch,” an additional membrane component may interact directly with the FcRn α-chain and/or β2m light-chain and influence the IgG-FcRn interaction resulting in a cooperative (PArtner Initiated) or competitive (PArtner Inhibited) Recycling process, or “PAIRed.” The human hemochromatosis protein (HFE) acts as a “PArtner Inhibited” regulator of iron absorption by competing with transferrin-bound iron (Tf-Fe) for transferrin receptor binding170. HFE function, like FcRn, is dependent on association with β2m171. In a similar fashion, Factor X may compete with IgG for binding to FcRn providing an additional regulatory component to control IgG homeostasis (Figure 8b).
The invariant chain (Ii), which promotes MHC Class II folding and endosomal targeting, also associates with FcRn in immortalized and bone marrow derived cells from mice and humans172. The Ii interacts with FcRn alone or the FcRn/β2m heterodimer. The FcRn/Ii complex can be isolated from cell lysates with IgG-beads at pH 6 suggesting that the Ii does not interfere with IgG binding to FcRn and is not displaced upon IgG binding. The Ii appears to shift the distribution of FcRn toward late endosomes/lysosomes (LAMP-1 positive compartments) suggesting a potential mechanism for the delivery of IgG immune complexes to the lysosome. The interacting amino acid residues on both the Ii and FcRn/β2m are unknown as well as the biological relevance of this interaction in vivo. A detailed molecular understanding of the Ii-FcRn/β2m-IgG interaction is warranted as this complex may regulate the IgG-FcRn interaction.
Alternatively, an additional membrane component may induce affinity between IgG and FcRn at physiological pH, resulting in FcRn-mediated endocytosis of the ternary Factor X-FcRn-IgG complex (Figure 8c). Again, even weak affinity at pH 7.4 would be sufficient to capture serum IgG. At some point along the recycling pathway, Factor X may dissociate from FcRn while IgG remains bound due to its high affinity at low pH. The binary FcRn-IgG complex may be trafficked back to the plasma membrane resulting in release of IgG from FcRn due to the elevated pH and absence of Factor X.
A major question that remains is why FcBP fusion proteins and IgG are recycled and transcytosed by human FcRn in vitro, if in fact an alternative regulatory process exists? We postulate that there may be cross-species differences in the Factor X interaction with FcRn, β2m, and/or IgG in the in vitro MDCK cell model (or other cell models) commonly used to study FcRn transport. MDCK cell systems over-expressing FcRn and β2m are widely used to study FcRn-mediated recycling and transcytosis of IgG29,151,166,173–176 and was also used to study the FcBP interaction with human FcRn151. However, the MDCK cell model has caveats. First, to detect both IgG and FcBP accumulation in MDCK cells by fluorescence FcRn must be over-expressed such that a high fraction of the receptor is present on the cell surface. In this model, when the culture medium is acidic, we rely on FcRn-dependent endocytosis not fluid-phase pinocytosis or alternative receptor mediated endocytosis processes. However, at sufficiently high enough ligand concentrations we would also expect some degree of fluid-phase uptake, although in this particular cell model we detect little to no non-specific IgG accumulation at pH 7.4 even at concentrations up to 25 μM151. Second, MDCK cells may not express Factor X or may express a species of Factor X that does not interact with human FcRn/β2M or human IgG. This is reminiscent of the lack of association between human FcRn and dog β2m in MDCK cells166. Thus, if Factor X exists, such in vitro models may not reflect the in vivo biology. The uptake and recycling mechanisms of IgG, albumin, FcBP fusions, or alternative FcRn-binding ligands should perhaps be evaluated in immortalized and/or primary human endothelial/epithelial cells and hematopoietic cells, as these cell types are thought to be a major contributor to FcRn function in vivo47,49 and possibly a more representative cell model.
The suggestions in this section are highly speculative; however, we would not be surprised that an additional regulatory component influences IgG homeostasis in combination or collaboration with FcRn, as has been suggested for the maternofetal transfer of IgG177. Our hypotheses are built upon published data and our observation that a synthetic peptide specific for human FcRn does not enable half-life extension of its fused cargos in vivo even though it mimics the IgG-FcRn interaction in single component model systems in vitro. These data suggest an alternative IgG-salvage component that cannot be mimicked by the FcBP and/or that has species-specific properties. However, we cannot rule out that subtle, unidentified differences in FcBP-FcRn binding kinetics, affinity, site, or mechanism contribute to its lack of function in vivo. Nonetheless, if an alternative IgG or FcRn regulatory component exits, its identification would have a significant impact on FcRn biology and provide new opportunities to engineer IgG, albumin, or alternative FcRn-targeted molecules for enhanced serum persistence.
1.8 Conclusion
In recent years a number of advancements in our understanding of FcRn biology have translated to the development of novel FcRn based drug delivery strategies. Most notably, the recently elucidated immunological functions of FcRn have been exploited to improve delivery and efficacy of protein subunit vaccines129 and neutralizing antibodies133,134 for treatment of infectious diseases. The continual exploitation of the classical roles of FcRn in regulating IgG homeostasis and transport across cellular barriers have also resulted in a number of novel engineering approaches that hijack FcRn-mediated recycling and transcytosis to improve protein half-life and delivery. Most approaches focus on engineering higher affinity IgG Fc-domains or albumins for use as therapeutics or fusion partners; however, a number of novel FcRn targeting strategies are in their infancy146,150,151,153. Engineered IgGs with higher affinity toward FcRn to improve half-life, a concept that originated almost 20 years ago63, have finally been evaluated in humans65,67. The recent crystal structure of albumin and an albumin variant in complex with FcRn35,39 should accelerate the development of albumin based therapeutics and underlying FcRn-albumin biology, which has lagged IgG. New immunological functions of FcRn continue to emerge demonstrating the importance of this receptor in human health126. The future is bright for FcRn as a molecular target to improve the drug-like properties of proteins and macromolecule drug carriers. With continual discovery of the vast biological functions of FcRn, the development of new tools to study FcRn in vitro and in vivo, and clever engineering approaches and creative minds, we anticipate research in the field to have a profound impact on the development of novel therapeutic strategies to treat human disease.
Acknowledgments
We first and foremost would like to thank our FcRn colleagues who have made significant contributions to the field of FcRn biology over the last five decades. We would also like to thank Professor Sally Ward at the UT Southwestern Medical Center for reagents and helpful discussion. J.S. and F.C.S are grateful for support from the National Institutes of Health (NIH) Grant R21 EB015520. J.S. is grateful for past support from the NIH training grant T32 GM007175 and past fellowship support from the American Foundation for Pharmaceutical Education (AFPE), UCSF Graduate Dean’s Chancellor’s Fellowship, and the Pharmaceutical Research and Manufacturers of America (PhRMA) foundation. J.S. is also grateful for current fellowship support from the Stanford Molecular and Cellular Immunobiology NIH postdoctoral training grant 5 T32 AI072905.
Footnotes
J.S. and F.C.S. declare no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
1.10 References
- 1.Brambell FW, Hemmings WA, Morris IG. A theoretical model of gamma-globulin catabolism. Nature. 1964;203:1352–4. doi: 10.1038/2031352a0. [DOI] [PubMed] [Google Scholar]
- 2.Rodewald R. pH-dependent binding of immunoglobulins to intestinal cells of the neonatal rat. J Cell Biol. 1976;71:666–9. doi: 10.1083/jcb.71.2.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones EA, Waldmann TA. The mechanism of intestinal uptake and transcellular transport of IgG in the neonatal rat. J Clin Invest. 1972;51:2916–27. doi: 10.1172/JCI107116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodewald R, Kraehenbuhl JP. Receptor-mediated transport of IgG. J Cell Biol. 1984;99:159s–164s. doi: 10.1083/jcb.99.1.159s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature. 1989;337:184–7. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- 6.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 7.Challa DK, Velmurugan R, Ober RJ, Sally Ward E. FcRn: from molecular interactions to regulation of IgG pharmacokinetics and functions. Curr Top Microbiol Immunol. 2014;382:249–72. doi: 10.1007/978-3-319-07911-0_12. [DOI] [PubMed] [Google Scholar]
- 8.Rath T, et al. The immunologic functions of the neonatal Fc receptor for IgG. J Clin Immunol. 2013;33(Suppl 1):S9–17. doi: 10.1007/s10875-012-9768-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Junghans RP. Finally! The Brambell receptor (FcRB). Mediator of transmission of immunity and protection from catabolism for IgG. Immunol Res. 1997;16:29–57. doi: 10.1007/BF02786322. [DOI] [PubMed] [Google Scholar]
- 10.Ghetie V, Ward ES. Multiple roles for the major histocompatibility complex class I- related receptor FcRn. Annu Rev Immunol. 2000;18:739–66. doi: 10.1146/annurev.immunol.18.1.739. [DOI] [PubMed] [Google Scholar]
- 11.Anderson CL, et al. Perspective-- FcRn transports albumin: relevance to immunology and medicine. Trends Immunol. 2006;27:343–8. doi: 10.1016/j.it.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Andersen JT, Sandlie I. The versatile MHC class I-related FcRn protects IgG and albumin from degradation: implications for development of new diagnostics and therapeutics. Drug Metab Pharmacokinet. 2009;24:318–332. doi: 10.2133/dmpk.24.318. [DOI] [PubMed] [Google Scholar]
- 13.Ward ES, Velmurugan R, Ober RJ. Targeting FcRn for therapy: from live cell imaging to in vivo studies in mice. Immunol Lett. 2014;160:158–62. doi: 10.1016/j.imlet.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rath T, et al. Fc-fusion proteins and FcRn: structural insights for longer-lasting and more effective therapeutics. Crit Rev Biotechnol. 2013 doi: 10.3109/07388551.2013.834293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson CL. There’s been a Flaw in Our Thinking. Front Immunol. 2014;5:540. doi: 10.3389/fimmu.2014.00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson IA, Bjorkman PJ. Unusual MHC-like molecules: CD1, Fc receptor, the hemochromatosis gene product, and viral homologs. Curr Opin Immunol. 1998;10:67–73. doi: 10.1016/s0952-7915(98)80034-4. [DOI] [PubMed] [Google Scholar]
- 17.Ghetie V, et al. Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur J Immunol. 1996;26:690–6. doi: 10.1002/eji.1830260327. [DOI] [PubMed] [Google Scholar]
- 18.Wani MA, et al. Familial hypercatabolic hypoproteinemia caused by deficiency of the neonatal Fc receptor, FcRn, due to a mutant beta2-microglobulin gene. Proc Natl Acad Sci U S A. 2006;103:5084–5089. doi: 10.1073/pnas.0600548103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Israel EJ, Wilsker DF, Hayes KC, Schoenfeld D, Simister NE. Increased clearance of IgG in mice that lack beta 2-microglobulin: possible protective role of FcRn. Immunology. 1996;89:573–8. doi: 10.1046/j.1365-2567.1996.d01-775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci U S A. 1996;93:5512–6. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin WL, West AP, Jr, Gan L, Bjorkman PJ. Crystal structure at 2.8 A of an FcRn/heterodimeric Fc complex: mechanism of pH-dependent binding. Mol Cell. 2001;7:867–877. doi: 10.1016/s1097-2765(01)00230-1. [DOI] [PubMed] [Google Scholar]
- 22.Kim JK, Tsen MF, Ghetie V, Ward ES. Catabolism of the murine IgG1 molecule: evidence that both CH2-CH3 domain interfaces are required for persistence of IgG1 in the circulation of mice. Scand J Immunol. 1994;40:457–65. doi: 10.1111/j.1365-3083.1994.tb03488.x. [DOI] [PubMed] [Google Scholar]
- 23.Kim JK, Tsen MF, Ghetie V, Ward ES. Identifying amino acid residues that influence plasma clearance of murine IgG1 fragments by site-directed mutagenesis. Eur J Immunol. 1994;24:542–8. doi: 10.1002/eji.1830240308. [DOI] [PubMed] [Google Scholar]
- 24.Burmeister WP, Gastinel LN, Simister NE, Blum ML, Bjorkman PJ. Crystal structure at 2.2 A resolution of the MHC-related neonatal Fc receptor. Nature. 1994;372:336–43. doi: 10.1038/372336a0. [DOI] [PubMed] [Google Scholar]
- 25.Shields RL, et al. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem. 2001;276:6591–6604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 26.Huang X, Zheng F, Zhan CG. Binding structures and energies of the human neonatal Fc receptor with human Fc and its mutants by molecular modeling and dynamics simulations. Mol Biosyst. 2013;9:3047–58. doi: 10.1039/c3mb70231f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.West AP, Bjorkman PJ. Crystal structure and immunoglobulin G binding properties of the human major histocompatibility complex-related Fc receptor. Biochemistry. 2000;39:9698–708. doi: 10.1021/bi000749m. [DOI] [PubMed] [Google Scholar]
- 28.Martin WL, Bjorkman PJ. Characterization of the 2:1 complex between the class I MHC-related Fc receptor and its Fc ligand in solution. Biochemistry. 1999;38:12639–47. doi: 10.1021/bi9913505. [DOI] [PubMed] [Google Scholar]
- 29.Tesar DB, Tiangco NE, Bjorkman PJ. Ligand Valency Affects Transcytosis, Recycling and Intracellular Trafficking Mediated by the Neonatal Fc Receptor. Traffic. 2006;7:1127–1142. doi: 10.1111/j.1600-0854.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeLano WL, Ultsch MH, de Vos AM, Wells JA. Convergent solutions to binding at a protein-protein interface. Science. 2000;287:1279–1283. doi: 10.1126/science.287.5456.1279. [DOI] [PubMed] [Google Scholar]
- 31.Idusogie EE, et al. Mapping of the C1q binding site on rituxan, a chimeric antibody with a human IgG1 Fc. J Immunol. 2000;164:4178–84. doi: 10.4049/jimmunol.164.8.4178. [DOI] [PubMed] [Google Scholar]
- 32.Duncan AR, Winter G. The binding site for C1q on IgG. Nature. 1988;332:738–40. doi: 10.1038/332738a0. [DOI] [PubMed] [Google Scholar]
- 33.Simmons LC, et al. Expression of full-length immunoglobulins in Escherichia coli: rapid and efficient production of aglycosylated antibodies. J Immunol Methods. 2002;263:133–47. doi: 10.1016/s0022-1759(02)00036-4. [DOI] [PubMed] [Google Scholar]
- 34.Pincetic A, et al. Type I and type II Fc receptors regulate innate and adaptive immunity. Nat Immunol. 2014;15:707–16. doi: 10.1038/ni.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oganesyan V, et al. Structural Insights into Neonatal Fc Receptor-based Recycling Mechanisms. J Biol Chem. 2014;289:7812–24. doi: 10.1074/jbc.M113.537563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stapleton NM, et al. Competition for FcRn-mediated transport gives rise to short half-life of human IgG3 and offers therapeutic potential. Nat Commun. 2011;2:599. doi: 10.1038/ncomms1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaughn DE, et al. Identification of critical IgG binding epitopes on the neonatal Fc receptor. J Mol Biol. 1997;274:597–607. doi: 10.1006/jmbi.1997.1388. [DOI] [PubMed] [Google Scholar]
- 38.Andersen JT, et al. Structure-based mutagenesis reveals the albumin-binding site of the neonatal Fc receptor. Nat Commun. 2012;3:610. doi: 10.1038/ncomms1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt MM, et al. Crystal Structure of an HSA/FcRn Complex Reveals Recycling by Competitive Mimicry of HSA Ligands at a pH-Dependent Hydrophobic Interface. Structure. 2013;21:1966–78. doi: 10.1016/j.str.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 40.Sand KMK, et al. Dissection of the neonatal Fc receptor (FcRn)-albumin interface using mutagenesis and anti-FcRn albumin-blocking antibodies. J Biol Chem. 2014;289:17228–39. doi: 10.1074/jbc.M113.522565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaudhury C, Brooks CL, Carter DC, Robinson JM, Anderson CL. Albumin binding to FcRn: distinct from the FcRn-IgG interaction. Biochemistry. 2006;45:4983–90. doi: 10.1021/bi052628y. [DOI] [PubMed] [Google Scholar]
- 42.Andersen JT, Daba MB, Berntzen G, Michaelsen TE, Sandlie I. Cross-species binding analyses of mouse and human neonatal Fc receptor show dramatic differences in immunoglobulin G and albumin binding. J Biol Chem. 2010;285:4826–36. doi: 10.1074/jbc.M109.081828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou J, Johnson JE, Ghetie V, Ober RJ, Sally Ward E. Generation of Mutated Variants of the Human Form of the MHC Class I-related Receptor, FcRn, with Increased Affinity for Mouse Immunoglobulin G. J Mol Biol. 2003;332:901–913. doi: 10.1016/s0022-2836(03)00952-5. [DOI] [PubMed] [Google Scholar]
- 44.Zhou J, Mateos F, Ober RJ, Ward ES. Conferring the Binding Properties of the Mouse MHC Class I-related Receptor, FcRn, onto the Human Ortholog by Sequential Rounds of Site-directed Mutagenesis. J Mol Biol. 2005;345:1071–1081. doi: 10.1016/j.jmb.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 45.Vaughn DE, Bjorkman PJ. High-affinity binding of the neonatal Fc receptor to its IgG ligand requires receptor immobilization. Biochemistry. 1997;36:9374–80. doi: 10.1021/bi970841r. [DOI] [PubMed] [Google Scholar]
- 46.Ober RJ, Radu CG, Ghetie V, Ward ES. Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int Immunol. 2001;13:1551–1559. doi: 10.1093/intimm/13.12.1551. [DOI] [PubMed] [Google Scholar]
- 47.Akilesh S, Christianson GJ, Roopenian DC, Shaw AS. Neonatal FcR Expression in Bone Marrow-Derived Cells Functions to Protect Serum IgG from Catabolism. J Immunol. 2007;179:4580–4588. doi: 10.4049/jimmunol.179.7.4580. [DOI] [PubMed] [Google Scholar]
- 48.Zhu X, et al. MHC class I-related neonatal Fc receptor for IgG is functionally expressed in monocytes, intestinal macrophages, and dendritic cells. J Immunol. 2001;166:3266–3276. doi: 10.4049/jimmunol.166.5.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montoyo HP, et al. Conditional deletion of the MHC class I-related receptor FcRn reveals the sites of IgG homeostasis in mice. Proc Natl Acad Sci. 2009;106:2788–2793. doi: 10.1073/pnas.0810796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Y, Balthasar JP. Evaluation of a catenary PBPK model for predicting the in vivo disposition of mAbs engineered for high-affinity binding to FcRn. AAPS J. 2012;14:850–9. doi: 10.1208/s12248-012-9395-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garg A, Balthasar JP. Physiologically-based pharmacokinetic (PBPK) model to predict IgG tissue kinetics in wild-type and FcRn-knockout mice. J Pharmacokinet Pharmacodyn. 2007;34:687–709. doi: 10.1007/s10928-007-9065-1. [DOI] [PubMed] [Google Scholar]
- 52.Ward ES. Evidence to support the cellular mechanism involved in serum IgG homeostasis in humans. Int Immunol. 2003;15:187–195. doi: 10.1093/intimm/dxg018. [DOI] [PubMed] [Google Scholar]
- 53.Cianga C, Cianga P, Plamadeala P, Amalinei C. Nonclassical major histocompatibility complex I-like Fc neonatal receptor (FcRn) expression in neonatal human tissues. Hum Immunol. 2011;72:1176–87. doi: 10.1016/j.humimm.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 54.Borvak J, et al. Functional expression of the MHC class I-related receptor, FcRn, in endothelial cells of mice. Int Immunol. 1998;10:1289–98. doi: 10.1093/intimm/10.9.1289. [DOI] [PubMed] [Google Scholar]
- 55.Ober RJ. Exocytosis of IgG as mediated by the receptor, FcRn: An analysis at the single-molecule level. Proc Natl Acad Sci. 2004;101:11076–11081. doi: 10.1073/pnas.0402970101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ward ES, et al. From Sorting Endosomes to Exocytosis: Association of Rab4 and Rab11 GTPases with the Fc Receptor, FcRn, during Recycling. Mol Biol Cell. 2005;16:2028–2038. doi: 10.1091/mbc.E04-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gan Z, Ram S, Vaccaro C, Ober RJ, Ward ES. Analyses of the Recycling Receptor, FcRn, in Live Cells Reveal Novel Pathways for Lysosomal Delivery. Traffic. 2009;10:600–614. doi: 10.1111/j.1600-0854.2009.00887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weflen AW, et al. Multivalent immune complexes divert FcRn to lysosomes by exclusion from recycling sorting tubules. Mol Biol Cell. 2013;24:2398–405. doi: 10.1091/mbc.E13-04-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ober RJ, Martinez C, Vaccaro C, Zhou J, Ward ES. Visualizing the Site and Dynamics of IgG Salvage by the MHC Class I-Related Receptor, FcRn. J Immunol. 2004;172:2021–2029. doi: 10.4049/jimmunol.172.4.2021. [DOI] [PubMed] [Google Scholar]
- 60.Wu AM. Engineered antibodies for molecular imaging of cancer. Methods. 2013 doi: 10.1016/j.ymeth.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swiercz R, et al. Use of Fc-Engineered Antibodies as Clearing Agents to Increase Contrast During PET. J Nucl Med. 2014;55:1204–1207. doi: 10.2967/jnumed.113.136481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Medesan C, Matesoi D, Radu C, Ghetie V, Ward ES. Delineation of the amino acid residues involved in transcytosis and catabolism of mouse IgG1. J Immunol. 1997;158:2211–7. [PubMed] [Google Scholar]
- 63.Ghetie V, et al. Increasing the serum persistence of an IgG fragment by random mutagenesis. Nat Biotechnol. 1997;15:637–40. doi: 10.1038/nbt0797-637. [DOI] [PubMed] [Google Scholar]
- 64.Dall’Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn) J Biol Chem. 2006;281:23514–23524. doi: 10.1074/jbc.M604292200. [DOI] [PubMed] [Google Scholar]
- 65.Robbie GJ, et al. A Novel Investigational Fc-Modified Humanized Monoclonal Antibody, Motavizumab-YTE, Has an Extended Half-Life in Healthy Adults. Antimicrob Agents Chemother. 2013;57:6147–53. doi: 10.1128/AAC.01285-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zalevsky J, et al. Enhanced antibody half-life improves in vivo activity. Nat Biotechnol. 2010;28:157–159. doi: 10.1038/nbt.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng Y, et al. Translational pharmacokinetics and pharmacodynamics of an FcRn-variant anti-CD4 monoclonal antibody from preclinical model to phase I study. Clin Pharmacol Ther. 2011;89:283–90. doi: 10.1038/clpt.2010.311. [DOI] [PubMed] [Google Scholar]
- 68.Kuo TT, Aveson VG. Neonatal Fc receptor and IgG-based therapeutics. MAbs. 2011;3:422–30. doi: 10.4161/mabs.3.5.16983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gurbaxani B, Dostalek M, Gardner I. Are endosomal trafficking parameters better targets for improving mAb pharmacokinetics than FcRn binding affinity? Mol Immunol. 2013;56:660–674. doi: 10.1016/j.molimm.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 70.Acqua WFD, et al. Increasing the affinity of a human IgG1 for the neonatal Fc receptor: biological consequences. J Immunol. 2002;169:5171–80. doi: 10.4049/jimmunol.169.9.5171. [DOI] [PubMed] [Google Scholar]
- 71.Hinton PR, et al. An engineered human IgG1 antibody with longer serum half-life. J Immunol. 2006;176:346–56. doi: 10.4049/jimmunol.176.1.346. [DOI] [PubMed] [Google Scholar]
- 72.Petkova SB, et al. Enhanced half-life of genetically engineered human IgG1 antibodies in a humanized FcRn mouse model: potential application in humorally mediated autoimmune disease. Int Immunol. 2006;18:1759–1769. doi: 10.1093/intimm/dxl110. [DOI] [PubMed] [Google Scholar]
- 73.Yeung YA, et al. Engineering human IgG1 affinity to human neonatal Fc receptor: impact of affinity improvement on pharmacokinetics in primates. J Immunol. 2009;182:7663–7671. doi: 10.4049/jimmunol.0804182. [DOI] [PubMed] [Google Scholar]
- 74.Hinton PR, et al. Engineered human IgG antibodies with longer serum half-lives in primates. J Biol Chem. 2004;279:6213–6. doi: 10.1074/jbc.C300470200. [DOI] [PubMed] [Google Scholar]
- 75.Vaccaro C, Bawdon R, Wanjie S, Ober RJ, Ward ES. Divergent activities of an engineered antibody in murine and human systems have implications for therapeutic antibodies. Proc Natl Acad Sci. 2006;103:18709–18714. doi: 10.1073/pnas.0606304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Finch DK, et al. Whole-Molecule Antibody Engineering: Generation of a High-Affinity Anti-IL-6 Antibody with Extended Pharmacokinetics. J Mol Biol. 2011;411:791–807. doi: 10.1016/j.jmb.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 77.Bernett MJ, et al. Immune suppression in cynomolgus monkeys by XPro9523: An improved CTLA4-Ig fusion with enhanced binding to CD80, CD86 and neonatal Fc receptor FcRn. MAbs. 2013;5:384–96. doi: 10.4161/mabs.23976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Datta-Mannan A, et al. FcRn affinity-pharmacokinetic relationship of five human IgG4 antibodies engineered for improved in vitro FcRn binding properties in cynomolgus monkeys. Drug Metab Dispos. 2012;40:1545–55. doi: 10.1124/dmd.112.045864. [DOI] [PubMed] [Google Scholar]
- 79.Yeung YA, et al. A therapeutic anti-VEGF antibody with increased potency independent of pharmacokinetic half-life. Cancer Res. 2010;70:3269–77. doi: 10.1158/0008-5472.CAN-09-4580. [DOI] [PubMed] [Google Scholar]
- 80.Gurbaxani B, Dela Cruz LL, Chintalacharuvu K, Morrison SL. Analysis of a family of antibodies with different half-lives in mice fails to find a correlation between affinity for FcRn and serum half-life. Mol Immunol. 2006;43:1462–1473. doi: 10.1016/j.molimm.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 81.Datta-Mannan A, et al. Humanized IgG1 variants with differential binding properties to the neonatal Fc receptor: relationship to pharmacokinetics in mice and primates. Drug Metab Dispos. 2007;35:86–94. doi: 10.1124/dmd.106.011734. [DOI] [PubMed] [Google Scholar]
- 82.Elkon K, Casali P. Nature and functions of autoantibodies. Nat Clin Pract Rheumatol. 2008;4:491–8. doi: 10.1038/ncprheum0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li N, et al. Complete FcRn dependence for intravenous Ig therapy in autoimmune skin blistering diseases. J Clin Invest. 2005;115:3440–50. doi: 10.1172/JCI24394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Akilesh S, et al. The MHC class I-like Fc receptor promotes humorally mediated autoimmune disease. J Clin Invest. 2004;113:1328–33. doi: 10.1172/JCI18838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu Z, Lennon VA. Mechanism of intravenous immune globulin therapy in antibody-mediated autoimmune diseases. N Engl J Med. 1999;340:227–8. doi: 10.1056/NEJM199901213400311. [DOI] [PubMed] [Google Scholar]
- 86.Raghavan M, Chen MY, Gastinel LN, Bjorkman PJ. Investigation of the interaction between the class I MHC-related Fc receptor and its immunoglobulin G ligand. Immunity. 1994;1:303–315. doi: 10.1016/1074-7613(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 87.Liu L, et al. Amelioration of experimental autoimmune myasthenia gravis in rats by neonatal FcR blockade. J Immunol. 2007;178:5390–8. doi: 10.4049/jimmunol.178.8.5390. [DOI] [PubMed] [Google Scholar]
- 88.Getman KE, Balthasar JP. Pharmacokinetic effects of 4C9, an anti-FcRn antibody, in rats: implications for the use of FcRn inhibitors for the treatment of humoral autoimmune and alloimmune conditions. Journal of pharmaceutical sciences. 2005;94:718–29. doi: 10.1002/jps.20297. [DOI] [PubMed] [Google Scholar]
- 89.Vaccaro C, Zhou J, Ober RJ, Ward ES. Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels. Nat Biotechnol. 2005;23:1283–1288. doi: 10.1038/nbt1143. [DOI] [PubMed] [Google Scholar]
- 90.Patel Da, et al. Neonatal Fc receptor blockade by Fc engineering ameliorates arthritis in a murine model. J Immunol. 2011;187:1015–22. doi: 10.4049/jimmunol.1003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mezo AR, et al. Reduction of IgG in nonhuman primates by a peptide antagonist of the neonatal Fc receptor FcRn. Proc Natl Acad Sci U S A. 2008;105:2337–42. doi: 10.1073/pnas.0708960105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mezo AR, Sridhar V, Badger J, Sakorafas P, Nienaber V. X-ray Crystal Structures of Monomeric and Dimeric Peptide Inhibitors in Complex with the Human Neonatal Fc Receptor, FcRn. J Biol Chem. 2010;285:27694–27701. doi: 10.1074/jbc.M110.120667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Z, Fraley C, Mezo AR. Discovery and structure-activity relationships of small molecules that block the human immunoglobulin G-human neonatal Fc receptor (hIgG-hFcRn) protein-protein interaction. Bioorg Med Chem Lett. 2013;23:1253–6. doi: 10.1016/j.bmcl.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 94.Strauch EM, Fleishman SJ, Baker D. Computational design of a pH-sensitive IgG binding protein. Proc Natl Acad Sci U S A. 2014;111:675–80. doi: 10.1073/pnas.1313605111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.James LC, Keeble AH, Khan Z, Rhodes DA, Trowsdale J. Structural basis for PRYSPRY-mediated tripartite motif (TRIM) protein function. Proc Natl Acad Sci U S A. 2007;104:6200–5. doi: 10.1073/pnas.0609174104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Keeble AH, Khan Z, Forster A, James LC. TRIM21 is an IgG receptor that is structurally, thermodynamically, and kinetically conserved. Proc Natl Acad Sci U S A. 2008;105:6045–50. doi: 10.1073/pnas.0800159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sockolosky JT, Kivimäe S, Szoka FC. Fusion of a Short Peptide that Binds Immunoglobulin G to a Recombinant Protein Substantially Increases Its Plasma Half-Life in Mice. PLoS One. 2014;9:e102566. doi: 10.1371/journal.pone.0102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Capon DJ, et al. Designing CD4 immunoadhesins for AIDS therapy. Nature. 1989;337:525–31. doi: 10.1038/337525a0. [DOI] [PubMed] [Google Scholar]
- 99.Suzuki T, et al. Importance of neonatal FcR in regulating the serum half-life of therapeutic proteins containing the Fc domain of human IgG1: a comparative study of the affinity of monoclonal antibodies and Fc-fusion proteins to human neonatal FcR. J Immunol. 2010;184:1968–1976. doi: 10.4049/jimmunol.0903296. [DOI] [PubMed] [Google Scholar]
- 100.Fast JL, Cordes AA, Carpenter JF, Randolph TW. Physical Instability of a Therapeutic Fc Fusion Protein: Domain Contributions to Conformational and Colloidal Stability. Biochemistry. 2009;48:11724–11736. doi: 10.1021/bi900853v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bitonti AJ, et al. Pulmonary delivery of an erythropoietin Fc fusion protein in non-human primates through an immunoglobulin transport pathway. Proc Natl Acad Sci. 2004;101:9763–9768. doi: 10.1073/pnas.0403235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Czajkowsky DM, Hu J, Shao Z, Pleass RJ. Fc-fusion proteins: new developments and future perspectives. EMBO Mol Med. 2012;4:1015–28. doi: 10.1002/emmm.201201379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dumont JA, Low SC, Peters RT, Bitonti AJ. Monomeric Fc fusions: impact on pharmacokinetic and biological activity of protein therapeutics. BioDrugs. 2006;20:151–160. doi: 10.2165/00063030-200620030-00002. [DOI] [PubMed] [Google Scholar]
- 104.Powell JS, et al. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med. 2013;369:2313–23. doi: 10.1056/NEJMoa1305074. [DOI] [PubMed] [Google Scholar]
- 105.Jensen PF, et al. Investigating the interaction between the neonatal Fc receptor and monoclonal antibody variants by hydrogen/deuterium exchange mass spectrometry. Mol Cell Proteomics. 2014 doi: 10.1074/mcp.M114.042044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang W, et al. Monoclonal Antibodies with Identical Fc Sequences Can Bind to FcRn Differentially with Pharmacokinetic Consequences. Drug Metab Dispos. 2011;39:1469–77. doi: 10.1124/dmd.111.039453. [DOI] [PubMed] [Google Scholar]
- 107.Schlothauer T, et al. Analytical FcRn affinity chromatography for functional characterization of monoclonal antibodies. MAbs. 2013;5:576–86. doi: 10.4161/mabs.24981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Israel EJ, et al. Expression of the neonatal Fc receptor, FcRn, on human intestinal epithelial cells. Immunology. 1997;92:69–74. doi: 10.1046/j.1365-2567.1997.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kliwinski C, et al. Contribution of FcRn binding to intestinal uptake of IgG in suckling rat pups and human FcRn-transgenic mice. Am J Physiol Gastrointest Liver Physiol. 2013;304:G262–70. doi: 10.1152/ajpgi.00340.2012. [DOI] [PubMed] [Google Scholar]
- 110.Dickinson BL, et al. Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line. J Clin Invest. 1999;104:903–911. doi: 10.1172/JCI6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yoshida M, et al. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20:769–783. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 112.Low SC, Nunes SL, Bitonti AJ, Dumont JA. Oral and pulmonary delivery of FSH-Fc fusion proteins via neonatal Fc receptor-mediated transcytosis. Hum Reprod. 2005;20:1805–1813. doi: 10.1093/humrep/deh896. [DOI] [PubMed] [Google Scholar]
- 113.Israel E. Neonatal necrotizing enterocolitis, a disease of the immature intestinal mucosal barrier. Acta Paediatr. 1994;83:27–32. doi: 10.1111/j.1651-2227.1994.tb13238.x. [DOI] [PubMed] [Google Scholar]
- 114.Yoshida M, et al. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J Clin Invest. 2006;116:2142–2151. doi: 10.1172/JCI27821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hornby PJ, et al. Human and Non-Human Primate Intestinal FcRn Expression and Immunoglobulin G Transcytosis. Pharm Res. 2013 doi: 10.1007/s11095-013-1212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Patton JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6:67–74. doi: 10.1038/nrd2153. [DOI] [PubMed] [Google Scholar]
- 117.Spiekermann GM, et al. Receptor-mediated Immunoglobulin G Transport Across Mucosal Barriers in Adult Life: Functional Expression of FcRn in the Mammalian Lung. J Exp Med. 2002;196:303–310. doi: 10.1084/jem.20020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dumont JA, et al. Delivery of an erythropoietin-Fc fusion protein by inhalation in humans through an immunoglobulin transport pathway. J Aerosol Med. 2005;18:294–303. doi: 10.1089/jam.2005.18.294. [DOI] [PubMed] [Google Scholar]
- 119.Krzyzanski W, Jusko WJ, Wacholtz MC, Minton N, Cheung WK. Pharmacokinetic and pharmacodynamic modeling of recombinant human erythropoietin after multiple subcutaneous doses in healthy subjects. Eur J Pharm Sci. 2005;26:295–306. doi: 10.1016/j.ejps.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 120.Vllasaliu D, Alexander C, Garnett M, Eaton M, Stolnik S. Fc-mediated transport of nanoparticles across airway epithelial cell layers. J Control Release. 2011;158:479–86. doi: 10.1016/j.jconrel.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 121.Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548–58. doi: 10.1038/clpt.2008.170. [DOI] [PubMed] [Google Scholar]
- 122.Leyland-Jones B, et al. Pharmacokinetics, safety, and efficacy of trastuzumab administered every three weeks in combination with paclitaxel. J Clin Oncol. 2003;21:3965–71. doi: 10.1200/JCO.2003.12.109. [DOI] [PubMed] [Google Scholar]
- 123.Pridgen EM, et al. Transepithelial Transport of Fc-Targeted Nanoparticles by the Neonatal Fc Receptor for Oral Delivery. Sci Transl Med. 2013;5:213ra167–213ra167. doi: 10.1126/scitranslmed.3007049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu X, et al. The neonatal FcR-mediated presentation of immune-complexed antigen is associated with endosomal and phagosomal pH and antigen stability in macrophages and dendritic cells. J Immunol. 2011;186:4674–86. doi: 10.4049/jimmunol.1003584. [DOI] [PubMed] [Google Scholar]
- 125.Baker K, et al. Neonatal Fc receptor for IgG (FcRn) regulates cross-presentation of IgG immune complexes by CD8-CD11b+ dendritic cells. Proc Natl Acad Sci U S A. 2011;108:9927–32. doi: 10.1073/pnas.1019037108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Baker K, et al. Neonatal Fc Receptor Expression in Dendritic Cells Mediates Protective Immunity against Colorectal Cancer. Immunity. 2013;39:1095–1107. doi: 10.1016/j.immuni.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vidarsson G, et al. FcRn: an IgG receptor on phagocytes with a novel role in phagocytosis. Blood. 2006;108:3573–9. doi: 10.1182/blood-2006-05-024539. [DOI] [PubMed] [Google Scholar]
- 128.Mi W, et al. Targeting the neonatal fc receptor for antigen delivery using engineered fc fragments. J Immunol. 2008;181:7550–61. doi: 10.4049/jimmunol.181.11.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ye L, Zeng R, Bai Y, Roopenian DC, Zhu X. Efficient mucosal vaccination mediated by the neonatal Fc receptor. Nat Biotechnol. 2011;29:158–63. doi: 10.1038/nbt.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood. 2012;119:5640–9. doi: 10.1182/blood-2012-01-380121. [DOI] [PubMed] [Google Scholar]
- 131.Lu L, et al. An FcRn-targeted mucosal vaccine strategy effectively induces HIV-1 antigen-specific immunity to genital infection. J Virol. 2011;85:10542–53. doi: 10.1128/JVI.05441-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li Z, et al. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4388–93. doi: 10.1073/pnas.1012861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ko SY, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014;514:642–645. doi: 10.1038/nature13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bai Y, et al. Intracellular neutralization of viral infection in polarized epithelial cells by neonatal Fc receptor (FcRn)-mediated IgG transport. Proc Natl Acad Sci U S A. 2011;108:18406–11. doi: 10.1073/pnas.1115348108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gupta S, et al. The Neonatal Fc Receptor (FcRn) Enhances Human Immunodeficiency Virus Type 1 (HIV-1) Transcytosis across Epithelial Cells. PLoS Pathog. 2013;9:e1003776. doi: 10.1371/journal.ppat.1003776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sleep D, Cameron J, Evans LR. Albumin as a versatile platform for drug half-life extension. Biochim Biophys Acta. 2013;1830:5526–34. doi: 10.1016/j.bbagen.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 137.Elsadek B, Kratz F. Impact of albumin on drug delivery--new applications on the horizon. J Control Release. 2012;157:4–28. doi: 10.1016/j.jconrel.2011.09.069. [DOI] [PubMed] [Google Scholar]
- 138.Rosenstock J, Reusch J, Bush M, Yang F, Stewart M. Potential of albiglutide, a long-acting GLP-1 receptor agonist, in type 2 diabetes: a randomized controlled trial exploring weekly, biweekly, and monthly dosing. Diabetes Care. 2009;32:1880–6. doi: 10.2337/dc09-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Andersen JT, et al. Extending serum half-life of albumin by engineering neonatal Fc receptor (FcRn) binding. J Biol Chem. 2014;289:13492–502. doi: 10.1074/jbc.M114.549832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dennis MS, et al. Albumin binding as a general strategy for improving the pharmacokinetics of proteins. J Biol Chem. 2002;277:35035–35043. doi: 10.1074/jbc.M205854200. [DOI] [PubMed] [Google Scholar]
- 141.Andersen JT, et al. Extending half-life by indirect targeting of the neonatal Fc receptor (FcRn) using a minimal albumin binding domain (ABD) J Biol Chem. 2010;286:5234–41. doi: 10.1074/jbc.M110.164848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tijink BM, et al. Improved tumor targeting of anti-epidermal growth factor receptor Nanobodies through albumin binding: taking advantage of modular Nanobody technology. Mol Cancer Ther. 2008;7:2288–97. doi: 10.1158/1535-7163.MCT-07-2384. [DOI] [PubMed] [Google Scholar]
- 143.Pluen A, et al. Role of tumor-host interactions in interstitial diffusion of macromolecules: cranial vs. subcutaneous tumors. Proc Natl Acad Sci U S A. 2001;98:4628–33. doi: 10.1073/pnas.081626898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sofou S, Yu YB, Thurber GM, Schmidt MM, Wittrup KD. Antibody tumor penetration: Transport opposed by systemic and antigen-mediated clearance. Adv Drug Deliv Rev. 2008;60:1421–1434. doi: 10.1016/j.addr.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kenanova V, et al. Radioiodinated versus radiometal-labeled anti-carcinoembryonic antigen single-chain Fv-Fc antibody fragments: optimal pharmacokinetics for therapy. Cancer Res. 2007;67:718–26. doi: 10.1158/0008-5472.CAN-06-0454. [DOI] [PubMed] [Google Scholar]
- 146.Ying T, Chen W, Gong R, Feng Y, Dimitrov DS. Soluble Monomeric IgG1 Fc. J Biol Chem. 2012;287:19399–408. doi: 10.1074/jbc.M112.368647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gong R, Wang Y, Feng Y, Zhao Q, Dimitrov DS. Shortened engineered human antibody CH2 domains: increased stability and binding to the human neonatal receptor. J Biol Chem. 2011;286:27288–93. doi: 10.1074/jbc.M111.254219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ying T, et al. Engineered soluble monomeric IgG1 CH3 domain: generation, mechanisms of function, and implications for design of biological therapeutics. J Biol Chem. 2013;288:25154–64. doi: 10.1074/jbc.M113.484154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gehlsen K, et al. Pharmacokinetics of engineered human monomeric and dimeric CH2 domains. MAbs. 2012;4:466–74. doi: 10.4161/mabs.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ishino T, et al. Engineering a monomeric Fc domain modality by N-glycosylation for the half-life extension of biotherapeutics. J Biol Chem. 2013;288:16529–37. doi: 10.1074/jbc.M113.457689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sockolosky JT, Tiffany MR, Szoka FC. Engineering neonatal Fc receptor-mediated recycling and transcytosis in recombinant proteins by short terminal peptide extensions. Proc Natl Acad Sci U S A. 2012;109:16095–16100. doi: 10.1073/pnas.1208857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Seijsing J, et al. An engineered affibody molecule with pH-dependent binding to FcRn mediates extended circulatory half-life of a fusion protein. Proc Natl Acad Sci U S A. 2014;111:17110–5. doi: 10.1073/pnas.1417717111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Andersen JT, et al. Selection of nanobodies that target human neonatal Fc receptor. Sci Rep. 2013;3:1118. doi: 10.1038/srep01118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Moran GW, Leslie FC, Levison SE, Worthington J, McLaughlin JT. Enteroendocrine cells: neglected players in gastrointestinal disorders? Therap Adv Gastroenterol. 2008;1:51–60. doi: 10.1177/1756283X08093943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Janssen S, Depoortere I. Nutrient sensing in the gut: new roads to therapeutics? Trends Endocrinol Metab. 2013;24:92–100. doi: 10.1016/j.tem.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 156.Powner MB, McKenzie JAG, Christianson GJ, Roopenian DC, Fruttiger M. Expression of neonatal Fc receptor in the eye. Invest Ophthalmol Vis Sci. 2014;55:1607–15. doi: 10.1167/iovs.13-12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Proetzel G, Roopenian DC. Humanized FcRn mouse models for evaluating pharmacokinetics of human IgG antibodies. Methods. 2014;65:148–53. doi: 10.1016/j.ymeth.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Acqua WFD, et al. Increasing the Affinity of a Human IgG1 for the Neonatal Fc Receptor: Biological Consequences. J Immunol. 2002;169:5171–5180. doi: 10.4049/jimmunol.169.9.5171. [DOI] [PubMed] [Google Scholar]
- 159.Popov S, et al. The stoichiometry and affinity of the interaction of murine Fc fragments with the MHC class I-related receptor, FcRn. Mol Immunol. 1996;33:521–530. doi: 10.1016/0161-5890(96)00004-1. [DOI] [PubMed] [Google Scholar]
- 160.Merchant AM, et al. An efficient route to human bispecific IgG. Nat Biotechnol. 1998;16:677–81. doi: 10.1038/nbt0798-677. [DOI] [PubMed] [Google Scholar]
- 161.Labrijn AF, et al. Controlled Fab-arm exchange for the generation of stable bispecific IgG1. Nat Protoc. 2014;9:2450–63. doi: 10.1038/nprot.2014.169. [DOI] [PubMed] [Google Scholar]
- 162.Lewis SM, et al. Generation of bispecific IgG antibodies by structure-based design of an orthogonal Fab interface. Nat Biotechnol. 2014;32:191–8. doi: 10.1038/nbt.2797. [DOI] [PubMed] [Google Scholar]
- 163.Yu YJ, et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci Transl Med. 2011;3:84ra44. doi: 10.1126/scitranslmed.3002230. [DOI] [PubMed] [Google Scholar]
- 164.Andersen JT, et al. Extending Serum Half-life of Albumin by Engineering FcRn Binding. J Biol Chem. 2014;M114:549832. doi: 10.1074/jbc.M114.549832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kim J, et al. Beta 2-microglobulin deficient mice catabolize IgG more rapidly than FcRn- alpha-chain deficient mice. Exp Biol Med (Maywood) 2008;233:603–9. doi: 10.3181/0710-RM-270. [DOI] [PubMed] [Google Scholar]
- 166.Claypool SM, Dickinson BL, Yoshida M, Lencer WI, Blumberg RS. Functional reconstitution of human FcRn in Madin-Darby canine kidney cells requires co-expressed human beta 2-microglobulin. The Journal of biological chemistry. 2002;277:28038–50. doi: 10.1074/jbc.M202367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zuckier LS, Chang CJ, Scharff MD, Morrison SL. Chimeric human-mouse IgG antibodies with shuffled constant region exons demonstrate that multiple domains contribute to in vivo half-life. Cancer Res. 1998;58:3905–8. [PubMed] [Google Scholar]
- 168.Montaño RF, Morrison SL. Influence of the isotype of the light chain on the properties of IgG. J Immunol. 2002;168:224–31. doi: 10.4049/jimmunol.168.1.224. [DOI] [PubMed] [Google Scholar]
- 169.Gitlin D, Koch C. On the mechanisms of maternofetal transfer of human albumin and gamma-G globulin in the mouse. J Clin Invest. 1968;47:1204–9. doi: 10.1172/JCI105809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Salter-Cid L, et al. Transferrin receptor is negatively modulated by the hemochromatosis protein HFE: Implications for cellular iron homeostasis. Proc Natl Acad Sci. 1999;96:5434–5439. doi: 10.1073/pnas.96.10.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Santos M. Defective iron homeostasis in beta 2-microglobulin knockout mice recapitulates hereditary hemochromatosis in man. J Exp Med. 1996;184:1975–1985. doi: 10.1084/jem.184.5.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ye L, et al. The MHC class II-associated invariant chain interacts with the neonatal Fc gamma receptor and modulates its trafficking to endosomal/lysosomal compartments. J Immunol. 2008;181:2572–85. doi: 10.4049/jimmunol.181.4.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ramalingam TS, Detmer SA, Martin WL, Bjorkman PJ. IgG transcytosis and recycling by FcRn expressed in MDCK cells reveals ligand-induced redistribution. EMBO J. 2002;21:590–601. doi: 10.1093/emboj/21.4.590. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 174.Tzaban S, et al. The recycling and transcytotic pathways for IgG transport by FcRn are distinct and display an inherent polarity. J Cell Biol. 2009;185:673–684. doi: 10.1083/jcb.200809122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jerdeva GV, et al. Comparison of FcRn- and pIgR-mediated transport in MDCK cells by fluorescence confocal microscopy. Traffic. 2010;11:1205–20. doi: 10.1111/j.1600-0854.2010.01083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Claypool SM, et al. Bidirectional transepithelial IgG transport by a strongly polarized basolateral membrane Fcgamma-receptor. Mol Biol Cell. 2004;15:1746–1759. doi: 10.1091/mbc.E03-11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gafencu A, Heltianu C, Burlacu A, Hunziker W, Simionescu Mf. Investigation of IgG Receptors Expressed on the Surface of Human Placental Endothelial Cells. Placenta. 2003;24:664–676. doi: 10.1016/s0143-4004(03)00041-9. [DOI] [PubMed] [Google Scholar]
- 178.Pettersen EF, et al. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–12. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 179.Jonsson A, Dogan J, Herne N, Abrahmsén L, Nygren PA. Engineering of a femtomolar affinity binding protein to human serum albumin. Protein Eng Des Sel. 2008;21:515–27. doi: 10.1093/protein/gzn028. [DOI] [PubMed] [Google Scholar]