Abstract
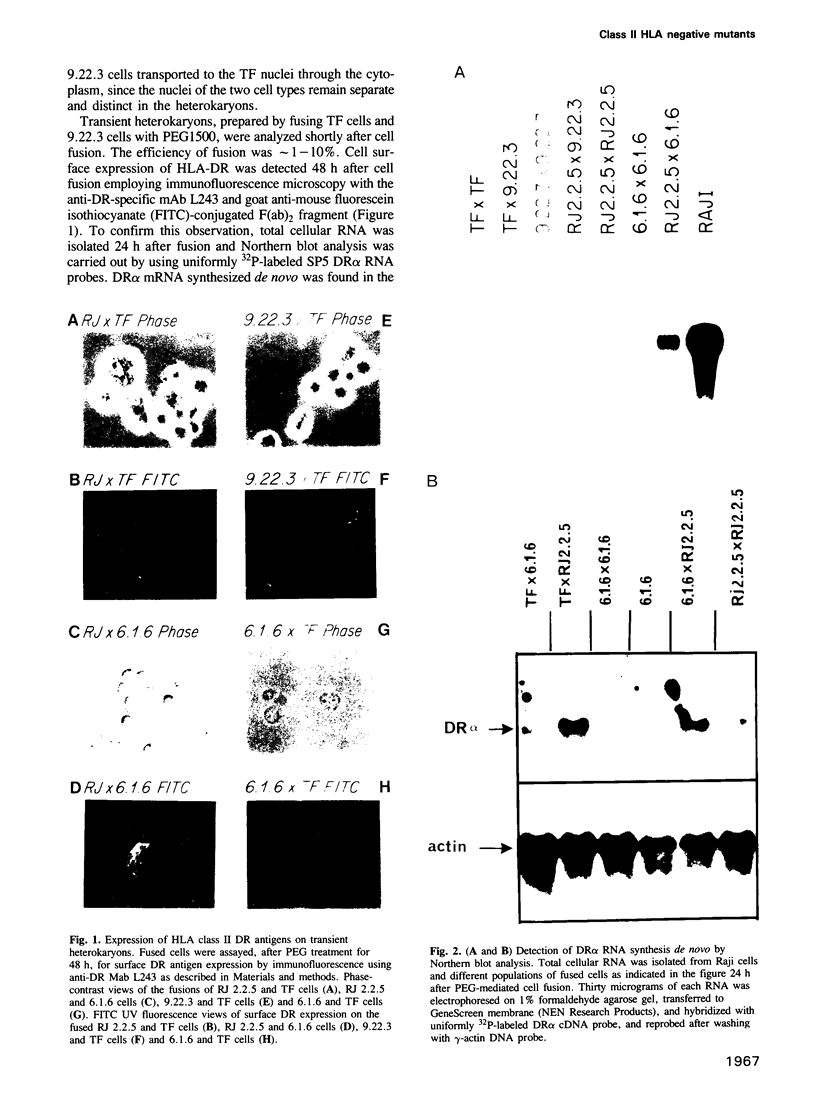
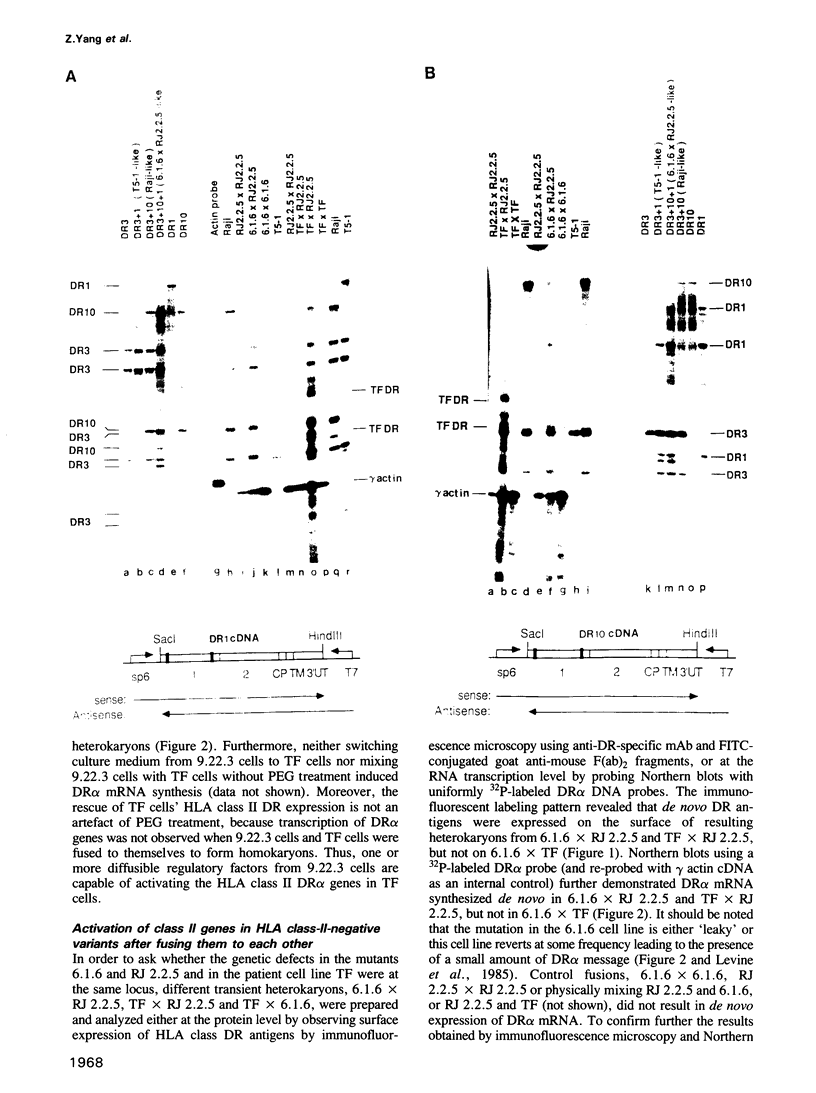
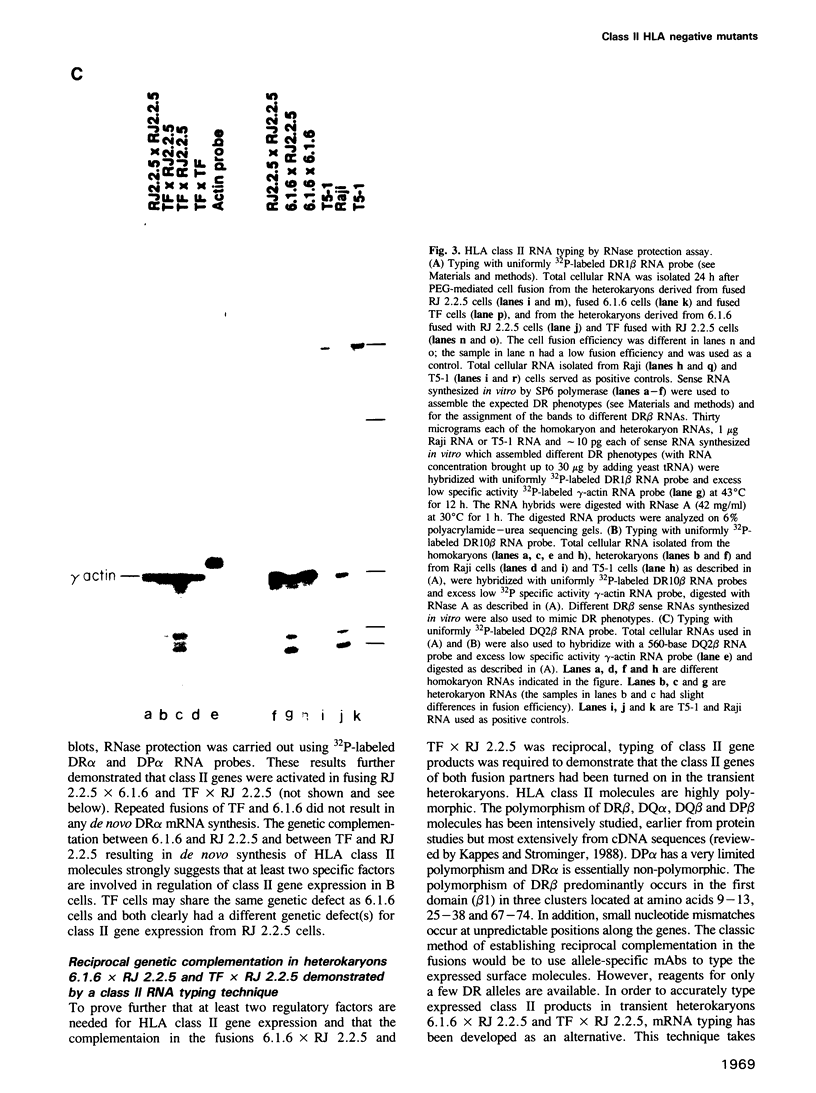
Heterokaryons were prepared and analyzed shortly after cell fusion using two mutant class-II-negative human B cell lines (RJ 2.2.5 and 6.1.6) and a cell line (TF) from a patient with a class-II-negative Bare Lymphocyte Syndrome. The resulting transient heterokaryons were analyzed by using an anti-HLA-DR monoclonal antibody to assess the cell surface expression of HLA-DR (the major subtype of class II antigens) by immunofluorescence microscopy and by using uniformly 32P-labeled SP6 RNA probes in Northern blots and RNase protection assays to assess mRNA synthesis. We find that class II gene expression in a B cell line from a Bare Lymphocyte Syndrome patient (TF) is rescued by a B cell line which expresses class II antigens indicating that this disease, at least in part, is caused by a defect(s) in a genetic locus encoding a factor(s) necessary for class II gene expression. Secondly, reciprocal genetic complementation was demonstrated in the heterokaryons 6.1.6 x RJ 2.2.5 and TF x RJ 2.2.5 (but not in TF x 6.1.6) by detection of cell surface DR by immunofluorescence microscopy and by a novel class II mRNA typing technique which allows characterization of distinct class II alleles. Thus, the two mutants generated in vitro have defects at two different genetic loci encoding specific regulatory factors necessary for human class II gene expression. One of these mutant cell lines, but not the other, complements the defect in the patient cell line, TF.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accolla R. S., Carra G., Guardiola J. Reactivation by a trans-acting factor of human major histocompatibility complex Ia gene expression in interspecies hybrids between an Ia-negative human B-cell variant and an Ia-positive mouse B-cell lymphoma. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5145–5149. doi: 10.1073/pnas.82.15.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accolla R. S. Human B cell variants immunoselected against a single Ia antigen subset have lost expression of several Ia antigen subsets. J Exp Med. 1983 Mar 1;157(3):1053–1058. doi: 10.1084/jem.157.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron M. H., Maniatis T. Rapid reprogramming of globin gene expression in transient heterokaryons. Cell. 1986 Aug 15;46(4):591–602. doi: 10.1016/0092-8674(86)90885-8. [DOI] [PubMed] [Google Scholar]
- Benacerraf B. Role of MHC gene products in immune regulation. Science. 1981 Jun 12;212(4500):1229–1238. doi: 10.1126/science.6165083. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Chiu C. P., Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell. 1983 Apr;32(4):1171–1180. doi: 10.1016/0092-8674(83)90300-8. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Pavlath G. K., Hardeman E. C., Chiu C. P., Silberstein L., Webster S. G., Miller S. C., Webster C. Plasticity of the differentiated state. Science. 1985 Nov 15;230(4727):758–766. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- Boss J. M., Strominger J. L. Cloning and sequence analysis of the human major histocompatibility complex gene DC-3 beta. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5199–5203. doi: 10.1073/pnas.81.16.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss J. M., Strominger J. L. Regulation of a transfected human class II major histocompatibility complex gene in human fibroblasts. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9139–9143. doi: 10.1073/pnas.83.23.9139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calman A. F., Peterlin B. M. Mutant human B cell lines deficient in class II major histocompatibility complex transcription. J Immunol. 1987 Oct 1;139(7):2489–2495. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dausset J. The major histocompatibility complex in man. Science. 1981 Sep 25;213(4515):1469–1474. doi: 10.1126/science.6792704. [DOI] [PubMed] [Google Scholar]
- Dorn A., Bollekens J., Staub A., Benoist C., Mathis D. A multiplicity of CCAAT box-binding proteins. Cell. 1987 Sep 11;50(6):863–872. doi: 10.1016/0092-8674(87)90513-7. [DOI] [PubMed] [Google Scholar]
- Dorn A., Durand B., Marfing C., Le Meur M., Benoist C., Mathis D. Conserved major histocompatibility complex class II boxes--X and Y--are transcriptional control elements and specifically bind nuclear proteins. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6249–6253. doi: 10.1073/pnas.84.17.6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch T., Zinn K., Maniatis T. Activation of the human beta-interferon gene requires an interferon-inducible factor. Mol Cell Biol. 1986 Mar;6(3):801–810. doi: 10.1128/mcb.6.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gillies S. D., Folsom V., Tonegawa S. Cell type-specific enhancer element associated with a mouse MHC gene, E beta. Nature. 1984 Aug 16;310(5978):594–597. doi: 10.1038/310594a0. [DOI] [PubMed] [Google Scholar]
- Gladstone P., Pious D. Identification of a trans-acting function regulation HLA-DR expression in a DR-negative B cell variant. Somatic Cell Genet. 1980 Mar;6(2):285–298. doi: 10.1007/BF01538802. [DOI] [PubMed] [Google Scholar]
- Gladstone, Pious D. Stable variants affecting B cell alloantigens in human lymphoid cells. Nature. 1978 Feb 2;271(5644):459–461. doi: 10.1038/271459a0. [DOI] [PubMed] [Google Scholar]
- Gustafsson K., Wiman K., Emmoth E., Larhammar D., Böhme J., Hyldig-Nielsen J. J., Ronne H., Peterson P. A., Rask L. Mutations and selection in the generation of class II histocompatibility antigen polymorphism. EMBO J. 1984 Jul;3(7):1655–1661. doi: 10.1002/j.1460-2075.1984.tb02026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Pujol-Borrell R., Chiovato L., Russell R. C., Doniach D., Bottazzo G. F. Aberrant expression of HLA-DR antigen on thyrocytes in Graves' disease: relevance for autoimmunity. Lancet. 1983 Nov 12;2(8359):1111–1115. doi: 10.1016/s0140-6736(83)90628-1. [DOI] [PubMed] [Google Scholar]
- Kaufman J. F., Auffray C., Korman A. J., Shackelford D. A., Strominger J. The class II molecules of the human and murine major histocompatibility complex. Cell. 1984 Jan;36(1):1–13. doi: 10.1016/0092-8674(84)90068-0. [DOI] [PubMed] [Google Scholar]
- Korman A. J., Boss J. M., Spies T., Sorrentino R., Okada K., Strominger J. L. Genetic complexity and expression of human class II histocompatibility antigens. Immunol Rev. 1985 Jul;85:45–86. doi: 10.1111/j.1600-065x.1985.tb01130.x. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. In vitro RNA synthesis with SP6 RNA polymerase. Methods Enzymol. 1987;155:397–415. doi: 10.1016/0076-6879(87)55027-3. [DOI] [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- Levine F., Erlich H. A., Mach B., Pious D. Transcriptional regulation of HLA class II and invariant chain genes. J Immunol. 1985 Jan;134(1):637–640. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa K., Doyle C., Strominger J. L. Sequence-specific interactions of nuclear factors with conserved sequences of human class II major histocompatibility complex genes. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4939–4943. doi: 10.1073/pnas.84.14.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa K., Strominger J. L. The HLA-DQ beta gene upstream region contains an immunoglobulin-like octamer motif that binds cell-type specific nuclear factors. Nucleic Acids Res. 1987 Oct 12;15(19):8057–8067. doi: 10.1093/nar/15.19.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pious D., Dixon L., Levine F., Cotner T., Johnson R. HLA class II regulation and structure. Analysis with HLA-DR3 and HLA-DP point mutants. J Exp Med. 1985 Oct 1;162(4):1193–1207. doi: 10.1084/jem.162.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Collins T., Gimbrone M. A., Jr, Cotran R. S., Gitlin J. D., Fiers W., Clayberger C., Krensky A. M., Burakoff S. J., Reiss C. S. Lymphocytes recognize human vascular endothelial and dermal fibroblast Ia antigens induced by recombinant immune interferon. Nature. 1983 Oct 20;305(5936):726–729. doi: 10.1038/305726a0. [DOI] [PubMed] [Google Scholar]
- Rijkers G. T., Roord J. J., Koning F., Kuis W., Zegers B. J. Phenotypical and functional analysis of B lymphocytes of two siblings with combined immunodeficiency and defective expression of major histocompatibility complex (MHC) class II antigens on mononuclear cells. J Clin Immunol. 1987 Mar;7(2):98–106. doi: 10.1007/BF00916003. [DOI] [PubMed] [Google Scholar]
- Salter R. D., Alexander J., Levine F., Pious D., Cresswell P. Evidence for two trans-acting genes regulating HLA class II antigen expression. J Immunol. 1985 Dec;135(6):4235–4238. [PubMed] [Google Scholar]
- Sherman P. A., Basta P. V., Ting J. P. Upstream DNA sequences required for tissue-specific expression of the HLA-DR alpha gene. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4254–4258. doi: 10.1073/pnas.84.12.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell G. D. Studies in histocompatibility. Science. 1981 Jul 10;213(4504):172–178. doi: 10.1126/science.7017931. [DOI] [PubMed] [Google Scholar]
- Tonnelle C., DeMars R., Long E. O. DO beta: a new beta chain gene in HLA-D with a distinct regulation of expression. EMBO J. 1985 Nov;4(11):2839–2847. doi: 10.1002/j.1460-2075.1985.tb04012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake C. T., Long E. O., Strubin M., Gross N., Accolla R., Carrel S., Mach B. Isolation of cDNA clones encoding HLA-DR alpha chains. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6979–6983. doi: 10.1073/pnas.79.22.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A. J., DeMars R., Trowbridge I. S., Bach F. H. Detection of a novel human class II HLA antigen. 1983 Jul 28-Aug 3Nature. 304(5924):358–361. doi: 10.1038/304358a0. [DOI] [PubMed] [Google Scholar]
- Widera G., Burkly L. C., Pinkert C. A., Böttger E. C., Cowing C., Palmiter R. D., Brinster R. L., Flavell R. A. Transgenic mice selectively lacking MHC class II (I-E) antigen expression on B cells: an in vivo approach to investigate Ia gene function. Cell. 1987 Oct 23;51(2):175–187. doi: 10.1016/0092-8674(87)90145-0. [DOI] [PubMed] [Google Scholar]
- Yang Z., Korman A. J., Cooper J., Pious D., Accolla R. S., Mulligan R. C., Strominger J. L. Expression of HLA-DR antigen in human class II mutant B-cell lines by double infection with retrovirus vectors. Mol Cell Biol. 1987 Nov;7(11):3923–3928. doi: 10.1128/mcb.7.11.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]
- de Préval C., Lisowska-Grospierre B., Loche M., Griscelli C., Mach B. A trans-acting class II regulatory gene unlinked to the MHC controls expression of HLA class II genes. Nature. 1985 Nov 21;318(6043):291–293. doi: 10.1038/318291a0. [DOI] [PubMed] [Google Scholar]
- van Mourik P., Zeijlemaker W. P. Improved hybridoma technology: spleen cell separation and soluble growth factors. Methods Enzymol. 1986;121:174–182. doi: 10.1016/0076-6879(86)21016-2. [DOI] [PubMed] [Google Scholar]