Abstract
Human T lymphocytes can be activated through either the antigen/MHC receptor complex T3-Ti (CD3-Ti) or the T11 (CD2) molecule to proliferate via an IL-2 dependent mechanism. To investigate the relationship of these pathways to one another, we generated and characterized Jurkat mutants which selectively express either surface CD3-Ti or CD2. Here we show that CD3-Ti- mutants fail to be stimulated by either pathway to increase phosphoinositide turnover, mobilize calcium or induce the IL-2 gene. The activation capacity of these mutants via CD2 as well as CD3-Ti can be restored following reconstitution of surface CD3-Ti expression upon appropriate DNA transfer (e.g. Ti beta subunit cDNA into Ti beta- Jurkat variants). Collectively, these results demonstrate that CD3-Ti and CD2 pathways are interdependent and that phosphoinositide turnover is linked to the CD3-Ti complex.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acuto O., Hussey R. E., Fitzgerald K. A., Protentis J. P., Meuer S. C., Schlossman S. F., Reinherz E. L. The human T cell receptor: appearance in ontogeny and biochemical relationship of alpha and beta subunits on IL-2 dependent clones and T cell tumors. Cell. 1983 Oct;34(3):717–726. doi: 10.1016/0092-8674(83)90528-7. [DOI] [PubMed] [Google Scholar]
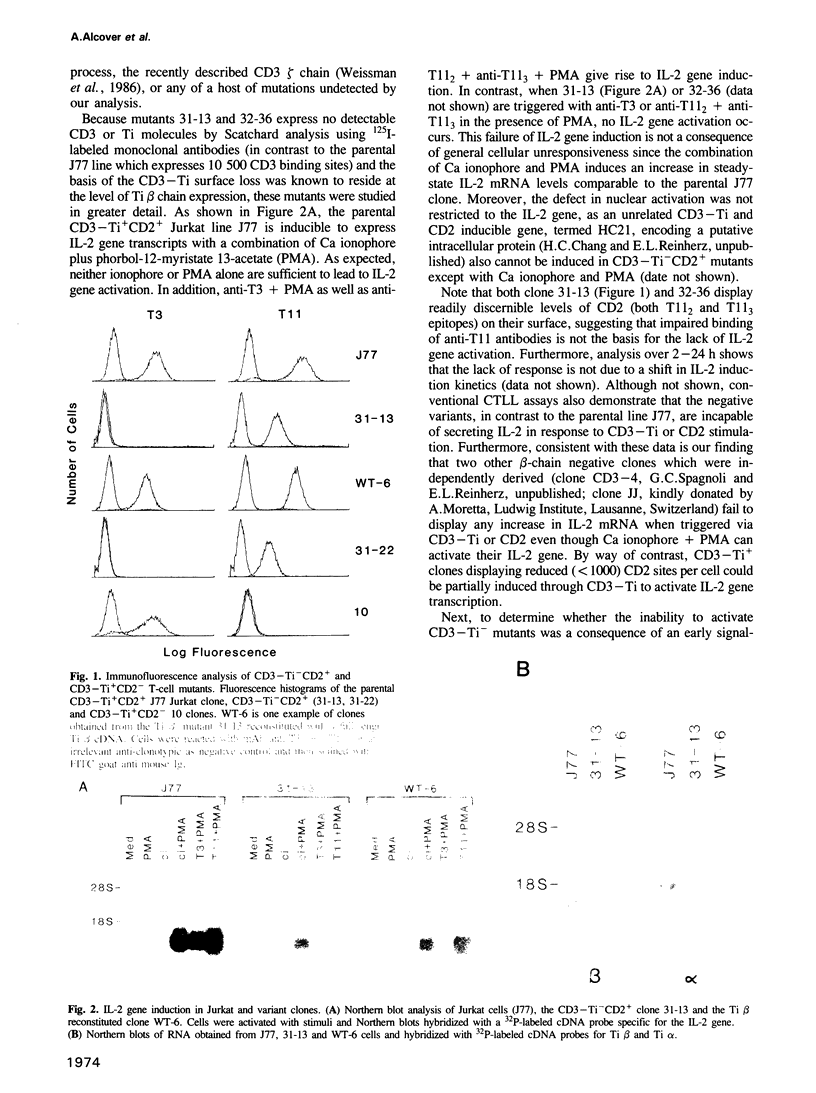
- Alcover A., Ramarli D., Richardson N. E., Chang H. C., Reinherz E. L. Functional and molecular aspects of human T lymphocyte activation via T3-Ti and T11 pathways. Immunol Rev. 1987 Feb;95:5–36. doi: 10.1111/j.1600-065x.1987.tb00498.x. [DOI] [PubMed] [Google Scholar]
- Alcover A., Weiss M. J., Daley J. F., Reinherz E. L. The T11 glycoprotein is functionally linked to a calcium channel in precursor and mature T-lineage cells. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2614–2618. doi: 10.1073/pnas.83.8.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
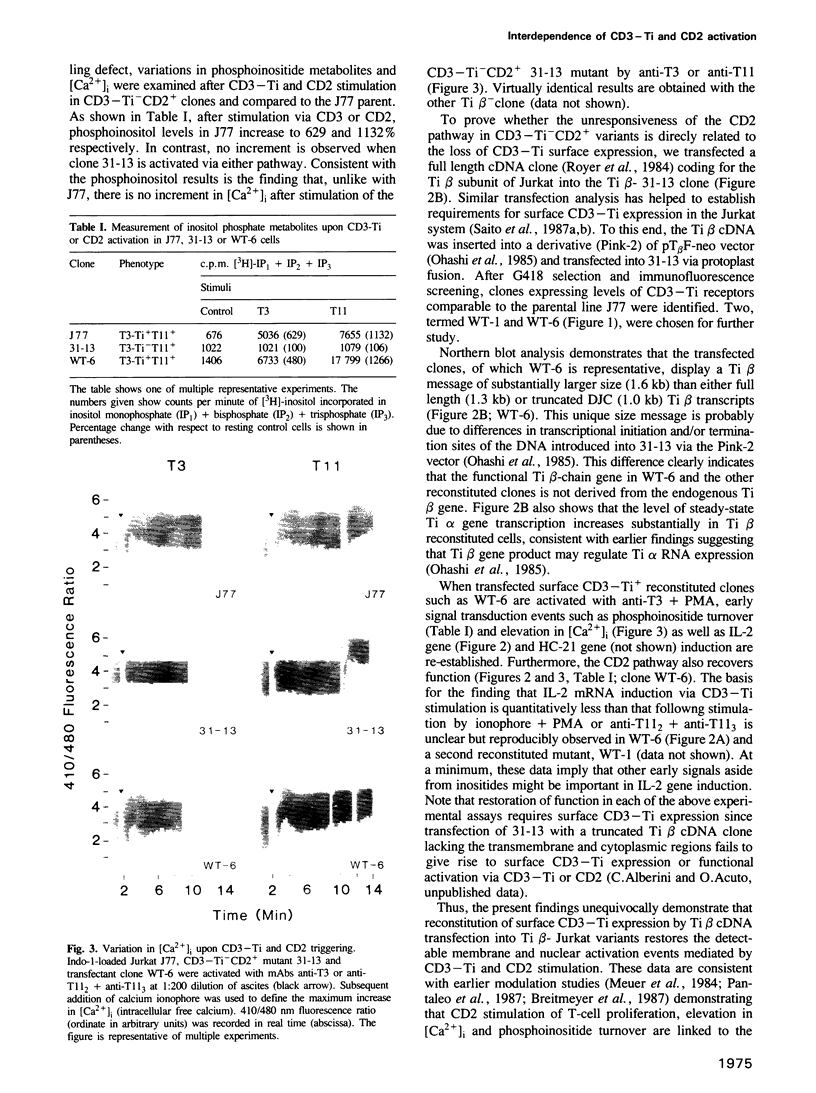
- Breitmeyer J. B., Daley J. F., Levine H. B., Schlossman S. F. The T11 (CD2) molecule is functionally linked to the T3/Ti T cell receptor in the majority of T cells. J Immunol. 1987 Nov 1;139(9):2899–2905. [PubMed] [Google Scholar]
- Fox D. A., Hussey R. E., Fitzgerald K. A., Bensussan A., Daley J. F., Schlossman S. F., Reinherz E. L. Activation of human thymocytes via the 50KD T11 sheep erythrocyte binding protein induces the expression of interleukin 2 receptors on both T3+ and T3- populations. J Immunol. 1985 Jan;134(1):330–335. [PubMed] [Google Scholar]
- Fox D. A., Schlossman S. F., Reinherz E. L. Regulation of the alternative pathway of T cell activation by anti-T3 monoclonal antibody. J Immunol. 1986 Mar 15;136(6):1945–1950. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gunter K. C., Germain R. N., Kroczek R. A., Saito T., Yokoyama W. M., Chan C., Weiss A., Shevach E. M. Thy-1-mediated T-cell activation requires co-expression of CD3/Ti complex. Nature. 1987 Apr 2;326(6112):505–507. doi: 10.1038/326505a0. [DOI] [PubMed] [Google Scholar]
- Hallcher L. M., Sherman W. R. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980 Nov 25;255(22):10896–10901. [PubMed] [Google Scholar]
- Imboden J. B., Stobo J. D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985 Mar 1;161(3):446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa U., Nishizuka Y. The role of protein kinase C in transmembrane signalling. Annu Rev Cell Biol. 1986;2:149–178. doi: 10.1146/annurev.cb.02.110186.001053. [DOI] [PubMed] [Google Scholar]
- Kroczek R. A., Gunter K. C., Germain R. N., Shevach E. M. Thy-1 functions as a signal transduction molecule in T lymphocytes and transfected B lymphocytes. Nature. 1986 Jul 10;322(6075):181–184. doi: 10.1038/322181a0. [DOI] [PubMed] [Google Scholar]
- Kuno M., Gardner P. Ion channels activated by inositol 1,4,5-trisphosphate in plasma membrane of human T-lymphocytes. Nature. 1987 Mar 19;326(6110):301–304. doi: 10.1038/326301a0. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Hodgdon J. C., Hussey R. E., Protentis J. P., Schlossman S. F., Reinherz E. L. Antigen-like effects of monoclonal antibodies directed at receptors on human T cell clones. J Exp Med. 1983 Sep 1;158(3):988–993. doi: 10.1084/jem.158.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Moretta A., Poggi A., Olive D., Bottino C., Fortis C., Pantaleo G., Moretta L. Selection and characterization of T-cell variants lacking molecules involved in T-cell activation (T3 T-cell receptor, T44, and T11): analysis of the functional relationship among different pathways of activation. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1654–1658. doi: 10.1073/pnas.84.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi A., Hawley R. G., Shulman M. J., Hozumi N. Transfer of a cloned immunoglobulin light-chain gene to mutant hybridoma cells restores specific antibody production. Nature. 1983 Mar 24;302(5906):340–342. doi: 10.1038/302340a0. [DOI] [PubMed] [Google Scholar]
- Ohashi P. S., Mak T. W., Van den Elsen P., Yanagi Y., Yoshikai Y., Calman A. F., Terhorst C., Stobo J. D., Weiss A. Reconstitution of an active surface T3/T-cell antigen receptor by DNA transfer. Nature. 1985 Aug 15;316(6029):606–609. doi: 10.1038/316606a0. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Morrison S. L., Herzenberg L. A., Berg P. Immunoglobulin gene expression in transformed lymphoid cells. Proc Natl Acad Sci U S A. 1983 Feb;80(3):825–829. doi: 10.1073/pnas.80.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G., Olive D., Poggi A., Pozzan T., Moretta L., Moretta A. Antibody-induced modulation of the CD3/T cell receptor complex causes T cell refractoriness by inhibiting the early metabolic steps involved in T cell activation. J Exp Med. 1987 Aug 1;166(2):619–624. doi: 10.1084/jem.166.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Meuer S., Fitzgerald K. A., Hussey R. E., Levine H., Schlossman S. F. Antigen recognition by human T lymphocytes is linked to surface expression of the T3 molecular complex. Cell. 1982 Oct;30(3):735–743. doi: 10.1016/0092-8674(82)90278-1. [DOI] [PubMed] [Google Scholar]
- Royer H. D., Acuto O., Fabbi M., Tizard R., Ramachandran K., Smart J. E., Reinherz E. L. Genes encoding the Ti beta subunit of the antigen/MHC receptor undergo rearrangement during intrathymic ontogeny prior to surface T3-Ti expression. Cell. 1984 Dec;39(2 Pt 1):261–266. doi: 10.1016/0092-8674(84)90003-5. [DOI] [PubMed] [Google Scholar]
- Royer H. D., Ramarli D., Acuto O., Campen T. J., Reinherz E. L. Genes encoding the T-cell receptor alpha and beta subunits are transcribed in an ordered manner during intrathymic ontogeny. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5510–5514. doi: 10.1073/pnas.82.16.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Weiss A., Gunter K. C., Shevach E. M., Germain R. N. Cell surface T3 expression requires the presence of both alpha- and beta-chains of the T cell receptor. J Immunol. 1987 Jul 15;139(2):625–628. [PubMed] [Google Scholar]
- Saito T., Weiss A., Miller J., Norcross M. A., Germain R. N. Specific antigen-Ia activation of transfected human T cells expressing murine Ti alpha beta-human T3 receptor complexes. Nature. 1987 Jan 8;325(7000):125–130. doi: 10.1038/325125a0. [DOI] [PubMed] [Google Scholar]
- Sayre P. H., Chang H. C., Hussey R. E., Brown N. R., Richardson N. E., Spagnoli G., Clayton L. K., Reinherz E. L. Molecular cloning and expression of T11 cDNAs reveal a receptor-like structure on human T lymphocytes. Proc Natl Acad Sci U S A. 1987 May;84(9):2941–2945. doi: 10.1073/pnas.84.9.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt-Verhulst A. M., Guimezanes A., Boyer C., Poenie M., Tsien R., Buferne M., Hua C., Leserman L. Pleiotropic loss of activation pathways in a T-cell receptor alpha-chain deletion variant of a cytolytic T-cell clone. Nature. 1987 Feb 12;325(6105):628–631. doi: 10.1038/325628a0. [DOI] [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell W. A., Brown M. H., Dunne J., Owen M. J., Crumpton M. J. Molecular cloning of the human T-lymphocyte surface CD2 (T11) antigen. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8718–8722. doi: 10.1073/pnas.83.22.8718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp M. A., Reinherz E. L. Differential expression of nuclear proto-oncogenes in T cells triggered with mitogenic and nonmitogenic T3 and T11 activation signals. J Immunol. 1987 Oct 1;139(7):2143–2148. [PubMed] [Google Scholar]
- Siliciano R. F., Pratt J. C., Schmidt R. E., Ritz J., Reinherz E. L. Activation of cytolytic T lymphocyte and natural killer cell function through the T11 sheep erythrocyte binding protein. Nature. 1985 Oct 3;317(6036):428–430. doi: 10.1038/317428a0. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Cantrell D. A. Interleukin 2 regulates its own receptors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):864–868. doi: 10.1073/pnas.82.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T., Matsui H., Fujita T., Takaoka C., Kashima N., Yoshimoto R., Hamuro J. Structure and expression of a cloned cDNA for human interleukin-2. Nature. 1983 Mar 24;302(5906):305–310. doi: 10.1038/302305a0. [DOI] [PubMed] [Google Scholar]
- Truneh A., Albert F., Golstein P., Schmitt-Verhulst A. M. Early steps of lymphocyte activation bypassed by synergy between calcium ionophores and phorbol ester. Nature. 1985 Jan 24;313(6000):318–320. doi: 10.1038/313318a0. [DOI] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Hardy K., Manger B., Terhorst C., Stobo J. The role of the T3/antigen receptor complex in T-cell activation. Annu Rev Immunol. 1986;4:593–619. doi: 10.1146/annurev.iy.04.040186.003113. [DOI] [PubMed] [Google Scholar]
- Weissman A. M., Samelson L. E., Klausner R. D. A new subunit of the human T-cell antigen receptor complex. Nature. 1986 Dec 4;324(6096):480–482. doi: 10.1038/324480a0. [DOI] [PubMed] [Google Scholar]
- Yang S. Y., Chouaib S., Dupont B. A common pathway for T lymphocyte activation involving both the CD3-Ti complex and CD2 sheep erythrocyte receptor determinants. J Immunol. 1986 Aug 15;137(4):1097–1100. [PubMed] [Google Scholar]
- Yoshikai Y., Anatoniou D., Clark S. P., Yanagi Y., Sangster R., Van den Elsen P., Terhorst C., Mak T. W. Sequence and expression of transcripts of the human T-cell receptor beta-chain genes. Nature. 1984 Dec 6;312(5994):521–524. doi: 10.1038/312521a0. [DOI] [PubMed] [Google Scholar]